Abstract
Bag3, a nucleotide exchange factor of the heat shock protein Hsp70, has been implicated in cell signaling. Here we report that Bag3 interacts with the SH3 domain of Src, thereby mediating the effects of Hsp70 on Src signaling. Using several complementary approaches, we established that the Hsp70-Bag3 module is a broad-acting regulator of cancer cell signaling, including by modulating the activity of the transcription factors NF-kB, FoxM1 and Hif1α, the translation regulator HuR and the cell cycle regulators p21 and survivin. We also identified a small molecule inhibitor, YM-1, that disrupts Hsp70-Bag3 interaction. YM-1 mirrored the effects of Hsp70 depletion on these signaling pathways, and in vivo administration of this drug was sufficient to suppress tumor growth in mice. Overall, our results defined Bag3 as a critical factor in Hsp70-modulated signaling and offered a preclinical proof-of-concept that the Hsp70-Bag3 complex may offer an appealing anti-cancer target.
Introduction
The major heat shock protein Hsp70 (HspA1A) has also been implicated in cancer. Hsp70 is a stress-inducible molecular chaperone that participates in protection of the proteome from aggregation and promotes refolding and degradation of damaged polypeptides (1, 2). The levels of this protein are highly elevated in a variety of cancers, and correlate with tumor grade, metastasis, chemotherapy resistance, and subsequently, poor prognosis, suggesting that Hsp70 plays a specific role in cancer (3, 4). Importantly, while Hsp70 is critical for survival of cancer cells, it is dispensable for viability of non-transformed cells (5). Accordingly, Hsp70 knockout mice are healthy (6), and the inactivation of Hsp70 manifests serious problems only following a challenge with stress (6, 7), or acute inflammation (8).
To leverage these observations into therapeutics, several groups have explored the mechanisms by which cancer cells become addicted to Hsp70. On their path to tumorigenesis cells must escape apoptosis (9), anoikis (10) and the harsh conditions of the tumor microenvironment (11). Although elevated levels of Hsp70 have strong anti-apoptotic activity (12, 13), it is not yet clear whether this function is important for its role in cancer.
Unlike apoptosis, the finding that Hsp70 suppresses cellular senescence has established a clear connection to cancer. Senescence is defined as irreversible cell growth arrest that is associated with assorted cellular changes in morphology and gene expression (14, 15). Certain oncogenes may trigger senescence, oncogene-induced senescence (OIS), which provides defense against cancer. We have demonstrated that depletion of Hsp70 activates senescence in cells transformed by the oncogenes Her2, PIK3CA and RAS, but that it has minimal effects in normal cells (16, 17). Accordingly, Hsp70 knockout mice did not develop breast cancer upon expression of Her2 oncogene (5). Therefore, Hsp70 could be critical for the escape of transformed cells from OIS, a property that defines its role in tumor initiation.
These properties of Hsp70 suggest that it could be used as a drug target. There have been several attempts to develop inhibitors of Hsp70 for cancer treatment, including inhibitors of substrate binding, e.g. aptamers (18) or pifithrin μ (19–22), or compounds that interact with the ATPase domain, such as VER155008 (23), MAL3–101 (24), and YK-5 (25). However, development of these inhibitors has not reached the clinical trial stage.
In order to tailor inhibitor development to cancer, specific mechanisms underlying the effects of Hsp70 on tumor development should be defined. In search for these mechanisms, we focused on a nucleotide exchange factor Bag3. This protein contains the Bag domain that binds to a motif in the ATPase domain of Hsp70 proteins (26), as well as PxxP and WW domains (26), which may connect it to SH3 domains and PPxY motifs of signaling proteins (27, 28). Bag3 has been implicated in macroautophagy and aggresome formation (29–31). In both processes Bag3 was proposed to link complexes of Hsp70 with protein aggregates to the autophagic and aggresome machineries. While it is unknown how Bag3 interacts with the latter, in the process of autophagy Bag3 uses its WW domain to interact with one of the organizers of the autophagic vacuole SYNPO2 (30). In addition to its function in recruitment of the Hsp70-bound cargo, it was demonstrated that Bag3 can interact with signaling factors via its PxxP motif (e.g. with PLCγ) (32) or WW domain (e.g. with components of Hippo pathway LATS1 and AMOT1) (30). Since Bag3 has been implicated in cancer cell motility and invasion (33–35), there is a possibility that these effects result from regulation of signaling pathways by Bag3. Interestingly, in the previous studies the effects of Bag3 on signaling were not connected to effects of Hsp70 on these pathways. Here, we hypothesized that Bag3 could serve as a scaffold with the potential for integrating Hsp70 levels and multiple cancer signaling pathways, and thus mediate effects of Hsp70 on cancer.
Materials and Methods
Cell cultures
MCF10A, HEK293, MCF-7, HCT116, B16-F10, and HeLa were from ATCC and initially frozen for storage upon receipt. ATCC authenticates its cell lines using short tandem repeat analysis. Cells were resuscitated and passaged for less than 6 months during use in experiments. MCF10A cells expressing PIK3CA or Her2 were as described before (5, 16, 17). MCF10A cells were cultivated in Dulbecco's modified Eagle's medium-F12 medium supplemented with 5% horse serum, hydrocortisone (500 ng/ml), insulin (10 µg/ml), cholera toxin (100 ng/ml), and epidermal growth factor (20 ng/ml). HEK293T cells were maintained in DMEM supplemented with 10% heat inactivated fetal bovine serum (FBS). B16-F10, MCF7, and HeLa cells were cultivated in Dulbecco's modified Eagle's medium supplemented with 10% FBS. HCT116 cells were cultured in McCoy’s 5A medium with 10% FBS. All cultures contained 1% penicillin/streptomycin.
Mice and Xenografts
For xenografts, 500,000 MCF7 cells were mixed at a 1:1 ratio with Matrigel, and 1 million cells were injected subcutaneously of 6-week-old female NCR nude mice (Taconic). For the B16-F10 melanoma allografts, 200,000 cells were injected subcutaneously in hind legs of 6-week old C57BL6 female mice. Tumor growth was monitored every other day using caliper and calculated according to the formula L × W2 × π/6, where L is length and W is width.
Compounds and Antibodies
MG132 was purchased from Biomol (Farmingdale, NY) and puromycin from Sigma. YM-1 was developed and supplied by Dr. Gestwicki. The following antibodies were used: anti-Hsp70 was from Stressgen, anti-Bag3 was a gift from Dr. Takayama, anti-p21 and anti-Hif1α were from BD PharMingen, anti-β-actin, anti-Src, anti-p-Src (Tyr416), p-NFkB (Ser536), anti-IκBα, p-paxillin (Tyr118), p-p130Cas (Tyr165, Tyr249, Tyr410), and p130Cas were from Cell Signaling, anti-FoxM1,,anti-HuR, anti-survivin, and anti-Src were from Santa Cruz.
Immunoblotting
Immunoblotting and cell lysate preparations were done according to (5).
