Abstract
Background
Kaposi's sarcoma remains the most common cancer in Sub-Saharan Africa and the second most common cancer in HIV-infected patients worldwide. Since the introduction of highly active antiretroviral therapy (HAART), there has been a decline in its incidence. However, Kaposi's sarcoma continues to be diagnosed in HIV-infected patients.
Objectives
To assess the added advantage of chemotherapy plusHAART compared toHAART alone; and the advantages of different chemotherapy regimens in HAART and HAART naive HIV infected adults with severe or progressive Kaposi's sarcoma.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and GATEWAY, the WHO Clinical Trials Registry Platform and the US National Institutes of Health's Clinical Trials.gov for ongoing trials and the Aegis archive of HIV/AIDS for conference abstracts. An updated search was conducted in July 2014.
Selection criteria
Randomised trials and observational studies evaluating the effects of any chemotherapeutic regimen in combination with HAART compared to HAART alone, chemotherapy versus HAART, and comparisons between different chemotherapy regimens.
Data collection and analysis
Two review authors assessed the studies independently and extracted outcome data. We used the risk ratio (RR) with a 95% confidence interval (CI) as the measure of effect. We did not conduct meta-analysis as none of the included trials assessed identical chemotherapy regimens.
Medical Subject Headings (MeSH): Antineoplastic Agents [therapeutic use]; Doxorubicin [therapeutic use]; HIV Infections [*complications]; Liposomes; Randomized Controlled Trials as Topic; Sarcoma, Kaposi [*therapy; virology]; Skin Neoplasms [*therapy]; Tretinoin [therapeutic use]
MeSH check words: Humans
Plain Language Summary
Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults
Kaposi's sarcoma was the first tumor to be described in association with HIV infection and is an AIDS-defining condition. It is also known as Kaposi's sarcoma-associated herpes virus (KSHV) as Herpes virus 8 (HHV8) is recognized as an essential and necessary factor in the pathogenesis of KS. Nonetheless, not all HHV-8-infected individuals will develop the disease. The abnormal cells of KS form purple, red, or brown patches, plaques or tumors on the skin. There is no universally accepted system for staging Kaposi's sarcoma. The most commonly used staging system for AIDS-related KS in adults is the AIDS Clinical Trial Group (ACTG) staging.
This review evaluated the effects of highly active antiretroviral therapy (HAART) and chemotherapy, or different chemotherapy regimens for severe or progressive Kaposi's sarcoma in HIV infected adults.
We found six randomised controlled trials and three observational studies that assessed the effects of HAART plus chemotherapy compared with HAART alone; HAART plus chemotherapy compared with HAART plus another chemotherapy regimen; and chemotherapy compared with chemotherapy in the time before HAART was available. Of the nine included studies, seven included patients with a mix of mild to moderate (T0) Kaposi's sarcoma and severe (T1) Kaposi's sarcoma. There was no universal definition for what severity of disease was considered chemotherapy-requiring. For this review, we only extracted data for 792 HIV infected adults with severe Kaposi's sarcoma disease.
The findings from this review suggest that HAART plus chemotherapy may be beneficial in reducing disease progression compared to HAART alone in patients with severe or progressive Kaposi's sarcoma. For patients on HAART, in choosing among different chemotherapy regimens, there was no observed difference between liposomal doxorubcin, liposomal daunorubicin, and paclitaxel. The overall quality of evidence in this review can be described as moderate.
Background
Acquired immunodeficiency syndrome (AIDS)-defining cancers, including Kaposi's sarcoma, have become common comorbidities afflicting HIV-infected individuals (Casper 2011). Kaposi's sarcoma was the first tumour to be described in association with HIV infection and is an AIDS-defining condition. Kaposi's sarcoma remains the most common HIV-associated cancer in Sub-Saharan Africa and the second most common cancer in HIV-infected patients worldwide (Gantt 2010). Since the introduction of ’highly active antiretroviral therapy’ (HAART) or combination antiretroviral therapy (cART), there has been a rapid decline in the incidence of Kaposi's sarcoma, especially in Western countries where HAART is readily available (Di Lorenzo 2007; Jones 2000; Mocroft 2004). The reduction in incidence of Kaposi's sarcoma is less clear in resource poor settings (Semeere 2012). However, it continues to be diagnosed in HIV-infected patients, even among patients with effective viral load suppression and high CD4 counts (Khanlou 2000; Maurer 2007). Survival after a diagnosis of KS in the modern HAART era continues to be sub-optimal in resource poor settings (Freeman 2013). Together, incident cases and poor survival in some settings emphasises the importance of understanding the treatment options for severe or progressive Kaposi's sarcoma.
Description of the condition
Kaposi's sarcoma is thought to originate from herpes virus 8 (HHV8)-infected lymphatic endothelial cells and is also known as Kaposi's sarcoma-associated herpes virus (KSHV). HHV8 is recognised as an essential and necessary factor in the pathogenesis of Kaposi's sarcoma (Browning 1994; Chang 1994; Davis 1997). Nonetheless, not all HHV-8-infected individuals will develop the disease.
Kaposi's sarcoma lesions are comprised of both distinctive spindle cells that line lymph or blood vessels and a variable inflammatory infiltrate, suggesting that it may result from reactive hyper-proliferation induced by chronic inflammation and is not a true neoplasm (Martellotta 2009).The abnormal cells of Kaposi's sarcoma form purple or brown patches, plaques or tumours on the skin. Biopsy for definitive diagnosis is recommended to distinguish Kaposi's sarcoma from other skin conditions that can look similar, which may include bacillary angiomatosis, non-Hodgkin lymphoma, and cutaneous fungal or bacterial infections (Krown 2006).
There is no universally accepted system for staging AIDS-related Kaposi's sarcoma. The most commonly used staging system for AIDS-related Kaposi's sarcoma in adults was developed by the AIDS Clinical Trial Group (ACTG) of the National Institutes of Health (Krown 1989). It is important to note that this staging system is most frequently used in research. Clinically, patients may be grouped more generally by a clinician into either a) sick patients needing an immediate fast-acting intervention (clinically severe Kaposi's sarcoma) or b) those patients that can be treated with HAART alone (clinically mild to moderate Kaposi's sarcoma). There is continued debate in the literature as to the definition of the severity of KS that is truly requiring of immediate fast-acting intervention as compared to HAART alone, and different definitions have been used in different studies (Martin 2013). In ACTG staging, patients are categorised according to three parameters.
Extent of tumour (T): a favourable prognosis (T0) is associated with disease limited to the skin or with minimal involvement of the oral cavity. Those with associated lymphoedema, more extensive oral cavity involvement or other visceral disease are considered to have a poor prognosis (T1).
Immune status (I): the degree of immunosuppression from the HIV infection is an important prognostic factor. Patients with a CD4 count greater than 200 cells/μl are considered to have a favourable prognosis (I0), while those with a lower CD4 count are classified as having a poor prognosis (I1).
Severity of systemic illness (S): features associated with a poor risk included the following (S1): a history of opportunistic infection, thrush, B symptoms (fever, night sweats, significant weight loss, diarrhoea for more than two weeks). Patients without any of these factors have a more favourable prognosis (S0).
Description of the intervention
HAART has been a very important step in the treatment of AIDS-related Kaposi's sarcoma and has led to a substantial reduction in morbidity and mortality (Bower 2006; Carrieri 2003; Di Lorenzo 2007; Engels 2006; Grulich 2001). In addition to HAART, many other potential systemic and local therapeutic regimens exist, which have been studied in HIV-infected adults. Generally, more widespread disease, or disease affecting internal organs, is treated with systemic therapy (including interferon alpha, liposomal anthracyclines or paclitaxel). Liposomal anthracyclines were found in the previous version of this Cochrane review to have a superior response rate without an increase in toxic side effects (Dedicoat 2003), and have been adopted by many as first-line therapy for severe Kaposi's sarcoma (Di Trolio 2006). However, at this time there are no guidelines from the World Health Organization regarding liposomal anthracyclines as first-line therapy and access to these drugs is still very limited in the developing world. Since the prior Cochrane review, more studies have been published in this area, necessitating an update of the literature. With the advent of ART, local treatment is often reserved for patients who do not respond to systemictherapy.
Why it is important to do this review
The incidence of AIDS-related Kaposi's sarcoma remains high in many countries where HIV-1 is prevalent, especially in Sub-Saharan Africa. This review aims to identify high-quality studies of therapy for clinically severe or treatment-refractory Kaposi's sarcoma. Studies using HAART plus chemotherapy or different chemotherapy regimens in both the pre- andpost-HAART era will be included. The previous version of this review included HIV-1-infected adult patients with both mild and severe/progressive Kaposi's sarcoma (Dedicoat 2003). However, this update of the review will be restricted to severe or progressive Kaposi's sarcoma. Another ongoing Cochrane systematic review will address treatment for mild and moderate Kaposi's sarcoma in ART-naive HIV-infected individuals. We aim to present the best available evidence from randomised controlled trials and observational studies. The findings from our review will help to guide policy and practice on the treatment of AIDS-related Kaposi's sarcoma in adults with HIV infection.
Objectives
To assess the added advantage of chemotherapy plus HAART compared to HAART alone; and the advantages of different chemotherapy regimens in HAART and HAART naive HIV infected adults with severe or progressive Kaposi's sarcoma.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and observational studies with a comparison group.
Types of participants
HIV-infected adults diagnosed with Kaposi's sarcoma, considered to have the following.
-
Severe Kaposi's sarcoma, requiring anti-Kaposi's sarcoma therapy, defined as patients with:
ACTG T1 disease; or
-
where ACTG staging is not available or analysis by ACTG stage is unobtainable, defined as:
documented or clinically suspected pulmonary or gastrointestinal Kaposi's sarcoma;
oral Kaposi's sarcoma that interferes with chewing or swallowing;
Kaposi's sarcoma tumour-associated oedema that affects function;
tumour ulceration that is unresponsive to general local care;
life-threatening Kaposi's sarcoma, deemed by the study authors to require immediate anti-Kaposi's sarcoma therapy such as chemotherapy.
-
Progressive disease despite prior treatment. Progression is defined according to ACTG response criteria (Krown 1989) as:
an increase of 25% or more in the size of previously existing lesions;
the appearance of new lesions or new sites of disease;
a change in the character of 25% or more of the skin or oral lesions from macular to plaque-like or nodular;
the development of new or increasing tumor-associated edema or effusions also considered to represent disease progression.
Types of interventions
Any chemotherapeutic regimen in combination with HAART compared to HAART alone, chemotherapy versus HAART, and comparisons between chemotherapy regimens both the pre-HAART era and while on HAART. For this review, HAART is defined as a combination of three or more antiretroviral agents, either taken individually or in fixed-dose combinations, as opposed to single or dual-drug therapy.
Types of outcome measures
Primary outcomes
Mortality
Progression of Kaposi's sarcoma
Clinical response (complete, partial and no response), which includes assessment of the number of lesions, size and oedema (Krown 1989)
Secondary outcomes
Time to response
Adverse events (including toxicity, worsening of co-existent disease or both)
Kaposi's sarcoma immune reconstitution inflammatory syndrome (IRIS)
Adherence
Quality of life
Search methods for identification of studies
Electronic searches
We formulated a comprehensive and exhaustive search strategy in order to identify all relevant studies regardless of language or publication status (published, unpublished, in press and in progress) (Table 1; Table 2; Table 3). Full details of the Cochrane HIV/AIDS Review Group methods and the journals handsearched are published in the section on Collaborative Review Groups in The Cochrane Library (Cochrane HIV/AIDS Group 2011). We searched the Cochrane CENTRAL and MEDLINE databases on November 9, 2012 (1980 to 2012); EMBASE and GATEWAY on November 26, 2012 (1980 to 2014). An updated search of the databases was done on July 4, 2014.
Table 1. Cochrane CENTRAL search strategy.
| ID | Search |
|---|---|
| #1 | MeSH descriptor: [Sarcoma, Kaposi] explode all trees |
| #2 | kaposi or karposi or KS |
| #3 | MeSH descriptor: [Herpesvirus 8, Human] explode all trees |
| #4 | hhv-8 or hhv8 or KSHV or “human herpes virus 8” or “human herpesvirus 8” |
| #5 | #1 or #2 or #3 or #4 |
| #6 | MeSH descriptor: [HIV Infections] explode all trees |
| #7 | MeSH descriptor: [HIV] explode all trees |
| #8 | hiv or hiv-1* or hiv-2* or hiv1 or hiv2 or HIV INFECT* or HUMAN IMMUNODEFICIENCY VIRUS or HUMAN IMMUNEDEFICIENCY VIRUS or HUMAN IMMUNE-DEFICIENCY VIRUS or HUMAN IMMUNO-DEFICIENCY VIRUS or HUMAN IMMUN* DEFICIENCY VIRUS or ACQUIRED IMMUNODEFICIENCY SYNDROME or ACQUIRED IMMUNEDEFICIENCY SYNDROME or ACQUIRED IMMUNO-DEFICIENCY SYNDROME or ACQUIRED IMMUNE-DEFICIENCY SYNDROME or ACQUIRED IMMUN* DEFICIENCY SYNDROME |
| #9 | MeSH descriptor: [Lymphoma, AIDS-Related] this term only |
| #10 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only |
| #11 | #6 or #7 or #8 or #9 or #10 |
| #12 | #5 and #11 from 1980 to 2012, in Trials (Word variations have been searched) |
Table 2. PubMed search strategy.
| Search | Query |
|---|---|
| #12 | Search ((#1 AND #2 AND #10)) AND (“1980/01/01”[Date - Publication] : “2012/11/0”[Date - Publication]) |
| #11 | Search (#1 AND #2 AND #10) |
| #10 | Search (#3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) |
| #9 | Search ((observational[tiab] AND (study[tiab] OR studies[tiab])) |
| #8 | Search (evaluation studies as topic[mh:noexp] OR (evaluation[tiab] AND (study[tiab] OR studies[tiab])) |
| #7 | Search (cross-sectional studies[mh] OR cross section*[tiab]) |
| #6 | Search (case control studies[mh] OR case control[tiab]) |
| #5 | Search ((follow up[tiab] OR prospective[tiab] OR longitudinal[tiab] OR retrospective[tiab]) AND (study[tiab] OR studies[tiab])) |
| #4 | Search (cohort studies[mh] OR cohort[tiab] OR cohorts[tiab]) |
| #3 | Search (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) |
| #2 | Search sarcoma, kaposi[mh] OR kaposi*[tiab] OR karposi*[tiab] OR KS [tiab] OR herpesvirus 8, human[mh] OR human herpes virus 8[tiab] OR human herpesvirus 8[tiab] OR hhv-8[tiab] OR hhv8[tiab] OR KSHV[tiab] |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv-1*[tiab] OR hiv-2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno-deficiency virus[tiab] OR human immune-deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) ORacquired immunodeficiency syndrome [tiab] ORacquired immunedeficiency syndrome [tiab] OR acquired immuno-deficiency syndrome [tiab] ORacquired immune-deficiency syndrome [tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR “sexually transmitted diseases, Viral”[MeSH:NoExp]) |
Table 3. EMBASE search strategy.
| No. | Query |
|---|---|
| #16 | #1 AND #13 AND #14 AND [embase]/lim AND [1-1-1980]/sd NOT [26-1 1-2012]/sd |
| #15 | #1 AND #13 AND #14 |
| #14 | ’kaposi sarcoma’/exp OR ’kaposi sarcoma’ OR ’human herpesvirus 8′/exp OR ’human herpesvirus 8′ OR kaposi*:ab,ti OR karposi*:ab,ti OR ks:ab,ti OR ’human herpes virus 8′:ab,ti OR ’hhv-8′:ab,ti OR hhv8:ab,ti OR kshv:ab,ti |
| #13 | #7 OR #8 OR #9 OR #10 OR #11 OR #12 |
| #12 | ’observational study’/exp OR (observational NEXT/1 (study OR studies)):ab,ti |
| #11 | ’evaluation’/exp OR (evaluation NEXT/1 (study OR studies)):ab,ti |
| #10 | ’cross-sectional study’/exp OR (cross NEXT/1 section*):ab,ti |
| #9 | ’case control study’/exp OR ’case control’:ab,ti |
| #8 | ’cohort analysis’/exp OR ’cohort analysis’ OR ’longitudinal study’/exp OR ’longitudinal study’ OR ’prospective study’/exp OR ’prospective study’ OR ’follow up’/exp OR ’follow up’ OR cohort*:ab,ti OR ’longitudinal studies’:ab,ti OR ’prospective studies’:ab,ti |
| #7 | #2 NOT #6 |
| #6 | #3 NOT #5 |
| #5 | #3 AND #4 |
| #4 | ’human’/de |
| #3 | ’animal’/de OR ’nonhuman’/de OR ’animal experiment’/de |
| #2 | ’randomised controlled trial’/de OR ’randomised controlled trial (topic)’/exp OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR placebo*:ab,ti OR ’crossover procedure’/de OR ’double-blind procedure’/de OR 'single-blind procedure’/de OR (doubl* NEAR/3 blind*):ab,ti OR(singl* NEAR/3 blind*):ab,ti OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*) :ab,ti |
| #1 | ’human immunodeficiency virus infection’/exp OR ’human immunodeficiency virus’/exp OR ’human immunodeficiency virus’:ab,ti OR ’human immuno+deficiency virus’:ab,ti OR ’human immunedeficiency virus’:ab,ti OR ’human immune+deficiency virus’:ab,ti OR hiv:ab,ti OR ’hiv-1′:ab,ti OR ’hiv-2′:ab,ti OR ’acquired immunodeficiency syndrome’:ab, ti OR ’acquired immuno+deficiency syndrome’:ab,ti OR ’acquired immunedeficiency syndrome’:ab,ti OR ’acquired immune+deficiency syndrome’:ab,ti |
Cochrane Central Register of Controlled Trials (CENTRAL) 2012 (Issue 10);
MEDLINE;
EMBASE;
GATEWAY.
