Abstract
Purpose
Little prospective data exist on quality of life (QOL) after pelvic exenteration (PE). This ongoing study prospectively examines the QOL-changes following this radical procedure using a comprehensive battery of psychological instruments.
Methods
Since 2005, enrolled patients were interviewed (EORTC QLQ-C30, EORTC QLQ-CR38, EORTC QLQ-BLM30, BFI, BPI-SF, IADL, CES-D, IES-R) preoperatively and at 3, 6, and 12 months after PE for physical/psychological symptoms. Data were examined using repeated measure ANOVA.
Results
Sixteen women (3 anterior, 1 posterior, and 12 total PE’s), with more than one year of follow up, completed all scheduled interviews. Median age was 58 years (28–76). Overall QOL (F=6.3, p<0.02), ability to perform instrumental daily activities (F=6.8, p<0.02), body image (F=11.9, p<0.00) and sexual function (F=8.0, p<0.01) all declined at 3 months but were near baseline by 12 months after PE. Although, overall, physical function followed a similar trend (F=14.8, p<0.00), it did not return to baseline. At the 12-month interview, patients reported increased gastrointestinal symptoms (F=8.9, p<0.01) but significantly less stress-related ideation (F=6.1, p<0.03) compared to baseline. Pain levels did not change significantly during the study period (F=0.4, p<0.74).
Conclusions
Although patients report lingering gastrointestinal symptoms and some persistent decline in physical function after PE, most adjust well, returning to almost baseline functioning within a year. Providers can counsel patients that many, though not all, symptoms in the first 3 months following exenteration are likely to improve as they adapt to their changed health status. These preliminary results await confirmation of a larger analysis.
INTRODUCTION
Pelvic exenteration [PE] is a radical, but potentially curative, treatment strategy for advanced/recurrent gynecologic malignancies. This surgical procedure, initially reported by Brunschwig from Memorial Hospital in 1948 [1], consists of an en bloc resection of the pelvic organs. Improvements in surgical technique, perioperative care and better patient selection have contributed to the improved survival following this procedure since its original description [2]. Hence, a growing cohort of survivors faces considerable recovery challenges, creating a mandate for quality of life (QOL) research that may benefit these patients in their adjustment [3].
The sheer number of domains affected by PE, including physical and sexual function, body image, social roles, psychological well-being and treatment satisfaction and side-effects, among others, presents a special challenge to QOL researchers attempting to capture the full range of impacts that these patients may experience. It is, therefore, essential that the instruments used to measure the postexenterative QOL reflect its multifaceted nature. In addition, patients arrive at PE with varying treatment history, psychosocial background and expectations making it essential to assess the preoperative baseline functioning in order to determine the extent to which the postexenterative QOL ratings can be attributed to the procedure itself. Clearly, this longitudinal assessment of QOL is only attainable through prospective studies.
Unfortunately, the existing research has been limited by retrospective study designs, the use of non-validated instruments, and/or the incomplete assessment of the potential impacts [4–12]. The current study was designed to address the limitation of the existing literature by prospectively characterizing the physical/psychological function and overall QOL in gynecologic patients undergoing PE using well-validated instruments. To date, this study has the most comprehensive battery of psychological instruments prospectively examining QOL in this patient population.
METHODS
Study design
In this ongoing longitudinal observational study, we assess PE patients for baseline performance, initiate intensive surveillance for one year after surgery via periodic interviews, and maintain long-term follow-up through annual assessments for five years after surgery or until death using a battery of questionnaires. Because PE is an uncommon procedure which results in a lengthy study period, we elected to conduct an interim analysis on participants who have completed one year of follow-up to contribute to the much needed literature on that subject while more patients accrue. This report summarizes the results, thus far, of this ongoing study.
Patients scheduled for a total, anterior or posterior PE for a variety of gynecologic malignancies were interviewed for assessment of physical/psychological symptoms and overall QOL. Because no single comprehensive instrument with proven ability to assess all the relevant QOL domains in PE patients currently exists, we, therefore, assembled a battery of well-validated measures to address the different QOL domains relevant to this particular patient population. The initial selection of the relevant domains was generated through an extensive review of the literature and in-depth interviews with women who had undergone PE [13]. A panel of oncologists and psychologists then reviewed the initial items and made recommendations for changes, including supplementing the various instruments with additional 5-point Likert-scale questions. These questions were developed to assess the domains that were less adequately covered by the validated instruments, such as the patient’s pain expectation and experience, satisfaction with the recovery time and the counseling received, as well as other QOL issues relevant to this patient population. The study battery was administered preoperatively (T0) and at approximately 3 (T1), 6 (T2), and 12 months (T3) following exenterative surgery. The assessment intervals were selected to coincide with the scheduled postoperative clinic visits. This timeframe reflected the natural history of postoperative recovery within the study population.
Participating patients completed the questionnaires with the assistance of the study research assistant. Ability to speak and read English proficiently was a prerequisite for study participation. To minimize bias, family members were discouraged from assisting patients with the questionnaires. Breaks or rest periods were permitted. In cases where participants were unable to complete the questionnaires in one interview, a follow-up session was scheduled within a week. If the participant was unable to return to the clinic, telephone interviews were conducted.
