The genetic epidemiology of psychiatric disorders is undergoing a sea change. The flood of genome-wide association studies (GWAS) enabled by the advent of low-cost, high throughput genotyping and the emergence of global consortia to harness the combined power of genome wide data on tens of thousands of individuals is now producing a large volume of discoveries. With these discoveries, hypothesis-free methods of uncovering the genetic roots of psychiatric disorder are overtaking traditional hypothesis-driven approaches. What this transition means for research on gene-environment interactions (GxE) is hotly debated.1–5 Uher’s review addresses some of the contours of this debate. Here we seek to contextualize and expand on his points. Our goal is to make some sense of the ongoing conflict in psychiatric genetics between hypothesis-driven genetic research focused on candidate systems and hypothesis-free genetic research focused on data mining of the genome. We think part of the reason that arguments over how to conduct GxE research have grown so acrimonious is a lack of clarity over how genetic measurements are being used in GxE studies. In the paragraphs that follow, we frame the debate over how to conduct GxE research in terms of the substantive questions being asked and discuss implications of this framing for the conduct of GxE research within psychiatric epidemiology.
The Framework: Two Types of GxE Questions
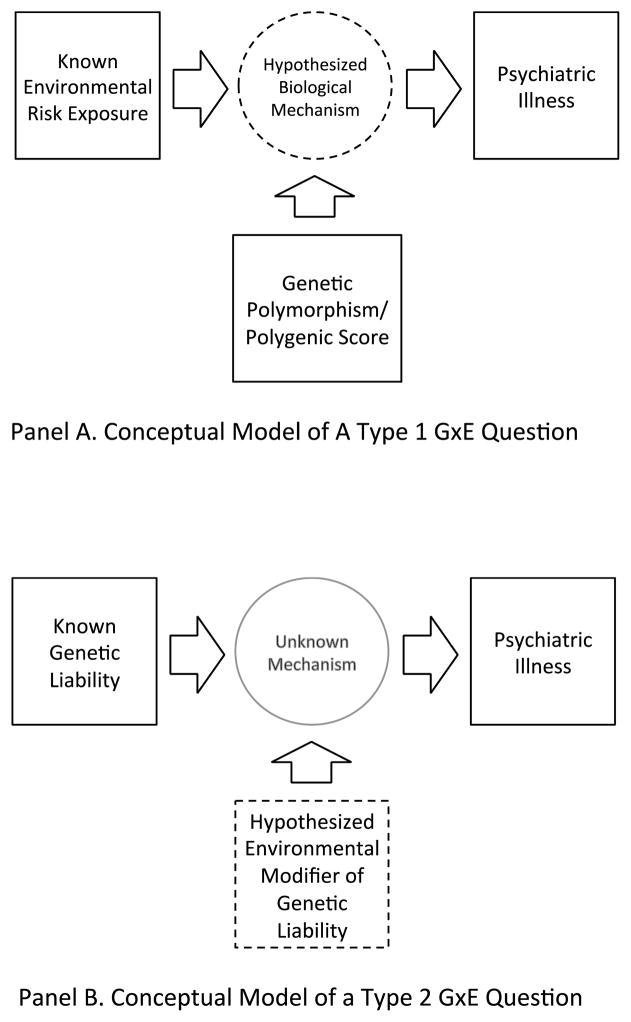
We propose that GxE research in psychiatric epidemiology addresses two different types of questions: “Type-1 Questions” about the biology through which an environmental exposure contributes to the pathogenesis of psychiatric illness; and “Type-2 Questions” about environmental conditions under which genetic liability to psychiatric illness is realized. Type-1 questions are fundamentally about what biological mechanisms mediate environmental risk. In research designs addressing Type-1 questions, genetic measurements function as proxies; polymorphisms in genes of known function are used to index individual differences in a biological pathway.6,7 GxE analysis tests the involvement of the biological pathway in the process through which the environment causes disease (Figure 1 Panel A).8 Type-2 questions are fundamentally about whether and how genetic risks are environmentally dependent. Polymorphisms already established as risk factors for psychiatric illness are examined under varying environmental circumstances. GxE analysis tests the involvement of an environmental factor in determining the pathogenicity of the genetic risk (Figure 1 Panel B). We view both types of questions as important in psychiatric epidemiology (see Box 1 for example cases).
