Summary
Background
Ibrutinib, an orally administered covalent inhibitor of Bruton tyrosine kinase (BTK), is an effective therapy for patients with relapsed chronic lymphocytic leukemia (CLL). We investigated the activity and safety of the combination of ibrutinib with the monoclonal antibody rituximab (iR) in patients with high-risk CLL.
Methods
In this single-arm, phase 2 studywe enrolled 40 patients with high-risk CLL at MD Anderson Cancer Center, Houston, Texas, USA. Patients with symptomatic CLL requiring therapy received 28 day cycles of once-daily ibrutinib 420 mg , together with rituximab (weekly during cycle 1, then once per cycle until cycle 6), followed by continuous single-agent ibrutinib. The primary endpoint was progression-free survival (PFS) in the intention-to-treat population. This study is registered with ClinicalTrials.gov, number NCT01520519 and is no longer accruing patients.
Findings
Between February 28, 2012 and September 11, 2012, we enrolled 40 CLL patients with high-risk disease features. 20 patients had del17p or TP53 mutations (16 previously treated, 4 untreated), 13 had relapsed CLL with del11q, and 7 patients a PFS < 36 months after frontline chemo-immunotherapy. Toxicity was mainly of mild to moderate severity (grade 1–2). 10 (25%) patients had diarrhea (grade 1 in 9 [22.5%] patients, grade 2 in 1 [2.5%]), bleeding events occurred in 14 (35%) patients (8 [20%] patients with grade 1, 5 [12.5%] patients grade 2, and 1 [2.5%] grade 3), nausea in 15 (37.5) patients (10 [25%] grade 1, 5 [12.5%] grade 2), and fatigue in 7 (17.5%) patients (4 [10%] grade 1, 3 [7.5%] grade 2). Grade 3 infections occurred in 4 patients (10%), no grade 4 or 5 infections occurred. At 18 months, the Kaplan Meier estimate of progression-free survival was 78% (95% CI 60.6–88.5) (del[17p] or TP53 mutation: 72%, 95% CI: 45.6–87.6)
Interpretation
Ibrutinib in combination with rituximab is a well-tolerated regimen for patients with high-risk CLL. It induces high rates of remissions and has positive impact on QOL in this difficult-to-treat patient population. These encouraging data merit further investigation of the added benefit of rituximab as combination partner for ibrutinib in an ongoing randomized trial, in which single-agent ibrutinib is compared to iR combination therapy (NCT02007044).
Funding
Pharmacyclics, Inc., Cancer Prevention and Research Institute of Texas (CPRIT), Leukemia & Lymphoma Society, NCI Grant P30 CA016672, MD Anderson’s Moon Shot Program in CLL, and MD Anderson Cancer Center Support Grant CA016672.
Introduction
The treatment of patients with chronic lymphocytic leukemia CLL is undergoing fundamental changes1 due to the emergence of new treatment modalities, such as kinase inhibitors targeting B cell receptor (BCR) signaling2, 3 and novel monoclonal antibodies4. CLL patients with high-risk cytogenetic abnormalities (deletion 17pTP53 mutation, or deletion 11q)5 can particularly benefit from new kinase inhibitors, given the high response rates to therapy with the BTK inhibitor ibrutinib2 and the PI3 kinase delta idelalisib in combination with rituximab3. Chemo-immunotherapy (CIT) regimens, such as fludarabine, cyclophosphamide, rituximab (FCR)6 or bendamustine in combination with rituximab (BR)7 are effective therapy options for younger patients with lower-risk CLL. However, patients with del(17p) or TP53 mutations respond poorly to CIT, typically have short remissions6, 7, and consequently a short median life expectancy of only 2 to 3 years after first-line therapy8. Patients with relapsed CLL and 11q deletion, who often have extensive lymphadenopathy9, are another subset with a poorer prognosis. These patients respond relatively well to chemo-immunotherapy regimens, but have shorter remission durations when compared to those of low-risk patients9, 10. Finally, patients with CLL relapsing early after frontline chemo-immunotherapy (i.e. after less than 24 to 36 months) are another challenging group of high-risk patients8, 10 with low response rates and a short survival when re-treated with chemo-immunotherapy8, 10.
Ibrutinib (previously called PCI-32765) is a potent (IC50, 0.5 nM), selective BTK inhibitor which inactivates BTK through irreversible covalent bonding to Cys-481 in the ATP binding domain of the BTK kinase11. For patients with CLL, ibrutinib is administered once-daily orally at a fixed dose of 420 mg continuously until disease progression or toxicity. Early stage clinical trials found single-agent ibrutinib to be particularly active in patients with chronic lymphocytic leukaemia (CLL)2, 12 and mantle cell lymphoma (MCL)13. The CLL data are based on a phase 1b/2 multicenter study of ibrutinib in 85 patients with relapsed or refractory CLL or SLL2. The authors report an ORR of 71%, an additional 15% (in the 840 mg cohort) or 20% (in the 420 mg cohort) of patients had a partial response (PR) with lymphocytosis (PRL). The response was independent of clinical and genomic risk factors present prior to treatment, including advanced-stage disease, number of prior treatments, or presence of 17p deletion. At 26 months, the estimated progression-free survival rate was 75% and the rate of overall survival was 83%. The results from this study led to a Breakthrough Therapy designation for ibrutinib by the U.S. Food and Drug Administration (FDA) in CLL patients with del 17p and the FDA approval of single-agent ibrutinib (Imbruvica™) for previously treated patients with CLL in February 2014. Another recent randomized clinical trial compared ibrutinib with the anti-CD20 antibody ofatumumab in patients with relapsed or refractory CLL or SLL14. The overall response rate was significantly higher in the ibrutinib group than in the ofatumumab group (42.6% versus 4.1%). An additional 20% of brutinib-treated patients had a partial response with lymphocytosis. At a median follow-up of 9.4 months, ibrutinib significantly improved PFS and OS when compared to ofatumumab14.