Measures used
The study battery consisted of the European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30) [14–16] version 3.0 and its colorectal cancer (EORTC QLQ-CR38) [17] and muscle invasive bladder cancer (EORTC QLQ-BLM30) [18,19] modules, the Brief Fatigue Inventory (BFI) [20], the Brief Pain Inventory-Short Form (BPI-SF) [21, 22], the Instrumental Activities of Daily Living (IADL) [23–26], the Center for Epidemiologic Studies Depression Scale (CES-D) [27, 28], and the Impact of Events Scale-Revised (IES-R) [29–31].
The EORTC QLQ-C30 broadly assesses health-related QOL in cancer patients. This instrument evaluates 5 major scales of function, including physical, role, emotional, social and cognitive functions, and overall QOL. In addition, three symptom scales measure fatigue, pain and emesis, and six single items assess the financial impact of the disease and its treatment, dyspnea, sleep disturbance, appetite, diarrhea and constipation [14].
The EORTC QLQ-C30 is a core instrument that covers a general range of QOL issues relevant to all patients with cancer. It is designed to be supplemented with more disease-specific modules which can assess aspects of QOL of particular importance to various patient subgroups. In this study, we chose the EORTC QLQ-CR38 [17] and the EORTC QLQ-BLM30 [18] modules because they contain scales examining QOL domains that are relevant to PE patients. The QLQ-C30 and its modules were scored according to the EORTC scoring manual [32]. Higher scores in a function scale or overall QOL indicate better function while higher scores in a symptom scale/item reflect worse symptomatology. A comprehensive review of the above instruments is beyond the scope of this article and is reported elsewhere [14–32].
Statistical analysis
The study was approved by our Institutional Review Board and all participants gave informed consent. The statistical package SPSS 17.0 (SPSS Inc, Chicago, IL) was used for data analysis. Scale/item score was expressed as the mean ± standard deviation. To examine the change over time, the mean scores of the patients completing the interview at the four time points were compared using repeated measure ANOVA. Pairwise comparisons were applied only after a significant F-test was detected (F-protection). This approach (post-hoc testing of ANOVAs) minimized type I error. A p value <0.05 was considered statistically significant.
RESULTS
Patient participation
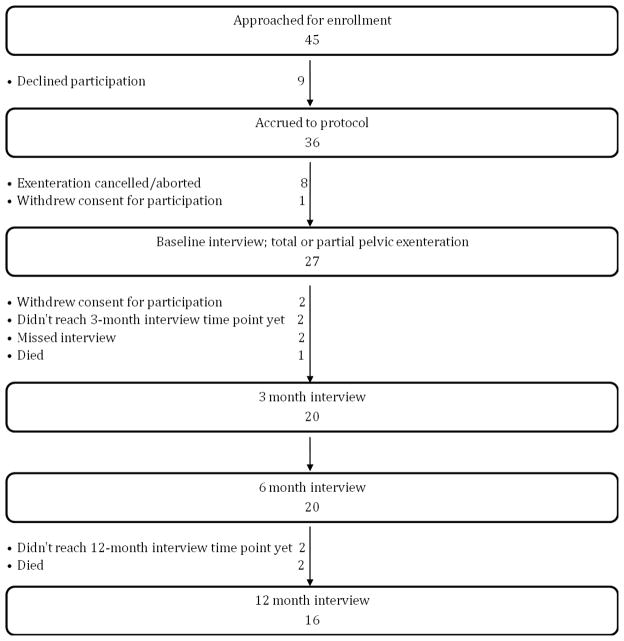
Since 2005, 36 women were accrued to this protocol and 27 of these undertook the preoperative baseline interview before undergoing PE as shown in Figure 1. Of these, 3 patients died of disease within one year of surgery and 4 completed less than one year of follow up. Of the 20 patients, with more than a year of follow up after PE at the time of this analysis, 16 (80%) were available for interview at the four time points and were included in this report (Figure 1). All the patients responded to all the instruments at all the time points. Reasons for non-participation in the study included time commitment required to complete the questionnaires (7 patients, including 2 patients that withdrew consent after the baseline interview) and feeling uncomfortable with the personal issues discussed in the questionnaires (1 patient). One patient got too emotional at the time of consent signing and was not enrolled, another cited health reasons and withdrew consent prior to the baseline interview, and another 2 patients gave no specific reason for declining participation. Reasons for aborting/cancelling the exenteration included metastatic disease found pre/intraoperatively (4 patients), exenteration was not required to clear the disease (2 patients), inability to obtain medical clearance or insurance-approval to undergo the procedure (1 patient each).
Figure 1.
Patient participation
Patient characteristics
Table 1 lists the social demographics and other characteristics of this patient cohort (3 anterior, 1 posterior, and 12 total PE’s). Age ranged 28–76 years with a median of 58 years. All patients had children except two (patients number 7 &16). Only 3 patients were taking medications for anxiety and/or depression at the baseline interview with another reporting prior use of such medications. Median time since the initial diagnosis of the gynecological cancer was 29 months (range, 2–222). Of note, patient number 16 underwent primary exenteration for cervical cancer (about 2 months following diagnosis) after prior pelvic radiation for rectal cancer. Following PE, eleven patients underwent a continent urinary diversion and four received ileal conduits. Eight patients had vaginal reconstruction (6 using gracilis flaps, another using fasciocutaneous Singapore flaps and one patient underwent rectus flaps vaginal reconstruction). Of 16 patients included in this analysis, 14 remained NED during the study period (including a patient whose disease status is unknown but who completed 5 years of follow up on this study after her exenterative surgery and likely has NED). Two patients recurred between their 6 and 12 month interviews.