Results
Bag3 mediates effects of Hsp70 on Src activity
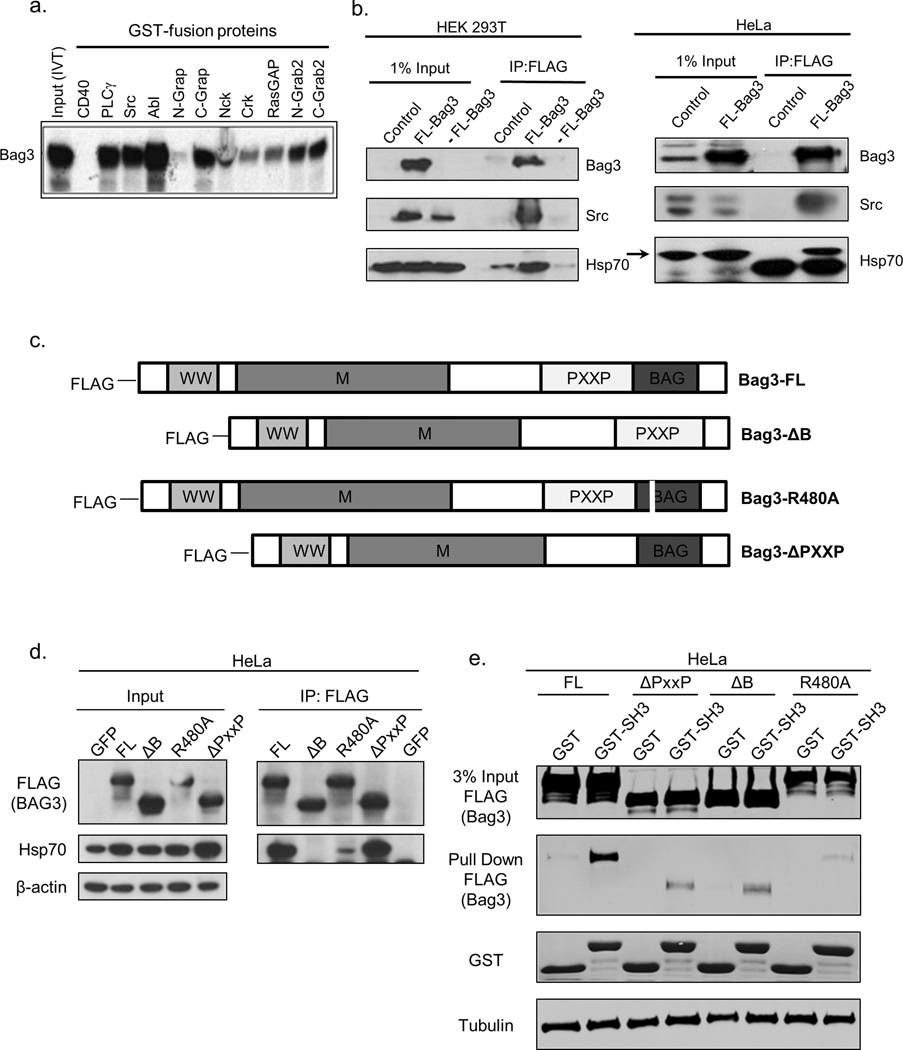
In order to uncover the role of Bag3 and its association with Hsp70 in cancer, we sought to identify SH3 domain-containing signaling factors that can interact with the PxxP motif of Bag3. Accordingly, we performed pull down experiments using a series of recombinant SH3 domains of various proteins fused to GST. GST-CD40 lacking the SH3 domain was used as a negative control. Lysates with in-vitro-translated S35-labeled Bag3 were passed over beads carrying various SH3-GST fusion proteins, and bound Bag3 was detected. In the pull down experiment, Bag3 interacted with SH3 domains of PLCγ, Src, c-Abl, c-Grap, Nck, Crk, RasGAP, n-Grab2, and c-Grab2 (Fig.1a). These proteins are components of cancer-related pathways, highlighting the potential of Bag3 as a master regulator of cancer signaling. Though any of these signaling proteins may contribute to cancer development, to explore mechanistically how Bag3 regulates signaling we chose Src as model.
Fig. 1. Bag3 interacts with Src and this interaction is regulated by Hsp70.
(a) Bag3 interaction with SH3 domains of various proteins, see Results. (b) Left panel: Co-immunoprecipitation of Bag3 with full length Src in 293T cells. Relatively low abundance of Src in this cell line necessitated overexpression of Src for IP as well as FLAG tagged Bag3. Cells were collected and lysed 24 hours after transfection. Negative controls included GFP only (control) or Src only (-FL-Bag3). Middle lane FL-Bag3 had expression of both FLAG-tagged Bag3 and Src. IP was done using FLAG antibody and blot was processed trueblot secondary antibody. Right panel: Co-immunoprecipitation of full length Src with FLAG-tagged Bag3 in HeLa cells. Cells were transfected with either FL-Bag3 or GFP (control), and immunoprecipitated using FLAG antibody. Association with Hsp70 is also shown (upper band indicated by arrow); lower band is the IgG heavy chain. (c) Schematic of Bag3 constructs used in this study, including wild type (FL), Bag domain deletion (ΔB), Bag domain point mutation (R480A), and PxxP domain deletion (ΔPxxP). All constructs contain an N-terminal FLAG tag. Domains are depicted including Bag domain (Hsp70 binding domain), PxxP motif, M domain (small heat shock protein binding domain), and WW domain. (d) Co-immunoprecipitation of Hsp70 with FLAG-tagged Bag3 constructs. HeLa cells were transfected with GFP (control) or indicated Bag3 constructs, and immuniprecipitated with anti-FLAG antibody. Hsp70 is the upper band indicated by the arrow. The large lower band in the Hsp70 IP blot is the IgG heavy chain. (e) GST pull down of Bag3 constructs with SH3 domain of Src. HeLa cells were transfected with indicated constructs, and after 24 hours, cell lysates were prepared and incubated with the purified GST-SH3 domain (from Src) bound to agarose beads or GST beads alone for 4 hours. Bag3 associated with the Src SH3 domain was analyzed via immunoblot with anti-FLAG antibody.
First we confirmed that the full length Src protein interacts with Bag3 in cells. FLAG-labeled Bag3 was co-expressed with Src in 293T cells, precipitated with anti-FLAG antibody, and Src was probed on the blot with anti-Src antibody. Fig.1b (left panel) shows that Src effectively associated with Bag3. A similar experiment was done in HeLa cells to detect association of Bag3 with endogenous Src (Fig.1b, right panel).
Since Bag3 is a co-factor of Hsp70, we further assessed how the ability to interact with Hsp70 influences association of Bag3 with the SH3 domain of Src. Various constructs of Bag3 were expressed, cell lysates were incubated with beads carrying recombinant SH3 domain of Src, and bound Bag3 molecules were detected by immunoblotting. Besides a full length Bag3 construct, we tested deletions of the PxxP and Bag domains (referred to as FL, ΔPxxP and ΔB, respectively), as well as an R480A mutation, which impairs Bag3 binding to Hsp70 (39) (Fig. 1c). The inability of the ΔB and R480A constructs to bind to Hsp70 was confirmed in the pull down experiment (Fig. 1d). The full length Bag3 effectively interacted with the SH3 domain of Src, and binding was strongly reduced by deletion of the PxxP domain, as expected (Fig.1e). Importantly, both ΔB and R480A constructs also demonstrated reduced binding to the Src-SH3 domain compared to the full length Bag3, and the effects of these mutations were comparable to the effect of the PxxP deletion (Fig.1e). Therefore, interaction with Hsp70 appears to be critical for binding of the PxxP domain of Bag3 with the SH3 domain of Src.
