Abstract
There is currently great interest in the phylogenetic origins of altruistic behaviour within the primate order. Considerable attention has been focused on chimpanzees, Pan troglodytes, because they are our closest living relatives and participate in a wide range of collective activities, including hunting and food sharing. Food sharing is of particular importance because it plays a critical role in the human foraging niche, but food sharing among adults is rare in nonhuman primates. Some research suggests that chimpanzees selectively share meat with reciprocating partners and allies, while other work indicates that chimpanzees primarily share to reduce harassment from other group members (tolerated theft). We examined the effects of kinship, relationship quality, reciprocity and the intensity of solicitations on the pattern of food transfers in six captive groups of chimpanzees. We observed events that occurred after the chimpanzees were provisioned with large frozen juice disks. These disks share some properties with prey carcasses: they are a valued, but limited, resource; they take a considerable period of time to consume; they can be monopolized by one individual, but bits can be broken off and transferred to others. Our analyses suggest that food transfers serve multiple functions for chimpanzees. Individuals may use food transfers to enhance the welfare of closely related group members, strengthen social relationships with favoured partners and reduce the costs of persistent solicitations.
Keywords: altruism, chimpanzee, food sharing, Pan troglodytes, prosociality, reciprocity, tolerated theft
Humans form larger, more complex and more cooperative societies than other vertebrate taxa, and this has generated interest in how we came to be such unusual creatures (Burkhart et al. 2009; Warneken & Tomasello 2009; Silk & Boyd 2010). Efforts to understand the phylogenetic origins of cooperation within the human lineage have focused on chimpanzees, Pan troglodytes, for several important reasons. First, chimpanzees are our closest living relatives. Second, chimpanzees participate in a wider range of collective activities than most other nonhuman primates. In the wild, male chimpanzees groom one another, form coalitions, jointly defend access to mates and patrol the borders of their territories (Muller & Mitani 2005). Female chimpanzees are less gregarious than males, but form lasting social bonds with other females (Boesch & Boesch-Achermann 2000; Gilby & Wrangham 2008; Langergraber et al. 2009). Adult females often share food with their offspring, particularly foods that are difficult for their offspring to procure or process on their own (McGrew 1975; Silk 1978; Nishida & Turner 1996). Third, chimpanzees are the only nonhuman primate species in which food is regularly shared among adults in the wild (Jaeggi & van Schaik 2011). Food sharing among chimpanzees is of particular interest because food sharing is a universal feature of human societies and plays a critical role in the human foraging niche (Kaplan et al. 2009).
Food sharing also occurs outside the primate order (Stevens & Gilby 2004). For example, males sometimes provide resources to prospective mates during courtship, carnivores sometimes hunt together and share access to kills, and in cooperatively breeding species, breeding females are sometimes provisioned by other group members. Although reciprocity and kin selection are most often invoked to explain food sharing, Stevens & Gilby (2004) emphasized the importance of others factors, such as by-product mutualism and group augmentation. They also discussed the possibility that food sharing may sometimes be a form of tolerated theft (Blurton Jones 1984; Moore 1984) or sharing under pressure (Wrangham 1975; Stevens & Stephens 2002; Stevens & Gilby 2004), as possessors share food in order to reduce the costs of defending resources against rivals.
Adult chimpanzees frequently hunt monkeys and small mammals, regularly share meat from the carcasses of captured prey (Muller & Mitani 2005) and occasionally share plant foods with other adults (McGrew 1975; Slocombe & Newton-Fisher 2005; Hockings et al. 2011). The evolutionary forces shaping food transfers among chimpanzees are the subject of much discussion. In the Taï Forest of Côte d’Ivoire and in Ngogo in the Kibale Forest of Uganda, males selectively transfer meat to males that have also transferred meat to them and to males that support them in agonistic interactions (Mitani & Watts 1999, 2001; Boesch & Boesch-Achermann 2000; Mitani 2006). Reciprocal transfers of plant foods have also been documented in captive chimpanzees (de Waal 1989, 1997; Jaeggi et al. 2010a). However, among chimpanzees at the Gombe Stream in Tanzania, Gilby (2006) found no correlation between meat transfer and grooming or proximity within dyads. He argued that males at Gombe give up parts of their kills ‘to avoid the costs of defending a food item against persistent beggars’. Thus, food transfers may be a form of tolerated theft (Blurton Jones 1984) or sharing under pressure (Wrangham 1975; Stevens & Stephens 2002; Stevens & Gilby 2004).
There have also been reports that males exchange meat for sex with receptive females. Such transfers have been reported to enhance immediate mating opportunities at Gombe (Stanford 1998) and future mating prospects, but not immediate ones, in the Taï Forest (Gomes & Boesch 2011). However, in a detailed review of patterns of food transfers and mating behaviour at several east African sites (Gombe, Kanyawara, Ngogo), Gilby et al. (2010) found no evidence that the presence of oestrous females increased the likelihood that males would hunt or preferentially transfer meat to receptive females, or that food transfers enhanced males’ short-term mating success. They concluded that ‘meat transfers in chimpanzees are rarely sexually motivated’ (page 51).