Table 1.
Patient characteristics
| Patient number |
Age (at surgery) |
Race / Ethnicity |
Married / Partnered |
Highest education received |
Religious affiliation |
Primary malignancy |
Type of exenteration |
Type of urinary diversion |
Vaginal reconstruction |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | White Non-Hispanic | No | College | Lutheran | Endometrial | Total | Continent | No |
| 2 | 62 | White Non-Hispanic | Yes | High School | Episcopalian | Vaginal | Total | Continent | No |
| 3 | 66 | White Non-Hispanic | Yes | College | Episcopalian | Endometrial | Total | Continent | Yes |
| 4 | 53 | White Non-Hispanic | No | College | Catholic | Cervical | Total | Continent | Yes |
| 5 | 50 | White Non-Hispanic | Yes | Graduate School | Catholic | Endometrial | Anterior | Continent | Yes |
| 6 | 76 | White Non-Hispanic | Yes | Grade School | Catholic | Vulvovaginal | Total | Incontinent | No |
| 7 | 60 | White Non-Hispanic | Yes | Graduate School | Catholic | Endometrial | Posterior | N/A | Yes |
| 8 | 41 | White Non-Hispanic | Yes | Unknown | Methodist | Cervical | Total | Continent | Yes |
| 9 | 57 | White Non-Hispanic | Yes | College | Catholic | Vaginal | Total | Continent | No |
| 10 | 46 | White Non-Hispanic | Yes | College | Catholic | Ovarian | Total | Incontinent | No |
| 11 | 68 | White Non-Hispanic | Yes | Graduate School | None | Cervical | Total | Incontinent | No |
| 12 | 59 | White Non-Hispanic | Yes | High School | Catholic | Vaginal | Anterior | Continent | No |
| 13 | 44 | White Hispanic | Yes | College | Catholic | Cervical | Total | Continent | Yes |
| 14 | 57 | White Non-Hispanic | Yes | College | Christian | Cervical | Anterior | Continent | Yes |
| 15 | 69 | White Hispanic | No | College | Catholic | Cervical | Total | Continent | No |
| 16 | 28 | White Non-Hispanic | No | College | None | Cervical | Total | Incontinent | Yes |
N/A: Not applicable.
Overall QOL & body Image/IADL/Sexual, role function & fatigue/Physical Function & gastrointestinal symptoms
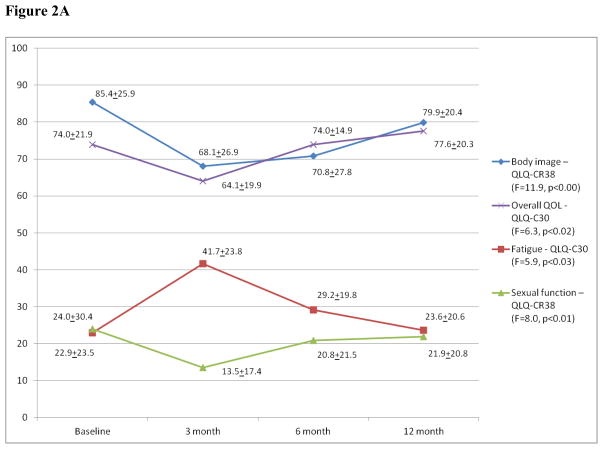
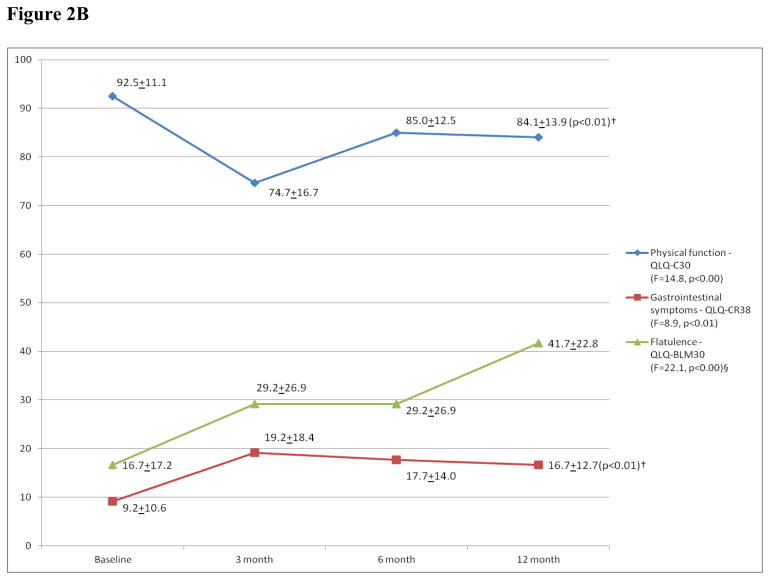
In general, QOL declined at 3 months but was at or near baseline by 12 months following PE as seen in the overall QOL and body image domains (Figure 2A) and in the ability to perform instrumental daily activities (T0: 20.7±0.8, T1: 19.4±1.6, T2: 20.0±0.7, T3: 19.9±1.5; F=6.8, p<0.02). A similar trend was noted in the sexual function (Figure 2A) and role function (Appendix Figure A1, online only) domains, overall mirroring the longitudinal change in the QLQ-C30 fatigue (Figure 2A) and the BFI (T0: 2.2±2.4, T1: 3.0±2.1, T2: 2.8 ±2.2, T3: 2.1±1.8; F=1.5, p<0.24) mean scores. The changes in the role function and the BFI mean scores did not reach statistical significance, however. Although, physical function also declined at 3 months postoperatively then improved, it did not return to baseline (Figure 2B). This partial recovery of physical function at the 12-month interview was accompanied by an increase in gastrointestinal symptoms, such as flatulence, when compared to baseline (Figure 2B). Although abdominal bloating increased significantly between baseline and 6 months, it was near baseline by 12 months after surgery (T0: 2.2±8.6, T1: 17.8±21.3, T2: 20.0±24.6, T3: 11.1±20.6; F=6.7, p<0.02, n=15; QLQ-BLM30).