Figure 1. Conceptual Models of Type 1 and Type 2 GxE Questions.
Panel A. Type 1 GxE Questions
In our framework, a Type 1 GxE question in psychiatric epidemiology is a questions about the biology through which an environmental exposure contributes to the pathogenesis of a psychiatric illness. The figure shows the conceptual model of a Type 1 GxE question. The GxE analysis is designed to test whether a specific biological mechanism mediates an environmental effect on illness. Because the hypothesized biological mechanism cannot be observed, a genetic polymorphism (or set of polymorphisms in the case of a polygenic score) is used as a quasi-experimental manipulation of the disease-relevant biology. (Genetic variation is known to influence the disease-relevant biology and it cannot be caused by the environmental exposure or the outcome.) If the pathogenic effect of the environment varies according to genotype, this provides evidence that the biological pathway affected by the gene connects the environmental risk with the psychiatric illness.
Panel B. Type 2 GxE Questions
In our framework, Type 2 GxE questions in psychiatric epidemiology are questions about the environmental conditions under which a genetic liability to a psychiatric illness is realized. The genetic liability may be a single variant or a polygenic score composed of many variants. The figure shows the conceptual model of a Type 2 GxE question. The GxE analysis is designed to test whether an identified genetic liability is amplified/mitigated by an environmental exposure. The mechanism through which the genetic liability interacts with the environmental exposure to cause illness is unknown.
Box 1. Examples of Type 1 and Type 2 GxE Questions: The Case of Depression.
Type 1 GxE Question Example
Environmental stress exposure is a risk factor for depression, but the mechanism through which stress causes depression is unknown. Altered serotonergic signaling in brain is hypothesized as a mechanism through which stress causes depression, but this hypothesis is difficult to test experimentally in humans. The gene encoding the serotonin transporter (5HTT) contributes to the regulation of stress response in rodents.39 A length polymorphism in that gene (5HTT-LPR) modifies its function40,41 and is associated with stress-dependent concentrations of serotonin in the cerebrospinal fluid of rhesus macaques42 and with threat-related reactivity of the amygdala in humans.43 On the basis of this evidence, one foundational GxE study used 5HTT-LPR as an instrument to measure individual differences in a difficult to observe biological substrate, serotonergic signaling in brain in response to stress.44 That GxE analysis examined the interaction of stressful live events with 5HTT-LPR in predicting depression. Framed as a Type 1 GxE question, that analysis tested the hypothesis that environmental stress contributes to the pathogenesis of depression via effects on serotonergic signaling in brain.
Type 2 GxE Question Example
Depression is known to be heritable. But not all individuals genetically predisposed to depression manifest illness. Environmental exposures are hypothesized to modify the effect of a genetic liability on depression. Although GWAS of depression have not detected replicable associations at the level of individual SNPs, results from GWAS and from genome-wide complex trait analysis indicate substantial and highly polygenic genetic influence on depression.26,45,46 On the basis of this evidence, a recent GxE analysis examined whether genetic liability to depression (as measured by a GWAS-derived polygenic score) was modified by exposure to stressful life events.33 Framed as a Type 2 GxE question, that analysis tested the hypothesis that genetically vulnerable individuals may be especially likely to develop depression when exposed to stress.
In our view, studies asking Type-1 GxE questions are important because the biology through which environmental exposures contribute to the pathogenesis of psychiatric illness can be hard to observe directly. Rapid advances in neuroscience and imaging technologies notwithstanding, it is difficult to watch brains work in real time. The problem is magnified when questions relate to developmentally sensitive or life-course cumulative environmental exposures, or environmental exposures that are impossible or unethical to simulate experimentally. Animal models offer one path to address this challenge. Human observational studies asking Type-1 GxE questions can complement research in model organisms by testing whether pathways implicated in animal studies play a parallel role in the etiology of psychiatric illness in humans. Together with human brain imaging studies and experiments with animals, human studies asking Type-1 GxE questions can advance a mechanistic understanding of environmental causation of psychiatric illness.