Along with MeSH terms and relevant keywords, we used the Cochrane highly sensitive search strategy for identifying reports of randomised controlled trials in MEDLINE and the Cochrane HIV/AIDS Group's existing validated strategies for identifying references relevant to HIV infection and AIDS. The search strategy was iterative, in that we searched the references of included studies for additional relevant references. There was no language restriction.
We also searched for unpublished and ongoing trials using relevant search terms in:
the US National Institutes of Health's Clinical Trials.gov (www.clinicaltrials.gov); and
the WHO Clinical Trials Registry Platform (ICTRP).
Searching other resources
We searched the Aegis archive of HIV/AIDS conference abstracts (http://www.aegis.org/) on 24 May 2013. Aegis includes abstracts for the following conferences:
International AIDS Society, International AIDS Conference (IAC), 1985 to 2006;
Conference on Retroviruses and Opportunistic Infections (CROI), 1994 to 2008;
European AIDS Society Conference, 2001 and 2003;
International AIDS Society, Conference on HIV Pathogenesis, Treatment and Prevention (IAS), 2001 to 2005;
British HIV/AIDS Association, 2001 to 2010;
US National HIV Prevention Conference, 1999, 2001, 2003, 2005 2007, 2009 and 2011.
We also searched the CROI and International AIDS Society websites for abstracts presented at conferences subsequent to those listed above (CROI, 2009 to 2012; IAC, 2008 to 2010; IAS, 2007 to 2011). In addition, we contacted experts in the field to identify further potentially eligible studies.
Data collection and analysis
Selection of studies
Two authors (GO and CO) independently read the titles and abstracts from the search output to identify potentially eligible studies. We obtained full-text articles for all citations identified as potentially eligible and two authors independently inspected these to establish the relevance of the article using the prespecified criteria. We resolved all disagreements by discussion and by contacting the third author (EF).
Data extraction and management
We designed a standardised data extraction form and two authors independently extracted data onto this. We extracted the following characteristics from each included study:
Administrative details: author(s); published or unpublished; year of publication; year(s) in which study was conducted.
Details of the study: study design; type, duration and completeness of follow-up; study location.
Details of participants: age; gender; clinical characteristics (e.g. baseline CD4 cell count, viral load, opportunistic infections).
Details of treatment.
Details of outcomes.
Details necessary for ’Risk of bias’ assessment.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each study using the ’Risk of bias’ assessment tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion. The Cochrane approach assesses risk of bias in individual studies across six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential biases.
Sequence generation (checking for selection bias)
Adequate: investigators described a random component in the sequence generation process, such as the use of random number table, coin tossing, card or envelope shuffling.
Inadequate: investigators described a non-random component in the sequence generation process, such as the use of odd or even date of birth, algorithm based on the day or date of birth, hospital or clinic record number.
Unclear: insufficient information to permit judgement of the sequence generation process.
Allocation concealment (checking for selection bias)
Adequate: participants and the investigators enrolling participants cannot foresee assignment (e.g. central allocation or sequentially numbered, opaque, sealed envelopes).
Inadequate: participants and investigators enrolling participants can foresee upcoming assignment (e.g. an open random allocation schedule, a list of random numbers), or envelopes were unsealed, non-opaque or not sequentially numbered.
Unclear: insufficient information to permit judgement of the allocation concealment or the method is not described.
Blinding (checking for performance bias and detection bias)
Adequate: blinding of the participants, key study personnel and outcome assessor and it is unlikely that the blinding could have been broken. Not blinded but a situation where non-blinding is unlikely to introduce bias.
Inadequate: no blinding or incomplete blinding when the outcome is likely to be influenced by lack of blinding.
Unclear: insufficient information to permit judgement of the adequacy or otherwise of the blinding.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
Adequate: no missing outcome data, reasons for missing outcome data are unlikely to be related to true outcome or missing outcome data are balanced in numbers across groups.
Inadequate: reasons for missing outcome data are likely to be related to true outcome, with either imbalance in numbers across groups or reasons for missing data.
Unclear: insufficient reporting of attrition or exclusions.
Selective reporting
Adequate: a protocol is available and clearly states that the primary outcome is the same as in the final trial report.
Inadequate: the primary outcome differs between the protocol and final trial report.
Unclear: no trial protocol is available or there is insufficient reporting to determine whether selective reporting is present.
Other sources of bias
Adequate: there is no evidence of bias from other sources.
Inadequate: there is potential bias present from other sources (e.g. early stopping of trial, fraudulent activity, extreme baseline imbalance or bias related to specific study design).
Unclear: insufficient information to permit judgement of other forms of bias.
For non-randomised studies, we used the Newcastle-Ottawa Scale to assess the risk of bias in three major areas: selection of study groups, comparability of groups and ascertainment of outcomes (Appendix 1).
Measures of treatment effect
For randomised controlled trials and observational studies, we calculated the risk ratio (RR) for dichotomous outcomes with a 95% confidence interval (CI). For continuous data we calculated the mean difference with a 95% CI.
Dealing with missing data
We did not impute missing data. We contacted the authors of the included studies if there were any missing or unclear data.
Assessment of heterogeneity
We planned to assess statistical heterogeneity by visually inspecting the forest plots to detect overlapping CIs, applying the Chi2 test (P value < 0.10 considered statistically significant) and by using the I2 statistic, where an I2 value of greater than 75% represents substantial heterogeneity. The studies differed significantly in terms of participants and interventions, precluding any meta-analysis.
Assessment of reporting biases
We did not explore the likelihood of reporting bias using funnel plots, since we did not combine any of the studies in a meta-analysis. However, we conducted a comprehensive search to identify all relevant studies.
Data synthesis
We have presented the results of individual studies narratively since meta-analysis was not possible.
Quality of evidence
We assessed the quality of evidence across each outcome measure using the GRADE approach. The quality rating across studies has four levels: high, moderate, low or very low. Randomised trials are categorised as high quality but can be downgraded; similarly, observational studies are categorised as low quality and can be downgraded or upgraded. We used the GRADEpro software to generate GRADE evidence profiles and ’Summary of findings’ tables.
Subgroup analysis and investigation of heterogeneity
We planned to explore heterogeneity by subgroup analyses. However, we did not combine any of the studies in a meta-analysis.
Results
Description of studies
Results of the search
See the PRISMA flow diagram (Figure 1) and the Characteristics of included studies table for details.
Figure 1. Study flow diagram.
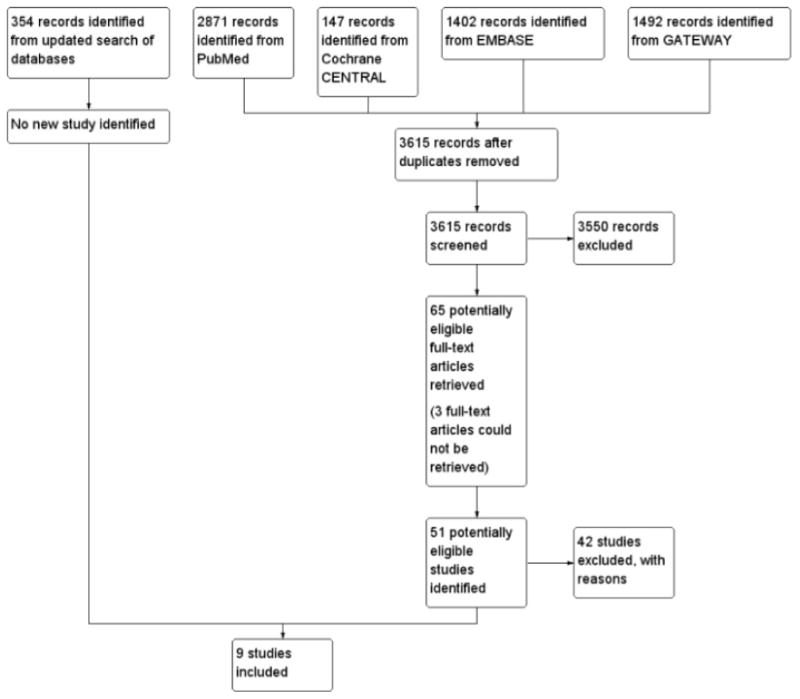
We conducted a search for studies in the various databases listed in Search methods for identification of studies in May 2013 and an updated search in July 2014. We obtained a total of 3615 titles and abstracts after de-duplication of references. Two authors independently scanned through the titles and abstracts using our prespecified inclusion criteria. We identified 65 potentially eligible studies and obtained the full-tex tarticles. Nine studies that met our inclusion criteria were included. We gave reasons for excluding any potentially eligible study. See Characteristics of excluded studies.
Included studies
Types of studies
We included six randomised controlled trials (Cianfrocca 2010; Cooley 2007; Gill 1996; Martin-Carbonero 2004; Mosam 2012; Olweny 2005) and three observational studies (Bower 2009; Grünaug 1998; Hernandez 1997). Where multiple papers were published on the same cohort of patients, we refer to the primary study only, but refer to additional publications in the Characteristics of included studies.
Types of participants
Of the nine included studies, seven had participants with a mix of T0 and T1 Kaposi's sarcoma (Bower 2009; Cianfrocca 2010; Cooley 2007; Hernandez 1997; Martin-Carbonero 2004; Mosam 2012; Olweny 2005). Two studies had only T1 disease patients (Gill 1996; Grünaug 1998). For all studies, we extracted data only for participants meeting criteria of severe disease, as defined above. All included studies did measure ACTG stage, and ultimately were able to provide results according to stage after communication with study authors. Therefore, for all included papers, only patients with T1 disease were included. Of note, the two studies included in the prior Cochrane review on Kaposi's sarcoma were not included in the current review due to a mix of Kaposi's sarcoma stages; T1 disease patients could not be separated from T0 (Northfelt 1998; Stewart 1998).
Participants in all the studies were HIV-infected adults with biopsy-proven Kaposi's sarcoma. Mosam 2012 included 100 patients with T1 Kaposi's sarcoma (out of a total of 112 treatmentnaive HIV-infected patients). It should be noted that T1 patients in this study were deemed by the author not to require “urgent” chemotherapy. Cianfrocca 2010 included 49 patients with T1 Kaposi's sarcoma out of a total of 73 patients aged 18 years and above. Bower 2009 included 254 patients of which 79 were T1 Kaposi's sarcoma patients (Letang 2013 included a later version of Bower's UK cohort of 129 T1 Kaposi's sarcoma patients). Cooley 2007 included 46 patients with T1 disease out of a total of 79 patients. Olweny 2005 included a total of 470 antiretroviral therapy (ART)-naive patients, of which 376 had T1 Kaposi's sarcoma. Martin-Carbonero 2004 included 10 patients with T1 disease out of a total of 28 HIV-infected patients with moderate to advanced Kaposi's sarcoma. Grünaug 1998 included 29 HIV-infected males with pulmonary (T1) Kaposi's sarcoma. Hernandez 1997 included 34 T1 Kaposi's sarcoma patients out of a total of 44 homosexual or bisexual men. Gill 1996 included 227 HIV-infected adults with T1 disease. All participants met this review's criteria of having severe KS.
Types of interventions
None of the included studies with the same study design compared similar interventions.
Mosam 2012 compared HAART plus doxorubicin, bleomycin and vincristine (ABV) versus HAART alone. There were a total of 100 T1 Kaposi's sarcoma patients, with 50 in the HAART plus ABV group and 50 in the HAART alone group. Chemotherapy was started within one month of initiation of HAART. Of note, when ABV was not available, oral etoposide was substituted.
Martin-Carbonero 2004 compared HAART plus pegylated liposomal doxorubicin (PLD) versus HAART alone. There were a total of 10 T1 Kaposi's sarcoma patients, with five in the HAART plus PLD group and five in the HAART alone group. HAART and chemotherapy were started simultaneously at the beginning of the study.
The Bower 2009 (Letang 2013, Bower 2014) UK cohort included patients on HAART alone as well as patients on HAART plus liposomal anthracycline. Of the 79 T1 patients in the study, there were a total of 73 patients with severe Kaposi's sarcoma in the HAART alone and HAART plus chemotherapy groups. Letang 2013, a pooled analysis of four cohort studies, included patients from the Bower 2009 UK cohort. There were 129 T1 disease patients out of a total of 213 patients, with 65 T1 patients in the HAART plus liposomal anthracycline group and 64 T1 patients in the HAART alone group. All T1 Kaposi's sarcoma patients in this study were meant to receive chemotherapy as per protocol; patients who received HAART alone were therefore exceptions. The original Bower 2009 cohort was not designed with the intent to compare these two groups.
Cianfrocca 2010 compared HAART plus paclitaxel versus HAART plus PLD. There were a total of 24 participants in the paclitaxel group and 25 in the PLD group. Participants were required to receive HAART for at least 14 days before study enrolment.
Cooley 2007 compared HAART plus pegylated liposomal doxorubicin versus HAART plus liposomal daunorubicin. Only 76 out of 80 patients in the overall study were on ART. There were a total of 46 T1 Kaposi's sarcoma patients with 34 in the PLD group and 12 in the liposomal daunorubicin group. The time interval between commencement of HAART and chemotherapy, and the type of HAART regimen received were not described.
Gill 1996 compared liposomal daunorubicin versus ABV in the pre-HAART era. There were a total of 227 participants, with 116 in the liposomal daunorubicin group and 111 in the ABV group. Olweny 2005 was a four-arm trial that compared supportive care versus supportive care plus oral etoposide, ABV and radiotherapy. There were a total of 378 ART-naive T1 Kaposi's sarcoma patients, of which 178 were in the oral etoposide (90 patients) and ABV (88 patients) groups.
