SUMMARY
Tumor-reactive T cells become unresponsive in advanced tumors. Here we have characterized a common mechanism of T cell unresponsiveness in cancer driven by the up-regulation of the transcription factor Forkhead box protein P1 (Foxp1), which prevents CD8+ T cells from proliferating and up-regulating Granzyme-B and interferon-γ (IFN-γ) in response to tumor antigens. Accordingly, Foxp1-deficient lymphocytes induced rejection of incurable tumors, and promoted protection against tumor re-challenge. Mechanistically, Foxp1 interacted with the transcription factors Smad2 and Smad3 in pre-activated CD8+ T cells in response to microenvironmental transforming growth factor-β (TGF-β), and was essential for its suppressive activity. Therefore, Smad2 and Smad3-mediated c-Myc repression requires Foxp1 expression in T cells. Furthermore, Foxp1 directly mediated TGF-β-induced c-Jun transcriptional repression, which abrogated T cell activity. Our results unveil a fundamental mechanism of T cell unresponsiveness different from anergy or exhaustion, driven by TGF-β signaling on tumor-associated lymphocytes undergoing Foxp1-dependent transcriptional regulation.
INTRODUCTION
Malignant progression promotes the selection of less immunogenic tumor variants (Vesely and Schreiber, 2013). However, clinical evidence supports that T cells exert immune pressure against the progression of even advanced cancers (Fridman et al., 2011; Zhang et al., 2003). In addition, de novo elicitation or re-activation of protective immunity is required for the effectiveness of several conventional or targeted anti-cancer therapies (Zitvogel et al., 2013). Still, established tumors are not spontaneously rejected by the immune system. Even when tumor cells remain immunogenic, the effector activity of tumor-reactive lymphocytes is weakened during malignant progression (Scarlett et al., 2012). In tumor-bearing hosts, two key mechanisms mediated by different transcriptional pathways (Crespo et al., 2013) render tumor-reactive lymphocytes unresponsive through defective T cell priming (anergy) (Zheng et al., 2012), or sustained exposure to suboptimal antigen concentrations (exhaustion) (Wherry, 2011).
Besides inherent T cell unresponsiveness, tumor, vascular, stromal and immune cells contribute to create an inflammatory and metabolically hostile environment where multiple immunosuppressive networks converge to abrogate residual T cell activity (Zou, 2005). Expression of the inhibitory receptors PD-1, LAG-3 and CTLA-4 (Baitsch et al., 2012) in leukocytes and tumor cells also contributes to maintain T cell inactivity. In addition, Indoleamine 2,3-dioxygenase (IDO) and its tolerogenic metabolites, immunosuppressive cytokines, or nitrogen-reactive species, all contribute to abrogate lingering lymphocyte activity in most solid tumors. Interestingly, some immunosuppressive pathways are more active in tumors infiltrated by activated T cells (Spranger et al., 2013), suggesting that these patients could be superior beneficiaries of immunotherapies targeting immunosuppression. Indeed, emerging clinical evidence supports that blockade of tolerogenic pathways unleashes anti-tumor immunity, but only in some patients (Pardoll and Drake, 2012). Understanding what is truly relevant for the abrogation of protective immunity in different cancers is needed for implementing more effective anti-tumor immunotherapies.
Transforming growth factor-β (TGF-β) is a lymphocyte inhibitor secreted by multiple cells and frequently overexpressed in aggressive cancers (Flavell et al., 2010; Wrzesinski et al., 2007). Tumors induce dendritic cells (DCs) to secrete TGF-β, promoting regulatory T cell (Treg) expansion and indirect suppression of T cell effectors (Ghiringhelli et al., 2005; Hanks et al., 2013). Conventional T cells also produce TGF-β. Interestingly, in some models, T cell-derived TGF-β (including TGF-β produced by Treg cells) is sufficient for anti-tumor T cell suppression, while ablation of TGF-β only in Treg cells has insignificant effects (Donkor et al., 2011). Furthermore, TGF-β can also suppress effector cytokines in anti-tumor CD8+ lymphocytes (Ahmadzadeh and Rosenberg, 2005). However, the pathways elicited by TGF-β signaling specifically in unresponsive tumor-reactive T cells and their overall impact, remain incompletely understood. TGF-β could inhibit T cell proliferation through Smad3 transcription factor-dependent repression of interleukin-2 (IL-2) (McKarns et al., 2004), and also through IL-2-independent mechanisms that involve Smad3 binding to the c-Myc promoter (Frederick et al., 2004). Still, it is unknown whether these pathways play a major role in tumor-induced immunosuppression, or whether other tumor-induced factors influence TGF-β-signaling.
Forkhead box (FOX) proteins are transcription factors with pleiotropic functions in the development and activity of immune cells. In naive T cells, constitutive expression of Foxp1 enforces quiescence by repressing the IL-7 receptor, implying that a cell-intrinsic, Foxp1-dependent transcriptional program actively maintains naive lymphocytes “at rest” (Feng et al., 2011; Hamilton and Jameson, 2012). Interestingly, Foxp1 is down-regulated in exhausted (but not in memory) CD8+ lymphocytes in chronic viral infections (Doering et al., 2012), but the functional relevance of this phenotype, or whether these patterns are recapitulated in anti-tumor T cells, remain completely unknown.
Here, we report that Foxp1 is universally up-regulated in human and mouse tumor-infiltrating effector T cells. Foxp1 mediates TGF-β signaling by interacting with Smad proteins as an obligate transcriptional co-repressor, and is required for dampening anti-tumor immunity. Our results identify a hitherto unknown mechanism of tumor-induced effector T cell suppression involving T cell-intrinsic transcriptional changes different from anergy or exhaustion.
RESULTS
Tumor-reactive T cells up-regulate Foxp1 in the tumor microenvironment
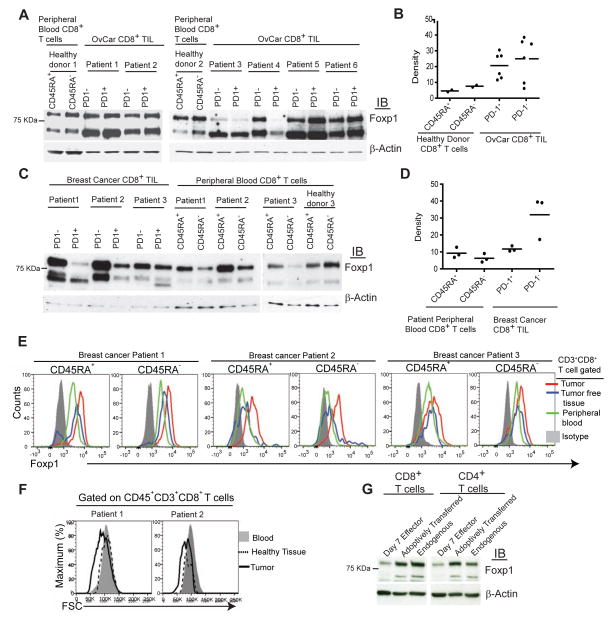
Foxp1 is down-regulated in exhausted CD8+ T cells in chronic viral infections (Doering et al., 2012). However, it has been shown to enforce quiescence in naïve lymphocytes (Feng et al., 2011), and its role in anti-tumor T cells remains to be determined. To define the expression of Foxp1 in human ovarian cancer-infiltrating lymphocytes, we used antibodies that detect two Foxp1 isoforms in mouse T cells: Constitutively expressed Foxp1a, which maintains naïve lymphocyte quiescence (Feng et al., 2011), and inducible Foxp1d. Accordingly, we also identified two predominant bands of ~80 and ~66 kDa in human T cells, consistent with the size of Foxp1.1 (the >97% similar human ortholog of mouse Foxp1a)I and the human counterpart of mouse Foxp1d, respectively (Figure 1A). Compared to activated (CD45RA−) or naïve (CD45RA+) T cells from the blood of different healthy donors, CD8+ tumor infiltrating lymphocytes (TILs) from 6 randomly selected patients overexpressed both Foxp1 isoforms, in both primary and metastatic ovarian tumors (Figures 1A and 1B, and Figure S1A). Foxp1 up-regulation in ovarian cancer was independent of the exhaustion status of T cells, because two different Foxp1 variants were expressed at higher (although variable) amounts independently of PD-1 expression, the inhibitory receptor associated with exhaustion in one third of ovarian cancer CD8+ TILs (Duraiswamy et al., 2013). Foxp1 overexpression was not restricted to ovarian TILs, because unexhausted (PD-1−) CD8+ lymphocytes sorted from 3 different breast cancer specimens showed much higher amounts of Foxp1 than CD45RA− and CD45RA+ T cells in peripheral blood from the same patients, although Foxp1 was only slightly up-regulated in PD-1+ CD8+ T cells in these samples (Figures 1C and 1D) Importantly, Foxp1 overexpression was not caused by mere homing to solid tissues, because Foxp1 was higher in intratumoral lymphocytes than in T cells in matching tumor-free tissue from multiple patients (Figure 1E and Figure S1B). In addition, Foxp1 overexpressing CD8+ lymphocytes harvested from ovarian tissue exhibited lower FSC (associated with smaller cell size), compared to their counterparts in peripheral blood from the same patient (Figure 1F).