Figure 2.
Figure 2A: Overall QOL/Body Image/Sexual Function/Fatigue*
*: Mean scores of various scales for all the patients at the four time points. Higher scores in a function scale or overall QOL indicate better function while higher fatigue scores reflect worsening fatigue; QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; QLQ-CR38: Colorectal cancer module of the QLQ-C30.
Figure 2B: Physical Function/Gastrointestinal symptoms*
*: Mean scores of two scales and a symptom item (§) for all the patients at the four time points. Higher physical function scores indicate better function while higher scores in a symptom scale/item reflect worse symptomatology; QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; QLQ-BLM-30: Muscle invasive bladder cancer module of the QLQ- C30; QLQ-CR38: Colorectal cancer module of the QLQ-C30.
†: Pairwise comparisons between T0 and T3 were statistically significant.
Psychological well-being
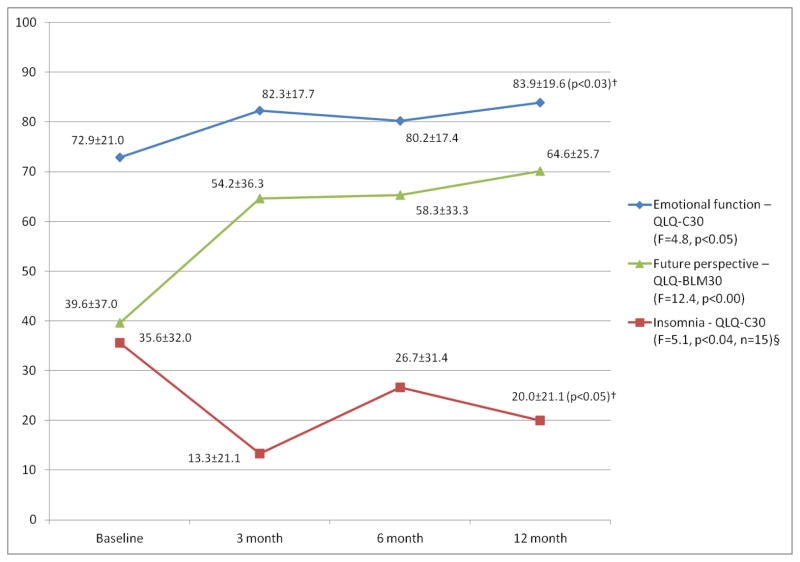
Overall, an improvement in psychological well-being was noted in the recovery period. Both emotional function and complaints of insomnia improved shortly after PE and, despite worsening slightly at 6 months, were significantly improved at the 12-month interview compared to baseline (Figure 3). In addition, both the patients’ perspective for the future (Figure 3) and stress-related ideation (T0: 3.1±2.3, T1: 1.7±1.3, T2: 2.1±2.2, T3: 1.7±2.4; F=6.1, p<0.03; IES-R) were worst at baseline and improved significantly during the study period. Although mean depression scores also declined postoperatively compared to baseline, overall, these changes did not reach statistical significance (T0: 10.4±8.2, T1: 7.3±6.0, T2: 7.4±8.8, T3: 7.3±9.0; F=3.1, p<0.10; CES-D).
Figure 3. Psychological well–being*.
*: Mean scores of two function scales and a symptom item (§) for all the patients at the four time points. Higher scores in a function scale indicate better function while higher insomnia scores reflect worsening insomnia. QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; QLQ-CR38: Colorectal cancer module of the QLQ-C30.
†: Pairwise comparisons between T0 and T3 were statistically significant.
Pain/Social & cognitive functions/Financial impact & other symptoms
No statistically significant changes in the mean pain scores were noted during the study period (T0: 1.3±1.7, T1: 1.5±1.4, T2: 1.4±1.6, T3: 1.1±1.4; F=0.4, p<0.74; BPI-SF). Similarly, the longitudinal changes in the social and cognitive function mean scores did not reach statistical significance (Appendix Figure A1, online only), nor did those of the financial impact domain and other symptoms (Appendix Figures A2 & A3, online only). Of note, considerable variability in diarrhea was noted during the study period; however, no statistically significant trend was detected (Appendix Figure A3, online only). The number of responses in the remaining QLQ-CR38 and QLQ-BLM30 domains/items was too small for meaningful comparisons.