In patients with CLL, ibrutinib causes mobilization of CLL cells from tissue sites into the peripheral blood15, resulting in lymphocytosis during the first weeks of therapy, which is variable among patients and directly related to the presence of the drug. On an intermittent dosing schedule, an increased absolute lymphocyte counts (ALC) rapidly dropped during the off-ibrutinib period, presumably due to increased tissue homing, and then increased again, once ibrutinib was re-started12. This lymphocytosis is asymptomatic and normalizes after a median time of 6.2 months16. It is due to the re-distribution of CLL cells from the tissue compartments into the peripheral blood12, 15 and therefore must not be confused with lymphocytosis due to disease progression. Preclinical models demonstrated that ibrutinib inhibits CLL cell survival and proliferation17, as well as leukaemia cell migration towards tissue homing chemokines (CXCL12, CXCL13) and integrin-mediated CLL cell adhesion18, 19; this resulted in transient redistribution lymphocytosis into the PB in an adoptive transfer CLL mouse model18. Given the redistribution lymphocytosis with single-agent ibrutinib, which can persist in a significant number of patients, the well-recognized efficacy of rituximab in clearing CLL cells from the peripheral blood, and the documented benefit of rituximab in chemotherapy combinations on PFS and OS in untreated patients with CLL6, we explored efficacy and safety of the iR combination therapy in high-risk CLL.
Methods
Patients
Forty patients with CLL or small lymphocyticlymphoma (SLL) with high-risk disease were enrolled onto a phase II study of ibrutinib and rituximab at MD Anderson Cancer Center between February and September, 2012. All patients were at least 18 years of age. Inclusion criteria included previously treated high-risk CLL (n=36), and untreated patients with 17p deletion or TP53 mutation (n=4), given the poor outcome of such patients with CIT. High-risk CLL was defined by the presence of a 17p deletion, TP53 mutation, or 11q deletion, according to the hierarchical model5. Patients with CLL and SLL who had short remission durations of less than 3 years after prior first-line CIT also fulfilled criteria for high-risk disease. Patients with 17p deletion or TP53 mutation were not required to have received prior therapy, given the poor outcome of such patients with CIT. For previously-treated patients, a 30 day wash out period from prior therapy was required. Patients were required to have an indication for therapy in accordance with the 2008 IWCLL criteria20, adequate renal and hepatic function, defined by an estimated creatinine clearance (CrCl) of > 30 mL/min, an alanine aminotransferase level of no more than 2.5 times the upper limit of the normal range, and absence of active infection. Patients with uncontrolled autoimmune haemolytic anemia (AIHA) or autoimmune thrombocytopenia (ITP), severe hematopoietic insufficiency (absolute neutrophil count < 500/µL and/or platelet count < 30,000/µL), bleeding diathesis or coagulopathy, recent hemorrhagic events or surgery, or concomitant treatment with warfarin were excluded.
Study design
This phase 2 clinical trial of ibrutinib and rituximab was developed by the investigators in collaboration with Pharmacyclics, Inc. and approved by The University of Texas MD Anderson Cancer Center institutional review board. Informed consent was obtained in accordance with institutional guidelines and the Declaration of Helsinki. Treatment consisted of ibrutinib (420 mg daily by mouth) continuously daily (one cycle = 28 days), in combination with weekly rituximab (375 mg/m2 intravenously) for weeks 1–4 (cycle 1) to front-load during time of highest intravascular CLL cell burden, then rituximab was given once every 4 weeks until cycle 6, followed by single-agent ibrutinib. Patients remained on treatment until disease progression or toxicities or complications precluded further therapy. Ibrutinib was held for any grade 3–4 toxicity until the adverse event returns to baseline or resolves complete. Rituximab was held for any Grade 4 toxicity or for any rituximab-related, clinically significant, unmanageable Grade 3 adverse events until the adverse event returns to baseline or resolves complete. Dose modifications were not permitted. Clinical and laboratory assessment were performed every week during cycle 1, then once every 4 weeks until cycle 6, and then every 3 months thereafter while patients remained on study.
Pretreatment evaluation
Pre-treatment evaluation included history, physical examination, complete blood count with differential, and chemistry profile consisting of serum creatinine, electrolytes, albumin, calcium, uric acid, lactate dehydrogenase, and alanine transaminase. Serum immunoglobulin (Ig) levels, β2-microglobulin levels, and peripheral T-cell lymphocyte subset analysis by flow cytometry were also measured. Bone marrow aspiration was performed before therapy, including infiltration assessment, immunophenotype by flow cytometry, Ig heavy chain gene mutation analysis by polymerase chain reaction. Standard metaphase karyotype analysis and genomic abnormalities were detected by fluorescent in situ hybridization (FISH) using standard CLL probes (Abbott Molecular).
Response and safety assessments, quality of life
Response assessment for PFS and overall response rate (ORR) included clinical assessment, along with radiologic examinations with computerized tomography (CT) scans of the chest, abdomen, and pelvisat baseline, after 3 or 6 months (time-point at the discretion of the treating physician), and after 12 months. Bone marrow aspirations and biopsies were performed and evaluated by morphology and flow cytometry at baseline and after 3, 6, and 12 months, and once every 12 months thereafter. Minimal residual disease (MRD) assessment was performed using 4-color flow cytometry. Quantitative MRD results were categorized as positive (≥ 0.01%) or negative (lower than 0.01%)21. Responses were evaluated according to the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) criteria20, with the exception that lymphocytosis was not the sole criterion for disease progression. Patients with persistent lymphocytosis, who otherwise were considered to have a partial response by all other measures, were considered to have a partial response with lymphocytosis. To assess for disease progression on therapy, best response, i.e. the true nadir was considered as baseline. For example, if a patient had normalization or major reduction of ALC on iR therapy, but later developed progressive lymphocytosis, this patient would be consider to have PD, especially in the context of other signs (clinical and laboratory) of disease progression, such as progressive cytopenias, increasing levels of LDH, and/or organomegaly and/or progressive lymphadenopathy. Safety monitoring was performed weekly for the first month, then monthly until month 6, and every 3 months thereafter. Adverse events were graded with the use of the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Hematologic toxic effects were graded according to the IWCLL system. The EORTC QLQ-C30 questionnaire was used to assess health-related quality of life (QOL) as detailed in the online supplement.
BTK probe assay, chemotaxis, plasma cytokine/chemokine assays, gene expression analysis
BTK target occupancy during iR treatment was tested using a fluorescently tagged derivative of ibrutinib (PCI-33380). Chemotaxis of CLL cells was assayed across polycarbonate Transwell inserts. Plasma levels of CCL3 and CCL4 before and during treatment were assessed by ELISA using Quantikine Kits (R&D Systems). For all other cytokines we used multiplex bead assays according to the manufacturer’s instructions (EMD Millipore, Billerica, MA, USA). For gene expression analyses, RNA was isolated from CD19-purified CLL cells from 10 randomly selected patients’ peripheral blood mononuclear cells (PBMC), collected at baseline and after 7 and 28 days on iR treatment. Gene expression was tested with HG U133 plus 2.0 oligonucleotide arrays from Affymetrix (Affymetrix, Santa Clara, CA) in 8 patients samples (2 patients had insufficient amounts of RNA), following the Affymetrix standard protocols. Further details are provided in the online supplement.