Figure 1. CD8+ T cells up-regulate Foxp1 in the TME.
(A) Foxp1 expression in PD-1− and PD-1+ CD8+ T cells flow cytometry-sorted from six freshly dissociated stage III and IV human ovarian carcinoma specimens (samples 4 and 5, primary tumors; samples 1, 2, 3, and 6, metastatic masses). CD45RA+ (resting or naïve) and CD45RA− (activated) CD8+ T cells from the peripheral blood of two healthy donors were sorted and analyzed in parallel in two independent experiments. (B) Normalization of Foxp1 protein expression in PD-1− and PD-1+ ovarian tumor-infiltrating CD8+ T cells with β-actin. (C) Foxp1 expression in PD-1− and PD-1+ CD8+ T cells flow cytometry-sorted from three freshly dissociated breast cancer specimens, as well as matching peripheral blood from the same patients and a healthy donor. (D) Densitometric normalization performed as in (B). (E) Intracellular staining for Foxp1 in CD3+CD8+ gated CD45RA+ and CD45RA− T cells from human breast tumors, tumor free tissues and peripheral blood from same patients. (F) Comparative FSC analysis of CD8+ T cells contained in a freshly dissociated advanced ovarian carcinoma and matching peripheral blood. (G) CD45.2+ naive T cell splenocytes were primed in vitro with bone marrow-derived DCs (BMDCs) pulsed with double irradiated (UV+gamma) ID8-Defb29-Vegf-a tumor cells for 7 days (day 7 effectors) and administered into the peritoneal cavity of congenic ID8-Defb29-Vegf-a tumor-bearing mice at day 23 after tumor challenge. Three days later, transferred (CD45.2+) and endogenous (CD45.1+) CD8+ and CD4+ T cells were flow cytometry-sorted from tumor ascites and analyzed by western blot. Representative of two independent experiments. IB, immunoblotting.
Because tumor-reactive T cells are contained in both the PD-1− and PD-1+ fractions of TILs (Matsuzaki et al., 2010), we next sought to define how tumor antigen-specific T cells up-regulate Foxp1 in the tumor microenvironment (TME). For that purpose, we activated mouse splenic T cells with DCs pulsed with immunogenic (UV- plus γ-irradiated) ID8-Defb29-Vegf-a cancer cells, a system that results in aggressive and widely disseminated orthotopic ovarian tumors (Cubillos-Ruiz et al., 2012; Cubillos-Ruiz et al., 2009). As reported, ~70% of de novo activated lymphocytes showed activation markers within 7 days (Nesbeth et al., 2009; Nesbeth et al., 2010) (not shown). Notably, recently primed CD8+ T cells exhibited lower amounts of Foxp1a compared to naïve T cells (not shown), while both Foxp1a and Foxp1d (Feng et al., 2011) were up-regulated in these cells in vivo in the TME within 3 days after adoptive transfer (Figure 1G). Universal ovarian microenvironmental factors such as hypoxia, PGE2, or Estradiol (Cubillos-Ruiz et al., 2010; Scarlett et al., 2012) had no measurable effect on Foxp1 up-regulation in activated T cells (Figures S1C and S1D). Incubation with tumor-derived regulatory DCs or Myeloid-Derived Suppressor Cells (MDSCs) also had negligible effects on the expression of any Foxp1 isoform (Figure S1D). In our hands, interleukin-6 (IL-6), IL-2, IL-23, IL-17, IL-15, IL-7 or vascular endothelial growth fator-a (Vegf-a) also did not affect Foxp1 amounts in activated T cells (Figure S1E). In contrast, signaling through the integrin ligand ICAM-1, the chemokine CXCL12 and, to a lesser extent, TGF-β, induced a modest but reproducible up-regulation of Foxp1, which was enhanced in an additive manner (Figure S1F). Correspondingly, independently of their activation status or antigen experience, CD45RA+ and CD45RA−, CD69+ and CD69−, or CD44low and CD44high human breast TILs from different patients exhibited comparable Foxp1 overexpression (Figure 1E, and Figures S1G and S1H). Therefore, TILs commonly up-regulate Foxp1 in response to cytokines, chemokines and integrin ligands overexpressed in the TME. At least in some tumors Foxp1 overexpression occurs independently of exhaustion markers, and to a greater amount than in quiescent (CD45RA+) peripheral T cells, implicating a potential role of this transcription factor in impairing anti-tumor immunity.
Foxp1 overexpression prevents tumor-reactive T cells from proliferating in the TME and eliciting tumor rejection
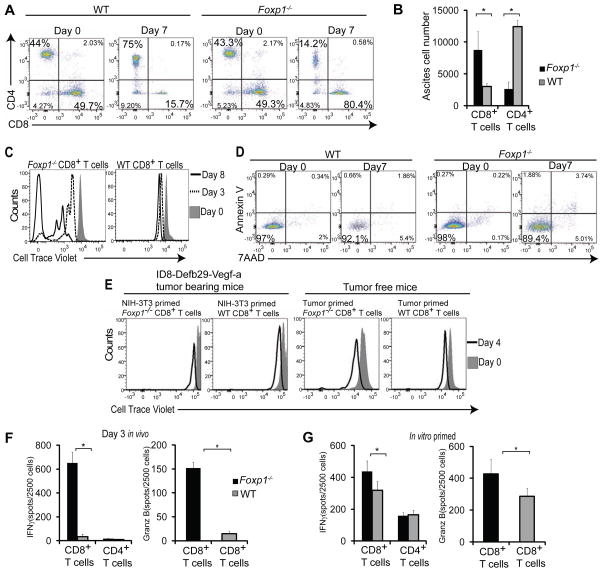
To elucidate the biological consequences of Foxp1 overexpression in anti-tumor T cells, we primed naïve Foxp1-deficient (from Foxp1f/f Cd4 cre+ mice (Feng et al., 2011)) and wild-type T cells (from Foxp1f/f littermates) with ID8-Defb29-Vegf-a-pulsed DCs. Ex vivo stimulation resulted in comparable CD8+ and CD4+ ratios in Foxp1+/+ and Foxp1−/− lymphocytes (Figure 2A and Figure S2A). When equal numbers of Foxp1-deficient and control tumor-reactive T cells were adoptively transferred into congenic tumor-bearing mice, the proportions and absolute numbers of CD8+ T cells lacking Foxp1 were ~4-fold increased (Figures 2A and 2B). Accumulation of Foxp1−/− CD8+ T cells in tumors was the result of their in vivo expansion, as Cell Trace Violet-labeled tumor antigen-primed Foxp1-deficient, but not control CD8+ T cells selectively proliferated in the TME for at least 8 days (Figure 2C and Figure S2B). Foxp1 overexpressing T cells did not undergo death, but remained unresponsive at tumor beds (Figures 2C and 2D, and Figures S2B–E). Importantly, selective expansion of Foxp1-deficient T cells was driven by response to cognate (tumor) antigen, because Foxp1−/− CD8+ T cells primed against irrelevant antigen, or against tumor antigens but transferred into tumor-free mice, did not proliferate (Figure 2E and Figure S2F).
Figure 2. Foxp1 expression regulates anti-tumor effector functions and proliferation of CD8+ T cells in the TME.
(A) Flow cytometric analysis of CD45.2+ Foxp1-deficient vs. wild-type T cells identically primed against tumor antigens as in Figure 1G. (B) Absolute cell number of these lymphocytes recovered from peritoneal wash 7 days after adoptive transfer into day 24 ID8-Defb29-Vegf-a tumor-bearing congenic mice. Representative of four independent experiments (p<0.05, Student’s t-test). (C) In vivo proliferation of tumor-reactive Foxp1-deficient vs. wild-type CD8+ T cells. Lymphocytes primed for 7 d against tumor antigens were labeled with cell trace violet, adoptively transferred into orthotopic advanced ovarian cancer-bearing congenic mice, and recovered from peritoneal wash 3 and 8 days later. Representative of three experiments. (D) Annexin V and 7AAD staining of tumor antigen-primed Foxp1-deficient and wild-type T cells recovered from tumor ascites at the indicated days after adoptive transfer in three independent experiments. (E) Proliferation of Foxp1−/− and WT T cells either primed with BMDCs pulsed with irradiated and UV-treated NIH-3T3 cells followed by transfer into day 24 ID8-Defb29-Vegf-a tumor-bearing congenic mice (left), or primed with tumor antigen and transferred into the peritoneal cavity of tumor free congenic mice (right). Representative of three experiments. (F) ELISPOT analysis of identically primed T cells, sorted from tumor ascites 3 d after adoptive transfer into advanced ID8-Defb29-Vegf tumor-bearing congenic mice, and re-stimulated with PMA and Ionomycin for 4 h. Representative of three independent experiments (G) IFN-γ and Granzyme B ELISPOT analysis of T cells primed against tumor antigens as above for 7 d. (p<0.05, Student’s t-test).