Although these studies provide very different interpretations of food transfers in chimpanzees, they are not necessarily incompatible. It is possible that food transfers may serve different functions in different contexts. Some food transfers may enhance the welfare of closely related group members, while others may strengthen alliances with favoured partners or enhance future mating prospects. In some cases, food transfers may reflect a trade-off between the benefits of monopolizing food items and the costs of defending them. Transfers may be made after persistent solicitations, but if possessors control the distribution of food, they may still be able to direct transfers selectively to reciprocating partners (Gurven 2004; Jaeggi et al. 2010a, b).
Here we examine the effects of kinship, relationship quality, reciprocity and the intensity of solicitations on the pattern of food transfers in six captive groups of chimpanzees. Our analyses focus on events that occurred after the chimpanzees were provisioned with large (30.5 × 12.5 cm) frozen juice disks. These frozen disks share some properties with prey carcasses: they are a valued, but limited, resource because only two or three are provided at one time to the group; they take considerable time to consume; they can be monopolized by one individual, but portions can be detached and transferred to other individuals, and more than one individual can feed on a disk at the same time.
METHODS
The study was conducted at the Michale E. Keeling Center for Comparative Medicine and Research (Bastrop, TX, U.S.A.). We observed all members of six groups that were housed in large outdoor compounds (22.86 m in diameter for a total of area of 410.25 m2) connected to indoor enclosures. The outdoor compounds contained enrichment devices (e.g. climbing structures, ropes and swings, barrels, and other toys). The chimpanzees had ad libitum access to primate chow and water during the day. Each group also received fruit and vegetable feeds four times per day, as well as additional food and material enrichment several times per week. The animals spent the majority of their time in the outdoor compounds, and during observations, the doors to the indoor enclosures were closed so that all chimpanzees in the group remained outside and in view.
The study groups ranged in size from 7 to 14 individuals (Table 1). The composition of social groups was stable over the course of our study, and most individuals had lived in the same group since the late 1970s. There were few young infants or juveniles in the study groups because females had been fitted with contraceptive devices to prevent conception. None of the females were pregnant or lactating during the study period, and all but five of the adult females were cycling. Information about kinship, dominance rank and female reproductive status were obtained from colony records.
Table 1.
Demographic composition of the six chimpanzee study groups
| Group | Adult male | Subadult male | Adult female | Subadult female | Immature | Total |
|---|---|---|---|---|---|---|
| C2 | 2 | 4 | 6 | 0 | 2 | 14 |
| C3 | 1 | 2 | 5 | 3 | 1 | 12 |
| C4 | 3 | 1 | 6 | 0 | 1 | 11 |
| C5 | 1 | 1 | 5 | 0 | 0 | 7 |
| C6 | 2 | 1 | 4 | 1 | 0 | 8 |
| C8 | 1 | 1 | 7 | 0 | 1 | 10 |
Definitions of age–sex categories based on Goodall (1986): adult male ≥16 years; adult female ≥14 years; subadult male 8–15 years; subadult female 8–13 years; immature <8 years.
Pant-grunts are submissive vocalizations that provide a clear indicator of dominance and subordinance in chimpanzees (Wroblewski et al. 2009). In the study population, pant-grunt vocalizations were monitored during social introductions and periods of high activity such as feeding and enrichment distribution. Individuals that received pant-grunts from all other same-sexed individuals and never directed them at other same-sexed individuals were categorized as high-ranking. Individuals that both pant-grunted to other same-sex individuals and received pant-grunts from other same-sex individuals were classified as middle-ranking. Individuals that pant-grunted to other same-sex individuals, but never received pant grunts from other same-sex individuals were categorized as low-ranking.
The groups included 28 mother–offspring pairs, 11 pairs of siblings (3 male–male, 6 male–female and 2 female–female pairs), three grandmother–grand–offspring dyads, and one uncle–niece dyad. Paternal relatedness was not known for all dyads and is not included in the analyses. For the purposes of these analyses, we assumed that siblings were related by 0.25 and that the single uncle–niece dyad was related by 0.125.
Observations of Food Transfers
We monitored events that occurred after the chimpanzees were provisioned with frozen juice disks. The disks were made by mixing fruit juice, water and peanuts together and freezing them in a round mould, which measured approximately 30.5 cm in diameter. The disks were removed from the mould before they were given to the chimpanzees. The chimpanzees were familiar with frozen juice as an enrichment treat. However, during the period of testing, frozen juice was not given as enrichment in order to maximize interest in the disks. Groups that contained seven to nine individuals were provisioned with two disks, and groups that contained at least 10 individuals were provisioned with three disks. The observer threw the disks into the compound in quick succession and began monitoring one disk. All coding was done in real time. The focal disk was chosen at random before the observation session began. Most observations were conducted in the morning, several hours after the chimpanzees had one of their regularly scheduled feedings. No more than one session was conducted per day on any group.
Observers noted the identity of the individual that first established possession of the disk and recorded all interactions that followed with that disk. If the disk was divided into more than one piece, the observer monitored the largest piece of the original disc. The observation session ended when the focal disk or the focal portion of the original disk was consumed fully.