Statistical Analysis
The primary endpoint was to assess the activity of the combination of ibrutinib and rituximab in high-risk CLL, with progression-free survival (PFS) as the primary endpoint. PFS was defined as the time between initiation of study treatment and the date of first documented disease progression or death from any cause. The secondary endpoints were overall survival (OS), overall response rate (ORR), response rates and survival in biological subgroups, rates of treatment-related and –unrelated adverse effects, quality of life (QOL), and changes in correlative biomarkers during treatment. We determined that with a sample size of 40 patients, if the new treatment improves the progression-free survival (PFS) in high-risk CLL by 2 months when compared to available data in a comparable group of patients treated with standard chemo-immunotherapy10, the power is > 80%. Progression free survival was defined as the time interval from treatment to progressive disease or death, whichever happened earlier. Patients in complete remission (CR), partial remission (PR) or stable disease (SD) are all counted as progression-free. Due to the characteristic activity of ibrutinib, which typically induces a transient lymphocytosis during the first months of therapy, which is due to a re-distribution of CLL cells from the tissue compartments (bone marrow, secondary lymphatic tissues) into the peripheral blood, increases in lymphocyte counts can be expected (although in this combination with rituximab are less likely) and was not considered as a sign of progressive disease. For interim analyses, the PFS was continuously monitored, and accrual would have been terminated early if the probability of improving PFS by 2 months is less than 0.01. The Department of Biostatistics provided and maintained a website (“Clinical Trial Conduct”) for enrolling patients on this study and evaluating the efficacy monitoring rules described above. This analysis shows the final data from our study, extracted on November 18, 2013. Descriptive statistics were used to summarize the patient cohorts at defined time-points. Fisher’s exact test and Wilcoxon rank test were used in the univariate analyses to compared groups. Wilcoxon signed ranks test for paired data was used to determine if there were differences in the distribution between time points in the QOL outcomes. The safety analysis was performed based on the intent-to-treat and efficacy analysis was analyzed only using the evaluable cohort (39 patients). Toxicity was reported by type, frequency and severity. Worst toxicity grades per patient were tabulated for selected adverse events and laboratory measurements. Survival times were estimated using the Kaplan-Meier method. Patients were censored for progression-free survival at the last clinical assessment before receipt of new anti-leukemia therapy, or after loss to follow-up, whichever occurred first. Patients were censored for overall survival at the last known alive date. Statistical analyses were conducted using STATA/SE version 12·1 statistical software (Stata, LP, College Station, TX) and GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California, 2013). This study is registered with ClinicalTrials.gov, number NCT01520519.
Role of the funding sources
The study was supported by Pharmacyclics, Inc. The clinical investigators were responsible for the design of the study protocol and analysis plan, together with Pharmacyclics. The investigators and their respective research teams collected all of the data, and compiled and analyzed the data. The investigators had full access to the data and analyses for compilation of this manuscript. JAB wrote the first draft of the manuscript, which was carefully reviewed by Pharmacyclics, Inc. and all co-authors, who approved the final submitted version. The corresponding author had full access to all of the data and had final responsibility to submit for publication.
Results
Patients and treatment
Patients were accrued between February 28, 2012 and September 11, 2012 at MD Anderson Cancer Center. Baseline characteristics of the forty patients are summarized in Table 1; they include a median age of 63 (range 35–82) and a median of 2 prior therapies. 20 patients had del17p or TP53 mutation (4 without prior therapy), and 13 patients had del11q. Thirty-two patients had unmutated IGHV genes, only one patient had mutated IGHV genes, the remaining seven patients had inconclusive IGHV results. At a median follow up of 16.8 months (range 2.3–20.5 months), 31 patients continue on therapy (14 of 20 with del17p or TP53 mutation) and 9 (22.5%) discontinued treatment. Among the 9 patients that discontinued, 2 patients died while on study, and 6 died after study discontinuation. One patient that died on study had an unrelated infectious complication (pneumonia and CNS abscesses) during the 2nd cycle of treatment, the other patient died in remission from unknown cause (during sleep). The 6 other patients that died after study discontinuation died from infectious complications or disease progression (for details please see supplemental Table S3). Two patients (5%) discontinued therapy because of toxicity (one grade 3 mucositis, one grade 3 recurrent ear and pulmonary infections). One patient stopped because of resistant pneumonia, and another with progressive COPD. Three patients (7.5%) had disease progression after 176, 395, and 429 days, one of them with Richter’s transformation. The post-remission treatments are detailed in the supplemental Table S3.
Table 1.
Patient characteristics.