Results of the supplementary questions
The patient’s pain expectation and experience as well as overall experience with a variety of other pertinent issues were also assessed using the Likert-scale questions. Tables 2 & 3 and Appendix Tables A1–A6 (online only) list the frequency distributions of the responses to those questions.
Tables 2. Patients’ overall experiences (number of patients/percentage)*.
How satisfied are you with the amount of time taking (or has taken) you to recover from surgery?
| 3 month | 6 month | 12 month (n=15) | ||||
|---|---|---|---|---|---|---|
| Not at all | 2 | 13% | 0 | 0% | 0 | 0% |
| A little | 3 | 19% | 2 | 13% | 0 | 0% |
| Moderately | 3 | 19% | 6 | 38% | 6 | 40% |
| Very | 5 | 31% | 7 | 44% | 6 | 40% |
| Extremely | 3 | 19% | 1 | 6% | 3 | 20% |
Percentages may not add up to 100% due to rounding.
Table 3. Patients’ overall experiences (number of patients/percentage)*.
How satisfied are you with the information that you received about the surgery?
| Baseline | 3 month | 6 month | 12 month | |||||
|---|---|---|---|---|---|---|---|---|
| Not at all | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| A little | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Moderately | 2 | 13% | 1 | 6% | 3 | 19% | 0 | 0% |
| Very | 5 | 31% | 8 | 50% | 8 | 50% | 10 | 63% |
| Extremely | 9 | 56% | 7 | 44% | 5 | 31% | 6 | 38% |
Percentages may not add up to 100% due to rounding.
DISCUSSION
This study stands out from previously published studies examining QOL after PE by being prospective, with establishment of a preoperative baseline QOL measurement, and by measuring a large number of QOL endpoints relevant to this unique patient population.
Several investigators have reported on the utility of incorporating information obtained through evaluating QOL into identifying rehabilitation needs, designing effective educational interventions and characterizing those patients that are most suitable for these interventions [33, 34]. Evaluating the impact of exenterative surgery on QOL may help providers to better direct mental health and supportive services and possibly avoid long-term sequelae.
The findings of this study suggest that, although QOL declines after PE, on longer follow up most patients adjust well both physically and psychologically with restoration of most domains defining QOL. For example, near pre-exenterative levels of function were achieved within 12 months of surgery in the overall QOL and body image domains (Figure 2A). This adaptation was also reflected in the improvement of the patients’ comfort with the changes in their bodies, with half of the patients reporting being very to extremely comfortable by 12 months following PE (versus only 19% at the 3-month interview) (Appendix Table A1, online only).
This study also examined pain expectations in patients undergoing PE. Although, almost two thirds (63%) of the patients expected to experience either large or extreme amount of pain when surveyed prior to the procedure (Appendix Table A2, online only), pain levels did not change significantly during the study period. While the expectation likely represents the patients’ perception of the degree of radicality of this procedure, the actual experience probably reflects the efficacy of modern pain control modalities following radical pelvic surgery. In this patient cohort, pain control was not a significant issue.
In general, satisfaction with the recovery time increased over time, with all the patients reporting being at least moderately satisfied with their recovery time by 12 months after surgery (Table 2). However, satisfaction with the information received was a common theme all through (Table 3), and likely reflects the extensive pre/postoperative counseling that these patients receive on our service both from providers and from prior PE patients.
Since the great majority of studies examining the postexenterative QOL have been retrospective [4–10], comparing our results to previous reports is difficult. Review of the published literature identified only one group that prospectively examined QOL in gynecological patients undergoing PE using validated instruments after establishing a preoperative baseline measurement [11, 12]. In those two reports, patients were evaluated using the Cancer Rehabilitation Evaluation System-Short Form (CARES-SF) for QOL assessment [35,36] and the body image evaluation measure by Strauss and Appelt [37]. Because of its extensive study battery, the current study is unique, however, as it examined QOL domains/items that were less systematically evaluated by those measures, such as mood, stress-related ideation, ability to perform instrumental daily activities, pain, fatigue and, finally, the patients’ overall experiences. This extensive study battery reflects the diverse challenges encountered by this unique patient population.
In the more recent report [12], QOL of patients who underwent Wertheim’s procedure was compared to that of those who underwent PE for cervical cancer. A subset of 31 PE patients, including those from the earlier report [11], completed the evaluation at all the time points (before surgery, and 4 & 12 months after PE). In contrast to our findings, body image mean scores continued to worsen over time in those 31 patients. Because postoperative adjuvant treatment may negatively impact body image as suggested by those investigators [12], the better outcome in the body image domain in our patient cohort 12 months after PE may be partly due to a less frequent use of postoperative adjuvant treatment in our study patients. The type of adjuvant treatment (chemotherapy versus radiotherapy) may have possibly contributed to the difference in the body image outcome, as well. Only about 31% of our patients received postoperative adjuvant treatment (mainly chemotherapy with no patients receiving postoperative radiation). On the contrary, about 44% of all the PE patients (n=62) in the study by Hawighorst-Knapstein et al [12] received postoperative adjuvant therapy with the majority receiving postoperative radiotherapy (either alone or in combination with chemotherapy). However, it is unclear exactly how many of that subset of 31 patients received adjuvant treatment [12].