All interactions between the possessor of the disk and other group members were recorded on a continuous basis. The observer recorded the identity of individuals that approached the possessor and kept track of their position relative to the possessor. We recorded all begging gestures (extending the hand towards the possessor) and the possessors’ responses to solicitors’ behaviour, which included turning away from the solicitor while remaining in the same location, moving away from the solicitor (avoid), threatening the solicitor or doing nothing (ignore).
Food transfers occurred in several different ways. In some cases, the possessor handed a piece of the disk to the solicitor. Following Boesch & Boesch (1989), these interactions are categorized as ‘active’ transfers. We recorded two types of ‘passive’ transfers: (1) ‘co-feeding’ occurred when the possessor maintained possession of the disk, but allowed another individual to lick or bite the disk while it was on the ground or in their hand; and (2) ‘collecting’ was recorded when the solicitor took pieces that had fallen off the disk while the possessor was eating it without resistance by the possessor (labelled ‘collect near’ by de Waal 1989 and ‘recovery’ by Boesch & Boesch 1989). We included both types of passive transfers in our analyses because it was possible for possessors to deter or prevent such transfers by threatening solicitors or moving the disk out of reach. ‘Forced transfers’ were recorded when one chimpanzee took food from a possessor who actively resisted the transfer. The analyses focused on the frequency and pattern of active and passive transfers.
Baseline Information about Social Behaviour
We also collected information about social behaviour in the group at times when provisioning events were not occurring. We conducted scan samples (Altmann 1974) of group members at 3 min intervals; scan sampling sessions lasted 60 min. On each scan, observers noted the activities of each group member in the same order. For the purposes of this study, the data of interest were (1) grooming, (2) sitting in contact and (3) sitting in arm’s reach. We completed a total of 313 1-hour scan sampling sessions (range 44–58 sessions per group).
Analysis Procedures
Analyses of food transfers were based on 95 provisioning sessions (15–17 sessions per group; N = 68 h). From the observational data, we extracted information about the identity of each possessor, the order of possession within each session, the duration of each possession, events that precipitated changes in possession, begging gestures, responses to begging gestures and types of food transfers.
Data derived from the scan samples were used to assess relationship quality. We tabulated the frequency with which each individual was in proximity, contact or grooming every other individual in the group. The frequencies of grooming, proximity and contact were positively correlated within dyads and do not represent independent sources of information about relationship quality. Following procedures previously developed for analyses of baboon social relationships, we created a composite index of sociality (CSI) for each dyad using the following formula.
In this equation, fixy is the rate of behaviour i for dyad xy, and is the mean rate of behaviour i across all dyads (see Silk et al. 2006). High values of the CSI represent dyads with stronger social relationships than the average dyad in their group, and low values represent dyads with weaker relationships than the average dyad in their group.
For analyses in which the individual is the unit of analysis, we examined the effects of individual characteristics (dominance rank category, sex and age) on patterns of behaviour, such as the number of possessions. Sex and dominance rank category are confounded because most adult and subadult males outrank all adult females. Because of ambiguities about the ranks of immature males and females, we excluded individuals below the age of 8 years in analyses of the effects of rank category. For analyses of the effects of the difference in dominance rank category between possessors and solicitors, we assumed that adult and subadult males outranked all females. When possessors outranked solicitors, we assigned the dyad a rank difference score of 2. When possessors held the same rank as solicitors, we assigned the dyad a rank difference score of 1. When possessors were subordinate to solicitors, we assigned the dyad a rank difference score of 0.
We used generalized linear mixed models (GLMM) for analyses in which individuals were sampled more than once and not all individuals contributed equally to the data set. For analyses in which the dyad was the unit of analysis, we treated the identity of the possessor as a random effect parameter. For analyses of the continuous variables, we conducted multilevel mixed effect linear regressions. For analyses of the number of food transfers, in which the outcome variable was a count variable, we conducted multilevel mixed effect Poisson regressions and included time of possession as an exposure variable. Standardized regression coefficients (β) are given throughout.
We used one-way ANOVA to evaluate the effects of categorical variables (e.g. age–sex class, dominance rank) on continuous outcome variables. All statistical analyses were conducted with Stata 11.2 (StatCorp, College Station, TX, U.S.A.). Where appropriate, means and standard errors are presented. All statistical tests are two tailed.
RESULTS
We begin by providing a general description of provisioning events and food transfers. Observations of provisioning events began when the disks were thrown into the enclosure and ended when the disk was fully consumed. This took on average 41.6 ± 2.1 min, and on average 2.7 ± 0.2 (range 1–10) different individuals were in possession of the focal portion of the disk as it was being consumed. The length of possessions declined as the order of possession increased and the disk shrank (mixed effects linear regression: β = −231.05 ± 50.55; Z = −4.57, N = 245 possessions, P < 0.001; Fig. 1).
Figure 1.