There were four intervention groups in Hernandez 1997: alpha-2 interferon, ABV, bleomycin and a no treatment group. Ten patients with limited Kaposi's sarcoma received alpha-2 interferon plus zidovudine, 24 patients with advanced Kaposi's sarcoma (12 in each group) received either intramuscular bleomycin or low-dose ABV, and 10 “poor risk” patients received no treatment due to financial constraint.
In Grünaug 1998, also performed in the pre-HAART era, 17 out of the 20 participants in the liposomal doxorubicin group received antiretroviral therapy. In the group that received conservative management, 4 out of the 9 participants had bleomycin and vinblastine or vincristine; five had no chemotherapy, and 2 of the 5 participants that did not receive chemotherapy had interferon alpha.
Types of outcome measures
The outcomes reported were mortality (Bower 2009; Cooley 2007; Grünaug 1998; Hernandez 1997; Mosam 2012; Olweny 2005), Kaposi's sarcoma immune reconstitution inflammatory syndrome (IRIS) (Bower 2009), tumour response (Cianfrocca 2010; Cooley 2007; Gill 1996; Hernandez 1997; Martin-Carbonero 2004; Mosam 2012; Olweny 2005), adverse events (Cianfrocca 2010; Cooley 2007; Gill 1996; Grünaug 1998; Hernandez 1997; Mosam 2012; Olweny 2005), time to response (Cooley 2007; Gill 1996; Grünaug 1998; Hernandez 1997; Olweny 2005) and quality of life (Cianfrocca 2010; Gill 1996; Olweny 2005). However, Cianfrocca and Olweny did not report quality of life according to disease stage. None of the included studies reported outcome data on adherence.
Settings
The studies were conducted in various settings including: Germany (Grünaug 1998), South Africa (Mosam 2012), Spain (Martin-Carbonero 2004), the UK (Bower 2009), the USA (Cianfrocca 2010; Cooley 2007; Gill 1996), Venezuela (Hernandez 1997) and Zimbabwe (Olweny 2005).
Risk of bias in included studies
For assessment results, please see the ’Risk of bias’ graph and summary (Figure 2; Figure 3), and the Newcastle-Ottawa quality assessment scale for included cohort studies (Appendix 2). The overall methodological quality of the studies was acceptable. We obtained additional information by contacting the study authors to be able to make informed assessments where necessary.
Figure 2.
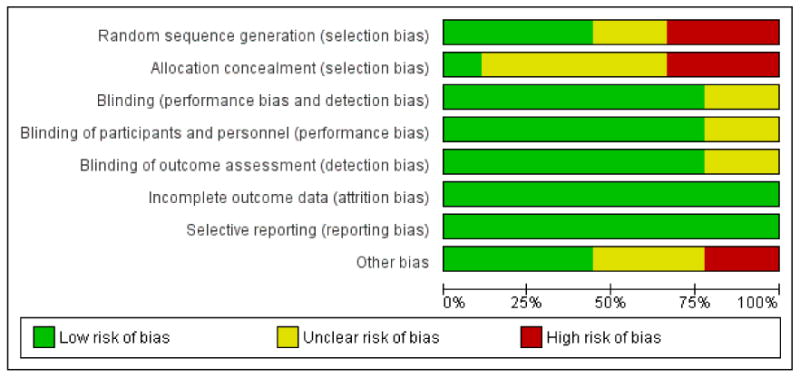
’Risk of bias’ graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
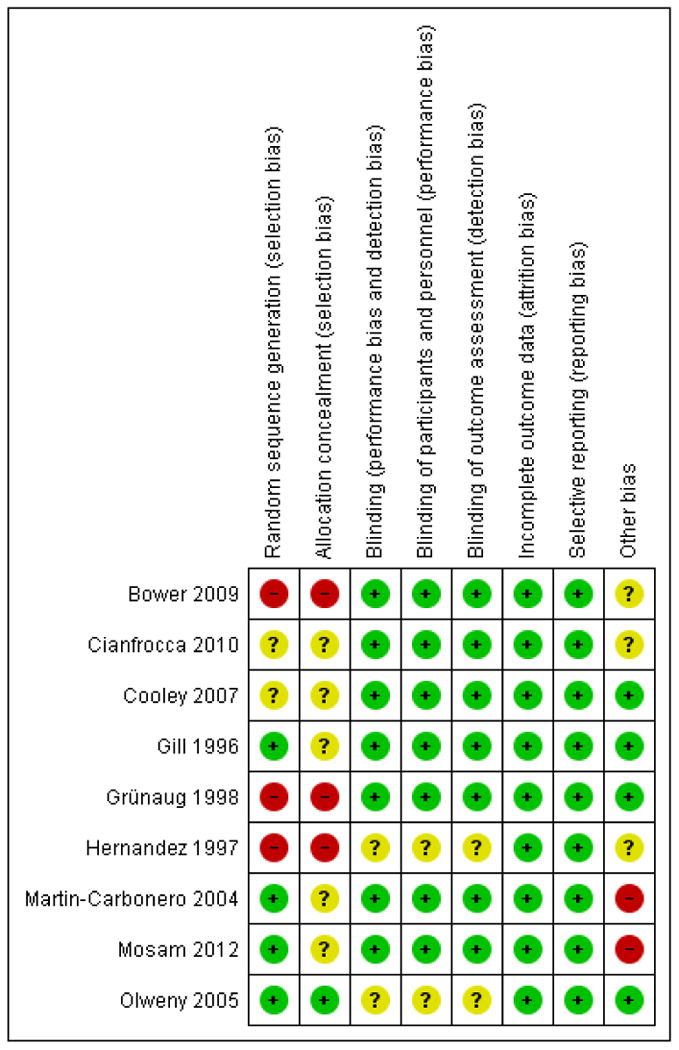
’Risk of bias’ summary: review authors’ judgements about each risk of bias item for each included study.
Allocation
We did not find information on the method of sequence generation and allocation concealment in any of the studies except Olweny 2005.
Blinding
There was no blinding to intervention in any of the studies. However, the reported outcomes were objective and not likely to be affected by lack of blinding. In the Cooley 2007 trial, an independent AIDS expert without knowledge of the patient evaluated the outcome.
Incomplete outcome data
There was no differential loss to follow-up in any of the studies.
Selective reporting
We did not find any evidence of selective outcome reporting in any of the studies.
Other potential sources of bias
The Cianfrocca 2010 trial was terminated prematurely due to slow accrual. There were some reported baseline imbalances in the Martin-Carbonero 2004 and Mosam 2012 studies. The Mosam 2012 study patients with T1 disease were deemed not to“urgently” require chemotherapy, and therefore may have been less sick than the T1 patients in other studies included in this review. All T1 KS patients in Bower 2009 were meant to receive chemotherapy as per protocol, patients who received HAART alone were therefore exceptions. We did not explore the potential for publication bias since we did not combine any of the studies in a meta-analysis. However, we conducted a comprehensive search to identify all relevant studies.
For non-randomised studies
(Grünaug 1998) had a cohort that was representative of HIV-infected adults with Kaposi's sarcoma and adjusted for potential confounders. Bower 2009 and Hernandez 1997 did not report adjusting for any other potential confounders for all outcomes of interest. All included observational studies described complete follow up of the study participants or characteristics of the participants lost to follow up (Appendix 2).
Effects of interventions
See: Summary of findings for the main comparison HAART + ABV compared to HAART alone for the treatment of severe Kaposi's sarcoma in HIV-infected adults; Summary of findings 2 HAART + pegylated liposomal doxorubicin compared to HAART alone for the treatment of severe Kaposi's sarcoma in HIV-infected adults; Summary of findings 3 HAART + liposomal anthracycline compared to HAART alone for the treatment of severe Kaposi's sarcoma in HIV-infected adults; Summary of findings 4 HAART + paclitaxel compared to HAART + pegylated liposomal doxorubicin for the treatment of severe Kaposi's sarcoma in HIV-infected adults; Summary of findings 5 HAART + pegylated liposomal doxorubicin compared to HAART + liposomal daunorubicin for the treatment of severe Kaposi's sarcoma in HIV-infected adults; Summary of findings 6 Liposomal daunorubicin compared to ABV for the treatment of severe Kaposi's sarcoma in HIV-infected adults; Summary of findings 7 Oral etoposide compared to ABV for the treatment of severe Kaposi's sarcoma in HIV-infected adults; Summary of findings 8 Bleomycin compared to ABV for the treatment of severe Kaposi's sarcoma in HIV-infected adults; Summary of findings 9 Liposomal doxorubicin compared to conservative management for the treatment of severe Kaposi's sarcoma in HIV-infected adults
Summary of Findings for the Main Comparison [Explanation].
HAART + ABV compared to HAART alone for the treatment of severe Kaposi's sarcoma in HIV-infected adults (Mosam 2012) – RCT
Patient or population: HIV-infected adults with severe (T1) Kaposi's sarcoma
Settings: Durban, South Africa
Intervention: HAART + ABV
Comparison: HAART alone
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| HAART alone | HAART + ABV | |||||
| Mortality | 240 per 1000 | 221 per 1000 (108 to 451) | RR 0.92 (0.45 to 1.88) | 100 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Progressive disease | 200 per 1000 | 20 per 1000 (2 to 150) | RR 0.1 (0.01 to 0.75) | 100 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Clinical response - complete response | 80 per 1000 | 160 per 1000 (51 to 498) | RR 2 (0.64 to 6.22) | 100 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Clinical response - partial response | 280 per 1000 | 479 per 1000 (283 to 815) | RR 1.71 (1.01 to 2.91) | 100 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Clinical response - stable disease | 160 per 1000 | 10 per 1000 (0 to 158) | RR 0.06 (0 to 0.99) | 100 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Clinical response - overall response (complete and partial) | 360 per 1000 | 641 per 1000 (418 to 979) | RR 1.78 (1.16 to 2.72) | 100 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Adverse events Follow-up: mean 12 months | 520 per 1000 | 458 per 1000 (307 to 686) | RR 0.88 (0.59 to 1.32) | 100 (1 study) | ⊕⊕⊕◯ moderate1 | |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
ABV: doxorubicin, bleomycin and vincristine; CI: confidence interval; HAART: highly active antiretroviral therapy; RCT: randomised controlled trial; RR: risk ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
There were very few events with very wide confidence intervals.
Additional Summary of Findings [Explanation].
HAART + pegylated liposomal doxorubicin compared to HAART alone for the treatment of severe Kaposi's sarcoma in HIV-infected adults (Martin-Carbonero 2004) – RCT
Patient or population: HIV-infected adults with severe (T1) Kaposi's sarcoma
Settings: Spain
Intervention: HAART + PLD
Comparison: HAART alone
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| HAART alone | HAART + PLD | |||||
| Clinical response | 0 per 1000 | 0 per 1000 (0 to 0) | RR 9 (0.61 to 133.08) | 10 (1 study) | ⊕⊕⊕◯ moderate1 | |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; HAART: highly active antiretroviral therapy; PLD: pegylated liposomal doxorubicin; RCT: randomised controlled trial; RR: risk ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
There were very few events with very wide confidence intervals.
HAART + liposomal anthracycline compared to HAART alone for the treatment of severe Kaposi's sarcoma in HIV-infected adults (Bower 2009) - cohort study
Patient or population: HIV-infected adults with severe (T1) Kaposi's sarcoma
Settings: United Kingdom
Intervention: HAART + liposomal anthracycline
Comparison: HAART alone
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| HAART alone | HAART + liposomal anthracycline | |||||
| Mortality | 62 per 1000 | 77 per 1000 (22 to 274) | RR 1.23 (0.35 to 4.38) | 129 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Kaposi's sarcoma IRIS | 125 per 1000 | 61 per 1000 (20 to 194) | RR 0.49 (0.16 to 1.55) | 129 (1 study) | ⊕⊕⊕⊕◯ moderate1 | |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; IRIS: immune reconstitution inflammatory syndrome; RR: risk ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
There were very few events with very wide confidence intervals.
HAART + paclitaxel compared to HAART + PLD for the treatment of severe Kaposi's sarcoma in HIV-infected adults (Cianfrocca 2010) – RCT
Patient or population: HIV-infected adults with severe (T1) Kaposi's sarcoma
Settings: USA
Intervention: HAART + paclitaxel
Comparison: HAART + PLD
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| HAART + PLD | HAART + paclitaxel | |||||
| Progression of Kaposi's sarcoma | 40 per 1000 | 42 per 1000 (3 to 629) | RR 1.04 (0.07 to 15.73) | 49 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Clinical response - complete response | 40 per 1000 | 83 per 1000 (8 to 860) | RR 2.08 (0.2 to 21.5) | 49 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Clinical response - partial response | 360 per 1000 | 374 per 1000 (180 to 781) | RR 1.04 (0.5 to 2.17) | 49 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Clinical response - stable disease | 400 per 1000 | 248 per 1000 (108 to 580) | RR 0.63 (0.27 to 1.45) | 49 (1 study) | ⊕⊕⊕◯ moderate1 | |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; HAART: highly active antiretroviral therapy; PLD: pegylated liposomal doxorubicin; RCT: randomised controlled trial; RR: risk ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
There were very few events with very wide confidence intervals.
HAART + pegylated liposomal doxorubicin compared to HAART + liposomal daunorubicin for the treatment of severe Kaposi's sarcoma in HIV-infected adults (Cooley 2007) – RCT
Patient or population: HIV-infected adults with severe (T1) Kaposi's sarcoma
Settings: USA
Intervention: HAART + pegylated liposomal doxorubicin
Comparison: HAART + liposomal daunorubicin
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| HAART + liposomal daunorubicin | HAART + PLD | |||||
| Progression of Kaposi's sarcoma | See comment | See comment | Not estimable | 46 (1 study) | ⊕⊕⊕◯ moderate1 | |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; HAART: highly active antiretroviral therapy; PLD: pegylated liposomal doxorubicin; RCT: randomised controlled trial; RR: risk ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
There were very few events with very wide confidence intervals.
Liposomal daunorubicin compared to ABV for the treatment of severe Kaposi's sarcoma in HIV-infected adults (Gill 1996) – RCT
Patient or population: HIV-infected adults with severe (T1) Kaposi's sarcoma
Settings: USA
Intervention: liposomal daunorubicin
Comparison: ABV
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| ABV | Liposomal daunorubicin | |||||
| Progression of Kaposi's sarcoma | 99 per 1000 | 77 per 1000 (34 to 180) | RR 0.78 (0.34 to 1.82) | 227 (1 study) | ⊕⊕⊕⊕◯ moderate1 | |
| Clinical response - complete response | 9 per 1000 | 26 per 1000 (3 to 245) | RR 2.87 (0.3 to 27.19) | 227 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Clinical response - partial response | 270 per 1000 | 224 per 1000 (143 to 354) | RR 0.83 (0.53 to 1.31) | 227 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Clinical response - overall response | 279 per 1000 | 251 per 1000 (162 to 385) | RR 0.9 (0.58 to 1.38) | 227 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Clinical response - stable disease | 577 per 1000 | 623 per 1000 (502 to 767) | RR 1.08 (0.87 to 1.33) | 227 (1 study) | ⊕⊕⊕◯ moderate1 | |
| Adverse events | 964 per 1000 | 974 per 1000 (925 to 1000) | RR 1.01 (0.96 to 1.06) | 227 (1 study) | ⊕⊕⊕◯ moderate1 | |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
ABV: doxorubicin, bleomycin and vincristine; CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
There were very few events with very wide confidence intervals.