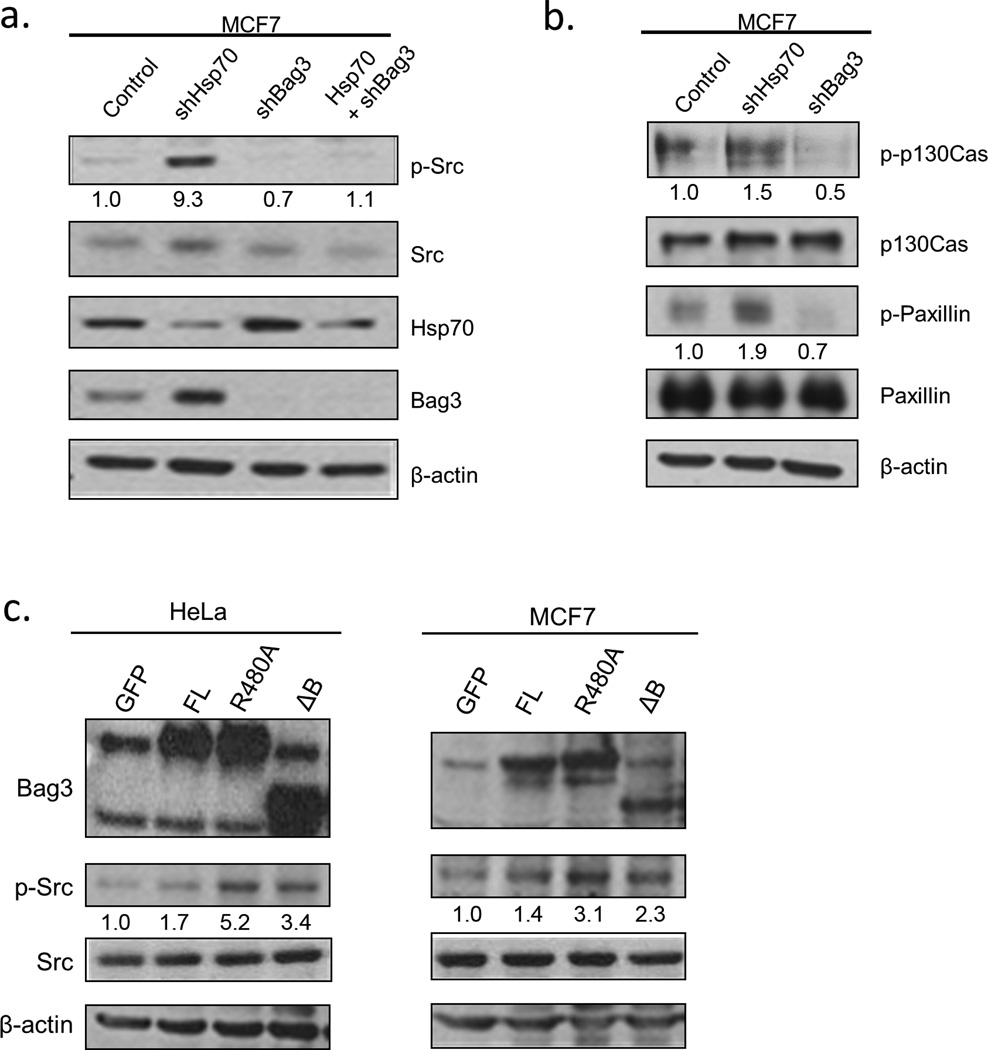
These findings, that the interaction of Bag3 with Src requires the association with Hsp70, suggest that Bag3 may serve as a scaffold, mediating the effects of Hsp70 on signaling pathways, including Src. In line with this hypothesis, knockdown of Hsp70 led to significant activation of Src, as judged by its Y416 phosphorylation (Fig.2a). Furthermore, depletion of Hsp70 increased phosphorylation of downstream Src targets paxillin and p130Cas (Fig. 2b), confirming Src activation. Interestingly, knockdown of Bag3 did not activate Src, however it prevented activation of Src upon depletion of Hsp70 (Fig. 2a). Therefore, (i) Hsp70 can regulate Src activity, and (ii) this regulation requires Bag3.
Fig. 2. Hsp70 affects Src activation in cells.
a) Hsp70 depletion activates Src in a Bag3-dependent manner. MCF7 cells were infected with control, shHsp70, shBag3, or dual shHsp70 and shBag3 retroviruses and immunobloted for activated Src (p-Y416). Values under p-Src blot represent relative phosphorylation compared to control. Levels of p-Src and total Src were normalized to β-actin and the ratio of normalized p-Src/Src are reported. (b) Effects of shHsp70 and shBag3 on the downstream Src targets. Cells were treated for 30 minutes with 1mmol/L sodium orthovanadate prior to lysis. Values under p-Paxillin and p-130Cas represent relative phosphorylation compared to control determined in a similar manner as p-Src. (c) Expression of Bag3 mutants that cannot bind Hsp70 leads to activation of Src. Bag3 constructs or GFP were transfected into HeLa or MCF7 cells. Cells were lysed after 24 hours and immunoblotted for activated Src (p-Y416). Representative blots of two experiments for each cell line are shown.
To further investigate the role of Hsp70-Bag3 module in Src regulation, we tested effects on Src of Bag3 mutants that are unable to bind Hsp70. While overexpression of the full length Bag3 did not affect Src activity, overexpression of either the ΔB or R480A constructs significantly activated endogenous Src in either HeLa or MCF7 cells (Fig.2c), further supporting the model.
Hsp70-Bag3 module affects NF-kB
Upon establishing the general mechanistic principle that Hsp70 and Bag3 can cooperate in direct regulation of signaling factors, we further sought to identify a spectrum of cancer-controlling signaling factors regulated by the Hsp70-Bag3 module. We used a combination of two general criteria for such regulation: the signaling factor should be affected (i) by Hsp70 and Bag3 depletion, and (ii) by overexpression of the ΔB or R480A Bag3 constructs but not of the normal Bag3.