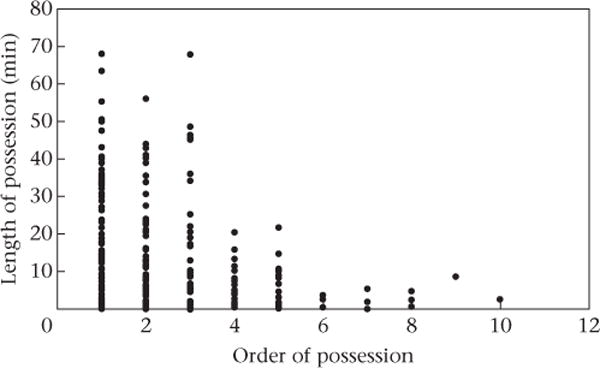
Effects of order of possession of fruit disks by chimpanzees on length of possessions.
Fifty-one of the 62 chimpanzees (82%) in the six study groups were in possession of disks at least once. Because the number of observation sessions per group varied (range 15–17), we computed the number of possessions per session and the average length of possessions (minutes per possession). There was no significant difference in the number of possessions per session across age–sex classes (one-way ANOVA: F5,56 = 0.73, P = 0.6066) or the length of possessions (F5,45 = 0.80, P = 0.5539; for definitions of age–sex classes see Table 1). There was no consistent effect of dominance rank category on the number of possessions per session for either sex (males: F2,17 = 0.48, P = 0.6266; females: F2,35 = 0.07, P = 0.9332). Similarly, dominance rank category did not consistently affect the length of possessions for males (F2,15 = 1.72, P = 0.2134) or females (F2,27 = 2.29, P = 0.1204).
Forty-five of the 51 chimpanzees (88%) that were in possession of disks were also involved in food transfers. Food transfers took several different forms. Nearly half of all transfers occurred when the possessor of the disk allowed another individual to co-feed from the disk while it was on the ground or in the possessor’s hand (49%). Another 37% of transfers occurred when solicitors collected pieces of food that had fallen off the disk. Active transfers of food to solicitors and forced thefts by solicitors were both uncommon (active transfers: 8%; forced thefts: 6%).
Begging gestures typically preceded food transfers. The rate of food transfers (number of active and passive transfers per minute of possession) was substantially higher when solicitors begged for food than when they remained passive (beg: 0.067 ± 0.01, N = 215; no beg: 0.01 ± 0.001, N = 266; F1,479 = 50.18, P < 0.001).
Possessors often resisted solicitations. In 69% of the 215 cases in which possessors were confronted with solicitors who begged from them, they resisted. When possessors resisted, they mainly responded by turning (41%) or moving away from solicitors (51%), but sometimes threatened them (8%).
The number of solicitations was linked to the nature of the possessors’ responses. For each possession, we calculated the rate of begging (number of gestures per minute of possession) by each solicitor and identified the most active form of response by the possessor towards the solicitor. We ranked responses in the following order: 1 = ignore, 2 = turn away, 3 = move away, 4 = threaten. Low rates of begging were likely to be ignored, while higher rates of begging gestures were associated with progressively more active types of responses (ß = 0.31 ± 0.04, Z = 7.28, N = 481 interactions, P < 0.001; Fig. 2).
Figure 2.
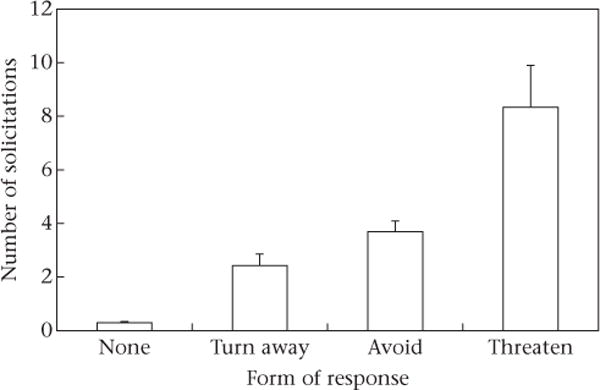
Relationship between number of solicitations directed towards possessors of fruit disks and the possessors’ responses.
Factors That Influence Food Transfers within Dyads
Based on previous studies of food transfers among chimpanzees, we expected food transfers to be biased in favour of close kin, particularly mothers and offspring. Chimpanzees might also reciprocate food transfers by transferring food most often to those that transferred food most often to them. Alternatively, food transfers might be part of a more generalized form of reciprocity in which individuals exchange various types of resources and services. If so, we would expect to find a positive relationship between food transfers and relationship quality. If food transfers are a form of tolerated theft or sharing under pressure, then food transfers may be influenced by the rank difference between possessor and solicitor or the number of solicitations directed towards possessors. The effectiveness of solicitations may interact with other variables, so we also examined the interactions between the frequency of solicitations and each of the other predictor variables. Kinship and relationship quality were positively correlated (N = 297 dyads, P = 0.34), but the degree of collinearity was low enough to include both variables in the models.