Although the presence of Foxp1 abrogated the proliferative capacity of tumor-reactive T cells in vivo, they retain their capacity to produce IL-2 and >20% of them show CD69 expression in vivo (Figures S2G and S2H). However, the expression of Foxp1 abolished the effector activity of anti-tumor lymphocytes after transfer into tumors (Figure 2F). Significantly higher numbers of Foxp1-deficient T cells sorted from peritoneal washes after 3 days in the TME reacted by secreting IFN-γ and cytolytic Granzyme-B in re-call ELISPOT analysis, compared to identically handled control CD8+ lymphocytes (Figure 2F). Importantly, superior effector activity in the absence of Foxp1 was amplified in the TME, compared to mild differences found after in vitro priming (Figure 2G). Collectively, these results indicate that the presence of Foxp1 in tumor-reactive T cells is sufficient to prevent their effector activity in the TME upon re-encounter with cognate tumor antigens.
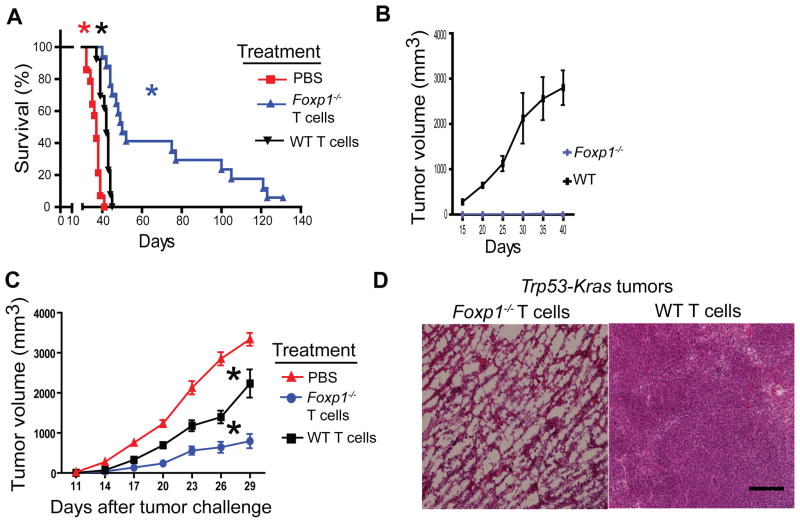
Accordingly, the adoptive transfer of tumor antigen-primed T cells lacking Foxp1 dramatically delayed the progression of established and aggressive ID8-Defb29-Vegf-a orthotopic tumors, while identically activated wild-type T cells only induced modest protection (Figure 3A). Notably, a fraction of mice treated with Foxp1-deficient T cells in every independent experiment did not show signs of disease >4 months after tumor challenge. To define whether Foxp1-deficient lymphocytes promoted long-term protection against tumor recurrences, we re-challenged these mice with ID8-Defb29-Vegf-a flank (axillary) tumors. As shown in Figure 3B, all long-term survivors rejected secondary tumors, while all control (naïve) mice developed >2 cm tumor masses. As expected, mice with selective ablation of Foxp1 in T cells also exhibited superior outcomes when they were directly challenged with orthotopic tumors (Figure S3A).
Figure 3. Foxp1 expression impairs the protective function of tumor-reactive T cells.
(A) On day 24 after ID8-Defb29-Vegf-a tumor challenge, 47 different CD45.1+ mice received 106 tumor antigen-primed (day 7) T cells from Foxp1-deficient (n=16) or wild-type (n=15) CD45.2+ mice. Sixteen additional control tumor-bearing mice were treated with PBS. Data pooled from three independent experiments. P<0.0001, Mantel-Cox test. (B) Four tumor-bearing mice surviving >60 d. after treatment with Foxp1−/− T cells and six control age-matched wild-type mice were re-challenged with 2 × 106 ID8-Defb29-Vegf-a tumor cells, administered into the axillary flank. Tumor growth was monitored in three independent experiments. P<0.003, Student’s t-test. (C) Naïve T cell splenocytes from Foxp1-deficient or wild-type mice were primed for 7 d with BMDCs pulsed with double (γ- plus UV- irradiated) MPKAS cells. Tumor-reactive T cells were delivered into tumors formed from this cell line (2 × 106 cells) in congenic mice (n=9 receiving Foxp1−/− and n=9 receiving wild-type T cells), at days 8 and 14 after flank tumor challenge. Eight additional flank tumor-bearing mice received PBS. Pooled from 3 independent experiments. P<0.005 between tumor volume of Foxp1-deficient T cells and either wild-type T cell or PBS treatment groups (Mann-Whitney). (D) Massive necrosis induced by intratumoral administration of Foxp1−/−, but not wild-type tumor-reactive T cells in C57BL/6 Trp53-Kras mice challenged with s.c. adenovirus-Cre to induce flank sarcomas. Palpable tumors were injected 3–4 times with 106 tumor antigen-primed Foxp1-deficient vs. wild-type T cells, at 5–6 d intervals. Representative of three independent experiments. Scale bar 200 μM.
To define whether the superior anti-tumor activity of Foxp1-deficient T cells is applicable to non-ovarian malignancies, we generated sarcoma cell lines (termed MPKAS) from tumors resulting from the administration of adenovirus-Cre into the flank of Trp53f/fLSL-KrasG12D/+ (Trp53-Kras) mice (Scarlett et al., 2012). We pulsed DCs with immunogenic (γ- plus UV-irradiated) tumor cells, and used them to de novo prime T cells. Again, the growth of MPKAS flank tumors was significantly delayed when Foxp1-deficient tumor-reactive T cells were administered directly into the tumor mass, compared to identically stimulated wild-type T cells (Figure 3C). In addition, intratumoral administration of Foxp1-defective, but not control tumor-reactive T cells, induced massive necrosis in flank tumors that were initiated with adenovirus-Cre in Trp53-Kras mice (Figure 3D and Figure S3B). Together, these results confirm that the expression of Foxp1 is sufficient to abrogate the protective activity of anti-tumor cytotoxic T cells in the TME, and identify an important mechanism of tumor-induced T cell unresponsiveness.
Foxp1 is required for TGF-β-induced inhibition of CD8+ T cells
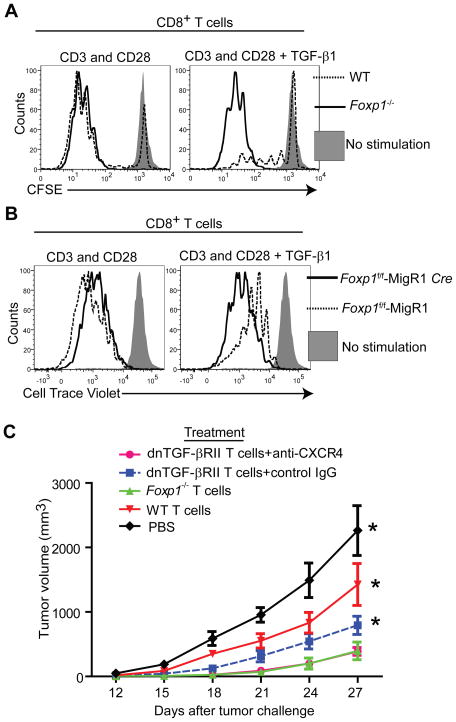
To determine how cytotoxic T cells acquire the capacity to expand and elicit immune protection against malignant progression in the absence of Foxp1, we focused on the activity of TGF-β, which is up-regulated in the microenvironment of multiple tumors. We observed that Foxp1-deficient CD8+ lymphocytes were resistant to TGF-β-mediated inhibition in multiple independent experiments, while wild-type T cell proliferation was abrogated in the presence of TGF-β (Figure 4A). Correspondingly, Foxp1−/− (and not Foxp1+/+) CD8+ T cells primed against tumor antigens in the presence of TGF-β, proliferated in vivo in the TME as effectively as without TGF-β (Figure S4A). Resistance to TGF-β was not the result of thymic selection or pre-activation artifacts in Foxp1-deficient CD8+ T cells, because retrovirus-Cre-induced excision of Foxp1 in CD8+ T cells from (Cd4-cre−) Foxp1f/f mice also allowed their expansion in the presence of TGF-β, while mocked-transduced lymphocytes remained inhibited, despite the robust pre-activation required for retroviral transduction (Figure 4B, and Figures S4B and S4C).