Oral etoposide compared to ABV for the treatment of severe Kaposi's sarcoma in HIV-infected adults (Olweny 2005) – RCT
Patient or population: HIV-infected adults with severe (T1) Kaposi's sarcoma
Settings: Zimbabwe
Intervention: oral etoposide
Comparison: ABV
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| ABV | Oral etoposide | |||||
| Mortality | 864 per 1000 | 933 per 1000 (846 to 1000) | RR 1.08 (0.98 to 1.19) | 178 (1 study) | ⊕⊕⊕◯ moderate1 | |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
ABV: doxorubicin, bleomycin and vincristine; CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
There were few events with wide confidence intervals.
Bleomycin compared to ABV for the treatment of severe Kaposi's sarcoma in HIV-infected adults (Hernandez 1997) - non-RCT
Patient or population: HIV-infected adults with severe (T1) Kaposi's sarcoma
Settings: Venezuela
Intervention: bleomycin
Comparison: ABV
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| ABV | Bleomycin | |||||
| Mortality | 917 per 1000 | 999 per 1000 (797 to 1000) | RR 1.09 (0.87 to 1.36) | 24 (1 study) | ⊕◯◯◯ very low1 | |
| Clinical response - complete response | See comment | See comment | Not estimable | 24 (1 study) | ⊕◯◯◯ very low1 | |
| Clinical response - partial response | 333 per 1000 | 37 per 1000 (3 to 620) | RR 0.11 (0.01 to 1.86) | 24 (1 study) | ⊕◯◯◯ very low1 | |
| Clinical response - stable disease | 667 per 1000 | 587 per 1000 (313 to 1000) | RR 0.88 (0.47 to 1.63) | 24 (1 study) | ⊕◯◯◯ very low1 | |
| Clinical response - progression | 0 per 1000 | 0 per 1000 (0 to 0) | RR 11 (0.67 to 179.29) | 24 (1 study) | ⊕◯◯◯ very low1 | |
| Adverse events | 0 per 1000 | 0 per 1000 (0 to 0) | RR 11 (0.67 to 179.29) | 24 (1 study) | ⊕◯◯◯ very low1 | |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
ABV: doxorubicin, bleomycin and vincristine; CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
There were very few events with very wide confidence intervals.
Liposomal doxorubicin compared to bleomycin + vinblastine, vincristine or single-agent antiretroviral therapy alone (conservative management) for the treatment of severe Kaposi's sarcoma in HIV-infected adults (Grünaug 1998) - non-RCT
Patient or population: HIV-infected adults with severe (T1) Kaposi's sarcoma
Settings: Germany
Intervention: liposomal doxorubicin
Comparison: conservative management
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Liposomal doxorubicin | Conservative management | |||||
| Mortality | 1000 per 1000 | 930 per 1000 (750 to 1000) | RR 0.93 (0.75 to 1.15) | 29 (1 study) | ⊕⊕⊕◯ moderate1 | |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
There were very few events.
Highly active antiretroviral therapy (HAART) plus chemotherapy versus HAART alone
Randomised controlled trials
HAART plus doxorubicin, bleomycin and vincristine (ABV) versus HAART alone
In Mosam 2012, in the HAART plus ABV group, ABV was started within one month of initiation of HAART. Of note, when ABV was not available, oral Etoposide was given. This alternative chemotherapy regimen was used in 31% of patients that received chemotherapy overall.
The HAART regimen consisted of a fixed-dose combination of stavudine (40 mg), lamivudine (150 mg) and nevirapine 200 mg.
The outcomes reported were:
Mortality
In the T1 Kaposi's sarcoma group, there were a total of 11 deaths out of 50 participants in the HAART plus ABV group compared to 12 deaths out of 50 in the HAART alone group (RR 0.92; 95% CI 0.45 to 1.88) (Analysis 1.1).
Progression of Kaposi's sarcoma
One out of 50 participants had progressive disease in the HAART plus ABV group, compared to 10 participants out of 50 with progressive disease in the HAART alone group(RR 0.10; 95% CI 0.01 to 0.75) (Analysis 1.2).
Clinical response (includes complete, partial and no response)
-
Complete response
Eight out of 50 participants had a complete response in the HAART plus ABV group, compared to four participants out of 50 with complete response in the HAART alone group(RR 2.0; 95% CI 0.64 to 6.22) (Analysis 1.3).
-
Partial response
Twenty-four participants in the HAART plus ABV group had a partial response compared to 14 participants in the HAART alone group(RR 1.71; 95% CI 1.01 to 2.91).
The overall response rate (complete and partial) in the HAART plus ABV group was 32 out of 50 participants compared to 18 in the HAART alone group(RR 1.78; 95% CI 1.16 to 2.72).
-
Stable disease (no response)
There were no participants with stable disease in the HAART plus ABV group and eight participants in the HAART alone group had stable disease.
Adverse events
Twenty-three participants in the HAART plus ABV group and 26 in the HAART alone group had grade 3 to 5 adverse events (including Kaposi's sarcoma immune reconstitution inflammatory syndrome (IRIS): four in the HAART plus ABV group and one patient in the HAART alone group (RR 0.88; 95% CI 0.59 to 1.32) (Analysis 1.4).
HAART plus pegylated liposomal doxorubicin (PLD) versus HAART alone
Martin-Carbonero 2004 included both T1 and T0 Kaposi's sarcoma patients. We present results only for participants with T1 Kaposi's sarcoma. HAART and chemotherapy were started simultaneously at the beginning of the study (there were two antiretroviral therapy (HAART) treatment-naive patients). The HAART regimen was protease inhibitor (PI) based, non-nucleoside reverse transcriptase (NNRTI) based or PI plus NNRTI.
The outcomes reported were:
Clinical response (includes complete, partial and no response)
There were a total of 10 T1 Kaposi's sarcoma patients, with five in the HAART plus PLD group and five in the HAART alone group. The authors combined the results for both partial and complete response. In the HAART plus PLD group, four participants had a complete/partial response, while none responded in the HAART alone group. PLD plus HAART appeared to increase the rate of both partial and complete response compared to HAART alone. However, this difference was not statistically significant (risk ratio (RR) 9; 95% confidence interval (CI) 0.61 to 133.08) (Analysis 2.1).
Prospective cohort study
HAART plus liposomal anthracycline versus HAART alone
In Bower 2009 (including Bower 2014 and Letang 2013), there were 163 participants in the HAART alone group (131 were antiretroviral-naive and 32were on HAART at the time of diagnosis), of which five participants had T1 disease. There were 73 patients in the HAART plus liposomal anthracycline group, of which 68 had T1 Kaposi's sarcoma. This study was not designed to compare different treatment regimens for patients with T1 disease. Specifically, the clinic followed guidelines that all T1 patients should be treated with HAART plus liposomal anthracyclines, therefore T1 patients who were treated with HAART alone were particular exceptions. Outcome data were not presented according to Kaposi's sarcoma staging. Updated data from the same cohort were similarly presented in Bower 2014. Letang 2013 included Bower's UK cohort of 213 patients, of which 129 had T1 Kaposi's sarcoma, with 65 patients in the HAART plus chemotherapy group and 64 in the HAART alone group. In order to ascertain outcome specific to stage, the data reported below were provided after communication with the author of Letang 2013, and represent unpublished Letang 2013 data.
The outcomes reported were:
Mortality/overall 12-month survival
A total of five out of 65 T1 participants in the HAART plus liposomal anthracycline group died at the end of 12months compared to four out of 64 participants in the HAART alone group (RR 1.23; 95% CI 0.35 to 4.38) (Analysis 3.1).
Kaposi's sarcoma IRIS
Four out of 65 T1 participants in the HAART plus liposomal anthracycline group developed Kaposi's sarcoma IRIS compared to eight out of 64 participants in the HAART alone group(RR 0.49; 95% CI 0.16 to 1.55) (Analysis 3.2).
HAART plus chemotherapy versus HAART plus another chemotherapy regimen
Randomised controlled trials
HAART plus paclitaxel versus HAART plus PLD
In Cianfrocca 2010, there were a total of 49 T1 Kaposi's sarcoma patients, with 24 in the paclitaxel group and 25 in the PLD group. Participants were required to receive HAART (PI, a non-nucleoside reverse transcriptase inhibitor without a PI or both) for at least 14 days before study enrolment. Mortality and adverse events results were not available according to Kaposi's sarcoma staging.
The outcomes reported were:
Progression of Kaposi's sarcoma
One out of 24 participants had progressive disease in the paclitaxel group, compared to one participant out of 25 in the PLD group(RR 1.04; 95% CI 0.07 to 15.73) (Analysis 4.1).
Clinical response (includes complete, partial and no response)
-
Complete response
Two out of 24 participants had a complete response in the paclitaxel group, compared to one participant out of 25 in the PLD group(RR 2.08; 95% CI 0.20 to 21.50) (Analysis 4.2).
-
Partial response
Nine participants had a partial response in both the paclitaxel and PLD groups(RR 1.04; 95% CI 0.50 to 2.17) (Analysis 4.2).
-
Stable disease (no response)
There were six participants out of 24 with stable disease in the paclitaxel group and 10 participants out of 25 in the PLD group(RR 0.63; 95% CI 0.27 to 1.45) (Analysis 4.2).
HAART plus pegylated liposomal doxorubicin (PLD) versus HAART plus liposomal daunorubicin
In Cooley 2007, there were a total of 46 T1 Kaposi's sarcoma patients, with 34 in the PLD group and 12 in the liposomal daunorubicin group. The time interval between HAART and chemotherapy and the HAART regimen was not described. 95% of patients in the overall trial received HAART.
The outcomes reported were:
Progression of Kaposi's sarcoma
There were no participants with progressive disease in either the PLD or liposomal daunorubicin groups.
Chemotherapy versus chemotherapy in the pre-HAART era
Randomised controlled trials
Liposomal daunorubicin versus doxorubicin, bleomycin and vincristine (ABV)
In Gill 1996, there were a total of 227 participants, with 116 in the liposomal daunorubicin group and 111 in the ABV group. During the course of the trial, 48 participants (41%) in the liposomal daunorubicin group received concomitant zidovudine therapy, 38 (33%) were treated with didanosine and 24 (21%) received zalcitabine. Among the participants in the ABV group, 47 (42%) received zidovudine, 29 (26%) received didanosine and 22 (20%) were treated with zalcitabine.
The outcomes reported were:
Mortality
Median survival time was 369 days for participants in the liposomal daunorubicin group and 342 days for participants in the ABV group. When the analysis was restricted to patients receiving prior zidovudine, survival was improved in the liposomal daunorubicin group as compared to the ABV group (p=0.26; individual level data not provided).
Progression of Kaposi's sarcoma
Nine out of 116 participants had progressive disease in the liposomal daunorubicin group, compared to 11 participants out of 111 in the ABV group(RR 0.78; 95% CI 0.34 to 1.82) (Analysis 6.1).
Clinical response (includes complete, partial and no response)
-
Complete response
Three out of 116 participants had a complete response in the liposomal daunorubicin group, compared to one participant out of 111 in the ABV group (RR 2.87; 95% CI 0.3 to 27.19)(Analysis 6.2).
-
Partial response
Twenty-six participants out of 116 in the liposomal daunorubicin group had a partial response compared to 30 participants out of 111 in the ABV group(RR 0.83; 95% CI 0.53 to 1.31) (Analysis 6.2).
-
Overall response
The overall response rate (complete and partial response) in the liposomal daunorubicin group was 29 out of 116 participants compared to 31 out of 111 in the ABV group (RR 0.90; 95% CI 0.58 to 1.38) (Analysis 6.2).
-
Stable disease (no response)
There were 72 participants out of 116 with stable disease in the liposomal daunorubicin group and 64 participants out of 111 in the ABV group(RR 1.08; 95% CI 0.87 to 1.33) (Analysis 6.2).
Adverse events (including toxicity and/or worsening of coexistent disease)
One hundred and thirteen out of 116 participants in the liposomal daunorubicin group and 107 out of 111 in the ABV group had clinical adverse events(RR 1.01; 95% CI 0.96 to 1.06) (Analysis 6.3).
The median time to treatment failure was 115 days in the liposomal daunorubicin group and 99 days in the ABV group.
Quality of life
This was reported in Gill 1996. The authors reported that although patients treated with ABV had a gradual decline in the combined QOL score, the differences between the two treatment arms were not statistically significant.
Oral etoposide versus ABV
In Olweny 2005, there were a total of 178 T1 Kaposi's sarcoma patients with 90 in the oral etoposide group and 88 in the ABV group. No participant received antiretroviral therapy.
The outcomes reported were:
Mortality
There were a total of 84 deaths out of 90 participants in the oral etoposide group compared to 76 deaths out of 88 in the ABV group(RR 1.08; 95% CI 0.98 to 1.19) (Analysis 7.1).
Clinical response (includes complete, partial and no response)
-
Complete response
There were no participants with complete response in either the oral etoposide or ABV group.
Non-randomised trials
Bleomycin versus ABV
In Hernandez 1997, there were 24 patients with severe Kaposi's sarcoma in the bleomycin and ABV groups(12 in each).
The outcomes reported were:
Mortality
All 12 participants in the bleomycin only group died compared to 11 deaths out of 12 in the ABV group(RR 1.09; 95% CI 0.87 to 1.36) (Analysis 8.1).
Clinical response (includes complete, partial and no response)
-
Complete response
None of the patients in either the bleomycin or ABV group had a complete response.
-
Partial response
None of the patients in the bleomycin group had a partial response compared to four patients in the ABV group(RR 0.11; 95% CI 0.01 to 1.86) (Analysis 8.2).
-
Stable disease
Seven patients in the bleomycin group compared to eight in the ABV group had stable disease(RR 0.88; 95% CI 0.47 to 1.63) (Analysis 8.2).
-
Progression
Five participants in the bleomycin group had progressive disease compared to none in the ABV group (RR 11; 95% CI 0.67 to 179.29) (Analysis 8.2).
Adverse events
There were five reported adverse events (fever) in the bleomycin only group and none in the ABV group(RR 11; 95% CI 0.67 to 179.29) (Analysis 8.3).
Time to mortality
The median survival time (in months) was 11 (6 to 20) in the bleomycin only group and 13 (7 to 36) in the ABV group.
Liposomal doxorubicin versus conservative management (defined as bleomycin plus vinblastine, or vincristine or single-agent antiretroviral therapy alone)
In Grünaug 1998, all 29 participants in the study were T1. There were 20 in the liposomal doxorubicin group and 9 in the group receiving conservative management. Seventeen out of 20 patients in the liposomal doxorubicin group received antiretroviral therapy, which was most likely single agent, as only a subset of these patients (2 out of the 17) received a combination of PI plus nucleosides. Of the nine patients in the group receiving conservative management, four had bleomycin and vinblastine or vincristine and five did not receive chemotherapy. Two out of the five that did not receive chemotherapy received interferon. Five of the nine patients in this group had antiretroviral therapy alone which appears to have been single agent therapy, although details on specific antiretrovirals used are lacking.
The outcomes reported were:
Mortality
There were 18 deaths out of 20 in the stealth liposomal doxorubicin group, while all nine patients receiving conservative management died(RR 0.93; 95% CI 0.75 to 1.15) (Analysis 9.1).
Adverse events
All 20 patients in the liposomal doxorubicin group were reported to have adverse events (grade 1 to 4).
Time to mortality (time after diagnosis of pulmonary Kaposi's sarcoma to death)
The mean survival time was 11.81 months (standard deviation (SD) 1.78) in the liposomal doxorubicin group and 4.44 months (SD 1.68) in the conservative management group. (Cox regression p<0.01).