That 31 patient subset was further divided into 3 subgroups: those without, those with one, and those with two ostomies [12]. Patients without or with one ostomy had comparable overall QOL and body image to those who underwent Wertheim procedure while those with two ostomies had significantly more problems in those domains. In addition, two-ostomy patients’ global QOL and body image scores worsened over time. Although stratifying PE patients according to the number of ostomies may reveal real differences in QOL, for a better definition of QOL, PE patients may be best stratified according to the type of diversion performed, as the impact of a urostomy on QOL may be different from that of a colostomy. Ideally, five distinct groups of PE patients ought to be studied: those with a continent urinary diversion (with or without a colostomy), those with an incontinent diversion (with or without colostomy) and those with a colostomy only. Gathering enough numbers for such analyses is definitely a challenge but may be feasible through multicenter collaboration.
Another study [9] of 25 women who underwent PE for a gynecologic or a urologic malignancy retrospectively examined the functional outcome and long-term QOL using the QLQ-C30 and its ovarian cancer module (EORTC QLQ-OV28) [38, 39]. That study showed lower physical function scores, compared to a historical control group of healthy women and compared to another with recently diagnosed advanced cervical cancer, but comparable overall QOL. Although comparing our results to that study is difficult due to the different study designs, the general theme of a lower physical function outcome with an acceptable overall QOL following PE is preserved, congruent with our findings. Because only a minority of the patients (n=7) in that report was surveyed in the first two years following PE, the current study provides more insight into the early postoperative challenges and adjustments that these patients undergo and suggests that satisfactory adaptation can be achieved much earlier in the recovery process.
In a more recent study, QOL significantly improved between the 4 and 16-month postoperative time points (the only time points evaluated) in 22 patients with recurrent pelvic malignancies (mostly colorectal cancer) who underwent total or posterior PE [40]. Because that study did not assess QOL preoperatively, it is plausible that a transient decline in QOL shortly after PE followed by a recovery on longer follow up (analogous to our findings) might have been demonstrated if a baseline QOL measurement had been established.
Finally, the results of this study suggest that adjustment following PE is an active ongoing process, as the majority of the patients returned to almost baseline level of function by 12 months after their surgery following an initial decline in QOL. This is consistent with a “response shift” where redefinition of life goals occurs as the patients adapt to their new health status [41–43]. Importantly, it is critical to emphasize when counseling patients that “baseline” refers to their QOL prior to undergoing PE and not to a state of perfect health.
Limitations of the current study should be noted. The relatively small number of patients included in this analysis could have obscured some longitudinal changes in some of the QOL domains. Assessing multiple outcomes within this sample is another limitation. This is, however, inherent to many QOL studies due to the multiple endpoints. In this analysis, procedures to minimize the type I error, compensating for the multiple comparisons, were employed. Despite these limitations, the conclusion that QOL, overall, recovers within about a year following PE is valid and, in general, is in agreement with that of others [40]. In addition, there is the possibility that data may not have been missing at random, but rather in a covariate-dependent fashion. For example, patients who miss an interview due to medical complications may have rated their QOL lower than patients who do not experience complications that interfere with study participation. Therefore, non-participation could have lead to a bias towards better outcomes. Given that the majority of patients completed the assessments at all the time points, such bias, if any, is probably minimal.
To conclude, most patients adjusted well within a year after PE in this patient cohort. To reap the benefits of this radical procedure, ideally, PE-candidates ought to have more than a year of expected survival. We hope that this initial analysis will lead to a better understanding of the physical and psychological sequelae that affect QOL following PE. We can then modify the informed consent process and tailor pre/postoperative support accordingly. To confirm these preliminary results, we will continue to examine the impact of PE on QOL using larger numbers of patients and longer follow up periods to verify that these trends remain stable.
Supplementary Material
Appendix Figure A1 (online only): Social, role and cognitive functions*
*: Mean scores of various scales for all the patients at the four time points. Higher scores indicate better function; QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire.
Appendix Figure A2 (online only): Financial impact and other symptoms*
*: Mean scores of various items and two scales (§) for all the patients at the four time points. Higher scores in a symptom scale/item reflect worse symptomatology; QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire.
Appendix Figure A3 (online only): Other symptoms (continued)*
*: Mean scores of various items and two scales (§) for all the patients at the four time points. Higher scores in a symptom scale/item reflect worse symptomatology; QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; QLQ-CR38: Colorectal cancer module of the QLQ-C30.
Appendix Tables A1 & A3–A6 (online only): Patients’ overall experiences (number of patients / percentage)*
Appendix Table A2 (online only): Patients’ pain expectations before pelvic exenteration (number of patients / percentage)
Research Highlights.
This ongoing prospective study examines quality of life (QOL) in patients undergoing pelvic exenteration (PE).