| Patients (N=40) | |
|---|---|
| Median age (range) | 63.2 (35 – 82) |
| Female | 14 (35 %) |
| Male | 26 (65 %) |
| Rai stage | |
| I / II | 7/4 |
| III | 6 |
| IV | 23 |
| Del (17p) or TP53 mutation, previously treated | 16 (40%) |
| Del (17p) or TP53 mutation, previously untreated | 4 (10 %) |
| Del (11q) | 13 (32.5 %) |
| Del (13q) | 5 (12.5 %) |
| FISH negative | 2 (5 %) |
| IGHV gene (unmutated / mutated / unknown) | 32 / 1 / 7 |
| Early relapse after chemo-immunotherapy | 7 |
| Number of prior treatments (mean, range) | 1.9 (1 – 3) |
| Time to relapse, months (mean, range) | 14.4 ( 2 – 24) |
| Median (range) | |
| Prior treatments | 2.0 (0 – 8) |
| Fludarabine, cyclophosphamide, rituximab (FCR) | 28 |
| Monoclonal antibodies (rituximab, ofatumumab, alemtuzumab) | 18 |
| Bendamustine, rituximab (BR) | 9 |
| Other regimen | 25 |
| Cycles completed | 15 (2 – 21) |
| Follow-up time (months) | 16.8 (2.3 – 20.5) |
| Hemoglobin (g/dL) | 11.7 (6.7 – 15.6) |
| Platelets (103/µL) | 91.5 (36 – 242) |
| White blood cell count (103/µL) | 22.4 (2.2 – 297.8) |
| Absolute lymphocytes (103/µL) | 19.9 (0.4 – 277) |
| beta-2-microglobulin (mg/L) | 4.15 (2.2 – 12.3) |
Treatment responses
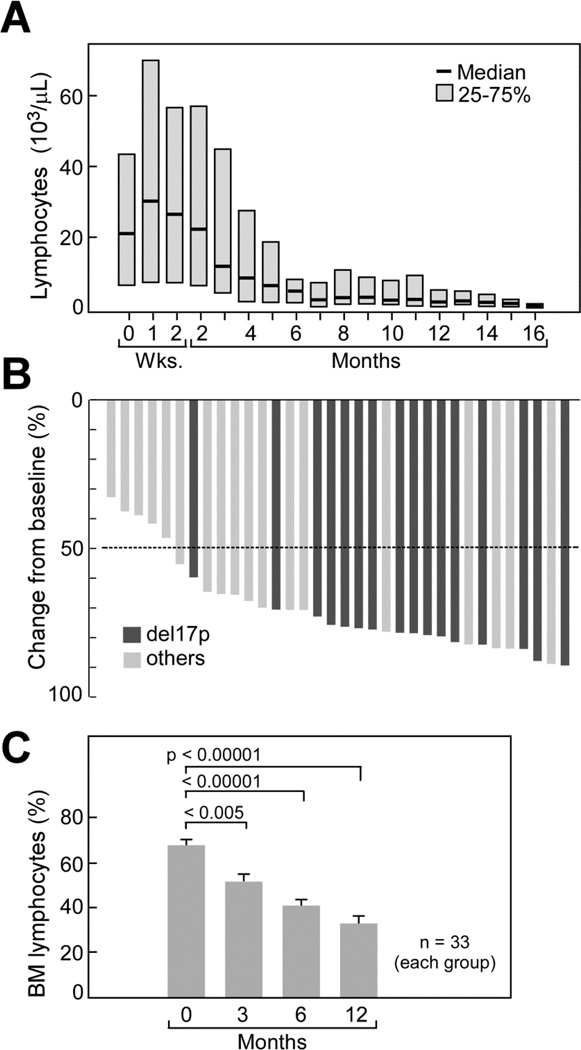
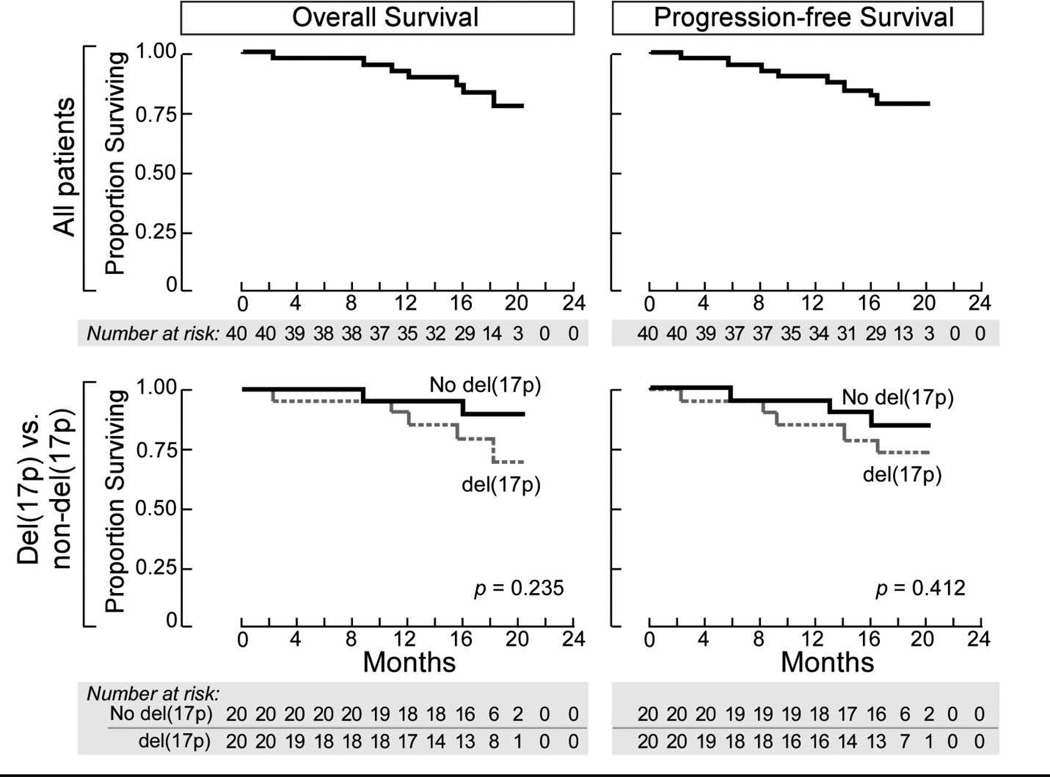
Thirty-nine patients were evaluable for response assessment per 2008 IWCLL guidelines: 34 (87%) achieved partial remission (PR), and three (8%) complete remission (CR) as best response, accounting for an ORR of 95%. Notably, two of three patients that achieved a CR were patients with del17p or TP53 mutation who were previously untreated. One patient in CR was negative for MRD by flow cytometry. The ORR in the 20 patients with del17p or TP53 mutation was 90% (16 PR, 2 CR). The median time to achieve a response was 5.72 months (IQR 5.32–6.87), and the median duration of response was 15.44 months (IQR 10.55–18.27). The two patients that did not respond were patients that discontinued therapy early, after 5 or 2 cycles of treatment, due to toxicity (mucositis, patient 3 in Table S3) and infectious complications (patient 5 in Table S3). Blood lymphocytosis peaked at a median of one week of iR therapy, and then continuously declined (Figure 1A). CT scans at 6 months and 12 months (Figure 1B) revealed more than 50% reduction in lymphadenopathy in 29 of 34 evaluable patients (85%). At 18 months, an estimated 78% of patients were free of progression (95% confidence interval [CI]: 60.58–88.45); in patients with del(17p) or TP53 mutation, the PFS was 72% (95% CI: 45.56–87.55). The estimated OS of all patients at 18 months was 84% (95% CI: 67.22–92.39); for patients with del(17p) or TP53 mutation, the estimated OS was 78% (95% CI: 51.99–91.36, Figure 2). In 38 evaluable patients, the lymphocyte count normalized (absolute lymphocyte count, <4×109 cells/L) or was reduced by 50% from baseline after 7 months (Figure 1A). This coincided with a notable reduction in lymphnode size (Figure 1B) and spleen size (supplemental Figure S1). Sustained improvement in cytopenias, defined as improvement by more than 50% from baseline or a hemoglobin level higher than 11 g/dL, or a platelet count higher than 100,000/µL lasting for ≥2 cycles without transfusion or administration of growth factors, was observed in 15 of 24 patients with baseline thrombocytopenia, and 15 of 17 with anemia (supplemental Figure S2). Serial bone marrow evaluations revealed a significant decrease in bone marrow infiltration by the CLL cells (Figure 1C). Due to the early and more complete resolution of lymphocytosis when compared to single-agent ibrutinib2, cases of partial remissions with lymphocytosis were infrequent at earlier time points (3 cases [8%] after 6 cycles), and absent after 12 months of treatment.