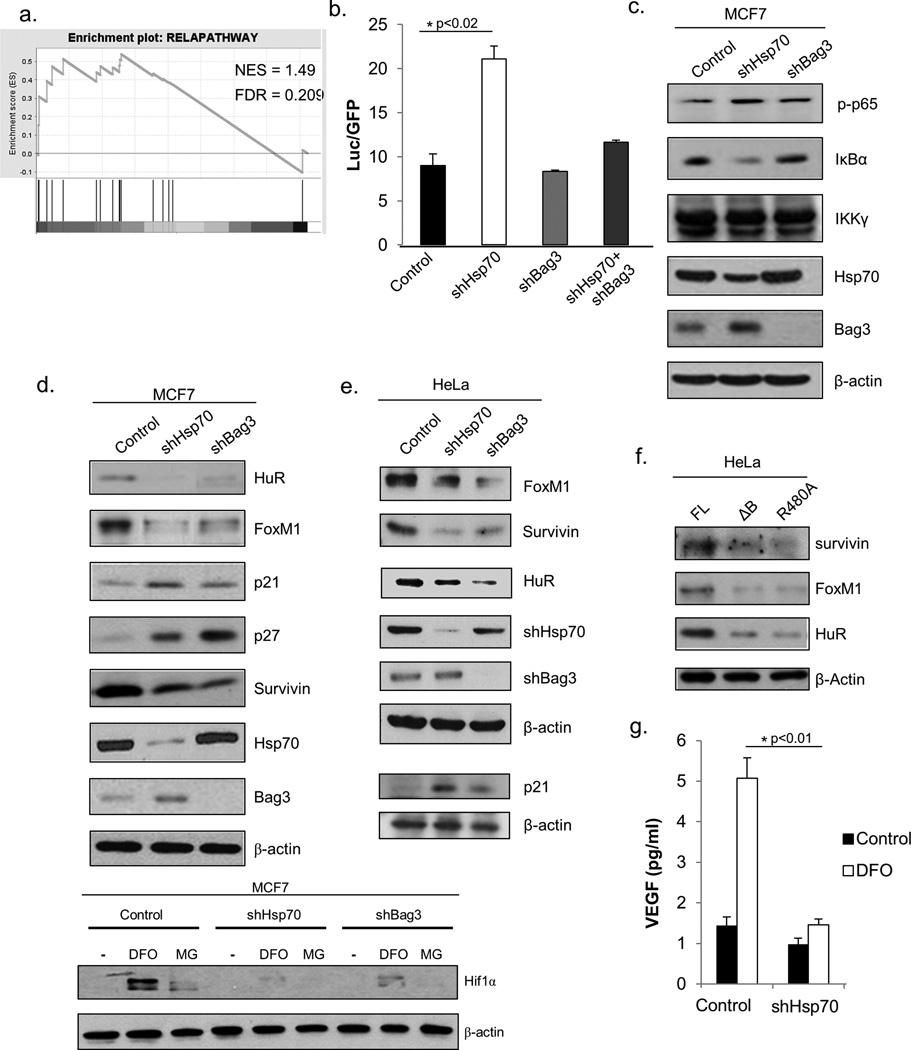
In this search, we utilized our prior data, where we used microarray analysis to compare gene expression in control and Hsp70-depleted MCF7 cells (Supplement Table S1), followed by Gene Set Enrichment Analysis (GSEA). The GSEA-based signal pathway analysis software was developed by the Broad Institute. According to their manual, pathways that show a false discovery rate (FDR) less than 0.25 are significant enough to warrant validation. Our analysis indicated that a large number of pathways were predicted to be up- or down-regulated by Hsp70. For example, the proteasome and ubiquitin pathways were strongly upregulated and received the highest scores (see Supplemental Fig. S1a). Another pathway that received a high score was NF-kB (Fig. 3a) and related pathways, such as the IL-1R, or TNF pathways (Supplement Fig. S1b). Indeed, using a luciferase reporter under the control of NF-kB, we confirmed that NF-kB activity was increased in Hsp70-depleted cells, (Fig.3b). Also, levels of IκB were reduced while phosphorylation of the p65 subunit of NF-kB was elevated (Fig.3c).
Fig. 3. Hsp70 depletion affects multiple signaling pathways.
(a) Hsp70 depletion activates NF-kB pathway. GSEA RelA pathway enrichment plot. NES-Normalized Enrichment Score. FDR-False Discover Rate. (b) Effect of Hsp70 on NF-kB depends on Bag3. MCF7 cells were infected with Rel-A luciferase lentiviral reporter, followed by infection with shBag3, shHsp70, or empty vector control retroviruses, as indicated. Levels of luciferase activity were measured and normalized to GFP. Results are shown as means and ±SEM of triplicate experiments. Dual depletion of Hsp70 and Bag3 results are mean and ±SEM of duplicate experiments. (c) Effect of Hsp70 or Bag3 depletion on NF-κB pathway members in MCF7 cells analyzed by immunoblotting. P-p65 is p-NF-κBp65-Ser536. (d) Effect of Hsp70 and Bag3 on cancer related pathways in MCF7 cells. Portions of cells were treated with DFO (100µmol/L, 4h) or MG132 (5µmol/L, 4h). Blots are representative of triplicate experiments. (e) Effect of shHsp70 and shBag3 on pathways in HeLa cells day 6 after infection with retroviruses. Effect on p21 is seen at day 7.Blots are examples from triplicate experiments. (f) Expression of Bag3 mutants that cannot bind Hsp70 leads to inactivation of survivin, FoxM1, and Hur. Bag3 constructs were transfected into HeLa cells, and after 48 hours, cells were lysed and immunoblotted for indicated proteins. Blots are representative of triplicate experiments. (g) Levels of VEGF were determined in supernatant as described (39).
Here, we tested if Bag3 is involved in regulation of NF-kB by Hsp70. Accordingly, Bag3 was depleted in MCF7 cells using shRNA, and activity of NF-kB was assessed using the luciferase reporter. Unlike Hsp70, depletion of Bag3 had little apparent effect on the activation of NF-kB (Fig. 3b), the levels of phosphorylated NF-kB p65 subunit (p-S536) and of IκB (Fig. 3c). On the other hand, in cells with Bag3 depletion, the ability of the knockdown of Hsp70 to activate NF-kB was strongly reduced (Fig. 3b). Therefore, as with Src regulation, the Hsp70-Bag3 module is critical for regulation of NF-kB.
Notably, it was previously reported that that Bag3 regulates NF-kB by controlling IKKγ stability (40), but we were unable to detect any change in IKKγ levels following Bag3 depletion (Fig. 3c). On the other hand, recent proteomics study (41) indicated that Bag3 can directly interact with IkB, which is consistent with our finding that the Hsp70-Bag3 module affects IkB levels (Fig. 3b and 3c).
Effects of the Hsp70-Bag3 module on other cancer-related signaling factors
Since the unbiased approach with GSEA produced only limited information about signaling network regulated by the Hsp70-Bag3 module, in further search for factors regulated by this module, we utilized a more biased approach based on hints obtained in our prior findings. Accordingly, we decided to test if the regulation of previously identified Hsp70-controlled signaling factors also involved Bag3. In studies of the oncogene-induced senescence and tumor initiation, we reported that depletion of Hsp70 affects two important signaling proteins that control growth of cancer cells, the cell cycle inhibitor p21, and the regulator of mitosis, survivin (5, 17). To test if Bag3 is also involved in control of these factors, we investigated whether depletion of Bag3 in MCF7 using shRNA affects levels of p21 and survivin. As with knockdown of Hsp70, Bag3 targeting resulted in decreased levels of survivin, and accumulation of p21 (Fig.3d, upper panel). Similar effects of Hsp70 and Bag3 depletion were seen in HeLa cells (Fig. 3e) and the colorectal carcinoma cell line, HCT116 (Supplemental Figure 2), demonstrating that these effects are related to multiple cancer types. Bag3 depletion paralleled the effects of Hsp70 knockdown in all three cell lines, further supporting the idea that Hsp70 and Bag3 are co-regulators of these pathways. In line with this suggestion, overexpression of the ΔB and R480A Bag3 mutants, but not normal Bag3, in HeLa cells mimicked effects of Hsp70 depletion on survivin (Fig.3f). In this experiment we were unable to assess effects on Bag3 mutants on p21, since levels of this protein in HeLa cells were undetectable.
Since both p21 and survivin have a common transcriptional regulator, FoxM1, we tested whether the Hsp70-Bag3 module can control this cancer-regulating factor. Indeed, depletion of either Hsp70 or Bag3 led to strong downregulation of FoxM1 (Fig.3d, upper panel) and upregulation of its distinct downstream target, the cell cycle inhibitor p27 (42). Furthermore, while the levels of FoxM1 were unaltered in normal Bag3 and empty vector control conditions, the ΔB or R480A Bag3 mutants caused strong down-regulation of FoxM1 (Fig. 3f), indicating the importance of Hsp70-Bag3 interaction in expression of this major transcription factor. Since FoxM1 plays multiple roles in OIS, metastasis and invasion (43), these findings further support a broad role for the Hsp70-Bag3 module in tumorigenesis.