An extensive battery of psychological measures is used to assess multiple QOL endpoints relevant to these patients.
Despite some persistent decline in physical function and lingering gastrointestinal symptoms, in general, QOL recovers within a year after PE.
Acknowledgments
Research Support: Dr Hurley received funding from NIH grant number NCI K07 CA109236
The authors would like to thank James Hollenberg, MD, for his critical review of the manuscript.
Footnotes
This original research was presented, in part, at the 41st Annual Meeting of the Society of Gynecologic Oncologists, San Francisco, California, March 14–17, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer. 1948;1:177–83. doi: 10.1002/1097-0142(194807)1:2<177::aid-cncr2820010203>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Crowe PJ, Temple WJ, Lopez MJ, et al. Pelvic exenteration for advanced pelvic malignancy. Semin Surg Oncol. 1999;17:152–60. doi: 10.1002/(sici)1098-2388(199910/11)17:3<152::aid-ssu3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. American Society of Clinical Oncology. J Clin Oncol. 1996;14:671–9. doi: 10.1200/JCO.1996.14.2.671. [DOI] [PubMed] [Google Scholar]
- 4.Vera M. Quality of life following pelvic exenteration. Gynecol Oncol. 1981;12:355–66. doi: 10.1016/0090-8258(81)90136-0. [DOI] [PubMed] [Google Scholar]
- 5.Andersen B, Hacker N. Psychosexual adjustment following pelvic exenteration. Obstet Gynecol. 1983;61:331–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Corney R, Crowther M, Everett H, et al. Psychosexual dysfunction in women with gynaecological cancer following radical pelvic surgery. Br J Obstet Gynaecol. 1993;100:73–8. doi: 10.1111/j.1471-0528.1993.tb12955.x. [DOI] [PubMed] [Google Scholar]
- 7.Gleeson N, Baile W, Roberts W, et al. Surgical and psychosexual outcome following vaginal reconstruction with pelvic exenteration. Eur J Gynaecol Oncol. 1994;15:89–95. [PubMed] [Google Scholar]
- 8.Mirhashemi R, Averette H, Lambrou N, et al. Vaginal reconstruction at the time of pelvic exenteration: a surgical and psychosexual analysis of techniques. Gynecol Oncol. 2002;87:39–45. doi: 10.1006/gyno.2002.6780. [DOI] [PubMed] [Google Scholar]
- 9.Roos E, de Graeff A, van Eijkeren M, et al. Quality of life after pelvic exenteration. Gynecol Oncol. 2004;93:610–4. doi: 10.1016/j.ygyno.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Ungar L, Palfalvi L, Novak Z. Primary pelvic exenteration in cervical cancer patients. Gynecol Oncol. 2008;111(2 Suppl):S9–S12. doi: 10.1016/j.ygyno.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 11.Hawighorst-Knapstein S, Schönefussrs G, Hoffmann S, et al. Pelvic exenteration: effects of surgery on quality of life and body image--a prospective longitudinal study. Gynecol Oncol. 1997;66:495–500. doi: 10.1006/gyno.1997.4813. [DOI] [PubMed] [Google Scholar]
- 12.Hawighorst-Knapstein S, Fusshoeller C, Franz C, et al. The impact of treatment for genital cancer on quality of life and body image--results of a prospective longitudinal 10-year study. Gynecol Oncol. 2004;94:398–403. doi: 10.1016/j.ygyno.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Carter J, Chi D, Abu-Rustum N, et al. Brief report: total pelvic exenteration--A retrospective clinical needs assessment. Psychooncology. 2004;13:125–31. doi: 10.1002/pon.766. [DOI] [PubMed] [Google Scholar]
- 14.Aaronson N, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 15.Fossa S. Quality of life assessment in unselected oncologic out-patients: A pilot study. Int J Oncol. 1994;4:1393–7. doi: 10.3892/ijo.4.6.1393. [DOI] [PubMed] [Google Scholar]
- 16.Hjermstad M, Fossa S, Bjordal K, et al. Test/retest study of the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire. J Clin Oncol. 1995;13:1249–54. doi: 10.1200/JCO.1995.13.5.1249. [DOI] [PubMed] [Google Scholar]
- 17.Sprangers M, te Velde A, Aaronson N. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer. 1999;35:238–47. doi: 10.1016/s0959-8049(98)00357-8. [DOI] [PubMed] [Google Scholar]
- 18.Sprangers M, Cull A, Groenvold M, et al. The European Organization for Research and Treatment of Cancer approach to developing questionnaire modules: an update and overview. EORTC Quality of Life Study Group. Qual Life Res. 1998;7:291–300. doi: 10.1023/a:1024977728719. [DOI] [PubMed] [Google Scholar]
- 19.Guren M, Wiig J, Dueland S, et al. Quality of life in patients with urinary diversion after operation for locally advanced rectal cancer. Eur J Surg Oncol. 2001;27:645–51. doi: 10.1053/ejso.2001.1195. [DOI] [PubMed] [Google Scholar]
- 20.Mendoza T, Wang X, Cleeland C, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 21.Tittle M, McMillan S, Hagan S. Validating the brief pain inventory for use with surgical patients with cancer. Oncol Nurs Forum. 2003;30:325–30. doi: 10.1188/03.ONF.325-330. [DOI] [PubMed] [Google Scholar]