Discussion
Summary of main results
We included six randomised trials involving 610 HIV-infected adults with severe Kaposi's sarcoma (Cianfrocca 2010; Cooley 2007; Gill 1996; Martin-Carbonero 2004; Mosam 2012; Olweny 2005), and three observational studies involving a total of 182 HIV-infected adults with severe Kaposi's sarcoma (Bower 2009; Grünaug 1998; Hernandez 1997). None of the included studies with the same study design compared similar interventions. Of the nine included studies, seven included patients with a mix of mild to moderate (T0) and severe (T1) Kaposi's sarcoma. However, this review was restricted to severe disease only, therefore we only extracted data for T1 Kaposi's sarcoma participants. There was no universal consensus in included studies on the severity of disease that is truly requiring of chemotherapy.
In the previous version of this Cochrane review (Dedicoat 2003), two large randomised trials (total of 499 T0 and T1 Kaposi's sarcoma patients) were pooled: Northfelt 1998, comparing pegylated liposomal doxorubicin (PLD) to doxorubicin, bleomycin and vincristine (ABV), and Stewart 1998, comparing PLD to bleomycin and vincristine. However, we excluded these studies from our update of the review because the results were not available according to Kaposi's sarcoma stage. The pooled analysis showed similar mortality and adverse events in both arms, but clinical response, which included complete and partial response, favoured PLD.
Of the trials comparing highly active antiretroviral therapy (HAART) plus chemotherapy to HAART alone for patients with T1 Kaposi's sarcoma, only Mosam 2012, comparing HAART plus ABV to HAART alone, showed a significant reduction in disease progression in the HAART plus ABV group. There also appeared to be a reduction in mortality and adverse events but these were not statistically significant. It should be noted that the sickest patients requiring urgent chemotherapy were excluded from this trial, so it is possible that this is an underestimate of the reduction in morbidity and possibly mortality associated with ABV when added to HAART. Another trial involving 10 patients and comparing HAART plus pegylated liposomal doxorubicin to HAART alone did not show any significant benefit in clinical response, but was of very small sample size (Martin-Carbonero 2004). The Bower 2009 cohort included patients on HAART alone and also on HAART plus liposomal anthracycline but was not designed to compare these two groups. A subset of this cohort was presented in Letang 2013 which showed a non-statistically significant reduction in Kaposi's sarcoma immune reconstitution inflammatory syndrome (IRIS) in patients that received HAART plus liposomal anthracyclines, but no difference in mortality between groups. All T1 Kaposi's sarcoma patients in this study were meant to receive chemotherapy as per protocol; patients who received HAART alone were therefore exceptions.
Of the studies comparing HAART plus chemotherapy to HAART plus a different chemotherapy regimen, Cianfrocca 2010, involving 49 T1 patients and comparing paclitaxel versus pegylated liposomal doxorubicin (PLD) in patients on HAART, did not demonstrate a difference between the two groups in disease progression or clinical response. Another trial involving 46 T1 disease patients, comparing pegylated liposomal doxorubicin to liposomal daunorubicin, showed no participants with progressive Kaposi's sarcoma disease in either group (Cooley 2007). We did not identify any randomised controlled studies from the modern HAART era that directly compared HAART plus liposomal anthracyclines to HAART plus ABV. In addition, we did not identify any studies that evaluated timing of chemotherapy, i.e. whether there was a benefit to starting chemotherapy prior to HAART as compared to simultaneous administration.
Other studies compared different chemotherapy regimens in patients from the pre-HAART era: Gill 1996, involving 227 patients, compared liposomal daunorubicin to ABV and showed no significant difference with the use of liposomal daunorubicin compared to ABV in disease progression and overall response rate. The exclusion of the Northfelt 1998 and Stewart 1998 studies is discussed above. Olweny 2005, a trial involving 178 patients comparing oral etoposide versus ABV in patients not on antiretroviral therapy, demonstrated no significant difference in mortality between groups. Hernandez 1997, a prospective non-randomised trial (24 patients) comparing bleomycin to ABV in the pre-HAART era, demonstrated a higher mean survival time and no reported adverse events in the ABV group. However, there was no significant difference in disease progression between the two groups. An additional retrospective study, involving 29 patients in the pre-HAART era, showed a non-statistically significant overall mortality benefit for liposomal doxorubicin compared to conservative management consisting of either bleomycin plus vinblastine, vincristine or single-agent antiretroviral therapy alone (Grünaug 1998). Liposomal doxorubicin also showed a significant survival time benefit.
Overall completeness and applicability of evidence
We included all studies that met the inclusion criteria for this review. The trials included HIV-infected adults with severe or progressive Kaposi's sarcoma, as defined by AIDS Clinical Trial Group T1 or progressive disease. We identified no studies on the relative timing of HAART in relationship to chemotherapy. Most of the included studies were not designed or powered specifically to address outcomes for patients with severe or progressive Kaposi's sarcoma. We were not able to do a subgroup analysis to assess if there were particular subgroups within the population of patients with T1 KS that would benefit more or less from chemotherapy. Some of the outcomes addressed in the studies could not be reported here because they were not presented according to disease severity (i.e. mixed T0 and T1). The studies were conducted in both resource-poor and rich settings. Therefore, the findings from this review are applicable to various settings, but identified major gaps in the literature as above.
Quality of the evidence
We assessed the quality of evidence using the GRADE approach and presented this in the ’Summary of findings’ tables (Summary of findings for the main comparison; Summary of findings 2; Summary of findings 3; Summary of findings 4; Summary of findings 5; Summary of findings 6; Summary of findings 7; Summary of findings 8; Summary of findings 9). The overall quality of evidence in this review can be described as moderate. We downgraded the quality of evidence due to the small size of many of the included studies and the low numbers of events.
Potential biases in the review process
We conducted a comprehensive search of databases and conference proceedings and we contacted experts in the field to ensure that all relevant completed, unpublished or ongoing studies were identified. There was no language restriction. We also contacted study authors where possible for clarification where this was required and for unpublished data where necessary. Unpublished data was included in the review. We minimised potential bias in the conduct of this review by having at least two authors independently scan through the search output, extract data and assess the methodological quality of each study.
Agreements and disagreements with other studies or reviews
The only data from comparison of liposomal anthracyclines with ABV come from the pre-HAART era; in contrast to the prior Cochrane review Dedicoat 2003, the single study in this comparison included here did not show a definitive advantage from liposomal anthracyclines over ABV. However, two large randomised controlled trials on this topic had to be excluded due to their mix of mild and severe Kaposi's sarcoma patients.
Authors' conclusions
Implications for practice
The findings from this review suggest that highly active antiretroviral therapy (HAART) plus chemotherapy may be beneficial in reducing disease progression compared to HAART alone in patients with severe or progressive Kaposi's sarcoma. For patients on HAART, when choosing from different chemotherapy regimens, there was no observed difference between liposomal doxorubicin, liposomal daunorubicin and paclitaxel.
Implications for research
Future studies should be designed and powered specifically to address outcomes for patients with severe or progressive Kaposi's sarcoma and the results should be presented according to disease severity. The delineation of what severity of disease truly needs chemotherapy in addition to HAART, and who may be treated with HAART alone, has yet to be firmly established. While this review demonstrates that there is at least a treatment response advantage to adding chemotherapy to HAART in patients with T1 disease, this does not necessarily mean that ACTG tumour staging is the most appropriate cut-off for clinicians to use when deciding who to treat with chemotherapy. For example, there may be subgroups of T1 patients that could be treated with HAART alone, or subgroups within T0 that require immediate anti-KS therapy. The studies included in this review were not powered in such a way to allow us to perform sub-group analyses. There is also a need for research on the relative timing of HAART in relation to chemotherapy for severe Kaposi's sarcoma, and the potential role of chemotherapy in preventing or treating Kaposi's sarcoma immune reconstitution inflammatory syndrome.
Characteristics of Studies.
| Characteristics of included studies [ordered by study ID] | |
|---|---|
| Bower 2009 | |
| Methods | Prospective cohort study (single centre) |
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes | This study was conducted in the UK. Median duration of follow-up was 4 years and maximum 12 years. (Letang 2013 included Bower's UK cohort of 213 patients of which 129 had T1 KS; Bower 2014 contains updated data from the same Bower 2009 cohort which has already been included in the review) |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not a randomised controlled trial |
| Allocation concealment (selection bias) | High risk | Not a randomised controlled trial |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential loss to follow-up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | All T1 KS patients were meant to receive chemotherapy as per protocol, patients who received HAART alone were therefore exceptions |
| Cianfrocca 2010 | |
|---|---|
| Methods | RCT (individual) |
| Participants | There were a total of 49 T1 KS patients with 24 in the paclitaxel group and 25 in the pegylated liposomal doxorubicin group Inclusion criteria:
|
| Interventions | Paclitaxel (100 mg/m2), infused intravenously over 3 hours every 14 days or pegylated liposomal doxorubicin (20 mg/m2), infused intravenously over 30 to 60 minutes every 21 days. At baseline, 53 of the 73 patients were receiving a combination HAART regimen containing either a protease inhibitor (N = 20), a non-nucleoside reverse transcriptase inhibitor without a protease inhibitor (N = 21) or both (N = 12) |
| Outcomes |
|
| Notes | The trial was terminated prematurely because of slow accrual |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant differential loss to follow-up. Intention-to-treat analysis described |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective outcome reporting |
| Other bias | Unclear risk | Trial terminated prematurely due to slow accrual |
| Cooley 2007 | |
|---|---|
| Methods | Double-blinded, multicentre RCT |
| Participants | 79 patients with AIDS-related KS. 46 patients had T1 disease with 34 in the PLD group and 12 in the liposomal daunorubicin group Inclusion criteria:
|
| Interventions | Pegylated liposomal doxorubicin (20 mg/m2; n = 60) versus liposomal daunorubicin (40 mg/m2; n = 20) as a 60-minute intravenous infusion every 2 weeks for 6 cycles Patients were assessed at < 30 days before treatment, at each of the 6 treatment cycles and at the end of the study 76 patients in the study received HAART |
| Outcomes | Tumour response: complete response (CR), partial response (PR), progressive disease (PD), stable disease (SD); time to progression; survival; adverse events recorded and graded using the National Cancer Institute Common Toxicity Criteria |
| Notes | This study was conducted in the USA |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | An independent AIDS expert without the knowledge of the patient evaluated the outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent AIDS expert evaluated the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no differential loss to follow-up |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective outcome reporting |
| Other bias | Low risk | No significant baseline differences between arms |
| Gill 1996 | |
|---|---|
| Methods | RCT (individual) |
| Participants |
Inclusion criteria:
|
| Interventions | Patients received liposomal daunorubicin at a dose of 40 mg/m2 infused over 30 to 60 minutes, or a regimen of doxorubicin 10 mg/m2, bleomycin 15 U and vincristine 1 mg (ABV) every 2 weeks intravenously on an outpatient basis. Cycles were repeated every 14 days provided absolute neutrophil count (ANC) was >0.75 x 109/l and the platelet count >75 x 109/l. Patients whose ANC decreased to less than 0.75 x 109/l had chemotherapy withheld and could receive G-CS F to enable resumption of chemotherapy once the ANC had returned to > 0.75 x 109/l During the course of the trial, 48 liposomal daunorubicin patients (41%) received concomitant zidovudine therapy, 38 (33%) were treated with didanosine and 24 (21%) received zalcitabine. Among the ABV patients, 47 (42%) received zidovudine, 29 (26%) received didanosine and 22 (20%) were treated with zalcitabine |
| Outcomes |
|
| Notes | This study was conducted in the USA |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted-block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| All outcomes | ||
| Blinding of participants and personnel (performance bias) | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| All outcomes | ||
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential loss to follow-up |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective outcome reporting |
| Other bias | Low risk | No significant baseline differences between arms |
| Grünaug 1998 | |
|---|---|
| Methods | Retrospective, cohort, multicentre study |
| Participants | 29 AIDS patient with bronchoscopy or histologically confirmed pulmonary KS patients. All participants had T1 KS |
| Interventions | Group 1: (n = 20) stealth liposomal doxorubicin (SL-DOX) 20 mg/m2 every 2nd week Group 2: (n = 9) no SL-DOX (conservative management) Of the 20 patients in group 1, 17 had ARV therapy; of the 9 patients in group 2, 4 had bleomycin and vincristine or vinblastine while the remaining 5 patients had no chemotherapy |
| Outcomes | Survival, clinical response |
| Notes | This study was conducted in Munich, Germany |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not a randomised controlled trial |
| Allocation concealment (selection bias) | High risk | Not a randomised controlled trial |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential loss to follow-up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Low risk | No other potential sources of bias identified |
| Hernandez 1997 | |
|---|---|
| Methods | Prospective non-randomised trial |
| Participants |
Inclusion criteria:
|
| Interventions |
|
| Outcomes | Clinical response: complete remission, partial remission, stable disease, progression; mortality; survival; adverse events |
| Notes | This study was conducted in Venezuela |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not a randomised controlled trial |
| Allocation concealment (selection bias) | High risk | Not a randomised controlled trial |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not described |
| All outcomes | ||
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential loss to follow-up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Unclear risk | No other potential sources of bias identified |
| Martin-Carbonero 2004 | |
|---|---|
| Methods | Randomised, open-label, multicentre study |
| Participants |
Inclusion criteria:
|
| Interventions | 25 treatment and non-treatment-naive HIV patients with moderate to advanced KS randomly assigned to receive:
|
| Outcomes | Response rate: complete response, partial response, disease progression |
| Notes | This study was conducted in Spain. Duration of follow-up was 48 weeks |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention-to-treat analysis done. No differential loss to follow-up |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective outcome reporting |
| Other bias | High risk | The prevalence of previous opportunistic infections was higher in patients allocated to the pegylated liposomal doxorubicin group |
| Mosam 2012 | |
|---|---|
| Methods | Open-label, prospective, single-centre RCT |
| Participants |
Inclusion criteria:
|
| Interventions | HAART alone or HAART and chemotherapy
|
| Outcomes |
Toxicities were graded using the Division of AIDS (DAIDS) toxicity scale Adherence was assessed by a 7-day recall questionnaire at week 2, week 4 and then monthly Previously validated quality of life (QOL) questionnaires (EORTC QOL-30) measured 6 functioning scales |
| Notes | Although the planned chemotherapy protocol was ABV, oral etoposide (50 to 100 mg for 1 to 21 days of a 28-day cycle) was used as an alternative therapy in the event of difficulties with the chemotherapy drug supply or intravenous administration during the protocol. Etoposide was started at 50 mg daily but could be escalated to 100 mg in patients in subsequent cycles with inadequate KS tumour regression and no limiting toxicities This study included a mix of T0 and T1 participants, but only T1 disease was included in our analysis (data provided by communication with the author) Results from Bihl 2007 were included in this study Study was conducted in South Africa. Duration of follow-up was 12 months |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computed-generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding. However, the reported outcomes are objective and not likely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors used an intention to treat analysis. There was no differential loss to follow-up |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective outcome reporting |
| Other bias | High risk | There was some baseline imbalance. Patients randomised to CXT but not receiving chemotherapy had low baseline CD4 counts (median, 77 cells/ml), compared with those receiving chemotherapy (median, 249 cells/ml). When ABV was not available, oral Etoposide was given. This alternative chemotherapy regimen was used in 31% of patients that received chemotherapy overall |
| Olweny 2005 | |
|---|---|
| Methods | 4-arm, randomised, open-label RCT |
| Participants |
Inclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome:
|
| Notes | Study was conducted in a resource-poor setting: Harare, Zimbabwe. Patients were followed up until death |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated randomisation cards |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding, reported primary outcome (QOL) is subjective. However, the other reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, reported primary outcome (QOL) is subjective. However, the other reported outcomes are objective and not likely to be affected by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding, reported primary outcome (QOL) is subjective. However, the other reported outcomes are objective and not likely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors imputed missing data; no differential loss to follow-up |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective outcome reporting |
| Other bias | Low risk | Differences between arms were adjusted for in the analysis |
ABV: doxorubicin, bleomycin and vincristine
AIDS: acquired immunodeficiency syndrome
ANC: absolute neutrophil count
ART: antiretroviral therapy ARV: antiretroviral
ACTG: AIDS Clinical Trial Group
AZT: zidovudine CXT: chemotherapy
ECOG: Eastern Cooperative Oncology Group
ELISA: enzyme-linked immunosorbent assay
G-CSF: granulocyte colony-stimulating factor
HAART: highly active antiretroviral therapy
HIV: human immunodeficiency virus
IRIS: immune reconstitution inflammatory syndrome
IV: intravenous
KPS: Karnofsky performance status
KS: Kaposi's sarcoma
LVEF: left ventricular ejection fraction
PLD: pegylated liposomal doxorubicin RNA: ribonucleic acid
QOL: quality of life
RCT: randomised controlled trial
SL-DOX: stealth liposomal doxorubicin
Characteristics of excluded studies [ordered by study ID].