Based on our prior study that connected the stress response to the translation factor HuR, another major player in cancer development (36, 44), we also suggested that Hsp70-Bag3 module may affect this pathway. Depletion of either Hsp70 or Bag3 led to significant down-regulation of HuR (Fig.3d, upper panel). Furthermore, while the levels of HuR were unaltered in normal Bag3 and empty vector control conditions, the ΔB or R480A Bag3 mutants caused strong down-regulation of HuR, indicating that interaction between Hsp70 and Bag3 is important for regulation of this major cancer regulator (Fig.3f). Additionally, we considered whether one of HuR’s translational targets, Hif1α, which is yet another major transcription factor involved in cancer, might also be down-regulated. Since levels of Hif1α depend on the availability of oxygen, control, Hsp70-depleted and Bag3-depleted cells were treated with either hypoxia mimetic deferoxamine (DFO), or proteasomal inhibitor MG-132, both of which stabilize Hif1α. Hsp70-depleted or Bag3-depleted MCF7 cells had significantly reduced levels of Hif1α compared to control cells (Fig.3d, lower panel). Furthermore, Hsp70 depletion strongly reduced expression of a major Hif1 downstream target gene VEGF (Fig. 3g). Therefore, it appears that the entire signaling network that controls multiple processes in cancer development, including growth, invasion and angiogenesis, involving HuR, Hif1α and VEGF is regulated by Bag3 and Hsp70. It should be noted that only with Src did we identify a direct connection to the Hsp70-Bag3 module biochemically. With other pathways, genetic data do not establish the direct interaction, and therefore these interactions could be mediated by additional factors that can bind to WW or PxxP motifs on Bag3.
In summary, the Hsp70-Bag3 module is involved in regulating a complex network of pathways, including Src, NF-kB, FoxM1, survivin, p21, p27, HuR, Hif1α and most likely other pathways, all of which play a role in cancer development. When individually considered, these pathways could have cancer-promoting or cancer-repressing effects. Nevertheless, we know that in breast cancer models, depletion of Hsp70 suppresses both tumor initiation and progression (5), indicating that the net outcome of this complex Hsp70-Bag3-controlled signaling network to be pro-oncogenic.
Disruption of the Hsp70-Bag3 complex by a small molecule mimics effects of Hsp70 depletion on signaling and cancer
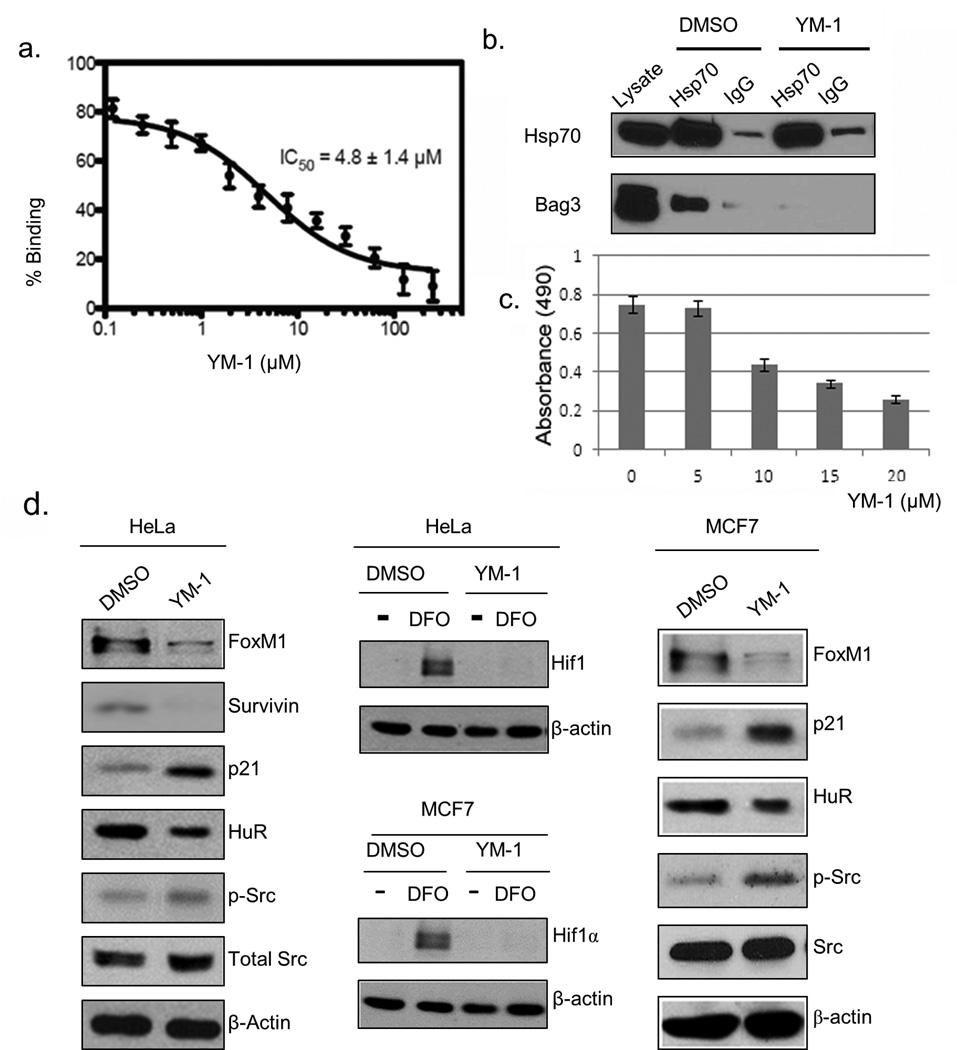
Here, we predicted that blocking interaction between Hsp70 and Bag3 could mimic depletion of Hsp70 and have anti-cancer effects. We focused on an inhibitor of Hsp70, termed YM-1 (45, 46). To test if YM-1 might disrupt the Hsp70-Bag3 interaction, we immobilized Hsp70 on beads and measured its binding to fluorescently-labeled Bag3 using a flow cytometry protein interaction assay (FCPIA). YM-1 strongly inhibited the interaction between Hsp70 and Bag3 (IC50 ~ 5 µmol/L; Fig.4a). This activity was consistent with the ability of YM-1 to bind a conserved, allosteric pocket in Hsp70, which traps the chaperone in an ADP-bound form that has weak affinity for Bag3 (45, 46). To test if YM-1 can disrupt the Hsp70-Bag3 interaction in cells, Hsp70 was immunoprecipitated from HeLa cells treated with YM-1 or a vehicle control, and immunoblotted for Bag3. YM-1 almost completely blocked binding of Hsp70 to Bag3 in cells (Fig.4b), indicating that YM-1 is a good chemical probe for addressing the importance of the Hsp70-Bag3 interaction in cancer signaling.
Fig. 4. YM-1 inhibits the Hsp70-Bag3 interaction and mimics effects of Hsp70 depletion on signaling.