Studies asking Type-2 GxE questions are important because the identification of environmental conditions that modify genetic risks can guide the development of interventions to prevent and treat psychiatric illness. Twin and family studies indicate that a substantial portion of the population burden of psychiatric morbidity has genetic roots. To date, gene-hunting studies have made only slow progress toward identifying the molecular basis of this genetic influence. There is ongoing debate about where this missing heritability may be hiding.9–12 As Uher notes, modification of genetic influences on psychiatric illness by environmental conditions is one likely candidate. As a complement to hypothesis-free genome-wide discovery research, studies asking Type-2 GxE questions advance understanding of genetic causation of psychiatric illness.
Implications
The type of GxE question being asked should inform the strategy used to select genetic measurements for study. A major area of contention in the design of GxE studies is whether there should be prior evidence for a “main-effect” of genotype on the psychiatric illness being studied. For studies asking Type-1 GxE questions, this seems unnecessary. Studies asking Type-1 GxE questions use the measured ‘G’ as a proxy for variation in a biological pathway through which an environmental risk is hypothesized to cause a psychiatric illness. The prior evidence that seems most important in this case is evidence that the genotype being measured is associated with variation in the biological pathway implicated in the hypothesis. In contrast, for studies asking Type-2 GxE questions, prior evidence for a main-effect of genotype on the psychiatric illness under study seems sensible. Studies asking Type-2 GxE questions use the measured ‘G’ as an index of inherited risk for the psychiatric illness. Therefore, evidence for a genetic main effect is precisely what is needed to motivate the study design. An important exception is the case in which the whole genome is to be scanned, as in genome-wide gene-environment interaction studies (GWIS).13,14 As Uher notes in his review, it is entirely possible that some genetic variants that influence psychiatric illness in an environmentally dependent manner will show no association with psychiatric illness in the general population.2,15 GWIS provides a statistically rigorous means to investigate such variants.
Requiring evidence for genetic main effects—on variation in a biological substrate in the case of Type-1 GxE questions; on risk for psychiatric illness in the case of Type-2 GxE questions—can help to focus epidemiologic inquiry. But limiting the number of variants to be examined in GxE research is not sufficient to address the power problem that is the primary obstacle to progress in GxE research.16 One means to address the power problem is to increase sample size. Uher suggests several useful strategies to accomplish this, including the leveraging of old tools, like registry data, and new tools, like Google Street View, to generate environmental measurements for individuals who have already been genotyped in large-scale consortium projects. As a complement to strategies that address the power problem by increasing sample size, we suggest the approach of increasing the size of the genetic effect being analyzed.
Psychiatric disorders are complex phenotypes. They are influenced by many different genetic factors. Rather than being present or absent, genetic risk is distributed along a continuum.17 Many different variants each contribute small increments in risk.18 The collective influence of these variants can be summarized in a single, quantitative index, a “genetic risk score.”19,20 Genetic risk scores have conceptual and empirical advantages over single variant approaches to measuring genetic risk. Conceptually, genetic risk scores are aligned with the understanding of psychiatric disorders as highly polygenic.21 Empirically, genetic risk scores have the statistically desirable property of being continuously and normally distributed and, because they measure the combined influence of many variants, they capture a larger genetic effect as compared to single variant measures.22
Genetic risk scores can be used in studies asking both Type-1 and Type-2 GxE questions. For Type-1 GxE questions, genetic risk scores can be derived from hypothesis-based genetic analyses to capture variation in a biological substrate. Neuroscientists are beginning to use such genetic risk scores in main effect studies,7,23 but we are not aware of any published studies that use genetic risk scores to ask Type-1 GxE questions. This is a promising frontier. Individual polymorphisms typically predict only a small amount of variation in measured biology. Polygenic scores for blood biomarkers and neural phenotypes may increase the statistical power of some Type-1 GxE analyses. For Type-2 GxE questions, genetic risk scores should be derived from hypothesis-free discovery studies. This approach is in wide use in main-effect genetic research in psychiatry.19,24–28 And there are also examples of this type of genetic risk score being used to ask Type-2 GxE questions—about how physical activity and diet modify genetic risk for obesity29,30 and about how stress exposures modify genetic risk for smoking31,32 and depression.33 Much more work is needed in this area, especially to address questions about the timing of environmental exposures, a point Uher highlighted in his review. For obesity and smoking, our work suggests that genetic risks discovered in GWAS manifest early in life, and that these early manifestations mediate genetic influence on adult outcomes.34,35 This raises the question of whether environments that intersect these apparent “sensitive” periods have the potential to modify genetic risk in lasting ways.32
Conclusions
Gene-environment interactions have been a highly contentious topic in psychiatric epidemiology in the past several years. Hopefully, this acrimonious phase in our history is coming to a close. We think the future looks promising. We are cautiously optimistic about the prospects of GWIS. But we think the lowest hanging fruit lies elsewhere. Resources like the ENCODE databases36,37 and tools like Cytoscape38 offer new approaches to identify genetic variants that can be used to ask Type-1 GxE questions. In parallel the ever-growing library of GWAS discoveries for psychiatric disorders and the extraordinary efforts of the Psychiatric Genomics Consortium to make their GWAS results publicly available is making possible new opportunities to ask Type-2 GxE questions, especially those that utilize polygenic measures of genetic risk. In concluding his review, Uher articulated a vision of big-science gene-environment interaction research that delivers real benefit to society through personalized therapies. We whole-heartedly support that vision. We hope that in the months and years to come, psychiatric epidemiology can leave behind the old debates about gene-environment interaction research and move forward to ask new questions.