| Study | Reason for exclusion |
|---|---|
| Asiimwe 2012 | This was a cohort of 17 patients with prevalent KS and 18 patients with incident KS in Tororo, Uganda. There was limited access to chemotherapy. All patients initially received the same ART regimen (NNRTI) and patients were only switched to a PI if they were considered treatment failures |
| Autier 2005 | This was a retrospective, observational study of docetaxel in anthracycline-pretreated AIDS-related KS. There was no comparison group |
| Bihl 2007 | This was a subset of the Mosam 2012 study, which has already been included in the review |
| Bodsworth 2001 | This was a phase III, vehicle-controlled, multi-centred study of topical alitretinoin gel 0.1% in cutaneous AIDS-related KS. The participants included were a mix of visceral and cutaneous KS. There was no relevant comparison for this review |
| Bonhomme 1991 | This was a randomised controlled trial of topical treatment of epidemic KS with all-trans-retinoic acid. The authors included participants with different stages of KS. Outcomes were not separated by stage of the disease |
| Bonhomme 1994 | This was a randomised trial assessing the treatment of AIDS-associated KS with oral tretinoin. We could not retrieve the full-text article |
| Borok 2010 | This was a cohort of 90 ART-naive adults with biopsy-proven KS. All patients received ART, and then patients received additional treatment at the discretion of their provider with chemotherapy in 56% of cases and adjunctive radiotherapy in 24% of cases. Outcomes were not separated by stage of the disease |
| Cattelan 2005 | We could not obtain the full-text article to help us make a decision |
| Duvic 2000 | This was an open-label, within-patient, controlled, dose-escalating phase 1 and 2 clinical trial to evaluate the efficacy and safety of topical alitretinoin gel in cutaneous KS. There was no relevant comparison for this review |
| Gelmann 1987 | This was a randomised trial of combination chemotherapy versus recombinant alpha interferon for KS. There was no relevant comparison for this review |
| Gill 1991 | This was a randomised trial of systemic treatment of AIDS-related KS. Patients with extensive mucocutaneous KS or visceral involvement were randomised to treatment with low-dose adriamycin (doxorubicin, 20 mg/m2) alone (31 cases) or in combination with bleomycin and vincristine (ABV) (30 cases). Results were not presented by KS disease stage. Attempts to contact the study author were not successful |
| Harrison 1998 | Biopsy confirmation of KS was not an absolute requirement for inclusion in the study |
| Hernandez 1996 | This is an observational study evaluating the treatment of epidemic KS with bleomycin or combination of doxorubicin, bleomycin and vincristine. Results for outcomes are not reported by stage of KS disease |
| Ireland-Gill 1992 | This was an observational study of the treatment of AIDS-related KS using bleomycin-containing combination chemotherapy regimens. We could not obtain the full-text article. The staging of KS was not mentioned in the abstract and the results presented were not separated by KS stage |
| Koon 2011 | This phase-II study of 23 HIV patients with KS had an intra-patient vehicle control design comparing topically administered halofuginone, an angiogenesis inhibitor that inhibits collagen type-I and matrix metalloproteinases (MMPs), with vehicle ointment. There was no relevant comparison for this review |
| Krigel 1988 | This was an prospective, observational study of the treatment of epidemic KS with a combination of interferon-alpha 2b and etoposide. There was no comparison group |
| Lane 1989 | This was a phase II, randomised, placebo-controlled trial of zidovudine in patients with HIV infection and KS. Results are not available by KS disease stage |
| Lasso 2003 | This was a retrospective review of HIV patients with KS seen at a public hospital and at a HIV clinic and subjected to antiretroviral treatment and chemotherapy. Results are not available by KS disease stage |
| Letang 2013 | Letang et al followed 4 cohorts of HIV-positive patients diagnosed with KS in a) Chelsea and Westminster Hospital, London, UK; b) King Edward VIII Hospital, Durban, South Africa; c) Parirenyatwa Hospital Kaposi Sarcoma Clinic, Harare, Zimbabwe; and d) Manhica Health Research Centre, Manhica, Mozambique. Only the UK cohort included participants who were exposed to the intervention of interest (ART plus chemotherapy). The UK cohort study is already included in this review. See Bower 2009 (IRIS and mortality data were provided by Letang 2013) |
| Lichterfeld 2005 | This was an observational study of treatment of HIV-1-associated KS with pegylated liposomal doxorubicin and HAART simultaneously. Participants with early-stage KS received etoposide while those with late-stage KS received ABV |
| Lim 2005 | This was an observational study of a weekly dose of docetaxel in the treatment of advanced-stage AIDS-related KS. There was no comparison group |
| Monticelli 2000 | This study presented either a case series or cohort (design not specified) of 18 HIV patients diagnosed with KS between 1994 and 1997 in Argentina. Staging of KS was specified as stages I to IV, but definition of these stages was not clearly laid out (another paper which used ACTG staging was referenced) |
| Mussini 2008 | This is a cohort study evaluating the effects of different HAART regimens on AIDS-related KS. Staging of KS and results by disease stage are not available |
| Nguyen 2008 | This was a cohort study characterising the predictors of clinical response in patients with KS. Multiple HAART and chemotherapy regimens were used. Comparison not clear |
| Northfelt 1998 | This was a randomised clinical trial of pegylated liposomal doxorubicin versus doxorubicin, bleomycin and vincristine in the treatment of AIDS-related KS. Staging of KS for included participants (mixed T0, T1) and results by KS disease stage are not available. We contacted the author to provide additional information but were not successful |
| Noy 2005 | This RCT of 202 HIV patients with KS compared synthetic dipeptide IM862 (L-glutamine L-tryptophan) plus ART to placebo plus ART. Patients with symptomatic visceral disease or who required chemotherapy were excluded |
| Nunez 2001 | This was a prospective, non-comparative study of response to liposomal doxorubicin and clinical outcome of HIV-1-infected patients with KS receiving highly active antiretroviral therapy. There was no comparison group |
| Opravil 1999 | Biopsy confirmation of KS was not an absolute requirement. Patients were allowed to change regimen if they did not tolerate the regimen to which they had been randomised. The comparison groups were not well matched for severity of disease |
| Osoba 2001 | This was a randomised trial of the effect of pegylated liposomal doxorubicin versus doxorubicin, bleomycin and vincristine on health-related quality of life in AIDS-related KS: staging of KS and results by disease stage are not available |
| Palmieri 2006 | This was a cohort study of evaluating the effects of HAART + liposomal anthracycline for treating pulmonary KS. All participants received the same treatment. There was no comparison group |
| Ramirez-Amador 2002 | This was a randomised clinical trial of intralesional vinblastine versus 3% sodium tetradecyl sulfate for the treatment of oral KS. There was no relevant comparison for this review |
| Rosenthal 2002 | This was an observational study of the efficacy and tolerance of liposomal daunorubicin in combination with HAART versus HAART alone in the treatment of AIDS-associated KS. The authors did not present results by comparison of the two groups |
| Shepherd 1998 | This was a prospective, randomised trial of 2 dose levels of interferon alfa with zidovudine for the treatment of KS associated with HIV infection. There was no relevant comparison for this review |
| Singh 2008 | This was a phase 1 dose study of hypofractionated radiation therapy in the treatment of epidemic KS: a prospective randomised trial |
| Stelzer 1993 | This was a randomised, prospective, phase 1 dose study of radiation therapy for AIDS-associated KS |
| Stewart 1998 | This was a randomised, comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related KS. We contacted the author to obtain additional information. However, he no longer has access to the original data |
| Strother 2010 | This was a retrospective analysis of the efficacy of gemcitabine for previously treated AIDS-associated KS in western Kenya. There was no comparison group |
| Tavio 1996 | This was a prospective, multi-institutional Italian study that evaluated the efficacy and toxicity of combination chemotherapy with doxorubicin, bleomycin and vindesine (ABVi) in patients with progressive and extensive HIV-related KS. There was no comparison group |
| Tulpule 2002 | This was a multicentre, phase 2 trial of low-dose paclitaxel in patients with advanced AIDS-related KS. All participants were given the same dose of paclitaxel |
| Uthayakumar 1996 | This was a randomised, cross-over comparison of liposomal daunorubicin versus observation for early KS. Only participants with T0 KS were included |
| Walmsley 1999 | This was a 12-week, multicentre, randomised, double-blind, vehicle-controlled safety and efficacy evaluation of topical alitretinoin 0.1% gel applied to cutaneous KS lesions conducted in HIV-infected patients. Staging of KS was not available and the results were not available by staging |
| Zhong 2012 | This was an observational study of etoposide, vincristine, doxorubicin and dexamethasone (EVAD) combination chemotherapy as second-line treatment for advanced AIDS-related KS. There was no comparison group |
ABV: doxorubicin, bleomycin and vincristine
ACTG: AIDS Clinical Trial Group AIDS: acquired immunodeficiency syndrome
ART: antiretroviral therapy
HAART: highly active antiretroviral therapy
HIV: human immunodeficiency virus
IRIS: immune reconstitution inflammatory syndrome
KS: Kaposi's sarcoma NNRTI: non-nucleoside reverse transcriptase inhibitor
PI: protease inhibitor
RCT: randomised controlled trial
Data and Analyses.
| Comparison 1. HAART + ABV versus HAART alone (RCT) | ||||
|---|---|---|---|---|
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2 Progressive disease | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 0.1 [0.01, 0.75] |
| 3 Clinical response | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Complete response | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 2.0 [0.64, 6.22] |
| 3.2 Partial response | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 1.71 [1.01, 2.91] |
| 3.3 Stable disease | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 0.06 [0.00, 0.99] |
| 3.4 Overall response (complete and partial) | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 1.78 [1.16, 2.72] |
| 4 Adverse events | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 0.88 [0.59, 1.32] |
Comparison 2. HAART + pegylated liposomal doxorubicin versus HAART alone (RCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response | 1 | 10 | Risk Ratio (M-H, Fixed, 95% CI) | 9.0 [0.61, 133.08] |
Comparison 3. HAART + liposomal anthracycline versus HAART alone (cohort study).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 129 | Risk Ratio (M-H, Fixed, 95% CI) | 1.23 [0.35, 4.38] |
| 2 KS IRIS | 1 | 129 | Risk Ratio (M-H, Fixed, 95% CI) | 0.49 [0.16, 1.55] |
Comparison 4. HAART + paclitaxel versus HAART + pegylated liposomal doxorubicin (RCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progression of KS | 1 | 49 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.07, 15.73] |
| 2 Clinical response | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Complete response | 1 | 49 | Risk Ratio (M-H, Fixed, 95% CI) | 2.08 [0.20, 21.50] |
| 2.2 Partial response | 1 | 49 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.50, 2.17] |
| 2.3 Stable disease | 1 | 49 | Risk Ratio (M-H, Fixed, 95% CI) | 0.63 [0.27, 1.45] |
Comparison 5. HAART + pegylated liposomal doxorubicin versus HAART + liposomal daunorubicin (RCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progression of KS | 1 | 46 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Liposomal daunorubicin versus ABV (RCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progression of KS | 1 | 227 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.34, 1.82] |
| 2 Clinical response | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Complete response | 1 | 227 | Risk Ratio (M-H, Fixed, 95% CI) | 2.87 [0.30, 27.19] |
| 2.2 Partial response | 1 | 227 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.53, 1.31] |
| 2.3 Overall response | 1 | 227 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.58, 1.38] |
| 2.4 Stable disease | 1 | 227 | Risk Ratio (M-H, Fixed, 95% CI) | 1.08 [0.87, 1.33] |
| 3 Adverse events | 1 | 227 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.96, 1.06] |
Comparison 7. Oral etoposide versus ABV (RCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 178 | Risk Ratio (M-H, Fixed, 95% CI) | 1.08 [0.98, 1.19] |
Comparison 8. Bleomycin versus ABV (non-RCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 24 | Risk Ratio (M-H, Fixed, 95% CI) | 1.09 [0.87, 1.36] |
| 2 Clinical response | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Complete response | 1 | 24 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Partial response | 1 | 24 | Risk Ratio (M-H, Fixed, 95% CI) | 0.11 [0.01, 1.86] |
| 2.3 Stable disease | 1 | 24 | Risk Ratio (M-H, Fixed, 95% CI) | 0.88 [0.47, 1.63] |
| 2.4 Progression | 1 | 24 | Risk Ratio (M-H, Fixed, 95% CI) | 11.0 [0.67, 179.29] |
| 3 Adverse events | 1 | 24 | Risk Ratio (M-H, Fixed, 95% CI) | 11.0 [0.67, 179.29] |
Comparison 9. Liposomal doxorubicin versus conservative management (non-RCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 29 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.75, 1.15] |
What’s New
Last assessed as up-to-date: 13 August 2014.
| Date | Event | Description |
|---|---|---|
| 4 July 2014 | New citation required and conclusions have changed | New searches. Review updated and conclusions changed. |
History
Protocol first published: Issue 2, 2001
Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 10 November 2008 | Amended | Converted to new review format. |
Main results.
We included six randomised trials and three observational studies involving 792 HIV-infected adults with severe Kaposi's sarcoma. Seven studies included patients with a mix of mild to moderate (T0) and severe (T1) Kaposi's sarcoma. However, this review was restricted to the subset of participants with severe Kaposi's sarcoma disease.
Studies comparing HAART plus chemotherapy to HAART alone showed the following: one trial comparing HAART plus doxorubicin, bleomycin and vincristine (ABV) to HAART alone showed a significant reduction in disease progression in the HAART plus ABV group (RR 0.10; 95% CI 0.01 to 0.75, 100 participants); there was no statistically significant reduction in mortality and no difference in adverse events. A cohort study comparing liposomal anthracyclines plus HAART to HAART alone showed a non-statistically significant reduction in Kaposi's sarcoma immune reconstitution inflammatory syndrome in patients that received HAART plus liposomal anthracyclines (RR 0.49; 95% CI 0.16 to 1.55, 129 participants).
Studies comparing HAART plus chemotherapy to HAART plus a different chemotherapy regimen showed the following: one trial involving 49 participants and comparing paclitaxel versus pegylated liposomal doxorubicin in patients on HAART showed no difference in disease progression. Another trial involving 46 patients and comparing pegylated liposomal doxorubicin versus liposomal daunorubicin showed no participants with progressive Kaposi's sarcoma disease in either group.