Figure 4. Foxp1 is required for TGF-β-induced suppression of CD8+ T cells.
(A) Proliferation analysis of cell trace violet-labeled Foxp1-deficient and wild-type T cells, stimulated for 5 days with CD3 and CD28 beads, in the presence or the absence of 5 ng/mL of TGF-β1. Cells were stained for CD8, Annexin V and 7AAD. Representative of four independent experiments. (B) T cells from the spleen and lymph nodes of Foxp1f/f were transduced with Cre-recombinase expressing MigR1-GFP retroviruses or the empty vector. GFP+ (excised) CD8+ T cells were flow cytometry-sorted after 48 hours, cell trace violet-labeled and CD3 and CD28-stimulated for 4–5 days, in the presence or the absence of TGF-β1 (5 ng/mL). Representative of three independent experiments. (C) Growth kinetics of MPKAS sarcomas (n=6/group) intratumorally treated at days 7 and 10 with 2×106 tumor antigen-primed (6 d.) T cells that were dnTGF-βRII (pre-incubated for 30 min with 5 μg/ml of anti-mouse CXCR4 or control IgG; both from R&D), Foxp1−/−, or WT. Additional controls received PBS.
Next, we aimed to define whether resistance to TGF-β was sufficient to explain the superior anti-tumor protection elicited by Foxp1-deficient CD8+ T cells. For that purpose, we treated ID8-Defb29-Vegf-a tumor-bearing mice with tumor antigen-primed T cells carrying a dominant-negative TGF-βR type II (dnTGF-βRII), in which TGF-β signaling is blocked (Chen et al., 2005; Gorelik and Flavell, 2000). As shown in Figure S4D, dnTGF-βRII T cells elicited survival increases higher than those induced by identically primed T cells from wild-type littermates, despite the fact that Foxp1 was still up-regulated in the TME (Figure S4E). Correspondingly, MPKAS sarcoma-reactive dnTGF-βRII T cells also elicited superior effects against flank tumor growth, compared to wild-type lymphocytes (Figure 4C). Most importantly, combined blockade of CXCL12 signaling and TGF-β resistance prevented the up-regulation of Foxp1 in the TME (Figure S4F), resulting in anti-tumor effects equivalent to the administration of Foxp1-deficient T cells (Figure 4C and Figure S4G). Therefore, although the blatant superiority of Foxp1-deficient T cells is not fully recapitulated by TGF-β-resistance alone, these results confirm that TGF-β is nevertheless a major contributor to anti-tumor T cell unresponsiveness. Therefore, the capacity of Foxp1−/− lymphocytes to overcome TGF-β-mediated inhibition is relevant for their enhanced activity in the TME, and combined TGF-β and CXCL12 signals cause Foxp1 overexpression in at least some tumors.
We then aimed to elucidate the mechanism whereby Foxp1-deficient CD8+ T cells become resistant to TGF-β-mediated inhibition. In the canonical TGF-β pathway, binding of TGF-β to a TGF-βRI and TGF-βRII receptor dimer drives phosphorylation and nuclear translocation of Smad2 and Smad3 molecules, which interact with co-repressors to suppress T cell function (Siegel and Massague, 2003). We therefore first ruled out repression of either the TGF-βR or downstream Smad2 and Smad3 by Foxp1, at either resting or activated stages (Figures 5A and 5B). Furthermore, Smad2 and Smad3 phosphorylation occurred as effectively in control T cells as in Foxp1-deficient lymphocytes (Figures 5B and 5C). Smad4-dependent nuclear translocation of Smad2 and Smad3 (Siegel and Massague, 2003) was also unaffected by the absence of Foxp1, as shown by immunofluorescent analysis of Foxp1-deficient CD8+ T cells treated with TGF-β (Figure 5D). Collectively, these data indicate that Foxp1 is required for TGF-β-mediated inhibition of tumor-reactive T cells, through a mechanism that takes place downstream of Smad2 and Smad3 translocation, which is sufficient to explain why Foxp1−/− lymphocytes remain responsive in the TME and exert superior anti-tumor protection.
Figure 5. Foxp1 deficiency does not affect TGF-β-induced nuclear translocation of Smad signaling molecules in CD8+ T cells.
(A) Western blot analysis of TGF-βRII expression in Foxp1-deficient and wild-type CD8+ T cells under various stimulation conditions. (B) Expressions of Smad2 (upper band), Smad3 (lower band) and phosphorylated Smad2 (p-Smad2) in Foxp1-deficient and wild-type CD8+ T cells at rest or CD3 and CD28-activated for 24 hours, in the presence or the absence of TGF-β1 (5 ng/mL). (C) Expression of p-Smad3 (Ser423 and Ser425) in Foxp1-deficient and wild-type CD8+ T cells stimulated in vitro with CD3 and CD28-beads for 24 h, followed by TGF-β1 (5ng/ml) treatment for 30 minutes. (D–E) Confocal microscopy analysis of resting and CD3 and CD28-stimulated (24 h) Foxp1-deficient and wild-type CD8+ T cells (+/−TGF-β1; 5 ng/ml). Representative of three independent experiments. Scale bar 10 μM.
Foxp1 is a component of Smad nuclear repression complex
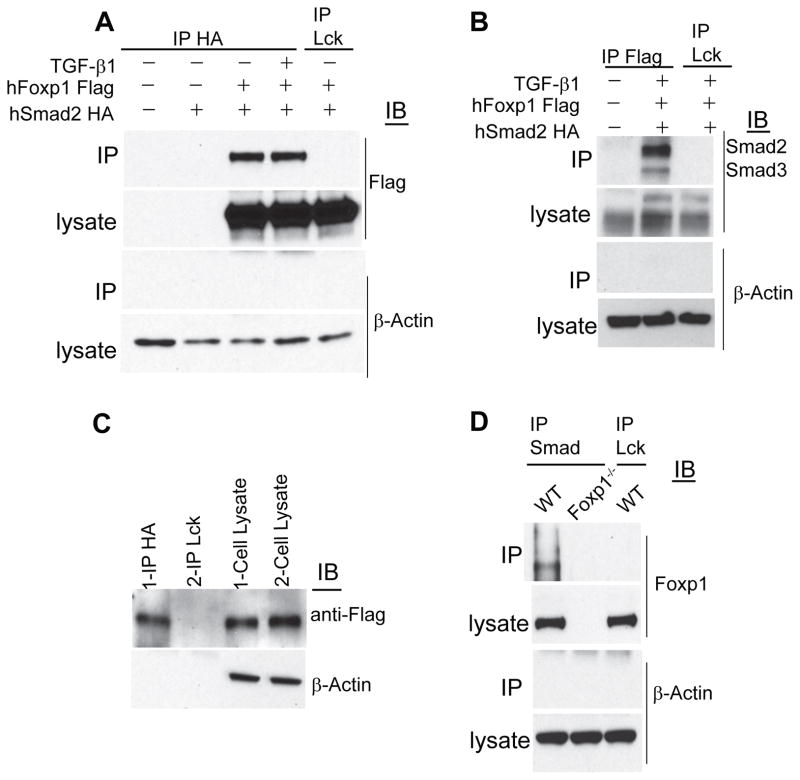
To define whether Foxp1 interacts with Smad2 and Smad3 proteins in the nucleus after TGF-β-induced translocation, we next performed confocal microscopy analysis of primary human and mouse CD8+ T cells. As shown in Figure 5E, Foxp1 co-localized with Smad2 and Smad3 in the nucleus of T cells after TGF-β signaling. Because in some cell types, Smad2 and Smad3 partner with other transcriptional repressors such as TGIF and CtBP1 (Postigo et al., 2003; Siegel and Massague, 2003), we hypothesized that Foxp1 could be a necessary component of the transcriptionally repressive Smad2 and Smad3 complex in CD8+ T cells. To confirm the physical interaction between Smad and Foxp1, we stably co-transfected HeLa cells with Flag-tagged human Foxp1 and hemagglutinin (HA)-tagged human Smad2. Western blot analysis confirmed that immunoprecipitates of tagged Smad2 contained a band corresponding to Flag-tagged Foxp1 that was recognized by α-Flag antibodies (Figure 6A). Co-immunoprecipitation of Foxp1 and Smad2 was specific because abundant proteins such as β-actin were not pulled-down, and immunoprecipitates of irrelevant (α-Lck) Abs did not contain Foxp1 (Figure 6A). Correspondingly, reverse (α-Flag Foxp1) immunoprecipitates, but not irrelevant pull-downs, contained interacting Smad2 and Smad3 proteins in independent experiments (Figure 6B).