For active transfers, the full model was highly significant. Kinship, the rank difference between the possessor and solicitor, and the frequency of begging had significant positive effects on the number of active transfers (Table 2, model 1). Relationship quality, direct reciprocity and all of the interaction terms were nonsignificant. We first removed all of the interaction terms and examined the linear effects of the predictor variables on active transfers. In this model, kinship, direct reciprocity, begging frequency and the rank difference between the possessor and recipient all had significant, positive effects on the frequency of active transfers (Table 2, model 2). Akaike’s Information Criterion (AIC) value for model 2 was lower than that for the full model (model 1: 186.9; model 2: 183.9). The elimination of the nonsignificant relationship quality term reduced the AIC value slightly (183.4; Table 2, model 3). These models indicate that possessors made more active transfers to close kin than to distant kin or nonkin, to those from whom they received the most active transfers, and to those that begged most frequently. Possessors were more likelyto make active transfers to those that ranked below them than to those who held the same rank or ranked above them. Direct reciprocity had a substantially smaller impact on active transfers than did other variables.
Table 2.
Factors that influence active food transfers within dyads
| Standardized coefficient |
SE | Z | P | Wald χ2 | P | |
|---|---|---|---|---|---|---|
| Model 1 | 38.96 | <0.001 | ||||
| Kinship | 0.75 | 0.30 | 2.53 | 0.011 | ||
| Relationship quality | 0.30 | 0.51 | 0.59 | 0.554 | ||
| Direct reciprocity | −0.69 | 0.60 | −1.15 | 0.250 | ||
| Rank difference | 0.88 | 0.40 | 2.22 | 0.027 | ||
| Begging frequency | 1.09 | 0.34 | 3.16 | 0.002 | ||
| Kinship*begging frequency | 0.25 | 0.38 | 0.66 | 0.510 | ||
| Relationship quality*begging frequency | −0.44 | 0.31 | −1.43 | 0.153 | ||
| Direct reciprocity*begging frequency | 0.47 | 0.31 | 1.51 | 0.132 | ||
| Rank difference*begging frequency | −0.28 | 0.35 | −0.81 | 0.419 | ||
| Model 2 | 29.59 | <0.001 | ||||
| Kinship | 0.83 | 0.22 | 3.74 | < 0.001 | ||
| Relationship quality | −0.23 | 0.19 | −1.19 | 0.234 | ||
| Direct reciprocity | 0.24 | 0.12 | 2.05 | 0.041 | ||
| Rank difference | 0.67 | 0.30 | 2.27 | 0.023 | ||
| Begging frequency | 0.80 | 0.17 | 4.81 | <0.001 | ||
| Model 3 | ||||||
| Kinship | 0.66 | 0.17 | 3.93 | <0.001 | ||
| Direct reciprocity | 0.20 | 0.11 | 1.83 | 0.067 | ||
| Rank difference | 0.79 | 0.30 | 2.67 | 0.008 | ||
| Begging frequency | 0.81 | 0.17 | 4.77 | <0.001 |
For passive transfers, the full model was highly significant. Kinship, relationship quality, the rank difference between the possessor and solicitor, and the frequency of begging significantly increased the number of passive transfers (Table 3, model 1). In addition, there were significant interactions between the frequency of begging and all of the other variables. When the nonsignificant term was excluded from the model, the AIC value declined (model 1: 741.3; model 2: 739.7).
Table 3.
Factors that influence passive food transfers within dyads
| ß | SE | Z | P | Wald χ2 | P | |
|---|---|---|---|---|---|---|
| Model 1 | 234.61 | <0.001 | ||||
| Kinship | 0.58 | 0.08 | 7.37 | <0.001 | ||
| Relationship quality | 0.28 | 0.13 | 2.09 | 0.037 | ||
| Direct reciprocity | −0.06 | 0.11 | −0.58 | 0.562 | ||
| Rank difference | 0.20 | 0.10 | 1.86 | 0.063 | ||
| Begging frequency | 0.81 | 0.09 | 8.81 | <0.001 | ||
| Kinship*begging frequency | −0.18 | 0.09 | −1.97 | 0.049 | ||
| Relationship quality*begging frequency | −0.23 | 0.07 | −3.40 | 0.001 | ||
| Direct reciprocity*begging frequency | 0.25 | 0.06 | 4.16 | <0.001 | ||
| Rank difference*begging frequency | −0.21 | 0.09 | −2.50 | 0.012 | ||
| Model 2 | 233.86 | <0.001 | ||||
| Kinship | 0.57 | 0.08 | 7.34 | <0.001 | ||
| Relationship quality | 0.28 | 0.13 | 2.06 | 0.039 | ||
| Rank difference | 0.21 | 0.11 | 1.95 | 0.051 | ||
| Begging frequency | 0.81 | 0.09 | 8.85 | <0.001 | ||
| Kinship*begging frequency | −0.18 | 0.09 | −1.96 | 0.050 | ||
| Relationship quality*begging frequency | −0.23 | 0.07 | −3.39 | 0.001 | ||
| Direct reciprocity*begging frequency | 0.23 | 0.04 | 6.04 | 0.001 | ||
| Rank difference*begging frequency | −0.21 | 0.09 | −2.53 | 0.011 |
Taken together this set of models indicates that the frequency of passive sharing is increased among kin and close associates. High levels of begging also increase the frequency of passive sharing. Passive sharing is more common when possessors outrank solicitors than vice versa. High frequencies of begging tended to reduce the positive impact of relationship quality and rank differences on the number of passive transfers, while frequent begging amplified the positive effects of direct reciprocity on passive transfers.