Figure 1. Changes in lymphocyte counts (A)lymph node sizes (B) and bone marrow (C) during iR therapy.
(A) Trended absolute lymphocyte counts (ALC) in CLL patients during therapy with ibrutinib and rituximab. The horizontal axis shows the time of treatment. Please note the early, transient lymphocytosis, which peaked during the first week of therapy, and then continuously declined and resolved. (B) Best responses in 34 evaluable patients evaluated by computed tomography scan for change from baseline in the sum of the largest diameter of each target lesion; negative values indicate tumor shrinkage. Among the 34 patients with measurable lesions both at baseline and after baseline, all 34 patients (100%) had improvements in lymphadenopathy, as assessed by changes in the sums of the products of the perpendicular dimensions (SPD) of index lesions. The dashed line shows the percentage change that represents the criterion for lymphadenopathy response.(C) The bar diagrams depict the mean (+/− SEM) relative numbers of bone marrow lymphocytes before therapy and after 3, 6, and 12 months of iR therapy. There was a continuous significant decline in marrow lymphocytes, indicating improvements in marrow infiltration by the CLL cells.
Figure 2. Kaplan–Meier curves for overall survival (OS) and progression-free survival (PFS).
The top panels show the probability of overall survival (left hand panel) and progression-free survival (right hand panel) for all 40 patients. The bottom graphs depict OS and PFS with respect to the del(17p) status; patients without del(17p) are indicated as solid line, and patients carrying del(17p) as hatched line. Tick marks indicate censored data.
Quality of life (QOL)
Comparing differences in HRQOL between baseline and during therapy, we noted that iR-treated patients showed significant improvement in overall health and quality of life after 6 and 12 months (table 2, and supplemental table 1), which coincided with a significant weight gain at 3 and 6 months (supplemental Figure S3).
Table 2.
Quality of life in CLL patients before (baseline) and after 6 months of treatment with iR. P values correspond to comparison of Z scores. There were significant improvements in global health, functional and symptom scales, and other items.
| EORTC – QOL- C30 v.3 Measure |
Mean Baseline (n=39) |
SD | Mean 6 months (n=36) |
SD | Mean difference (95% CI) |
p- value |
|---|---|---|---|---|---|---|
| Global health status | 70.9 | 18.8 | 84.2 | 12.4 | 13.3 (6.1, 20.5) | ≤.001 |
| Functioning scale | ||||||
| Physical | 88 | 17.8 | 97.6 | 6.8 | 9.6 (3.6, 15.6) | .003 |
| Role | 90.2 | 19.0 | 98.1 | 7.7 | 7.9 (1.4, 14.4) | .02 |
| Social | 81.6 | 22.9 | 97.2 | 8.4 | 15.6 (7.9, 23.3) | ≤.001 |
| Emotional | 83.8 | 14.5 | 97.7 | 5.8 | 13.9 (9.0, 18.2) | ≤.001 |
| Cognitive | 92.3 | 12.6 | 98.6 | 4.7 | 6.3 (2.1, 10.5) | .006 |
| Symptom scale | ||||||
| Nausea/vomiting | 20.5 | 14 | 1.4 | 4.7 | −19.1 (−23.8, −14.4) | ≤.001 |
| Pain | 13.2 | 25.1 | 3.7 | 12.7 | −9.5 (−18.4, −0.6) | .04 |
| Fatigue | 24.8 | 23.7 | 4.9 | 13.4 | −19.9 (−28.5, −11.3) | ≤.001 |
| Single item | ||||||
| Insomnia | 24.8 | 26.2 | 7.4 | 16.1 | −17.4 (−29.2, −7.6) | ≤.001 |
| Appetite loss | 10.2 | 17.4 | 0 | 0 | −10.2 (−15.7, −4.7) | ≤.001 |
| Diarrhea | 2.6 | 9 | 11.1 | 19.5 | 8.5 (1.53, 15.5) | ≤.01 |
| Constipation | 6 | 15 | 1.8 | 11.1 | −4.2 (−10.1, 1.74) | .18 |
| Dyspnea | 19.6 | 27.3 | 2.8 | 9.3 | −16.8 (−25.9, −7.7) | ≤.001 |
| Financial impact | 12 | 20.9 | 3.7 | 10.6 | −8.3 (−15.7, −0.9) | .04 |
Safety
Treatment was well tolerated, with respiratory infections (8 cases of pneumonia [grade 2, 3] and 9 cases of upper respiratory infections [grade 2]) being the most common adverse events (AE). There were also grade 2–3 possibly related AE of mucositis (n=3), and peripheral neuropathy (n=5), and one case of a grade 2 subdural hematoma in a 70 year old male, who fell and subsequently developed headaches. This patient did not require any intervention; ibrutinib was initially held, and later restarted. Milder toxicities included grade 1–2 diarrhea (n=17), bruising and rash (n=15), nausea or acid reflux (n=10, grade 1, 2), fatigue (n=7), and bone pain, myalgia/arthralgia (n=13). Hematologic toxicities included grade 2–3 neutropenia (n=4) and anemia (n=2). The related and unrelated toxicities are summarized in the supplemental Figure S4 and in the supplemental Tables S3, S4.
Gene expression response of CLL cells during iR therapy
We observed a relatively homogenous gene expression response of CLL cells during iR therapy. Supplemental Figure S7A displays heat maps that illustrate the most down-regulated genes in CLL cells after 7 and 28 days of iR therapy relative to baseline gene expression. The genes with known and potentially relevant function are annotated on Figure S7A, and include the chemokine CCL3, early growth response protein genes 1,2, and 3 (EGR 1,2,3), and CD72. We provide more details in the supplemental Tables S4–7 where we display the most up- and down-regulated genes after 7 and 28 days of therapy. Interestingly, we found that the pattern of down-regulated genes mirrors the genes up-regulated in CLL cells in nurselike cell cocultures22 previously characterized as BCR-regulated genes, as illustrated in Figure S7B.