Studies comparing different chemotherapy regimens in patients from the pre-HAART era showed the following: in the single RCT comparing liposomal daunorubicin to ABV, there was no significant difference with the use of liposomal daunorubicin compared to ABV in disease progression (RR 0.78; 95% CI 0.34 to 1.82, 227 participants) and overall response rate. Another trial involving 178 participants and comparing oral etoposide versus ABV demonstrated no difference in mortality in either group. A non-randomised trial comparing bleomycin alone to ABV demonstrated a higher median survival time in the ABV group; there was also a non-statistically significant reduction in adverse events and disease progression in the ABV group (RR 11; 95% CI 0.67 to 179.29, 24 participants). An additional non-randomised study showed a non-statistically significant overall mortality benefit from liposomal doxorubicin as compared to conservative management consisting of either bleomycin plus vinblastine, vincristine or single-agent antiretroviral therapy alone (RR 0.93; 95% CI 0.75 to 1.15, 29 participants). The overall quality of evidence can be described as moderate quality. The quality of evidence was downgraded due to the small size of many of the included studies and small number of events.
Acknowledgments
This research project has been conducted as part of the academic requirements of the MSc in Clinical Epidemiology, Stellenbosch University (www.sun.ac.za/clinepi).
We want to thank Joy Oliver of the South African Cochrane Centre for helping with the detailed search.
We would like to thank the World Health Organization and the Harry Crossley Foundation for providing financial assistance in the preparation of this review.
Dr. Freeman is supported by the NIH T32AR007098 Training Grant.
Sources of Support: Internal sources:
Centre for Evidence-based Health Care, Stellenbosch University, South Africa.
External sources:
Harry Crossley Foundation, South Africa.
World Health Organization, Switzerland.
National Institutes of Health, USA.
Footnotes
- Gbabe Oluwatoyin and Charles Okwundu wrote the protocol with insightful input from Esther Freeman and Martin Dedicoat.
- Gbabe Oluwatoyin, Charles Okwundu and Esther Freeman reviewed the search outputs, selected studies for inclusion, located copies of relevant studies, contacted study authors and extracted data.
- Gbabe Oluwatoyin wrote the review under the supervision of Charles Okwundu.
- Esther Freeman and Martin Dedicoat provided input into the draft review.
- Gbabe Oluwatoyin has no known conflict of interest.
- Charles Okwundu has no known conflict of interest.
- Martin Dedicoat has no known conflict of interest.
- Esther Freeman has no known conflict of interest.
Differences Between Protocol and Review: This is an update of a previous Cochrane systematic review. In this update, we have included data only for participants with severe Kaposi's sarcoma. Data for participants with mild or moderate Kaposi's sarcoma will be presented in a separate review (Freeman E. et al, “Treatment for mild and moderate Kaposi's sarcoma in ART-naive HIV-infected individuals,” Cochrane review in progress).
References to studies included in this review
- Bower M, Dalla Pria A, Coyle C, Andrews E, Tittle V, Dhoot S, et al. Prospective stage-stratified approach to AIDS-related Kaposi's sarcoma. Journal of Clinical Oncology. 2014;32(5):409–14. doi: 10.1200/JCO.2013.51.6757. [DOI] [PubMed] [Google Scholar]
- Bower M, Weir J, Francis N, Newsom-Davis T, Powles S, Crook T, et al. The effect of HAART in 254 consecutive patients with AIDS-related Kaposi's sarcoma. AIDS. 2009;23(13):1701–6. doi: 10.1097/QAD.0b013e32832d080d. * Bower 2009 {published and unpublished data} [DOI] [PubMed] [Google Scholar]
- Letang E, Lewis JJ, Bower M, Mosam A, Borok M, Campbell TB, et al. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS. 2013;27(10):1603–13. doi: 10.1097/QAD.0b013e328360a5a1. PUBMED: 23462220. [DOI] [PubMed] [Google Scholar]
- Cianfrocca M, Lee S, Von Roenn J, Tulpule A, Dezube BJ, Aboulafia DM, et al. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer. 2010;116(16):3969–77. doi: 10.1002/cncr.25362. [PUBMED: 20564162] Cianfrocca 2010 {published and unpublished data} [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley T, Henry D, Tonda M, Sun S, O’Connell M, Rackoff W. A randomized, double-blind study of pegylated liposomal doxorubicin for the treatment of AIDS-related Kaposi's sarcoma. The Oncologist. 2007;12(1):114–23. doi: 10.1634/theoncologist.12-1-114. [PUBMED: 17227906] Cooley 2007 {published data only} [DOI] [PubMed] [Google Scholar]
- Gill PS, Wernz J, Scadden DT, Cohen P, Mukwaya GM, von Roenn JH, et al. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi's sarcoma. Journal of Clinical Oncology. 1996;14(8):2353–64. doi: 10.1200/JCO.1996.14.8.2353. [PUBMED: 8708728] Gill 1996 {published data only} [DOI] [PubMed] [Google Scholar]
- Grünaug M, Bogner JR, Loch O, Goebel FD. Liposomal doxorubicin in pulmonary Kaposi's sarcoma: improved survival as compared to patients without liposomal doxorubicin. European Journal of Medical Research. 1998;21(3):13–9. Grünaug 1998 {published data only} [PubMed] [Google Scholar]
- Hernandez DE, Perez JR. Systemic treatment modalities in the management of AIDS-related Kaposi's sarcoma. Journal of the European Academy of Dermatology and Venereology. 1997;9:44–9. Hernandez 1997 {published data only} [Google Scholar]
- Martin-Carbonero L, Barrios A, Saballs P, Sirera G, Santos J, Palacios R, et al. Pegylated liposomal doxorubicin plus highly active antiretroviral therapy versus highly active antiretroviral therapy alone in HIV patients with Kaposi's sarcoma. AIDS (London, England) 2004;18(12):1737–40. doi: 10.1097/01.aids.0000131385.60974.b9. [PUBMED: 15280789] Martin-Carbonero 2004 {published and unpublished data} [DOI] [PubMed] [Google Scholar]
- Bihl F, Mosam A, Henry LN, Chisholm JV, 3rd, Dollard S, Gumbi P, et al. Kaposi's sarcoma-associated herpesvirus-specific immune reconstitution and antiviral effect of combined HAART/chemotherapy in HIV clade C-infected individuals with Kaposi's sarcoma. AIDS (London, England) 2007;21(10):1245–52. doi: 10.1097/QAD.0b013e328182df03. PUBMED: 17545700. [DOI] [PubMed] [Google Scholar]
- Mosam A, Shaik F, Uldrick TS, Esterhuizen T, Friedland GH, Scadden DT, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. Journal of Acquired Immune Deficiency Syndromes. 2012;60(2):150–7. doi: 10.1097/QAI.0b013e318251aedd. * [PUBMED: 22395672] Mosam 2012 {published and unpublished data} [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olweny CL, Borok M, Gudza I, Clinch J, Cheang M, Kiire CF, et al. Treatment of AIDS-associated Kaposi's sarcoma in Zimbabwe: results of a randomized quality of life focused clinical trial. International Journal of Cancer. 2005;113:632–9. doi: 10.1002/ijc.20606. Olweny 2005 {published and unpublished data} [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Asiimwe F, Moore D, Were W, Nakityo R, Campbell J, Barasa A, et al. Clinical outcomes of HIV-infected patients with Kaposi's sarcoma receiving nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy in Uganda. HIV Medicine. 2012;13(3):166–71. doi: 10.1111/j.1468-1293.2011.00955.x. [PUBMED: 22112164] Asiimwe 2012 {published data only} [DOI] [PubMed] [Google Scholar]
- Autier J, Picard-Dahan C, Marinho E, Grossin M, Yeni P, Leport C, et al. Docetaxel in anthracycline-pretreated AIDS-related Kaposi's sarcoma: a retrospective study. British Journal of Dermatology. 2005;152(5):1026–9. doi: 10.1111/j.1365-2133.2005.06452.x. [PUBMED: 15888164] Autier 2005 {published data only} [DOI] [PubMed] [Google Scholar]
- Bihl F, Mosam A, Henry LN, Chisholm JV, 3rd, Dollard S, Gumbi P, et al. Kaposi's sarcoma-associated herpesvirus-specific immune reconstitution and antiviral effect of combined HAART/chemotherapy in HIV clade C-infected individuals with Kaposi's sarcoma. AIDS (London, England) 2007;21(10):1245. doi: 10.1097/QAD.0b013e328182df03. [PUBMED: 17545700] Bihl 2007 {published data only} [DOI] [PubMed] [Google Scholar]
- Bodsworth NJ, Bloch M, Donnell D, Yocum R, The International Panretin Gel KS Study Group Phase III vehicle controlled multi-centered study of topical alitretinoin gel 0.1% in cutaneous AIDS related Kaposi's sarcoma. American Journal of Clinical Dermatology. 2001;2(2):77–87. doi: 10.2165/00128071-200102020-00004. Bodsworth 2001 {published data only} [DOI] [PubMed] [Google Scholar]
- Bonhomme L, Fredj G, Averous S, Szekely AM, Ecstein E, Trumbic B, et al. Topical treatment of epidemic Kaposi's sarcoma with all-trans-retinoic acid. Annals of Oncology. 1991;2(3):234–5. doi: 10.1093/oxfordjournals.annonc.a057916. [PUBMED: 2043496] Bonhomme 1991 {published data only} [DOI] [PubMed] [Google Scholar]
- Bonhomme L, Fredj G, Ecstein E, Maurisson G, Farabos C, Misset JL, et al. Treatment of AIDS-associated Kaposi's sarcoma with oral tretinoin. American Journal of Hospital Pharmacy. 1994;51(19):2417–9. [PUBMED: 7847408] Bonhomme 1994 {published data only} [PubMed] [Google Scholar]
- Borok M, Fiorillo S, Gudza I, Putnam B, Ndemera B, White IE, et al. Evaluation of plasma human herpesvirus 8 DNA as a marker of clinical outcomes during antiretroviral therapy for AIDS-related Kaposi sarcoma in Zimbabwe. Clinical Infectious Diseases. 2010;51(3):342–9. doi: 10.1086/654800. [PUBMED: 20572760] Borok 2010 {published data only} [DOI] [PubMed] [Google Scholar]
- Cattelan AM, Calabro ML, De Rossi A, Aversa SM, Barbierato M, Trevenzoli M, et al. Long-term clinical outcome of AIDS-related Kaposi's sarcoma during highly active antiretroviral therapy. International Journal of Oncology. 2005;27(3):779–85. [PUBMED: 16077928] Cattelan 2005 {published data only} [PubMed] [Google Scholar]
- Duvic M, Friedman-Kien AE, Looney DJ, Miles SA, Myskowski PL, Scadden DT, et al. Topical treatment of cutaneous lesions of acquired immunodeficiency syndrome-related Kaposi sarcoma using alitretinoin gel: results of phase 1 and 2 trials. Archives of Dermatology. 2000;136(12):1461–9. doi: 10.1001/archderm.136.12.1461. [PUBMED: 11115156] Duvic 2000 {published data only} [DOI] [PubMed] [Google Scholar]
- Gelmann E, Longo D, Lane C, Fauci A, Masur H, Wesley M, et al. Combination chemotherapy of disseminated Kaposi's sarcoma in patients with the acquired immune deficiency syndrome. American Journal of Medicine. 1987;62:456–62. doi: 10.1016/0002-9343(87)90445-1. Gelmann 1987 {published data only} [DOI] [PubMed] [Google Scholar]
- Gill PS, Rarick M, McCutchan JA, Slater L, Parker B, Muchmore E, et al. Systemic treatment of AIDS related Kaposi's sarcoma: results of a randomized trial. American Journal of Medicine. 1991;90:427–33. Gill 1991 {published data only} [PubMed] [Google Scholar]
- Harrison M, Harrington KJ, Tomlinson DR, Stewart JS. Response and cosmetic outcome of two fractionation regimens for AIDS related Kaposi's sarcoma. Radiotherapy and Oncology. 1998;46(1):23–8. doi: 10.1016/s0167-8140(97)00141-2. Harrison 1998 {published data only} [DOI] [PubMed] [Google Scholar]
- Hernandez DE, Perez JR. Advanced epidemic Kaposi's sarcoma: treatment with bleomycin or combination of doxorubicin, bleomycin, and vincristine. International Journal of Dermatology. 1996;35(11):831–3. doi: 10.1111/j.1365-4362.1996.tb02990.x. [PUBMED: 8915746] Hernandez 1996 {published data only} [DOI] [PubMed] [Google Scholar]
- Ireland-Gill A, Espina BM, Akil B, Gill PS. Treatment of acquired immunodeficiency syndrome-related Kaposi's sarcoma using bleomycin-containing combination chemotherapy regimens. Seminars in Oncology. 1992;19(2 Suppl 5):32–6. [PUBMED: 1384142] Ireland-Gill 1992 {published data only} [PubMed] [Google Scholar]
- Koon HB, Fingleton B, Lee JY, Geyer JT, Cesarman E, Parise RA, et al. Phase II AIDS Malignancy Consortium trial of topical halofuginone in AIDS-related Kaposi sarcoma. Journal of Acquired Immune Deficiency Syndromes. 2011;56(1):64–8. doi: 10.1097/QAI.0b013e3181fc0141. [PUBMED: 21068672] Koon 2011 {published data only} [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krigel RL, Slywotzky CM, Lonberg M, Green MD, Andes WA, Kempf R, et al. Treatment of epidemic Kaposi's sarcoma with a combination of interferon-alpha 2b and etoposide. Journal of Biological Response Modifiers. 1988;7(4):359–64. [PUBMED: 3049944] Krigel 1988 {published data only} [PubMed] [Google Scholar]
- Lane CL, Falloon J, Walker RE, Deyton L, Kovacs JA, Masur H, et al. Zidovudine in patients with human immunodeficiency virus (HIV) infection and Kaposi's sarcoma. Annals of Internal Medicine. 1989;111:41–50. doi: 10.7326/0003-4819-111-1-41. Lane 1989 {published data only} [DOI] [PubMed] [Google Scholar]
- Lasso M, Perez J, Noriega L, Malebran A, Espinoza S. Kaposi sarcoma in HIV patients: response to antiretroviral treatment and chemotherapy. Revista Medica de Chile. 2003;131(5):483–90. Sarcoma de Kaposi y VIH: tratamiento antirretroviral y quimioterapia en 32 pacientes. [PUBMED: 12879808] Lasso 2003 {published data only} [PubMed] [Google Scholar]
- Letang E, Lewis JJ, Bower M, Mosam A, Borok M, Campbell TB, et al. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS. 2013;27(10):1603–13. doi: 10.1097/QAD.0b013e328360a5a1. [PUBMED: 23462220] Letang 2013 {published and unpublished data} [DOI] [PubMed] [Google Scholar]
- Lichterfeld M, Qurishi N, Hoffmann C, Hochdorfer B, Brockmeyer NH, Arasteh K, et al. Treatment of HIV-1-associated Kaposi's sarcoma with pegylated liposomal doxorubicin and HAART simultaneously induces effective tumor remission and CD4+ T cell recovery. Infection. 2005;33(3):140–7. doi: 10.1007/s15010-005-4099-z. [PUBMED: 15940415] Lichterfeld 2005 {published data only} [DOI] [PubMed] [Google Scholar]