(a) YM-1 disrupts the Hsp70-Bag3 interaction by FCPIA. Experiments were performed in triplicate, normalized and combined; the error bars represent the standard error of the mean. (b) YM-1(5µmol/L for 2 hours) disrupts Hsp70-Bag3 interaction in cells. Co-immunoprecipitation of Hsp70 and Bag3 from HeLa cell lysates showed that YM-1 inhibits formation of the Hsp70-Bag3 complex. (c) Effects of YM-1 on viability of MCF7 cells. Cells were incubated with indicated concentrations of YM-1 for 48 hours, and viability was assessed by the MTT assay. (d) YM-1 mimics effects of Hsp70 depletion on signaling pathways. Left panel: Hela cells were treated with YM-1 (5 µmol/L) for 48 hours, and cell lysates analyzed for levels of indicated components of signaling pathways by immunoblotting. Top middle panel: HeLa cells treated with YM-1 were also treated with DFO (100µmol/L) before lysis to visualize effect on Hif1. Lower middle panel-MCF7 cells were treated with YM-1 (10 µmol/L) in the presence of DFO (100µmol/L) and then lysed to evaluate levels of Hif1. Right panel: MCF7 cells treated with YM-1 (10 µmol/L, 48 h)
Next, MCF7 and HeLa cells were treated with YM-1 and the activities of signaling pathways were assessed. In order to determine the appropriate dosage, a range of concentrations were tested (Supplemental Figure 3). The concentrations of YM-1 used for testing effects on signaling pathways are described in the legend to Fig. 4, but represent the minimal dose necessary to see an effect in the appropriate cell line. As predicted, YM-1 diminished the FoxM1 and Hif1α pathways, while activating Src (Fig.4c), further supporting a role for the Hsp70-Bag3 interaction in cancer signaling. As a further test of this model, we examined the effects of the small molecule inhibitor myricetin, which inhibits Hsp70, through a different mechanism that involves the Hsp40 family of co-chaperones (47), and does not hinder Bag3 binding to Hsp70. Unlike YM-1, myricetin had no significant effects on the signaling pathways (Table 1), highlighting the specific role of the Hsp70-Bag3 interaction.
| shHsp70 | YM-1 | Myricetin | |
|---|---|---|---|
| FoxM1 | ↓ | ↓ | - |
| p21 | ↑ | ↑ | - |
| HuR | ↓ | ↓ | - |
| Hif1 | ↓ | ↓ | - |
| p-Src | ↑ | ↑ | - |
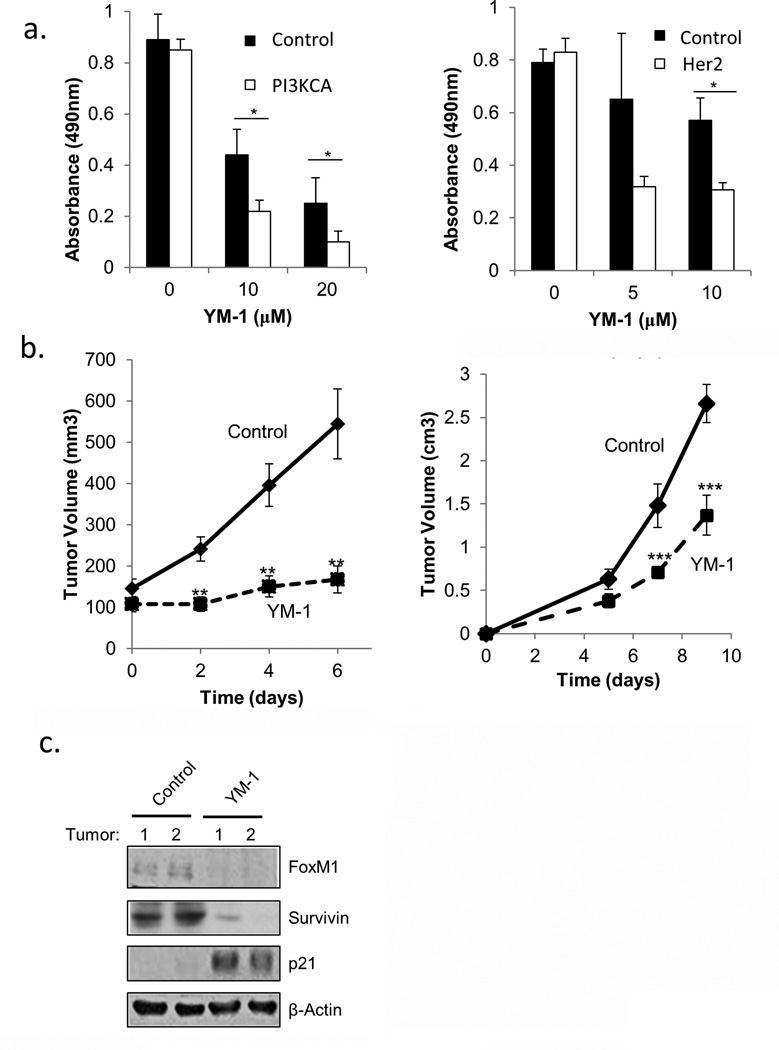
Since many of the Hsp70-Bag3-regulated pathways affect cell growth and survival, and depletion of Hsp70 is detrimental for cancer cells specifically due to oncogene signaling (5), we compared effects of YM-1 on viability of non-transformed MCF10A human breast epithelial cells and MCF10A derivatives transformed with either Her2 or PIK3CA oncogenes (Fig. 5a). Here, cells expressing oncogenes were more sensitive to YM-1 than untransformed MCF10A cells (Fig. 5a).
Fig. 5. YM-1 inhibits tumor growth.
(a) Oncogene-expressing cells are more sensitive to YM-1 than un-transformed cells. MTS assay showing effect of YM-1 on wild type (control) or transformed MCF10A cell viability. Means and ± SEM of triplicate experiments are shown. * p<0.05 (b) Top panel: Effect of YM-1 on MCF7 xenograft growth. Nude mice were subcutaneously injected bilaterally in hind flanks with MCF7 cells in matrigel. Once tumors were palpable mice were randomly selected and injected with saline (control: n=7 mice) or YM-1 (25mg/kg; n=7 mice) once every other day for three days. Plot shows tumor growth over time of control tumors (12 tumors/7 mice treated with saline; two tumors did not take) and tumors treated with YM-1 (14 tumors/7 mice treated with YM-1). Solid lines are control tumors. Dotted lines are YM-1 treated. Error bars designate ±SEM. ** p<0.001. Bottom panel: Effect of YM-1 on growth of B16-F10 melanoma. On day 7 after injection of melanoma cells mice were randomly selected and injected with saline (control: n=7 mice) or YM-1 (25mg/kg; n=8 mice) once every other day three times. Tumors were measured using calipers and volume was calculated. Plot shows tumor growth over time of control tumors and tumors treated with YM-1. Solid lines are control tumors. Dotted lines are YM-1 treated. Means±SEM are shown. *** p<0.01 by Student’s t-test.(c) Two control and two YM1-treated MCF7 tumors from above mice were taken after last injection and analyzed by immunoblot for effect on FoxM1 pathway. (d) Same as (c) but analysis was done by immunohistochemistry.
To test the effects of YM-1 in vivo, we used the xenograft model with MCF7 cells, which we previously showed lose the ability to develop tumors upon depletion of Hsp70 (5). MCF7 cells were transplanted subcutaneously into nude mice, and when tumors reached the palpable stage, animals were injected intraperitoneally with 25mg/kg YM-1 or saline. The injections were done three times every second day, and tumor growth was monitored by caliper. Fig.5b shows that administration of YM-1 dramatically inhibited tumor growth. There were no apparent effects of the drug on the health and vitality of the animals at the effective doses used. In fact, they were more alert than untreated animals due to the lower tumor burden. The inhibition of tumor growth by the drug closely correlated with suppression of FoxM1, down-regulation of survivin and induction of p21 (Figs.5c, Supplemental Fig. S4). The Hif1α pathway could not be assessed in this experiment since the levels of Hif1 were undetectable in either naïve or YM-1-treated animals. We also tested effects of YM-1 on a distinct tumor model, allograft tumor of B16 melanoma cells. B16 cells were transplanted subcutaneously into nude mice, and at day 7 post-transplantation (day 0 on the graph Fig. 5b, right panel), animals were injected intraperitoneally with 25mg/kg YM-1 or saline. The injections were done three times every second day, and tumor growth was monitored by caliper. As seen on Fig. 5b, administration of YM-1 significantly reduced tumor growth. Therefore, YM-1 showed a predictable response in animals, which correlated with strong suppression of growth of two distinct tumor types with minimal normal tissue toxicity.