Other laboratory markers
The mean beta-2-microglobulin (β2M) level before therapy was 5.2 mg/L (n=40), which significantly decreased during iR therapy to 3.2 mg/L after 3 months (n=37), to 2.3 mg/L after 6 months (n=35), to 2.7 mg/L after 12 months (n=35), and to 2.6 mg/L after 18 months (n=5, supplemental Figure S8A). The fact that β2M level plateau rather than normalize over time is of interest and may be due to residual disease. IgG and IgA immunoglobulin levels did not significantly change during iR therapy over 18 months of follow up. We noted a decline in IgM levels, which was not statistically significant (supplemental Figure S8B). Mean pre-treatment IgM levels of 110 mg/dL (n=39) dropped to 72 mg/dL after 3 months (n=37), to 66 mg/dL after 6 and 12 months (n=36 and 35, respectively), and to 29 mg/dL after 18 months (n=4). T cells and T cell subset numbers also changed during iR therapy (supplemental Figure S8E–G). Elevated numbers of CD3+ cells prior to treatment (mean: 2820/µL, n=30) remained at 3029/µL after 3 months (n=36), but then declined to 1919 after 6 months (n=33), and to 1606 after 12 months (n=32). This was due to changes in both, CD4+ and CD8+ T cells. Mean CD3+ CD4+ cell counts significantly decreased during iR therapy from 1369/µL prior to treatment (n=35) to 1240/µL after 3 months (n=37), to 841/µL after 6 months (n=33), and to 681/µL after 12 months (n=32, p=0.014). Mean CD3+ CD8+ cell counts slightly increased from 1527/µL prior to therapy (n=35) to 1649/µL after 3 months (n=37), but then declined to 1090/µL after 6 months (n=33), and 885/µL after 12 months (n=32). T cell counts in individual patients before and during iR therapy are depicted in the Supplement (supplemental Figures S5, S6).
BTK occupancy and chemotaxis
We found that iR treatment resulted in a full occupancy of BTK at both time points (supplemental Figures S9A). To explore iR therapy effects on CLL cell migration in vivo, serial blood CLL samples were tested in chemotaxis assays. We found that iR therapy significantly reduced CLL cell chemotaxis toward CXCL12 and CXCL13 (supplemental Figures S9B). For example, the mean (±SEM) relative numbers of CLL cells from 6 patients migrating toward CXCL13 decreased from 1906 (±556) before therapy to 666 (±133) after 14 days and 1109 (±401) after 28 days of therapy.
Plasma cytokine and chemokine levels
Serial plasma samples were assayed for changes in cytokine and chemokine levels at earlier and later time points. We found significant reductions in plasma levels of a number of cytokines that are either CLL cell-derived (CCL3, CCL4) or originate from cells in the microenvironment (CXCL13). For example, after 14 and 28 days of iR treatment, elevated median plasma levels of CCL3 and CCL4 significantly decreased from 139.6 (±40.4) pg/mL to 6.9 (±0.9) pg/mL, and from 578.6 (±103.9) pg/mL to 68.7 (±7.2) pg/mL, respectively (figure 7C). Plasma levels of CCL19 TNFα (Figure 5C), and CCL21 (data not shown) were also significantly reduced after 1 month. These chemokines, in concert with CXCL12 and CXCL13, are involved in B cell homing into lymph nodes. CXCL13 plasma levels decreased significantly from 94.6 (±16.3) pg/mL to 32.26 (±6.9) pg/mL after 1 month and to 28.7 (±6.8) pg/mL after 3 months of iR therapy. Notably, levels of CXCL12, a chemokine secreted by mesenchymal stromal cells remained stable during treatment (Figure S9).
Discussion
The combination of ibrutinib and rituximab induces a high rate of responses in our patients with high-risk CLL, with an ORR of 95% at a median follow-up of 17 months. In this study, responses are durable with 78% of patients free of disease progression, and 84% remained alive at 18 months. One of the most distinguishing findings in this study is the short duration of ibrutinib-associated redistribution lymphocytosis (Figure 1A). Consequently, there are fewer cases of patients with persistent lymphocytosis, even at early time points, and, compared to the reported single-agent experience, objective remissions are achieved in a higher proportion of patients. Specifically, the ORR with iR was more than 20% higher than with single agent ibrutinib (95% ORR with iR vs. 71%2 or 42.6%14 with single-agent ibrutinib in two different trials). Cross trial comparisons have limitations, but this earlier and more complete clearance of CLL cells from the peripheral blood presumably is due to the addition of rituximab. While 20% of patients treated with 420 mg ibrutinib daily as single agent had persistent lymphocytosis at 12 months2, 16, persistent lymphocytosis was rare after 6 months of iR (8%), and absent after 12 months of combination therapy (Figure 1A). Similar findings of a blunted and shortened duration of lymphocytosis recently were reported for the combination of the PI3Kδ inhibitor idelalisib with rituximab in patients with relapsed CLL3.
The relevance of clearance of CLL cells from the PB during ibrutinib therapy remains largely unknown. Woyach and colleagues recently provided data demonstrating that persistent lymphocytosis during ibrutinib therapy was not associated with an adverse outcome16, suggesting that elimination of CLL cells from the PB, traditionally a key response criterion, may not be a necessary goal of ibrutinib-based therapy. In this study we evaluated the activity of ibrutinib in combination with rituximab. It is known that rituximab, which has very limited clinical activity in CLL as a single agent, substantially increases PFS and OS when added to fludarabine and cyclophosphamide in previously untreated patients with CLL6, 23. Its role as combination partner with ibrutinib, beyond effective clearance of PB from CLL cells cannot be further defined in this study and is currently addressed in an ongoing randomized trial of ibrutinib versus iR (NCT02007044).