Acknowledgments
DWB is supported by grants from the US National Institute on Aging T32-AG00029, P30 AG028716-08. SI received support from National Institute of Child Health & Human Development Grants HD061298 and HD077482 and the Jacobs Foundation and is grateful to the Yad Hanadiv Rothschild Foundation for the award of a Rothschild Fellowship.
Footnotes
Conflict of Interest. On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–9. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dick DM. Gene-environment interaction in psychological traits and disorders. Annu Rev Clin Psychol. 2011;7:383–409. doi: 10.1146/annurev-clinpsy-032210-104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boardman JD, Domingue BW, Blalock CL, Haberstick BC, Harris KM, McQueen MB. Is the gene-environment interaction paradigm relevant to genome-wide studies? the case of education and body mass index. Demography. 2013 doi: 10.1007/s13524-013-0259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Sayed AM, Koenen KC, Galea S. Rethinking our public health genetics research paradigm. Am J Public Health. 2013;103:S14–S18. doi: 10.2105/AJPH.2012.301127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009;32:225–47. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2011;36:1940–7. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–90. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 9.Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: genetic interactions create phantom heritability. Proc Natl Acad Sci U S A. 2012;109:1193–8. doi: 10.1073/pnas.1119675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson G. Rare and common variants: twenty arguments. Nat Rev Genet. 2011;13:135–45. doi: 10.1038/nrg3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era--concepts and misconceptions. Nat Rev Genet. 2008;9:255–66. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- 12.Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, et al. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet. 2010;11:446–50. doi: 10.1038/nrg2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murcray CE, Lewinger JP, Gauderman WJ. Gene-environment interaction in genome-wide association studies. Am J Epidemiol. 2009;169:219–26. doi: 10.1093/aje/kwn353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelis MC, Tchetgen EJT, Liang L, Qi L, Chatterjee N, Hu FB, et al. Gene-environment interactions in genome-wide association studies: a comparative study of tests applied to empirical studies of type 2 diabetes. Am J Epidemiol. 2012;175:191–202. doi: 10.1093/aje/kwr368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manuck SB, McCaffery JM. Gene-environment interaction. Annu Rev Psychol. 2014;65:41–70. doi: 10.1146/annurev-psych-010213-115100. [DOI] [PubMed] [Google Scholar]
- 16.Khoury MJ, Wacholder S. Invited commentary: from genome-wide association studies to gene-environment-wide interaction studies-025efchallenges and opportunities. Am J Epidemiol. 2009;169:227–30. doi: 10.1093/aje/kwn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visscher PM, Goddard ME, Derks EM, Wray NR. Evidence-based psychiatric genetics, aka the false dichotomy between common and rare variant hypotheses. Mol Psychiatry. 2012;17:474–85. doi: 10.1038/mp.2011.65. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–51. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wray NR, Goddard ME, Visscher PM. Prediction of individual genetic risk to disease from genome-wide association studies. Genome Res. 2007;17:1520–8. doi: 10.1101/gr.6665407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plomin R, Haworth CMa, Davis OSP. Common disorders are quantitative traits. Nat Rev Genet. 2009;10:872–8. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- 22.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9:e1003348. doi: 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stice E, Yokum S, Burger K, Epstein L, Smolen A. Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J Neurosci. 2012;32:10093–100. doi: 10.1523/JNEUROSCI.1506-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntosh AM, Gow A, Luciano M, Davies G, Liewald DC, Harris SE, et al. Polygenic risk for schizophrenia is associated with cognitive change between childhood and old age. Biol Psychiatry. 2013:1–6. doi: 10.1016/j.biopsych.