Discussion
Here we investigated potential mechanisms of regulation of cancer signaling by Hsp70. We specifically focused on Bag3, the member of the Bag family proteins which functions in Hsp70 nucleotide exchange. The focus was defined by prior reports that Bag3 can interact with signaling molecules, via its PxxP and WW domains. In other words, via its Bag domain Bag3 may regulate the chaperone activity of Hsp70 by controlling the ADP/ATP exchange, and via PxxP and WW motifs it can also regulate activities of important signaling pathways. Therefore Bag3 may potentially be able to integrate the Hsp70 chaperone network with a network of signaling pathways. Using both biochemical and genetic approaches, we demonstrated that effects of Hsp70 in regulation of signaling are mediated by Bag3. Surprisingly, while depletion of Bag3 had similar effects on some signaling pathways as depletion of Hsp70 (e.g. on FoxM1 or Hif1), in other cases, depletion of Bag3 did not affect signaling but was necessary for Hsp70 effects (e.g. with NF-kB or Src). Therefore, in control of signaling pathways by Bag3-Hsp70 pair each individual target may be subject to specific regulatory mechanisms. Although more work is needed, we suggest that these differences could be related to specific ways in which the signaling modules interact with Bag3. For example, while the Bag3 regulation of Src is mediated by the PxxP domain, the interaction with other pathways could be through the WW or M-domains.
We undertook several unbiased and biased approaches to dissect cancer-related pathways regulated by the Hsp70-Bag3 module. We observed that Hsp70-Bag3 control activities of multiple signaling factors that are involved in cancer development. The number of the factors was relatively large, consistent with a broad “hub” role for Hsp70 in cancer. We validated a subset of these pathways, and found that Hsp70 depletion down-regulates Hif1 and FoxM1, while up-regulating NF-κB pathways. Interestingly, the former effects would presumably be anti-cancerous, while the latter is likely to be cancer-promoting. These disparate responses to the same stimulus should not be very surprising because similar phenomena have been observed with other anti-cancer treatments. For example, radiation triggers anti-cancer p53 pathway and also cancer-promoting NF-κB and c-Abl (48, 49). In this case and in the case of Hsp70 depletion, the net effect is anti-cancerous.
Previously, Hsp70 knockout was shown to prevent development of Her2-positive tumors in a mouse model by suppressing OIS (5), indicating that Hsp70 plays an important role at the early stages of tumor development. Our findings that the Hsp70-Bag3 module affects pathways regulating angiogenesis and invasion (e.g. Hif1 or FoxM1), suggest that Hsp70 may play a broader role in cancer development. While it has been reported that Hsp70 is seen at elevated levels in low grade tumors (50) curiously, the levels of Hsp70 increase further in high grade cancers (3, 4), consistent with distinct role(s) of Hsp70 in later stages of cancer progression, such as invasion.
Overall, this work pointed to a key role for the Hsp70-Bag3 complex in cancer, and suggests that this pair may represent a promising drug target. Of the available Hsp70 inhibitors, YM-1 is a unique probe because it specifically traps the ADP-bound form of Hsp70 (45). These observations suggested to us that YM-1 might block the Hsp70-Bag3 interaction because Bag3 has a weak affinity for the ADP-bound form of Hsp70 and it normally favors client release (Gestwicki, manuscript in preparation). Thus, although the known binding site for YM-1 is more than 20Å from the contact surface between Hsp70 and Bag3, allosteric regulation of Hsp70 by YM-1 would be predicted to interrupt NEF binding. Accordingly, YM-1 has a mechanism of action that is unique among the known Hsp70 inhibitors, which positions it to destabilize Hsp70-Bag3 activities in cancer cells, leading to suppression of cancer-promoting signaling pathways. Consistently, YM-1 had anti-cancer activity in cells and in a xenograft model. These results validate the Hsp70-Bag3 complex as a potential anti-cancer target.
Supplementary Material
Footnotes
Authors do not have conflict of interest regarding this work.
References
- 1.Michels AA, Kanon B, Konings AW, Ohtsuka K, Bensaude O, Kampinga HH. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. Journal of Biological Chemistry. 1997;272:33283–33289. doi: 10.1074/jbc.272.52.33283. [DOI] [PubMed] [Google Scholar]
- 2.Stege GJ, Li GC, Li L, Kampinga HH, Konings AW. On the role of hsp72 in heat-induced intranuclear protein aggregation. International Journal of Hyperthermia. 1994;10(5):659–674. doi: 10.3109/02656739409022446. [DOI] [PubMed] [Google Scholar]
- 3.Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23(16):2907–2918. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- 4.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31(3):164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Meng L, Hunt C, Yaglom JA, Gabai VL, Sherman MY. Heat shock protein Hsp72 plays an essential role in Her2-induced mammary tumorigenesis. Oncogene. 2011;30(25):2836–2845. doi: 10.1038/onc.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt CR, Dix DJ, Sharma GG, et al. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol. 2004;24(2):899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Gall JM, Bonegio RG, et al. Induction of heat shock protein 70 inhibits ischemic renal injury. Kidney Int. 2011;79(8):861–870. doi: 10.1038/ki.2010.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McConnell KW, Fox AC, Clark AT, et al. The role of heat shock protein 70 in mediating age-dependent mortality in sepsis. J Immunol. 2011;186(6):3718–3725. doi: 10.4049/jimmunol.1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Kim YN, Koo KH, Sung JY, Yun UJ, Kim H. Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol. 2012;2012:306879. doi: 10.1155/2012/306879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136(5):823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5(22):2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 13.Beere H, Wolf B, Cain K, et al. Heat shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biology. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 14.Itahana K, Campisi J, Dimri GP. Mechanisms of cellular senescence in human and mouse cells. Biogerontology. 2004;5(1):1–10. doi: 10.1023/b:bgen.0000017682.96395.10. [DOI] [PubMed] [Google Scholar]
- 15.Shay JW, Roninson IB. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004;23(16):2919–2933. doi: 10.1038/sj.onc.1207518. [DOI] [PubMed] [Google Scholar]
- 16.Yaglom JA, Gabai VL, Sherman MY. High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res. 2007;67(5):2373–2381. doi: 10.1158/0008-5472.CAN-06-3796. [DOI] [PubMed] [Google Scholar]
- 17.Gabai VL, Yaglom JA, Waldman T, Sherman MY. Heat shock protein Hsp72 controls oncogene-induced senescence pathways in cancer cells. Mol Cell Biol. 2009;29(2):559–569. doi: 10.1128/MCB.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rerole AL, Gobbo J, De Thonel A, et al. Peptides and aptamers targeting HSP70: a novel approach for anticancer chemotherapy. Cancer Res. 2011;71(2):484–495. doi: 10.1158/0008-5472.CAN-10-1443. [DOI] [PubMed] [Google Scholar]