DISCUSSION
Our results suggest that food transfers may serve multiple functions for chimpanzees. Individuals may use food transfers to provision closely related group members, strengthen relationships with close associates, reciprocate food transfers that they have received in the past and reduce the costs of persistent solicitations. Thus, decisions about whether to initiate, tolerate or resist food transfers may reflect a complex, albeit unconscious, calculus in which individuals weigh the value of the resources they control, the nature and quality of their relationship to solicitors, the past behaviour of solicitors and the costs of resisting solicitations.
Several lines of evidence are consistent with the view that food transfers mainly occur as a response to persistent solicitations, which constitute a form of harassment. First, food transfers were typically preceded by begging gestures. Second, the frequency of begging gestures was significantly related to the frequency of both active and passive transfers within dyads. Third, low levels of begging were likely to be ignored, while higher levels of begging were associated with progressively more active types of responses that seemed to reflect the possessors desire to resist solicitations.
Despite this, there is also evidence that possessors can control the distribution of food, a critical prerequisite for behavioural strategies based on nepotism, reciprocity or selective tolerance (Jaeggi et al. 2010a, b). For example, more active transfers were directed to close kin and reciprocating partners than to others when the frequency of begging was held constant. Similarly, passive transfers were selectively directed to close kin and close associates. Moreover, the negative interactions between begging frequency and several of the main predictor variables suggest that frequent begging can have an inhibitory effect on passive food transfers to close kin, close associates and subordinates. The positive interaction between begging frequency and direct reciprocity of passive transfers suggests that begging may prompt partners to reciprocate.
It is not clear whether the patterns observed here are also characteristic of wild populations of chimpanzees in which meat is the main food item exchanged among adults and males monopolize possessions. Our study groups contained fewer mature males than most wild chimpanzee groups, and more of our data was based on food transfers by females than is the case in studies of meat sharing in the wild. It seems likely that relationship quality might have a greater influence in free-ranging communities than it did in our groups, as free-ranging males often form strong bonds with unrelated males (Mitani 2006). It is also possible that differences in chimpanzees’ interest in meat and the frozen juice disks may influence the patterns of food distribution. This may be, for example, why adult males did not monopolize access to the disks, and why dominance rank did not have a greater influence on food transfers. Finally, the per-capita number of disks distributed in each session was higher than the per-capita number of kills in most hunting parties. The relative abundance of these items may have altered patterns of transfers in some way.
The pattern of food transfers in chimpanzees is relevant to ongoing discussions of the motives underlying cooperation in chimpanzees and other primates and of the origins of human concern for the welfare of others. In humans, food sharing and other forms of cooperation extend beyond networks of close kin and reciprocating partners (Hill et al. 2011; Alvard 2012). In a range of experimental contexts, people from a wide range of cultures behave as if they prefer outcomes that benefit others, even when there is no opportunity for nepotism, reciprocity or reputational gains (e.g. Henrich et al. 2004, 2010). Thus, while humans are clearly motivated by self-interest, they also feel concern for the welfare of others and this concern motivates them to act in ways that go beyond motives derived from direct reciprocity and kinship.
It is difficult to determine whether similar motives underlie food transfers in chimpanzees. However, the pattern of these transfers seems to suggest that they are motivated more by kinship, relationship quality, reciprocity and the avoidance of conflict than by motives associated with other evolutionary processes (Chudek & Henrich 2010). Thus, chimpanzees preferentially transfer food to kin, reciprocating partners, close associates and perhaps to potential mates. If possessors were strongly motivated by concerns for the welfare of others that go beyond the standard evolutionary models, it seems likely that they would spontaneously offer food to others more often, respond more enthusiastically to solicitations, and extend generosity beyond kin and close associates. Thus, food transfers seem compatible with (genetic) self-interest, and standard evolutionary models seem sufficient to explain the evolution of food transfers in chimpanzees.
The patterns of food sharing are largely consistent with the results of experimental studies that were designed to probe the motives that underlie prosociality. There are important methodological differences in these studies, and some dispute about which set of studies provides the most informative insight about chimpanzees’ prosocial preferences (de Waal 2009; Jaeggi et al. 2010b; Melis & Semmann 2010; Silk & House 2011). However, a comparison of chimpanzees’ behaviour across the full range of experiments indicates that chimpanzees perform helpful responses on behalf of others approximately half of the time, even though they are paired with familiar group members and the cost of helping is very low (Silk & House 2011). Thus, the experimental evidence suggests that chimpanzees are considerably less strongly motivated by concern for the welfare of others than humans are.