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Lencz T, Knowles E, Davies G, Guha S, Liewald DC, Starr JM, et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the cognitive genomics consortium (cogent) Mol Psychiatry. 2014;19:168–74. doi: 10.1038/mp.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium. Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18:497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, Nurnberger JI, et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014 doi: 10.1038/nature13595. [Internet] [cited 2014 Jul 22]; advance online publication. Available from: http://www.nature.com/nature/journal/vaop/ncurrent/full/nature13595.html. [DOI] [PMC free article] [PubMed]
- 29.Li S, Zhao JH, Luan J, Ekelund U, Luben RN, Khaw K-T, et al. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from epic-norfolk prospective population study. PLoS Med. 2010;7:1–9. doi: 10.1371/journal.pmed.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367:1387–96. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyers JL, Cerdá M, Galea S, Keyes KM, Aiello AE, Uddin M, et al. Interaction between polygenic risk for cigarette use and environmental exposures in the detroit neighborhood health study. Transl Psychiatry. 2013;3:e290. doi: 10.1038/tp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belsky DW, Moffitt TE, Caspi A. Genetics in population health science: strategies and opportunities. Am J Public Health. 2013;103 (Suppl 1):S73–83. doi: 10.2105/AJPH.2012.301139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peyrot WJ, Milaneschi Y, Abdellaoui A, Sullivan PF, Hottenga JJ, Boomsma DI, et al. Effect of polygenic risk scores on depression in childhood trauma. Br J Psychiatry J Ment Sci. 2014 doi: 10.1192/bjp.bp.113.143081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belsky DW, Moffitt TE, Houts R, Bennett GG, Biddle AK, Blumenthal JA, et al. Polygenic risk, rapid childhood growth, and the development of obesity: evidence from a 4-decade longitudinal study. Arch Pediatr Adolesc Med. 2012;166:515–21. doi: 10.1001/archpediatrics.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belsky DW, Moffitt TE, Baker TB, Biddle AK, Evans JP, Harrington H, et al. Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: evidence from a 4-decade longitudinal study. JAMA Psychiatry. 2013;70:534–42. doi: 10.1001/jamapsychiatry.2013.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Encode T, Consortium P. A user’s guide to the encyclopedia of dna elements (encode) PLoS Biol. 2011;9:e1001046. doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandenbergh DJ, Schlomer GL. Finding genomic function for genetic associations in nicotine addiction research: the encode project’s role in future pharmacogenomic analysis. Pharmacol Biochem Behav. 2014 doi: 10.1016/j.pbb.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy DL, Li Q, Engel S, Wichems C, Andrews A, Lesch KP, et al. Genetic perspectives on the serotonin transporter. Brain Res Bull. 2001;56:487–94. doi: 10.1016/s0361-9230(01)00622-0. [DOI] [PubMed] [Google Scholar]
- 40.Heils A, Teufel A, Petri S, Seemann M, Bengel D, Balling U, et al. Functional promoter and polyadenylation site mapping of the human serotonin (5-ht) transporter gene. J Neural Transm Gen Sect. 1995;102:247–54. doi: 10.1007/BF01281159. [DOI] [PubMed] [Google Scholar]
- 41.Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 42.Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, et al. Early experience and serotonin transporter gene variation interact to influence primate cns function. Mol Psychiatry. 2002;7:118–22. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- 43.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 44.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-htt gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 45.Demirkan A, Penninx BWJH, Hek K, Wray NR, Amin N, Aulchenko YS, et al. Genetic risk profiles for depression and anxiety in adult and elderly cohorts. Mol Psychiatry. 2011;16:773–83. doi: 10.1038/mp.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lubke GH, Hottenga JJ, Walters R, Laurin C, de Geus EJC, Willemsen G, et al. Estimating the genetic variance of major depressive disorder due to all single nucleotide polymorphisms. Biol Psychiatry. 2012;72:707–9. doi: 10.1016/j.biopsych.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]