Besides BTK, ibrutinib can also interfere with the activity of other kinases that contain a modifiable cysteine residue homologous to Cys-481 in BTK, such as interleukin-2 inducible tyrosine kinase (ITK)24. ITK is expressed in NK and T cells, and Kohrt et al. recently reported that ibrutinib antagonizes rituximab’s anti-lymphoma activity in a mouse model, based on ibrutinib’s inhibition of FcR-stimulated NK cell function, specifically antibody-dependent cell-mediated cytotoxicity (ADCC)25. However, the relative contribution and importance of NK cells, monocytes, or other effector cells for CD20 antibody-mediated killing of B cells remains controversial. Other mechanisms of rituximab-induced toxicity towards malignant B cells, which are independent of NK cell function (via induction of direct cell death and complement-dependent cytotoxicity) have been documented. The rapid clearance of CLL cells from the PB in this trial suggest that any antagonistic activity of ibrutinib does not play a significant role in blood stream clearance of CLL cells. Beyond that, to define if the addition of rituximab impacts PFS or OS, we will need to wait for mature data from the randomized trial of ibrutinib versus iR. If PFS or OS are prolonged with the addition of rituximab, this would suggest a lack of ibrutinib-rituximab antagonism in CLL in the human disease setting.
The PFS of 78% (95% CI: 60.58–88.45; 72% PFS [95% CI: 45.56–87.55]in patients with del[17p] or TP53 mutation) and OS of 84% (95% CI: 67.22–92.39; 78% OS [95% CI: 51.99–91.36]in patients with del[17p] or TP53 mutation) at 18 months (Figure 2) compare very favorably to other therapy options for similarly high-risk patients with CLL, especially in the relapsed disease setting. In a retrospective single-center analysis of 174 patients, median PFS and OS in CLL patients with del(17p) was higher with ibrutinib therapy when compared to any other therapy26, and the high response rate observed in this study supports the conclusion that, among the approved therapies, ibrutinib alone or in combination with rituximab represents a highly effective treatment for high-risk CLL. Besides PFS and OS, improvement in QOL has become one of the major end points in clinical trials with novel anticancer agents, especially in incurable diseases. CLL has a profound impact on QOL, which is particularly low in patients with advanced stage disease, older age, fatigue, co-morbidity, and active treatment27. Eichhorst reported moderate improvement in QOL after FC therapy28. Our finding of a significant improvement of QOL during iR therapy supports the notion that effective CLL therapy improves QOL.
The gene expression profile data (Figure S7) demonstrate on-target down-regulation of BCR- and NFκB-regulated genes which are considered central for CLL cell survival and interactions within the lymphoid tissue microenvironment29. For example, the chemokine CCL3, which is up-regulated in CLL cells after nurselike cell co-culture and after BCR triggering22, can attract accessory cells, such as T cells and monocytes into the lymphoid tissues, fostering the influx of cells that support the survival and growth of the CLL clone. CLL cells isolated from CLL lymph nodes contain high levels of CCL329, and high CCL3 plasma levels predict for inferior outcome in CLL30. Importantly, down-modulation of CCL3 during iR therapy was seen at the gene expression level and in patients’ plasma samples, serving as a cross validation of the data, and corroborating the value of CCL3 as a plasma biomarker for responses to kinase inhibitors that target BCR signalling such as ibrutinib18 and idelalisib31.
The biologically most interesting correlative findings in this study are the normalization of high PB T cell counts, and the normalization of a number of cytokines/chemokines, as well as β2 microglobulin (β2M) levels. T cell numbers of both, the CD4 and CD8 sub-sets are increased in untreated patients with CLL, and the normalization of these cell populations during iR therapy (Figure S8) can be interpreted as a sign of co-evolution and interdependence between T cells and CLL cells. Significant reductions in plasma levels of TNFα, the lymph node homing chemokines CCL19 and CXCL13, as well as β2M (Figure S9) provide further insight into the targets and consequences of iR therapy. Together with the inhibition of chemotaxis of CLL cells isolated from patients on iR therapy (Figure S9B), which was expected from the pre-clinical work18, 19 and which is due to direct effects of BTK inhibition on chemokine receptor signalling18, 19, the reduction of CCL19 and CXCL13 plasma levels (Figure S9C) points towards additional mechanism for redistribution of tissue CLL cells into the PB during ibrutinib therapy. CCL19 and CXCL13 are important chemokines that coordinate normal and malignant B cell homing into secondary lymphoid tissues32, and their down-modulation during iR therapy is interpreted as additional, indirect mechanism for CLL cell mobilization by ibrutinib. Significant reduction of elevated TNFα and β2M levels support the important role of these proteins in disease progression and prognosis, but there are no data to suggest a mechanism of regulation of these proteins by iR therapy.
In summary, our data demonstrate that the combination of ibrutinib and rituximab is an effective therapy for CLL patients with high-risk disease, where it induces high ORR and durable remissions in the majority of patients. Treatment is well tolerated and is associated with significant improvements in QOL. The correlative studies demonstrate that this therapy normalizes multiple cellular and plasma markers of disease activity. A randomized study of ibrutinib versus ibrutinib plus rituximab is ongoing and will determine whether the addition of rituximab, besides abridging the initial lymphocytosis, improves PFS and/or OS.
Research in context
Systematic review
We searched PubMed for articles published in English after January 1, 2005 to identify agents used to treat patients with high-risk chronic lymphocytic leukaemia (alemtuzumab, chlorambucil, rituximab, ofatumumab, obinutuzumab, fludarabine, cyclophosphamide, and lenalidomide). We identified primary publications for these agents6, 8, 23, 33 which show that low response rates and short remissions are a major problem. Only single-agent ibrutinib trials have been published to date12, 14, 34, 35. To our knowledge, no other data are available for ibrutinib combination therapy.
Interpretation
We have shown that ibrutinib in combination with rituximab is active and safe for the treatment of patients with high-risk chronic lymphocytic leukemia. Larger randomized trials are warranted to confirm these findings.
Supplementary Material
Table 3.