Knowledge of the pattern of naturalistic food transfers may inform our design of experimental studies of chimpanzee social preferences (Jaeggi et al. 2010b). For instance, the observation that active transfers are uncommon in chimpanzees suggests that it may be profitable to focus on situations in which there are opportunities for passive transfers. Subjects might be given the opportunity to monopolize food or consume it within range of potential solicitors (see de Waal 2000). By varying the quantity, quality and monopolizability of food systematically, it might be possible to evaluate trade-offs between access to desirable food items and the costs of solicitation. Careful analysis of naturalistic behavioural data and well-designed experiments will both contribute to a more complete understanding of the motives and preferences that shape altruistic behaviour in chimpanzees.
Acknowledgments
This work was supported by grants from the MacArthur Foundation Preferences Network. S.F.B. was funded in part by National Science Foundation (NSF) grant SES 0729244 and National Institutes of Health (NIH) Institutional Research and Academic Career Development grant K12 GM00680-05 to Emory University. Support for the Bastrop chimpanzee colony comes from NIH 8U42OD011197-12. The University of Texas MD Anderson Cancer Center is fully accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and this work was approved under Animal Care Protocol 07-92-03887. All experiments described in this paper comply with the laws of the country in which they were performed. We thank Adrian Jaeggi and Ian Gilby for their comments on the manuscript.
References
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:229–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Alvard M. Human sociality. In: Mitani JC, editor. Evolution of Primate Societies. Chicago: University of Chicago Press; 2012. pp. 585–603. [Google Scholar]
- Blurton Jones NG. A selfish origin for human food sharing: tolerated theft. Ethology and Sociobiology. 1984;5:1–3. [Google Scholar]
- Boesch C, Boesch H. Hunting behavior of wild chimpanzees in the Taï National Park. American Journal of Physical Anthropology. 1989;78:547–573. doi: 10.1002/ajpa.1330780410. [DOI] [PubMed] [Google Scholar]
- Boesch C, Boesch-Achermann H. The Chimpanzees of the Taï Forest: Behavioural Ecology and Evolution. Oxford: Oxford University Press; 2000. [Google Scholar]
- Burkhart JM, Hrdy SB, van Schaik CP. Cooperative breeding and human cognitive evolution. Evolutionary Anthropology. 2009;18:175–186. [Google Scholar]
- Chudek M, Henrich J. Cultureegene coevolution, norm-psychology and the evolution of human prosociality. Trends in Cognitive Sciences. 2010;15:218–226. doi: 10.1016/j.tics.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Gilby IC. Meat sharing among the Gombe chimpanzees: harassment and reciprocal exchange. Animal Behaviour. 2006;71:953–963. [Google Scholar]
- Gilby IC, Wrangham RW. Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behavioral Ecology and Sociobiology. 2008;62:1831–1842. [Google Scholar]
- Gilby IC, Emery Thompson M, Ruane JD, Wrangham R. No evidence of short-term exchange of meat for sex among chimpanzees. Journal of Human Evolution. 2010;59:44–53. doi: 10.1016/j.jhevol.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Gomes CM, Boesch C. Wild chimpanzees exchange meat for sex on a long-term basis. PLoS One. 2011;4:e5116. doi: 10.1371/journal.pone.0005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J. The Chimpanzees of Gombe. Cambridge, Massachusetts: Belknap Press; 1986. [Google Scholar]
- Gurven M. To give and to give not: the behavioral ecology of human food transfers. Behavioral and Brain Sciences. 2004;27:543–583. [Google Scholar]
- Henrich J, Boyd R, Bowles S, Camerer C, Fehr E, Gintis H, editors. Foundations of Human Sociality: Economic Experiments and Ethnographic Evidence from Fifteen Small-scale Societies. New York: Oxford University Press; 2004. [Google Scholar]
- Henrich J, Ensminger J, McElreath R, Barr A, Barrett C, Bolyanatz A, Cardenas JC, Gurven M, Gwako E, Henrich N, et al. Markets, religion, community size, and the evolution of fairness and punishment. Science. 2010;327:1480–1484. doi: 10.1126/science.1182238. [DOI] [PubMed] [Google Scholar]
- Hill KR, Walker RS, Božičević M, Eder J, Headland T, Hewlett B, Hurtado AM, Marlowe F, Weissner P, Wood B. Coresidence patterns in hunter-gatherer societies show unique human social structure. Science. 2011;331:1286–1289. doi: 10.1126/science.1199071. [DOI] [PubMed] [Google Scholar]
- Hockings KJ, Humle T, Anderson JR, Biro D, Sousa C, Ohashi G, Matsuzawa T. Chimpanzees share forbidden fruit. PLoS One. 2011;2:e886. doi: 10.1371/journal.pone.0000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi AV, van Schaik CP. The evolution of food sharing in primates. Behavioral Ecology and Sociobiology. 2011;65:2125–2140. [Google Scholar]