Figure 6. Foxp1 interacts with the TGF-β-induced Smad repressor complex.
(A) HeLa cells were transiently transfected with HA-tagged human Smad2 (Smad2-HA) and Flag-tagged human Foxp1.1 (Foxp1-Flag), treated or not with 5 ng/ml of TGF-β1 for 12 hours, cross-linked and lysed. HA was immunoprecipitated (IP) from the extracted proteins, followed by immunoblotting for Flag. HA IP from un-transfected, Smad2-HA only transfected HeLa cell lysates, and irrelevant (α-Lck) IgG IP from Smad2-HA and Foxp1-Flag transfected cell lysates were used as IP controls. Representative of four independent experiments. (B) Reverse IP with Flag and immunoblotting for Smad2 and Smad3 from transiently transfected HeLa cells described above. Representative of two independent experiments. (C) Primary human CD8+ T cells were electroporated with Smad2-HA and Foxp1-Flag. After 6–12 hours, cells were CD3 and CD28-stimulated for 24 h (+/− 5 ng/ml TGF-β1), cross-linked, and lysed. IP was carried out with anti-HA antibody and irrelevant IgG followed by immunoblotting for Flag. (D) Endogenous Smad2 and Smad3 was IPed from the whole cell lysates of wild-type and Foxp1-deficient T cells CD3 and CD28-activated in the presence of TGF-β1 (5 ng/ml). IP with irrelevant IgG was simultaneously performed. IPed proteins were immunoblotted and probed with anti-Foxp1 antibodies. Representative of three independent experiments. IP, Immunoprecipitation.
To verify that Smad2 and Foxp1 also interact in primary human T cells, we nucleofected CD3 and CD28 pre-activated lymphocytes from the peripheral blood of 2 different healthy donors with tagged Smad2 and Foxp1. Immunoprecipitation of Smad2-HA, but not of (irrelevant) Lck, again specifically pulled-down Flag-tagged Foxp1 and not β-actin (Figure 6C), confirming that Foxp1 physically binds to the Smad2 and Smad3 complex also in primary T cells.
Finally, to confirm the interaction between endogenous Smad2 and Foxp1 in primary lymphocytes, we CD3 and CD28-activated mouse T cell splenocytes (both Foxp1-deficient and control lymphocytes) in the presence of TGF-β, and performed new immunoprecipitation analysis. As expected, immunoprecipitates of α-Smad2 and Smad3 Abs (but not irrelevant α-Lck Abs) contained endogenous Foxp1 and not β-actin, and that occurred only when control Foxp1+ T cells were used (Figure 6D). These results demonstrate that Foxp1 physically interacts with the transcriptional repressor complex orchestrated by Smad2 and Smad3 in CD8+ T cells upon TGF-β-induced nuclear translocation.
Foxp1 mediates transcriptional repression of c-Myc and c-Jun in CD8+ T cells in response to TGF-β signaling
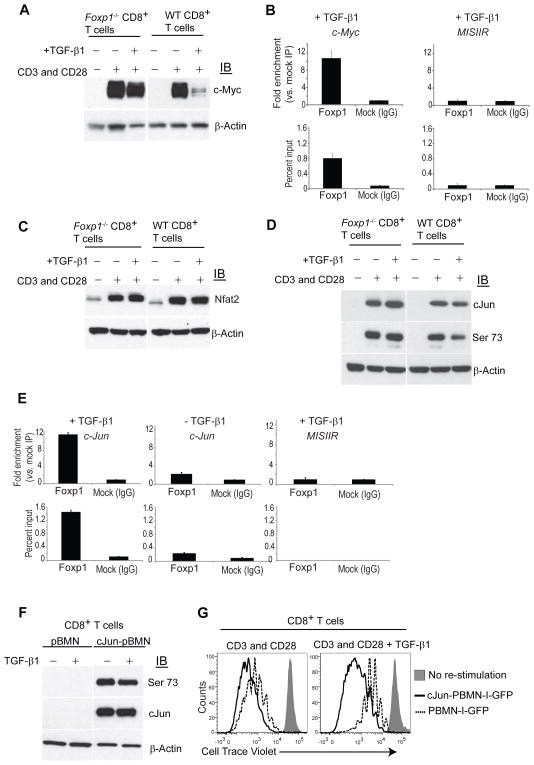
Because Foxp1-deficient CD8+ T cells are resistant to TGF-β-mediated inhibition, and because Foxp1 and Smad2 and Smad3 interact in the nucleus, our results imply that Foxp1 is a required element for the repressive effects of TGF-β. To confirm this proposition, we focused on the cell cycle promoter c-Myc, a known target of the TGF-β pathway that is transcriptionally repressed through direct binding of Smad to its promoter (Yagi et al., 2002). As shown in Figure 7A, TGF-β greatly diminished CD3 and CD28-induced c-Myc overexpression in Foxp1+ T cells, while it had negligible effects on identically treated Foxp1-deficient CD8+ lymphocytes. As expected, ERK inhibitors abrogated Myc up-regulation independently of Foxp1 (Figure S5A). To determine whether Foxp1 physically associates with the c-Myc promoter, we performed chromatin immunoprecipitation (ChIP) with specific Abs. As shown in Figure 7B, we observed enrichment of the fragment of the c-Myc promoter containing a reported Smad binding site (Yagi et al., 2002) in Foxp1-DNA precipitates, compared to control pull-downs with an irrelevant IgG. Supporting the specificity of the binding to the c-Myc promoter, no enrichment was found for the sequence of a control (MISIIR) promoter, primarily expressed in Mullerian epithelium (Figure 7B).
Figure 7. Foxp1 is required for TGF-β-induced repression of c-Myc and c-Jun in CD8+ T cells.
(A) Foxp1−/− and WT CD8+ T cells were CD3 and CD28-stimulated (+/−TGF-β1, 5 ng/ml) for 24 h. Proteins were isolated and immunoblotted for c-Myc. (B) Foxp1 binding to the c-Myc promoter. Chromatin was immunoprecipitated with anti-Foxp1 or control IgG from negatively immunopurified mouse CD8+ T cells activated for 24 hours (+TGF-β1; 5 ng/ml). Enrichment of the c-Myc promoter sequence in chromatin IPed with anti-Foxp1 Abs vs. irrelevant IgG (top) and percent of input samples before immunoprecipitation (2.5% gel input values; bottom) were quantified by Real-Time Q-PCR. The mouse MISIIR gene promoter (GenBank#AF092445) was used as an additional negative control. Representative of two independent experiments. (C–D); Western blot analysis of Foxp1−/− and WT stimulated with CD3 and CD28 (+/−TGF-β1, 5 ng/ml) for 24 h for Nfat 2 (C) or for Jun and phospho-(serine 73)-c-Jun (D). Blots were stripped and reprobed with β-actin as an endogenous normalization control. Representative of three independent experiments. (E) ChIP PCR of mouse CD8+ T cells activated for 24 hours (+/−TGF-β1; 5 ng/ml) for c-Jun promoter sequence as described above. Representative of two independent experiments. (F) CD45.2+ wild-type mouse T cells were transduced with murine c-Jun, and congenic CD45.1+ T cells were infected with the empty pBMN-I-GFP retroviral vector. GFP+CD8+ cells were flow cytometry-sorted based upon CD45.1 and CD45.2 after 48 hours and CD3 and CD28-re-stimulated for 24 hours (+/−TGF-β1; 5 ng/ml). Proteins isolated from cell lysates were immunoblotted for total and Ser73 phosphorylated c-Jun. Representative of two independent experiments. (G) Positively c-Jun-transduced CD45.2+ T cells and mocked-transduced CD45.1+ congenic T cells were flow cytometry-sorted based on GPF expression, pooled at 1:1 ratio, rested for one day, and labeled with cell trace violet. Proliferation in response to CD3 and CD28-stimulation for 3–5 days, in the presence or the absence of TGF-β1 (5 ng/mL), was quantified by flow cytometry. Gated on CD8+ T cells. Annexin V and 7ADD staining were performed to discard dead and apoptotic cells. Representative of three independent experiments.