- 19.Strom E, Sathe S, Komarov PG, et al. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol. 2006;2(9):474–479. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- 20.Leu JI, Pimkina J, Frank A, Murphy ME, George DL. A small molecule inhibitor of inducible heat shock protein 70. Mol Cell. 2009;36(1):15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leu JI, Pimkina J, Pandey P, Murphy ME, George DL. HSP70 inhibition by the small-molecule 2-phenylethynesulfonamide impairs protein clearance pathways in tumor cells. Mol Cancer Res. 2011;9(7):936–947. doi: 10.1158/1541-7786.MCR-11-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyata Y, Rauch JN, Jinwal UK, et al. Cysteine reactivity distinguishes redox sensing by the heat-inducible and constitutive forms of heat shock protein 70. Chem Biol. 2012;19(11):1391–1399. doi: 10.1016/j.chembiol.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massey AJ, Williamson DS, Browne H, et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother Pharmacol. 2010;66(3):535–545. doi: 10.1007/s00280-009-1194-3. [DOI] [PubMed] [Google Scholar]
- 24.Huryn DM, Brodsky JL, Brummond KM, et al. Chemical methodology as a source of small-molecule checkpoint inhibitors and heat shock protein 70 (Hsp70) modulators. Proc Natl Acad Sci U S A. 2011;108(17):6757–6762. doi: 10.1073/pnas.1015251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodina A, Patel PD, Kang Y, et al. Identification of an allosteric pocket on human hsp70 reveals a mode of inhibition of this therapeutically important protein. Chem Biol. 2013;20(12):1469–1480. doi: 10.1016/j.chembiol.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nature Cell Biol. 2001;3:E237–E241. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- 27.Ingham RJ, Colwill K, Howard C, et al. WW domains provide a platform for the assembly of multiprotein networks. Mol Cell Biol. 2005;25(16):7092–7106. doi: 10.1128/MCB.25.16.7092-7106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li SS. Specificity and versatility of SH3 and other proline-recognition domains: structural basis and implications for cellular signal transduction. Biochem J. 2005;390(Pt 3):641–653. doi: 10.1042/BJ20050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carra S, Seguin SJ, Lambert H, Landry J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem. 2008;283(3):1437–1444. doi: 10.1074/jbc.M706304200. [DOI] [PubMed] [Google Scholar]
- 30.Ulbricht A, Eppler FJ, Tapia VE, et al. Cellular mechanotransduction relies on tension-induced and chaperone-assisted autophagy. Curr Biol. 2013;23(5):430–435. doi: 10.1016/j.cub.2013.01.064. [DOI] [PubMed] [Google Scholar]
- 31.Gamerdinger M, Kaya AM, Wolfrum U, Clement AM, Behl C. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep. 2011;12(2):149–156. doi: 10.1038/embor.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doong H, Price J, Kim YS, et al. CAIR-1/BAG-3 forms an EGF-regulated ternary complex with phospholipase C-gamma and Hsp70/Hsc70. Oncogene. 2000;19(38):4385–4395. doi: 10.1038/sj.onc.1203797. [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki M, Homma S, Hishiya A, Dolezal SJ, Reed JC, Takayama S. BAG3 regulates motility and adhesion of epithelial cancer cells. Cancer Res. 2007;67(21):10252–10259. doi: 10.1158/0008-5472.CAN-07-0618. [DOI] [PubMed] [Google Scholar]
- 34.Kassis JN, Guancial EA, Doong H, Virador V, Kohn EC. CAIR-1/BAG-3 modulates cell adhesion and migration by downregulating activity of focal adhesion proteins. Exp Cell Res. 2006;312(15):2962–2971. doi: 10.1016/j.yexcr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Iwasaki M, Sugio A, et al. BAG3 (BCL2-associated athanogene 3) interacts with MMP-2 to positively regulate invasion by ovarian carcinoma cells. Cancer Lett. 2011;303(1):65–71. doi: 10.1016/j.canlet.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Gabai VL, Meng L, Kim G, Mills TA, Benjamin IJ, Sherman MY. Heat shock transcription factor Hsf1 is involved in tumor progression via regulation of hypoxia-inducible factor 1 and RNA-binding protein HuR. Mol Cell Biol. 2012;32(5):929–940. doi: 10.1128/MCB.05921-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim G, Meriin AB, Gabai VL, et al. The heat shock transcription factor Hsf1 is downregulated in DNA damage-associated senescence, contributing to the maintenance of senescence phenotype. Aging Cell. 2012;11(4):617–627. doi: 10.1111/j.1474-9726.2012.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blazer LL, Roman DL, Muxlow MR, Neubig RR. Use of flow cytometric methods to quantify protein-protein interactions. Current protocols in cytometry / editorial board, J Paul Robinson, managing editor [et al] 2010;Chapter 13(Unit 13 1):1–5. doi: 10.1002/0471142956.cy1311s51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gentilella A, Khalili K. BAG3 expression in glioblastoma cells promotes accumulation of ubiquitinated clients in an Hsp70-dependent manner. J Biol Chem. 2011;286(11):9205–9215. doi: 10.1074/jbc.M110.175836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ammirante M, Rosati A, Arra C, et al. IKK{gamma} protein is a target of BAG3 regulatory activity in human tumor growth. Proc Natl Acad Sci U S A. 2010;107(16):7497–7502. doi: 10.1073/pnas.0907696107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Yang LN, Cheng L, et al. Bcl2-associated athanogene 3 interactome analysis reveals a new role in modulating proteasome activity. Mol Cell Proteomics. 2013;12(10):2804–2819. doi: 10.1074/mcp.M112.025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang IC, Chen YJ, Hughes D, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25(24):10875–10894. doi: 10.1128/MCB.25.24.10875-10894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halasi M, Gartel AL. FOX(M1) news--it is cancer. Mol Cancer Ther. 2013;12(3):245–254. doi: 10.1158/1535-7163.MCT-12-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdelmohsen K, Gorospe M. Posttranscriptional regulation of cancer traits by HuR. Wiley Interdiscip Rev RNA. 2010;1(2):214–229. doi: 10.1002/wrna.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rousaki A, Miyata Y, Jinwal UK, Dickey CA, Gestwicki JE, Zuiderweg ER. Allosteric drugs: the interaction of antitumor compound MKT-077 with human Hsp70 chaperones. J Mol Biol. 2011;411(3):614–632. doi: 10.1016/j.jmb.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang AM, Miyata Y, Klinedinst S, et al. Activation of Hsp70 reduces neurotoxicity by promoting polyglutamine protein degradation. Nat Chem Biol. 2013;9(2):112–118. doi: 10.1038/nchembio.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang L, Miyata Y, Ung PM, et al. Chemical screens against a reconstituted multiprotein complex: myricetin blocks DnaJ regulation of DnaK through an allosteric mechanism. Chem Biol. 2011;18(2):210–221. doi: 10.1016/j.chembiol.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Shen B, Xia L, et al. Activation of nuclear factor kappaB in radioresistance of TP53-inactive human keratinocytes. Cancer Res. 2002;62(4):1213–1221. [PubMed] [Google Scholar]
- 49.Meltser V, Ben-Yehoyada M, Shaul Y. c-Abl tyrosine kinase in the DNA damage response: cell death and more. Cell Death Differ. 2011;18(1):2–4. doi: 10.1038/cdd.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10(2):86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.