- Jaeggi AV, Stevens JMG, van Schaik CP. Tolerant food sharing and reciprocity is precluded by despotism among bonobos but not chimpanzees. American Journal of Physical Anthropology. 2010a;143:41–51. doi: 10.1002/ajpa.21288. [DOI] [PubMed] [Google Scholar]
- Jaeggi AV, Burkhart JM, van Schaik CP. On the psychology of cooperation in humans and other primates: combining the natural history and experimental evidence of prosociality. Philosophical Transactions of the Royal Society B. 2010b;365:2723–2735. doi: 10.1098/rstb.2010.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HS, Hooper PL, Gurven M. The evolutionary and ecological roots of human social organization. Philosophical Transactions of the Royal Society B. 2009;364:3289–3299. doi: 10.1098/rstb.2009.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langergraber K, Mitani J, Vigilant L. Kinship and social bonds in female chimpanzees (Pan troglodytes) American Journal of Primatology. 2009;71:840–851. doi: 10.1002/ajp.20711. [DOI] [PubMed] [Google Scholar]
- McGrew WC. Patterns of plant food sharing by wild chimpanzees. In: Kondo S, editor. Contemporary Primatology: Fifth International Congress of Primatology Nagoya 1974. Basel: Karger; 1975. pp. 304–309. [Google Scholar]
- Melis A, Semmann D. How is human cooperation different? Philosophical Transactions of the Royal Society B. 2010;365:2664–2674. doi: 10.1098/rstb.2010.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani JC. Reciprocal exchange in chimpanzees and other primates. In: Kappeler PM, van Schaik CP, editors. Cooperation in Primates: Mechanisms and Evolution. Heidelberg: Springer-Verlag; 2006. pp. 101–113. [Google Scholar]
- Mitani JC, Watts DP. Demographic influences on the hunting behavior of chimpanzees. American Journal of Physical Anthropology. 1999;109:439–454. doi: 10.1002/(SICI)1096-8644(199908)109:4<439::AID-AJPA2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Mitani JC, Watts DP. Why do chimpanzees hunt and share meat? Animal Behaviour. 2001;61:915–924. [Google Scholar]
- Moore J. The evolution of reciprocal sharing. Ethology and Sociobiology. 1984;5:5–14. [Google Scholar]
- Muller MN, Mitani JC. Conflict and cooperation in wild chimpanzees. Advances in the Study of Behavior. 2005;35:275–331. [Google Scholar]
- Nishida T, Turner LA. Food transfer between mother and infant chimpanzees in the Mahale Mountains National Park, Tanzania. International Journal of Primatology. 1996;17:947–968. [Google Scholar]
- Silk JB. Patterns of food sharing among mother and infant chimpanzees at Gombe National Park, Tanzania. Folia Primatologica. 1978;29:129–141. doi: 10.1159/000155835. [DOI] [PubMed] [Google Scholar]
- Silk JB, Altmann J, Alberts SC. Social relationships among adult female baboons (Papio cynocephalus). I. Variation in the strength of social bonds. Behavioral Ecology and Sociobiology. 2006;61:183–195. [Google Scholar]
- Silk JB, Boyd R. From grooming to giving blood: the origins of human altruism. In: Kappeler P, Silk JB, editors. Mind the Gap: the Origins of Human Universals. Berlin: Springer-Verlag; 2010. pp. 223–244. [Google Scholar]
- Silk JB, House BR. The evolutionary foundations of human moral sentiments. Proceedings of the National Academy of Sciences, USA. 2011;108:10910–10917. doi: 10.1073/pnas.1100305108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocombe KE, Newton-Fisher NE. Fruit sharing between wild adult chimpanzees (Pan troglodytes schweinfurthii): a socially significant event? American Journal of Primatology. 2005;65:638–691. doi: 10.1002/ajp.20123. [DOI] [PubMed] [Google Scholar]
- Stanford CB. Chimpanzees and Red Colobus. Cambridge, Massachusetts: Harvard University Press; 1998. [Google Scholar]
- Stevens JR, Gilby IC. A conceptual framework for nonkin food sharing: timing and currency of benefits. Animal Behaviour. 2004;67:603–614. [Google Scholar]
- Stevens JR, Stephens DW. Food sharing: a model of manipulation by harassment. Behavioral Ecology. 2002;13:393–400. [Google Scholar]
- de Waal FBM. Food-sharing and reciprocal obligations in chimpanzees. Journal of Human Evolution. 1989;18:433–459. [Google Scholar]
- de Waal FBM. The chimpanzee’s service economy: food for grooming. Evolution and Human Behavior. 1997;18:375–386. [Google Scholar]
- de Waal FBM. Attitudinal reciprocity in food sharing among brown capuchin monkeys. Animal Behaviour. 2000;60:253–261. doi: 10.1006/anbe.2000.1471. [DOI] [PubMed] [Google Scholar]
- de Waal FBM. The Age of Empathy. New York: Crown Press; 2009. [Google Scholar]
- Warneken F, Tomasello M. Varieties of altruism in children and chimpanzees. Trends in Cognitive Sciences. 2009;13:397–402. doi: 10.1016/j.tics.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Wrangham RW. Ph.D. thesis. Cambridge University; 1975. Behavioural ecology of chimpanzees in Gombe National Park, Tanzania. [PubMed] [Google Scholar]
- Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Animal Behaviour. 2009;77:873–885. doi: 10.1016/j.anbehav.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