To identify additional Foxp1-dependent mechanisms that explain the superior activity of Foxp1-deficient T cells in the TME, we finally focused on concurrent activation of Nuclear Factor of Activated T cells (NFAT) and AP-1, a coordinated process needed for optimal T cell effector function (Macian et al., 2002). We found that CD3 and CD28-induced NFAT2 up-regulation was not affected by TGF-β signaling or the absence of Foxp1 expression (Figure 7C). In contrast, the expression of total c-Jun, a crucial component of the AP-1 transcriptional complex in T cells (Chen et al., 1998; Li et al., 2012), was increased in TGF-β-treated Foxp1−/− T cells, compared to wild-type lymphocytes (Figure 7D). Most importantly, c-Jun phosphorylation in CD3 and CD28-activated T cells was decreased upon TGF-β signaling, in a Foxp1-dependent manner (Figure 7D). Therefore, Foxp1 is required for TGF-β-induced reduction of the AP-1/NFAT ratio in CD8+ T cells, which is associated with T cell unresponsiveness (Macian et al., 2002). As demonstrated for c-Myc, we observed enrichment of c-Jun promoter sequences in Foxp1-DNA precipitates, suggesting that the TGF-β-induced complex inhibits c-Jun through transcriptional repression (Figure 7E). In addition, enrichment was higher when T cells were activated in the presence of TGF-β (Figure 7E). Together, our results indicate that the Foxp1-mediated inhibitory program triggered by TGF-β includes direct transcriptional suppression of at least two important transcription factors required for optimal T cell activation; namely, c-Myc and AP-1.
Restoring c-Jun expression overcomes Foxp1-dependent TGF-β-mediated inhibition in CD8+ T cells
To determine the unknown consequences of transcriptional repression of c-Jun in T cells, we retrovirally expressed c-Jun in mouse (Foxp1+) wild-type T cells. Sustained CD3 and CD28-mediated T cell activation, which is required for retroviral transduction, resulted in ablation of c-Jun expression in mock-transduced CD8+ T cells. As expected, identically pre-activated, positively transduced lymphocytes, expressed high amounts of c-Jun, and overexpression was unaffected by TGF-β signaling (Figure 7F). Ectopic expression of total c-Jun also resulted in high amounts of phosphorylated (activated) c-Jun, suggesting that transcriptional repression induced by the Foxp1and Smad complex is sufficient to impair AP-1-mediated T cell activation (Figure 7F). Supporting this, c-Jun-transduced CD8+ T cells overcame the inhibitory effects of TGF-β on CD3 and CD28-induced expansion (Figure 7G). Of note, mock transduced CD8+ lymphocytes also exhibited a reduction of their proliferative capacity upon TGF-β-mediated signaling, regardless of the sustained CD3 and CD28 pre-activation required for retroviral transduction (Figure 7G). Taken together, these results unravel an unknown mechanism of CD8+ T cell inhibition whereby Foxp1 represses the transcription of c-Jun, which impairs TCR-induced re-stimulation of CD8+ T cells in the presence of TGF-β.
DISCUSSION
We have identified a mechanism of T cell unresponsiveness driven by up-regulation of Foxp1 in tumor-associated CD8+ T cells. Foxp1 enforces proliferation arrest upon encounter with cognate tumor antigens in CD8+ lymphocytes in vivo. Additionally, Foxp1 impairs T cell effector functions by decreasing cytolytic Granzyme-B and IFN-γ in anti-tumor T cells. These effects are induced by multiple tumor microenvironmental factors that up-regulate Foxp1 in the nucleus of local lymphocytes, thus licensing physical interactions with Smad2 and Smad3 translocated in response to TGF-β signaling. Foxp1 is correspondingly required for the suppressive activity of TGF-β on CD8+ T cells, including transcriptional repression of c-Myc and c-Jun.
Overall, Foxp1 was overexpressed in tumor-infiltrating CD8+ T cells, compared to their counterparts in nearby tumor-free tissue, and regardless of antigenic experience. In the ovarian cancer microenvironment, Foxp1 overexpression occurs at similar concentrations in PD-1+ (recently activated or exhausted) vs. PD-1neg T cells, and also independently of recent activation (CD69 expression). In contrast, Foxp1 overexpression occurred to a lesser extent in breast cancer-infiltrating PD-1+ vs. PD-1− CD8+ T cells. This could reflect a different degree of exhaustion in PD-1+ T cells in the breast vs. the ovarian tumor microenvironment, and/or result from the potentially dissimilar immunogenicity of these malignancies. Elucidating the differences between exhaustion and Foxp1-driven transcriptional programs in different tumors provides exciting targets for future investigations.
We also found that tumor antigen-primed T cells up-regulate Foxp1 after homing to the TME, which is in contrast with sustained Foxp1 down-regulation exhibited by exhausted CD8+ lymphocytes in chronic viral infections (Doering et al., 2012). While multiple inhibitory mechanisms identified in chronic infections also play a role in the suppression of anti-tumor immunity, solid tumors orchestrate different microenvironments. Our results underscore the importance that these differences, along with specific tolerogenic networks in the TME (Cui et al., 2013; Watkins et al., 2011; Zou, 2005), and/or the weaker nature of tumor antigen-specific responses (Wang et al., 2006; Zhu et al., 2013), might have on tumor-induced immunosuppression.
Our results also provide some mechanistic clues to understand how TGF-β inhibits effector T cells in cancer and other pathological settings. Although Foxp1 is clearly involved in additional mechanisms of T cell unresponsiveness, we have identified Foxp1 as a necessary transcriptional co-repressor in the Smad inhibitory complex. Notably, Foxp1 expression does not promote T cell death in vivo. Instead, Foxp1 is required for TGF-β-dependent inhibition of both proliferative and effector (Granzyme-B and IFN-γ production) responses. Inhibition of T cell expansion involves direct transcriptional repression of c-Myc in CD8+ T cells. In addition, different concentrations of c-Jun are important for the intensity of T cell activation in the presence of TGF-β. Foxp1, by directly binding to the c-Jun promoter, represses c-Jun expression but not NFAT, resulting in decreased activated (phosphorylated) c-Jun and impairing T cell activation in CD8+ lymphocytes.
Our study shows that Foxp1-deficient CD8+ T cells have superior anti-tumor effector functions. Although Foxp1-deficient T cell immunotherapy prolonged survival and protected mice from secondary tumor challenges, we failed to recover adoptively transferred cells from tumor free mice. Thus, Foxp1-deficient T cells, besides directly targeting tumor cells, may boost host endogenous anti-tumor immunity (Nesbeth et al., 2009). It’s possible that Foxp1 has unique functions in naïve and memory T cells, such that differentiation and survival of memory T cells may requires Foxp1 expression. However, Foxp1 function in memory T cell differentiation remains unknown.
We mainly focused on CD8+ T cells in this study for several reasons. We observed enhanced persistence and activity of tumor antigen-primed Foxp1-deficient CD8+ T cells, but not CD4+ T cells. Furthermore, proliferation of Foxp1-deficient or WT CD4+ T cells was not affected by TGF-β. However, we acknowledge the potential importance of Foxp1 in CD4+ T cells, especially in generating host effector and memory populations, a focus of our future studies.
The molecular pathways by which Foxp1 impairs the effector activity of TILs demands further investigation, but Smad factors are known to bind to the Granzyme B and IFN-γ promoters (Thomas and Massague, 2005). It is therefore likely that Foxp1 also mediates these suppressive effects. Correspondingly, we found that adoptively transferred tumor-reactive Foxp1-deficient T cells are able to induce the regression of aggressive established tumors, without noticeable toxicity. Foxp1-dependent repression therefore emerges as fundamental mechanism of T cell unresponsiveness, different from other transcriptional programs such as anergy or exhaustion. Ablation of Foxp1 in tumor antigen-primed or chimeric receptor anti-tumor T cells (e.g., through TALEN or CRISPR technologies) could empower lymphocytes to resist immunosuppressive networks in solid tumors, thus allowing more effective clinical interventions.
EXPERIMENTAL PROCEDURES
Mice
Female C57BL/6 and congenic CD45.1+ Ly5.2 mice, aged 5–6 weeks were purchased from the Frederick Cancer Research Facility of the National Cancer Institute. Foxp1f/f and Foxp1f/f Cd4-Cre mice (Feng et al., 2011) were provided by Hui Hu and backcrossed with C57BL/6 mice for 12 generations. dnTGF-βRII mice (Chen et al., 2005; Gorelik and Flavell, 2000), procured from the Jackson Laboratories were used at 6 weeks age. Trp53-Kras (p53/K-ras) double transgenic mice in C57BL/5 background were described earlier (Scarlett et al., 2012). All animals were maintained in specific pathogen free barrier facilities and used in accordance with the institutional animal care and use guidelines of the Wistar Institute. Ovarian and sarcoma tumor-bearing mice were euthanized if they showed ruffled fur; lethargy, anorexia, reluctance to move; ocular discharge; or labored respiration.