- 22.Mendoza T, Chen C, Brugger A, et al. The utility and validity of the modified brief pain inventory in a multiple-dose postoperative analgesic trial. Clin J Pain. 2004;20:357–62. doi: 10.1097/00002508-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Lawton M, Brody E. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 24.Fillenbaum G. Screening the elderly. A brief instrumental activities of daily living measure. J Am Geriatr Soc. 1985;33:698–706. doi: 10.1111/j.1532-5415.1985.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 25.George L, Fillenbaum G. OARS methodology. A decade of experience in geriatric assessment. J Am Geriatr Soc. 1985;33:607–15. doi: 10.1111/j.1532-5415.1985.tb06317.x. [DOI] [PubMed] [Google Scholar]
- 26.Fillenbaum G. The Duke older Americans resources and services procedures. Hilldale, NJ: Lawrence Erlbaum Associates; 1988. Multidimensional functional assessment of older adults. [Google Scholar]
- 27.Radloff L. The CES-D scale: a self-report depressive scale for research in the general population. J Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 28.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46:437–43. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Weiss D, Marmar C. The impact of event scale – revised. In: Wilson J, Keane T, editors. Assessing psychological trauma and post-traumatic stress disorder. New York, NY: Guilford Press; 1997. pp. 399–411. [Google Scholar]
- 31.Thewes B, Meiser B, Hickie I. Psychometric properties of the Impact of Event Scale amongst women at increased risk for hereditary breast cancer. Psychooncology. 2001;10:459–68. doi: 10.1002/pon.533. [DOI] [PubMed] [Google Scholar]
- 32.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual. 3. European Organisation for Research and Treatment of Cancer; Brussels: 2001. [Google Scholar]
- 33.Cella D, Tulsky D. Quality of life in cancer: definition, purpose, and method of measurement. Cancer Invest. 1993;11:327–36. doi: 10.3109/07357909309024860. [DOI] [PubMed] [Google Scholar]
- 34.Bodurka-Bevers D, Basen-Engquist K, Carmack C, et al. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecol Oncol. 2000;78:302–8. doi: 10.1006/gyno.2000.5908. [DOI] [PubMed] [Google Scholar]
- 35.Schag C, Heinrich R. Development of a comprehensive quality of life measurement tool: CARES. Oncology (Williston Park) 1990;4:135–8. discussion 147. [PubMed] [Google Scholar]
- 36.Schag C, Ganz P, Heinrich R. CAncer Rehabilitation Evaluation System--short form (CARES-SF). A cancer specific rehabilitation and quality of life instrument. Cancer. 1991;68:1406–13. doi: 10.1002/1097-0142(19910915)68:6<1406::aid-cncr2820680638>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Strauss B, Richter-Appelt H. Fragebogen zur Beurteilung des eigenen Körpers (FbeK) Manual. Göttingen: Hogrefe; 1996. [Google Scholar]
- 38.Cull A, Howat S, Greimel E, et al. Development of a European Organization for Research and Treatment of Cancer questionnaire module to assess the quality of life of ovarian cancer patients in clinical trials: a progress report. Eur J Cancer. 2001;37:47–53. doi: 10.1016/s0959-8049(00)00369-5. [DOI] [PubMed] [Google Scholar]
- 39.Greimel E, Bottomley A, Cull A, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-OV28) in assessing the quality of life of patients with ovarian cancer. Eur J Cancer. 2003;39:1402–8. doi: 10.1016/s0959-8049(03)00307-1. [DOI] [PubMed] [Google Scholar]
- 40.Zoucas E, Frederiksen S, Lydrup ML, et al. Pelvic exenteration for advanced and recurrent malignancy. World J Surg. 2010;34:2177–84. doi: 10.1007/s00268-010-0637-7. [DOI] [PubMed] [Google Scholar]
- 41.Breetvelt I, Van Dam F. Underreporting by cancer patients: the case of response-shift. Soc Sci Med. 1991;32:981–7. doi: 10.1016/0277-9536(91)90156-7. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz C, Sprangers M. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med. 1999;48:1531–48. doi: 10.1016/s0277-9536(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 43.Wilson I. Clinical understanding and clinical implications of response shift. Soc Sci Med. 1999;48:1577–88. doi: 10.1016/s0277-9536(99)00050-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure A1 (online only): Social, role and cognitive functions*
*: Mean scores of various scales for all the patients at the four time points. Higher scores indicate better function; QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire.
Appendix Figure A2 (online only): Financial impact and other symptoms*
*: Mean scores of various items and two scales (§) for all the patients at the four time points. Higher scores in a symptom scale/item reflect worse symptomatology; QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire.
Appendix Figure A3 (online only): Other symptoms (continued)*
*: Mean scores of various items and two scales (§) for all the patients at the four time points. Higher scores in a symptom scale/item reflect worse symptomatology; QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; QLQ-CR38: Colorectal cancer module of the QLQ-C30.
Appendix Tables A1 & A3–A6 (online only): Patients’ overall experiences (number of patients / percentage)*
Appendix Table A2 (online only): Patients’ pain expectations before pelvic exenteration (number of patients / percentage)