- Lim ST, Tupule A, Espina BM, Levine AM. Weekly docetaxel is safe and effective in the treatment of advanced-stage acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2005;103(2):417–21. doi: 10.1002/cncr.20780. [PUBMED: 15578686] Lim 2005 {published data only} [DOI] [PubMed] [Google Scholar]
- Monticelli A, Lewi D, Salomon H, Pampuro S, Libonatti O, Jauregui Rueda H, et al. Regression of AIDS-related Kaposi's sarcoma following combined antiretroviral treatment. Revista Argentina de Microbiologia. 2000;32(4):206–8. [PUBMED: 11149154] Monticelli 2000 {published data only} [PubMed] [Google Scholar]
- Mussini C, Manzardo C, Johnson M, Monforte AD, Uberti-Foppa C, Antinori A, et al. Patients presenting with AIDS in the HAART era: a collaborative cohort analysis. AIDS. 2008 Nov 30;22(18):2461–9. doi: 10.1097/QAD.0b013e328314b5f1. Mussini 2008 {published data only} [DOI] [PubMed] [Google Scholar]
- Nguyen HQ, Magaret AS, Casper C. Persistent Kaposi sarcoma in the era of HAART: characterizing the predictors of clinical response. AIDS. 2008;22(8):937–45. doi: 10.1097/QAD.0b013e3282ff6275. Nguyen 2008 {published data only} [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Freidman-Kien A, et al. Pegylated liposomal doxorubicin versus doxorubicin, bleomycin and vincristine in the treatment of AIDS related Kaposi's sarcoma: results of a randomised phase III clinical trial. Journal of Clinical Oncology. 1998;16(7):2445–51. doi: 10.1200/JCO.1998.16.7.2445. Northfelt 1998 {published data only} [DOI] [PubMed] [Google Scholar]
- Noy A, Scadden DT, Lee J, Dezube BJ, Aboulafia D, Tulpule A, et al. Angiogenesis inhibitor IM862 is ineffective against AIDS-Kaposi's sarcoma in a phase III trial, but demonstrates sustained, potent effect of highly active antiretroviral therapy: from the AIDS Malignancy Consortium and IM862 Study Team. Journal of Clinical Oncology. 2005;23(5):990–8. doi: 10.1200/JCO.2005.11.043. [PUBMED: 15598977] Noy 2005 {published data only} [DOI] [PubMed] [Google Scholar]
- Nunez M, Saballs P, Valencia ME, Santos J, Ferrer E, Santos I, et al. Response to liposomal doxorubicin and clinical outcome of HIV-1-infected patients with Kaposi's sarcoma receiving highly active antiretroviral therapy. HIV Clinical Trials. 2001;2(5):429–37. doi: 10.1310/700b-9qt3-hgn9-q3fq. [PUBMED: 11673818] Nunez 2001 {published data only} [DOI] [PubMed] [Google Scholar]
- Opravil M, Hirschel B, Bucher HC, Luthy R. A randomized trial of interferon a2a and zidovudine versus bleomycin and zidovudine for AIDS related Kaposis sarcoma. Swiss HIV Cohort Study International Journal of STD and AIDS. 1999;10:369–75. Opravil 1999 {published data only} [PubMed] [Google Scholar]
- Osoba D, Northfelt DW, Budd DW, Himmelberger D. Effect of treatment on health-related quality of life in acquired immunodeficiency syndrome (AIDS)-related Kaposi's sarcoma: a randomized trial of pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine. Cancer Investigation. 2001;19(6):573–80. doi: 10.1081/cnv-100104284. [PUBMED: 11486699] Osoba 2001 {published data only} [DOI] [PubMed] [Google Scholar]
- Palmieri C, Dhillon T, Thirlwell C, Newsom-Davis T, Young AM, Nelson M, et al. Pulmonary Kaposi sarcoma in the era of highly active antiretroviral therapy. HIV Medicine. 2006;7(5):291–3. doi: 10.1111/j.1468-1293.2006.00378.x. [PUBMED: 16945073] Palmieri 2006 {published data only} [DOI] [PubMed] [Google Scholar]
- Ramirez-Amador V, Esquivel-Pedraza L, Lozada-Nur F, De la Rosa-Garcia E, Volkow-Fernandez P, Suchil-Bernal L, et al. Intralesional vinblastine vs. 3% sodium tetradecyl sulfate for the treatment of oral Kaposi's sarcoma. A double blind, randomized clinical trial. Oral Oncology. 2002;38(5):460–7. doi: 10.1016/s1368-8375(01)00100-2. [PUBMED: 12110340] Ramirez-Amador 2002 {published data only} [DOI] [PubMed] [Google Scholar]
- Rosenthal E, Poizot-Martin I, Saint-Marc T, Spano JP, Cacoub P. Phase IV study of liposomal daunorubicin (DaunoXome) in AIDS-related Kaposi sarcoma. American Journal of Clinical Oncology. 2002;25(1):57–9. doi: 10.1097/00000421-200202000-00012. [PUBMED: 11823698] Rosenthal 2002 {published data only} [DOI] [PubMed] [Google Scholar]
- Shepherd FA, Beaulieu R, Gelman K, Thuot CA, Sawaka C, Read S, et al. Prospective randomized trial of two dose levels of interferon alpha with zidovudine for the treatment of Kaposi's sarcoma associated with human immunodeficiency virus infection. A Canadian HIV clinical trials network study. Journal of Clinical Oncology. 1998;16(5):1736–42. doi: 10.1200/JCO.1998.16.5.1736. Shepherd 1998 {published data only} [DOI] [PubMed] [Google Scholar]
- Singh NB, Lakier RH, Donde B. Hypofractionated radiation therapy in the treatment of epidemic Kaposi sarcoma--a prospective randomized trial. Radiotherapy and Oncology. 2008;88(2):211–6. doi: 10.1016/j.radonc.2008.03.009. [PUBMED: 18439694] Singh 2008 {published data only} [DOI] [PubMed] [Google Scholar]
- Stelzer KJ, Griffin TW. A randomized prospective trial of radiation therapy for AIDS-associated Kaposi's sarcoma. International Journal of Radiation Oncology, Biology, Physics. 1993;27(5):1057–61. doi: 10.1016/0360-3016(93)90523-x. [PUBMED: 8262827] Stelzer 1993 {published data only} [DOI] [PubMed] [Google Scholar]
- Stewart S, Jablonowski H, Goebel FD, Arasteh K, Spittle M, Rios A, et al. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi's sarcoma. Journal of Clinical Oncology. 1998;16(2):683–91. doi: 10.1200/JCO.1998.16.2.683. Stewart 1998 {published data only} [DOI] [PubMed] [Google Scholar]
- Strother RM, Gregory KM, Pastakia SD, Were P, Tenge C, Busakhala N, et al. Retrospective analysis of the efficacy of gemcitabine for previously treated AIDS-associated Kaposi's sarcoma in western Kenya. Oncology. 2010;78(1):5–11. doi: 10.1159/000292356. [PUBMED: 20215784] Strother 2010 {published data only} [DOI] [PubMed] [Google Scholar]
- Tavio M, Vaccher E, Antinori A, Ammassari A, Cusini M, Fasan M, et al. Combination chemotherapy with doxorubicin, bleomycin, and vindesine for AIDS-related Kaposi's sarcoma. Cancer. 1996;77(10):2117–22. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2117::AID-CNCR23>3.0.CO;2-X. [PUBMED: 8640679] Tavio 1996 {published data only} [DOI] [PubMed] [Google Scholar]
- Tulpule A, Groopman J, Saville MW, Harrington W, Jr, Friedman-Kien A, Espina BM, et al. Multicenter trial of low-dose paclitaxel in patients with advanced AIDS-related Kaposi sarcoma. Cancer. 2002;95(1):147–54. doi: 10.1002/cncr.10634. [PUBMED: 12115328] Tulpule 2002 {published data only} [DOI] [PubMed] [Google Scholar]
- Uthayakumar S, Bower M, Money-Kyrle J, Muyshondt C, Youle M, Hannon F, et al. Randomized cross-over comparison of liposomal daunorubicin versus observation for early Kaposi's sarcoma. AIDS (London, England) 1996;10(5):515–9. doi: 10.1097/00002030-199605000-00010. [PUBMED: 8724043] Uthayakumar 1996 {published data only} [DOI] [PubMed] [Google Scholar]
- Walmsley S, Northfelt DW, Melosky B, Conant M, Friedman-Kien AE, Wagner B. Treatment of AIDS-related cutaneous Kaposi's sarcoma with topical alitretinoin (9-cis-retinoic acid) gel. Panretin Gel North American Study Group. Journal of Acquired Immune Deficiency Syndromes. 1999;22(3):235–46. doi: 10.1097/00126334-199911010-00004. [PUBMED: 10770343] Walmsley 1999 {published data only} [DOI] [PubMed] [Google Scholar]
- Zhong DT, Shi CM, Chen Q, Huang JZ, Liang JG, Lin D. Etoposide, vincristine, doxorubicin and dexamethasone (EVAD) combination chemotherapy as second-line treatment for advanced AIDS-related Kaposi's sarcoma. Journal of Cancer Research and Clinical Oncology. 2012;138(3):425–30. doi: 10.1007/s00432-011-1109-7. [PUBMED: 22160130] Zhong 2012 {published data only} [DOI] [PubMed] [Google Scholar]
Additional references
- Bower M, Palmieri C, Dhillon T. AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Current Opinion in Infectious Diseases. 2006;19(1):14–9. doi: 10.1097/01.qco.0000200295.30285.13. Bower 2006. [DOI] [PubMed] [Google Scholar]
- Browning PJ, Sechler JM, Kaplan M, Washington RH, Gendelman R, Yarchoan R, et al. Identification and culture of Kaposi's sarcoma-like spindle cells from the peripheral blood of human immunodeficiency virus-1-infected individuals and normal controls. Blood. 1994;84:2711–20. Browning 1994. [PubMed] [Google Scholar]
- Carrieri MP, Pradier C, Piselli P, Piche M, Rosenthal E, Heudier P, et al. Reduced incidence of Kaposi's sarcoma and of systemic non-Hodgkin's lymphoma in HIV-infected individuals treated with highly active antiretroviral therapy. International Journal of Cancer. 2003;103(1):142–4. doi: 10.1002/ijc.10790. Carrieri 2003. [DOI] [PubMed] [Google Scholar]
- Casper C. The increasing burden of HIV-associated malignancies in resource-limited regions. Annual Review of Medicine. 2011;62:157–70. doi: 10.1146/annurev-med-050409-103711. Casper 2011. [DOI] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin M, Lee F, Culpepper J, Knowles DM, et al. Identification of new human herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266(5192):1865–9. doi: 10.1126/science.7997879. Chang 1994. [DOI] [PubMed] [Google Scholar]
- Cochrane HIV/AIDS Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2011;(1) Art. No.: HIV. Cochrane HIV/AIDS Group 2011. [Google Scholar]
- Davis MA, Stürzl MA, Blasig C, Schreier A, Guo HG, Reitz M, et al. Expression of human herpesvirus 8-encoded cyclin D in Kaposi's sarcoma spindle cells. Journal of the National Cancer Institute. 1997;89:1868–74. doi: 10.1093/jnci/89.24.1868. Davis 1997. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo G, Konstantinopoulos PA, Pantanowitz L, Di Trolio R, De Placido S, Dezube BJ. Management of AIDS-related Kaposi's sarcoma. The Lancet Oncology. 2007;8(2):167–76. doi: 10.1016/S1470-2045(07)70036-0. Di Lorenzo 2007. [DOI] [PubMed] [Google Scholar]
- Di Trolio R, Di Lorenzo G, Delfino M, De Placido S. Role of pegylated lyposomal doxorubicin (PLD) in systemic Kaposi's sarcoma: a systematic review. International Journal of Immunopathology and Pharmacology. 2006;19(2):253–63. doi: 10.1177/039463200601900202. Di Trolio 2006. [DOI] [PubMed] [Google Scholar]
- Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20(12):1645–54. doi: 10.1097/01.aids.0000238411.75324.59. Engels 2006. [DOI] [PubMed] [Google Scholar]
- Freeman E, Semeere A, Wenger M, Mwebesa B, Asirwa FC, Busakhala N, et al. Pitfalls of Practicing Cancer Epidemiology in resource-limited settings: The case of survival after a diagnosis with Kaposi's sarcoma in sub-Saharan Africa. 14th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies; November 2013; Freeman 2013. [Google Scholar]
- Gantt S, Kakuru A, Wald A, Walusansa V, Corey L, Casper C, et al. Clinical presentation and outcome of epidemic Kaposi sarcoma in Ugandan children. Pediatric Blood Cancer. 2010;54(5):670–4. doi: 10.1002/pbc.22369. Gantt 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grulich AE, Li Y, McDonald AM, Correll PK, Law MG, Kaldor JM. Decreasing rates of Kaposi's sarcoma and non-Hodgkin's lymphoma in the era of potent combination anti-retroviral therapy. AIDS. 2001;15(5):629–33. doi: 10.1097/00002030-200103300-00013. Grulich 2001. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011 updated March 2011. Available from www.cochrane-handbook.orgHiggins 2011.
- Jones JL, Hanson DL, Dworkin MS, Jaffe HW. Incidence and trends in Kaposi's sarcoma in the era of effective antiretroviral therapy. Journal of Acquired Immune Deficiency Syndrome. 2000;24:270–4. doi: 10.1097/00126334-200007010-00013. Jones 2000. [DOI] [PubMed] [Google Scholar]
- Khanlou H, Stein T, Farthing C. Development of Kaposi sarcoma despite sustained suppression of HIV plasma viremia. Journal of Acquired Immune Deficiency Syndrome. 2000;23(4):361. doi: 10.1097/00126334-200004010-00017. Khanlou 2000. [DOI] [PubMed] [Google Scholar]
- Krown SE, Metroka C, Wernz JC. Kaposi's sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. Journal of Clinical Oncology. 1989;7(9):1201–7. doi: 10.1200/JCO.1989.7.9.1201. Krown 1989. [DOI] [PubMed] [Google Scholar]
- Krown SE. Clinical characteristics of Kaposi sarcoma. HIV InSite Knowledge Base Chapter. 2006 Feb; Krown 2006. [Google Scholar]
- Martellotta F, Berretta M, Vaccher E, Schioppa O, Zanet E, Tirelli U. AIDS-related Kaposi's sarcoma: state of the art and therapeutic strategies. Current HIV Research. 2009;7(6):634–8. doi: 10.2174/157016209789973619. Martellotta 2009. [DOI] [PubMed] [Google Scholar]
- Martin J, Laker-Oketta M, Walusana V, Orem J, Wabinga H, Bennett J, et al. Antiretrovirals for Kaposi's Sarcoma (ARKS): A randomized trial of protease inhibitor-based antiretroviral therapy for AIDS-associated Kaposi's sarcoma in sub-Saharan Africa. 14th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies; November 2013; Martin 2013. [Google Scholar]
- Maurer T, Ponte M, Leslie K. HIV-Associated Kaposi's Sarcoma with a High CD4 Count and a Low Viral Load. New England Journal of Medicine. 2007;357:1352–1353. doi: 10.1056/NEJMc070508. Maurer 2007. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Kirk O, Clumeck N, Gargalianos-Kakolyris P, Trocha H, Chentsova N, et al. The changing pattern of Kaposi sarcoma in patients with HIV, 1994-2003: the EuroSIDA Study. Cancer. 2004;100:2644–54. doi: 10.1002/cncr.20309. Mocroft 2004. [DOI] [PubMed] [Google Scholar]
- Semeere AS, Busakhala N, Martin JN. Impact of Antiretroviral Therapy on the Incidence of Kaposi's Sarcoma in Resource-rich and Resource-limited Settings. Current opinion in oncology. 2012;24(5):522–530. doi: 10.1097/CCO.0b013e328355e14b. Semeere 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Dedicoat M, Vaithilingum M, Newton RR. Treatment of Kaposis sarcoma in HIV-1 infected individuals with emphasis on resource poor settings. Cochrane Database of Systematic Reviews. 2003;(3):CD003256. doi: 10.1002/4651858. * Indicates the major publication for the study. [DOI] [PubMed] [Google Scholar]