T cell tumor antigen priming
T cells tumor-free Cd4-cre+Foxp1f/f (Foxp1-deficient) or Cd4-cre−Foxp1f/f control littermates mice were primed with tumor antigen-pulsed bone marrow dendritic cells (BMDCs) as described (Nesbeth et al., 2009) with slight variations. Briefly, day 6 BMDCs were pulsed overnight with γ-irradiated (10000 rad) and UV-treated (30 minutes) ID8-Defb29-Vegf-a, MPKAS tumor cells or NIH-3T3 fibroblasts at a 10:1 (DC:tumor cell) ratio. For Trp53-Kras flank tumors, DCs were primed with lysates from advanced dissociated tumors subjected to nine quick freeze and thaw cycles. Tumor antigen-pulsed BMDCs were co-cultured with Foxp1-deficient or WT T cells at a 1:10 (DC to T cell) ratio in the presence of IL-2 (10 U/ml) and IL-7 (1 ng/ml) (both from Peprotech) for 7 days. Antigen primed T cells on day 7 were either analyzed immediately (following Cell Trace Violet labeling in some experiments) or transferred into congenic autologous tumor bearing or tumor free mice.
Tumor induction and T cell immunotherapy
ID8-Defb29-Vegf-a ovarian tumors were induced in CD45.1+ congenic female mice as reported previously (Cubillos-Ruiz et al., 2009; Nesbeth et al., 2009). Briefly, 2×106 ID8-Defb29-Vegf-a tumor cells were injected intraperitoneally. Tumor bearing mice received 2×106 antigen primed T cells intraperitoneally on day 24 post tumor challenge and was evaluated for disease progression and survival. ID8-Defb29-Vegf-a flank tumors were induced by subcutaneous injection of 2×106 tumor cells in the axilla. MPKAS sarcomas were induced by injecting 1×105 tumor cells subcutaneously in the flank of 6–8 week old male mice. To induce flank tumors in Trp53-Kras transgenic mice, 108 pfu Adenovirus expressing Cre (Gene Transfer Vector Core, University of Iowa) was subcutaneously injected into the dorsolateral flank. Tumor antigen-primed T cells were intratumorally injected into palpable tumors three times (once per week), followed by measuring of tumor growth.
Immunoprecipitation
HeLa cells were transfected with HA-tagged human Smad2 (Smad2-HA) and Flag-tagged human Foxp1 (Foxp1-Flag) using Lipofectamine 2000 (Life Technologies). After six hours of transfection, cells were treated with TGF-β1 (5 ng/ml) or left untreated for 24 hours. CD3 and CD28 activated human CD8+ T cells were electroporated with human Smad2-HA and human Foxp1-Flag using Amaxa Nucleofector system (Lonza) according to the manufacturer’s protocols. Six hours later, electroporated cells were treated with TGF-β1 for a total of 24 hours. Mouse CD8+ T cells for Foxp1 immunoprecipitation were in vitro stimulated with CD3 and CD28 microbeads (Invitrogen) in the presence of TGF-β1 for 24 hours. Cells were treated with Dithiobis[succinimidyl propionate] (DSP; Thermo Scientific) to cross link proteins, lysed in RIPA buffer supplemented with a protease inhibitor (Roche) and HA or Smad 2 and 3 were immunoprecipitated using Protein A/G agarose (Millipore) using HA.11 (16B12) (Covance) or Smad2/3 (BD Transduction Laboratories) antibodies followed by immunoblotting for Flag (M2) (Sigma Aldrich) or Foxp1. For immunoprecipitating Flag, transfected HeLa cells were cross-linked, lysed in buffer containing 50 mM Tris HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, and 1% TRITON X-100, and IPed with Anti-Flag M2 affinity gel (Sigma Aldrich) as per manufacturer’s instruction. Immunoprecipitated proteins were resolved using SDS-PAGE and immunobloted for Smad2 and Smad3.
Immunoblotting
Cells were lysed in RIPA buffer (Pierce) supplemented with protease inhibitors (Roche) and phosphatase inhibitors (Sigma Aldrich) and 0.1mM Sodium Orthovanadate on ice. Proteins were resolved on SDS-PAGE and immunoblotted. Antibodies to Foxp1 (rabbit polyclonal, (Feng et al., 2011), a gift from Hui Hu), β-actin (Sigma Aldrich), TGF-βRII (K105), Smad2 and Smad3 (Smad2/3; D7G7), phospho-Smad2 (S465/467), phospho-Smad3 (S423/425), c-Jun (60A8), phospho-cJun (Ser73) (D47G9), c-Myc (D84C12), Nfat2 (D15F1) (all from Cell Signaling Technologies), and Flag-HRP (M2) (Sigma-Aldrich) were used for immunoblotting.
ChIP
ChIP assays were performed as reported previously (Yashiro-Ohtani et al., 2009), using rabbit polyclonal antibody to Foxp1. Input DNA and immunoprecipitated DNA were analyzed using the SYBR Green in a real-time PCR machine (Applied Biosystem). The sequence of primers used for promoter quantification were: c-Myc, 5′-CCTCACTCAGCTCCCCTCCT-3′ and 5′ CCCTCCCCTCCCTTCTTTTT-3′; c-Jun: 5′-AGTTGCACTGAGTGTGGCAGAG -3′ and 5′-AAGTCCGTCCGTCTGTCTGTCT-3′. The primers used for the quantification of the MISIIR promoter were 5′-CAGCCGTTAGGAGTTGTTAGGTG -3′ and 5′-ATGGTGTGCAGACATACATGCAG -3′ (all sequences designed to give approximately 80 bp amplicons). Results shown for each ChIP condition were analyzed in two ways: Using the percent input method, the amount of DNA recovered from the ChIP were divided by signals obtained from the input sample (signals calculated with 2.5% of the amount of chromatin used in the ChIP). Using the fold enrichment method, the ChIP signals were divided by the irrelevant antibody signals, representing the ChIP signal as the fold increase in signal relative to the background signal.
Statistical analysis
Mann-Whitney U tests were used for calculating differences between means of experimental groups, and the Logrank test was used when analyzing survival experiments. All statistical analysis was performed using GraphPad Prism 5.0 software. A p value of less than 0.05 was considered statistically significant.
Supplementary Material
Figure S1, related to Figure 1. Tumor microenvironment upregulate Foxp1 on infiltrating CD8+ T cells. (A) Densitometric normalization of Foxp1-expression on CD8+ T cells from tumors and peripheral bloods of human breast, ovarian cancer patients and healthy donors as described in Figure 1A&C. (B) Foxp1 mean fluorescent intensities of intracellular staining of CD3+CD8+ T cells from human breast tumors, tumor free tissues, and matching peripheral bloods of the same patients (p<0.05, Students t-test). (C) Human CD8+ T cells were CD3 and CD28-activated for 3 days, followed by incubation under normoxic or hypoxic (2% O2) conditions for 24 h and immunobloted for Foxp1. Representative of three independent experiments. (D) Mouse CD8+ T cells identically primed against ID8-Defb29-Vegf-a tumor antigens were incubated for 30 h with splenic MDSCs from advanced tumor-bearing mice, tumor ascities-derived CD11c+MHC-II+ DCs, PGE2 (1 μM), Estradiol (1 μM). (E–F) Foxp1-western blots of in vitro CD3 and CD28 stimulated mouse CD8+ T cells incubated with various cytokines as described in supplementary materials and methods. Representative data of 3 to 4 independent experiments. (G) Foxp1-expressions on CD3+CD8+PD1− T cells gated for CD69 from 4 independent human breast tumors and matching peripheral bloods. (H) Intracellular staining for Foxp1-expression in CD3+CD8+ T cells from human breast cancers gated for CD44 and CD69.
Figure S2, related to Figure 2. In vitro and in vivo survival and proliferation of Foxp1-deficient CD8+ T cells. (A) Representative data of Figure 2A shows CD4+ and CD8+ proportions among in vitro tumor antigen primed Foxp1-deficient and WT T cells before (day 0) and after adoptive transfer to ID8-Defb29-Vegf-a tumor (day 7). (B) Representative data for Figure 2C shows enhanced proliferation of tumor antigen primed, Cell Trace Violet labeled, Foxp1-deficient, but not WT CD8+ T cells in the tumor microenvironment. (C) Additional data for Figure 2D showing identical levels apoptosis and cell deaths of in vitro tumor antigen primed Foxp1-deficient and WT CD8+ T cells before transfer (day 0) and 7 days after transfer to the tumor microenvironment. (D) Annexin V and 7AAD staining of tumor antigen primed WT and Foxp1-deficient CD4+ T cells 3 and 7 days after adoptive transfer into ID8-Defb29-Vegf-a tumor bearing mice (E) Data in duplicates showing adoptively transferred, tumor antigen primed WT CD4+ T cells not proliferating in the ID8-Defb29-Vegf-a tumors. (F) Data in duplicate showing tumor antigen dependent proliferation of Foxp1-deficient but not WT CD8+ T cells. ID8-Defb29-Vegf-a tumor or NIH-3T3 fibroblast-derived antigen primed CD8+ T cells on day 7 were labeled with Cell Trace Violet and adoptively transferred into day 24 syngeneic tumor bearing CD45.1+ mice (left) or into the peritoneal cavity of healthy tumor free congenic mice (right). Cells were recovered on day 4 of transfer and analyzed for proliferation. Data representative of three independent experiments. (G) Intracellular IL-2 staining of tumor antigen primed Foxp1-deficient and WT CD8+ T cells 7 days after adoptive transfer into tumor ascities. Representative data of two independent experiments. (H) CD69 expression on tumor antigen primed WT CD8+ T cells 3 days after transfer into ID8-Defb29-Vegf-a tumors. Data representative of three independent experiments.