Related and unrelated adverse events
| Related adverse events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Lung Infection | 5 | 9 | 2 | |
| Diarrhea | 9 | 1 | ||
| Neutropenia | 1 | 1 | ||
| Fatigue | 4 | 3 | ||
| Upper respiratory infection | 3 | 10 | 1 | |
| Nausea/Acid reflux | 10 | 3 | ||
| Arthralgia | 8 | 3 | ||
| Transaminase increase | 1 | |||
| Bleeding events (Bruising/Rash/ Epistaxis) | 8 | 5 | ||
| Peripheral neuropathy | 1 | 1 | 1 | |
| Weight gain | 4 | |||
| Eye disorders (itching/watery eyes) |
1 | 2 | ||
| Mucositis | 1 | 1 | 1 | |
| Constipation | 1 | |||
| Alopecia | 1 | |||
| Atrial Fibrillation | 1 | 1 | ||
| Unrelated adverse events | Grade 1 | Grade 2 | Grade3 | Grade 4 |
| UTI (Urinary tract infection) | 3 | |||
| Insomnia | 4 | |||
| Headache | 3 | |||
| Anemia | 1 | 2 | ||
| Osteoporosis | 1 | |||
| Hot flashes | 2 | |||
| Constipation | 1 | 2 | ||
| Nausea/vomiting | 2 | |||
| Anxiety | 2 | |||
| Dry mouth | 1 | 1 | ||
| Dyspnea | 1 | |||
| Subdural Hematoma | 1 | |||
| Sepsis | 1 |
Acknowledgments
The authors thank the patients who participated in this trial and their families, and the study investigators and coordinators at MD Anderson, especially Benjamin Hayes for sample and data collection and Jeannice Theriot for expert editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Primary results were presented in part at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 8–11, 2012 and the 55th annual meeting of the American Society of Hematology, New Orleans, LA, December 7–10, 2013
Author Contributions
J.A.B. designed and supervised the trial and correlative studies, analyzed the data and wrote the paper; M.J.K., A.F., S.F., H.K., W.G.W., and S.O.B. contributed to the trial design, clinical patient management, sample collection, and clinical data analysis, and reviewed and approved the paper. E.H. and A.R. analyzed gene expression profiles, J.H., N.Y.R., and I.D.W. analyzed correlative samples, S.L., G.J., M.C., G.N.G., and X.H. performed statistical analyses of the data, and N.G. analyzed serial imaging studies.
Conflict of interest
JAB and SOB received research funding from Pharmacyclics, Inc.; JAB is a consultant for Janssen Pharmaceuticals, Inc.. All other authors declare that they have no conflict of interest.
References
- 1.Hallek M. Signaling the end of chronic lymphocytic leukemia: new frontline treatment strategies. Blood. 2013;122(23):3723–3734. doi: 10.1182/blood-2013-05-498287. [DOI] [PubMed] [Google Scholar]
- 2.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 5.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. The New England journal of medicine. 2000;343(26):1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 6.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 7.Fischer K, Cramer P, Busch R, Bottcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30(26):3209–3216. doi: 10.1200/JCO.2011.39.2688. [DOI] [PubMed] [Google Scholar]
- 8.Stilgenbauer S, Zenz T. Understanding and managing ultra high-risk chronic lymphocytic leukemia. Hematology / the Education Program of the American Society of Hematology American Society of Hematology. 2010;2010:481–488. doi: 10.1182/asheducation-2010.1.481. [DOI] [PubMed] [Google Scholar]
- 9.Dohner H, Stilgenbauer S, James MR, Benner A, Weilguni T, Bentz M, et al. 11q deletions identify a new subset of B-cell chronic lymphocytic leukemia characterized by extensive nodal involvement and inferior prognosis. Blood. 1997;89(7):2516–2522. [PubMed] [Google Scholar]
- 10.Badoux XC, Keating MJ, Wang X, O’Brien SM, Ferrajoli A, Faderl S, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117(11):3016–3024. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger JA, Buggy JJ. Emerging drug profiles: Bruton tyrosine kinase (BTK) inhibitor ibrutinib (PCI-32765) Leuk Lymphoma. 2013 doi: 10.3109/10428194.2013.777837. [DOI] [PubMed] [Google Scholar]
- 12.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31(1):88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with Ibrutinib in Relapsed or Refractory Mantle-Cell Lymphoma. The New England journal of medicine. 2013 doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger JA, Montserrat E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood. 2013;121(9):1501–1509. doi: 10.1182/blood-2012-08-452607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woyach JA, Smucker K, Smith LL, Lozanski A, Zhong Y, Ruppert AS, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123(12):1810–1817. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton’s tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011 doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 20.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rawstron AC, Villamor N, Ritgen M, Bottcher S, Ghia P, Zehnder JL, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21(5):956–964. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]
- 22.Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113(13):3050–3058. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 24.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohrt HE, Sagiv-Barfi I, Rafiq S, Herman SE, Butchar JP, Cheney C, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood. 2014;123(12):1957–1960. doi: 10.1182/blood-2014-01-547869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens DM, Ruppert AS, Jones J, Woyach J, Maddocks K, Jaglowski S, et al. Impact of targeted therapy on outcome of chronic lymphocytic leukemia patients with relapsed Del(17p13.1) karyotype at a single center. Leukemia. 2014 doi: 10.1038/leu.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanafelt TD, Bowen D, Venkat C, Slager SL, Zent CS, Kay NE, et al. Quality of life in chronic lymphocytic leukemia: an international survey of 1482 patients. British journal of haematology. 2007;139(2):255–264. doi: 10.1111/j.1365-2141.2007.06791.x. [DOI] [PubMed] [Google Scholar]
- 28.Eichhorst BF, Busch R, Obwandner T, Kuhn-Hallek I, Herschbach P, Hallek M. Health-related quality of life in younger patients with chronic lymphocytic leukemia treated with fludarabine plus cyclophosphamide or fludarabine alone for first-line therapy: a study by the German CLL Study Group. J Clin Oncol. 2007;25(13):1722–1731. doi: 10.1200/JCO.2006.05.6929. [DOI] [PubMed] [Google Scholar]
- 29.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivina M, Hartmann E, Kipps TJ, Rassenti L, Krupnik D, Lerner S, et al. CCL3 (MIP-1{alpha}) plasma levels and the risk for disease progression in chronic lymphocytic leukemia (CLL) Blood. 2010 doi: 10.1182/blood-2010-09-307249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3’-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger JA. Chemokines and chemokine receptors in chronic lymphocytic leukemia (CLL): from understanding the basics towards therapeutic targeting. Seminars in cancer biology. 2010;20(6):424–430. doi: 10.1016/j.semcancer.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99(10):3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 34.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. The Bruton’s Tyrosine Kinase (BTK) Inhibitor Ibrutinib (PCI-32765) Promotes High Response Rate, Durable Remissions, and Is Tolerable in Treatment Naïve (TN) and Relapsed or Refractory (RR) Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) Patients Including Patients with High-Risk (HR) Disease. New and Updated Results of 116 Patients in a Phase Ib/II Study Blood. 2012;120(21) Abstract 189. [Google Scholar]
- 35.O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.