Figure S3, related to Figure 3. Foxp1 impairs T cell anti-tumor responses. (A) Foxp1f/f Cd4 Cre (n=6) and control Foxp1f/f WT mice (n=6) were challenged with 2×106 ID8-Defb29-Vegf-a tumor cells intraperitoneally followed by observing survival. Data pooled from two independent experiments, P<0.01, Mantel-Cox test. (B) Additional data showing massive necrosis induced by intratumoral administration of Foxp1−/−, but not wild-type tumor-reactive T cells in Trp53-Kras mice challenged with s.c. adenovirus-Cre to induce flank sarcomas as described in Figure 3D. Scale bars 200 μM.
Figure S4, related to Figure 4. Foxp1-enhances CD8+ T cell susceptibility to TGF-β1. (A) Proliferation of Foxp1-deficient or WT T cells in vitro primed with ID8-Defb29-Vegf-a tumor antigens for 6 days, then treated with TGF-β1 (5 ng/ml) for 5 hours. Cells were then labeled with Cell Trace Violet and adoptively transferred into mice bearing day 24 syngeneic tumors. Cells were recovered after 4 days and analyzed for proliferation using flowcytometry. Reprentative data of three independent experiments. (B) Foxp1−/− or WT T cells were labeled with Cell Trace Violet and stimulated in vitro with CD3 and CD28 microbeads (+/− 5ng/ml TGF-β1) for 5 days as described in Figure 4A, surface stained for CD8+ and analyzed for proliferation using flow cytometry. (C) Response of Foxp1f/f CD8+ T cells transduced with MigR1GFP-Cre (Foxp1-deficient CD8+) or control MigR1GFP (Foxp1 WT CD8+) to TGF-β1 treatement as described in Figure 4B. (D) Orthotopic ID8-Defb29-Vegf-a tumor-bearing mice received tumor antigen-primed dnTGF-bRII (n=15) or wild-type T cells (n=15) on day 24 post-tumor challenge. Fifteen additional control tumor-bearing mice received PBS. Data pooled from three independent experiments. P<0.0001, Mantel-Cox test. (E) Foxp1 expression on dnTGFb-RII CD8+ T cells primed in vitro with ID8-Defb29-Vegf-a tumor antigens, recovered from peritoneal wash 3 days after intraperitoneal adoptive transfer into congenic tumor-bearing mice. Data representative of two independent experiments. (F) Foxp1 expression on MPKAS tumor antigen primed CD45.2+ dnTGFb-RII CD8+ T cells treated on day 6 of in vitro priming with 5 ug/ml anti mouse-CXCR-4 or Rat IgG and injected to intratumorally into congenic mice bearing day 10 orthotopic tumors. Drayining lymph nodes were collected three days after T cell injection, stained for intracellular Foxp1 and analyzed by flow cytometry. Data representative of two independent analysis. (G) Survival curves of MPKAS sarcoma-bearing mice receiving tumor antigen-primed dnTGFb-RII T cells pre-treated with neutralizing anti-mouse CXCR4 or control Rat IgG, Foxp1−/−, WT T cells, or PBS (n=6 mice per group).
Figure S5, related to Figure 7. Foxp1-regulation of c-Myc expression in CD8+ T cells. (A) Foxp1-deficient and WT CD8+ T cells were stimulated with CD3 and CD28 microbeads (+/− 5 ng/ml TGF-β1) in the presence or absence of IL-7 (5 ng/ml). For inhibition of ERK activation, MEK/Erk inhibitor U0126 (100 μM) was added to CD8+ T cells after 5 hours of CD3 and CD28 stimulation. Cells were lyzed after 24 hours and analyzed for c-Myc expression by western blotting. Data representative of 2 independent experiments (B) Meassurement of IL-7 amounts in ID8-Defb29-Vegf-a induced tumor ascites (total of 11 ascities samples).
HIGHLIGHTS.
Foxp1 mediates TGF-β-driven transcriptional repression in CD8+ T cells
Foxp1 suppresses anti-tumor T cell effector function in the tumor microenvironment
Foxp1-deficient lymphocytes induce durable rejection of incurable tumors
Foxp1 overexpression in anti-tumor T cells is independent of exhaustion status
Acknowledgments
Support for Shared Resources utilized in this study was provided by Cancer Center Support Grant (CCSG) CA010815 to The Wistar Institute. We thank H. Wang and H. Hu for providing transgenic models, antibodies and technical support, and Yashiro-Ohtani (Pear laboratory) for support with ChIP experiments. This study was supported by R01CA157664, R01CA124515, R01CA178687, U54CA151662, P30CA10815 and awards from the Breast Cancer Alliance (JT) and the Ovarian Cancer Research Fund (JRCG). MJA was supported by T32CA009171. APP was supported by Fundación Alfonso Martín Escudero. AJT was a nested Teal Scholar in DoD grant OC100059.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadzadeh M, Rosenberg SA. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174:5215–5223. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitsch L, Fuertes-Marraco SA, Legat A, Meyer C, Speiser DE. The three main stumbling blocks for anticancer T cells. Trends Immunol. 2012 doi: 10.1016/j.it.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Chen L, Glover JN, Hogan PG, Rao A, Harrison SC. Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifically to DNA. Nature. 1998;392:42–48. doi: 10.1038/32100. [DOI] [PubMed] [Google Scholar]
- Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25:214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Baird JR, Tesone AJ, Rutkowski MR, Scarlett UK, Camposeco-Jacobs AL, Anadon-Arnillas J, Harwood NM, Korc M, Fiering SN, et al. Reprogramming tumor-associated dendritic cells in vivo using microRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72:1683–1693. doi: 10.1158/0008-5472.CAN-11-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, Wang L, Nesbeth Y, Durant Y, Gewirtz AT, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119:2231–2244. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Martinez D, Scarlett UK, Rutkowski MR, Nesbeth YC, Camposeco-Jacobs AL, Conejo-Garcia JR. CD277 is a negative co-stimulatory molecule universally expressed by ovarian cancer microenvironmental cells. Oncotarget. 2010;1:329–338. doi: 10.18632/oncotarget.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, et al. Myeloid-Derived Suppressor Cells Enhance Stemness of Cancer Cells by Inducing MicroRNA101 and Suppressing the Corepressor CtBP2. Immunity. 2013 doi: 10.1016/j.immuni.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkor MK, Sarkar A, Savage PA, Franklin RA, Johnson LK, Jungbluth AA, Allison JP, Li MO. T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-beta1 cytokine. Immunity. 2011;35:123–134. doi: 10.1016/j.immuni.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Wang H, Takata H, Day TJ, Willen J, Hu H. Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nat Immunol. 2011;12:544–550. doi: 10.1038/ni.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick JP, Liberati NT, Waddell DS, Shi Y, Wang XF. Transforming growth factor beta-mediated transcriptional repression of c-myc is dependent on direct binding of Smad3 to a novel repressive Smad binding element. Mol Cell Biol. 2004;24:2546–2559. doi: 10.1128/MCB.24.6.2546-2559.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71:5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- Hamilton SE, Jameson SC. CD8 T cell quiescence revisited. Trends Immunol. 2012;33:224–230. doi: 10.1016/j.it.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks BA, Holtzhausen A, Evans KS, Jamieson R, Gimpel P, Campbell OM, Hector-Greene M, Sun L, Tewari A, George A, et al. Type III TGF-beta receptor downregulation generates an immunotolerant tumor microenvironment. J Clin Invest. 2013;123:3925–3940. doi: 10.1172/JCI65745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Spolski R, Liao W, Wang L, Murphy TL, Murphy KM, Leonard WJ. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature. 2012;490:543–546. doi: 10.1038/nature11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKarns SC, Schwartz RH, Kaminski NE. Smad3 is essential for TGF-beta 1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J Immunol. 2004;172:4275–4284. doi: 10.4049/jimmunol.172.7.4275. [DOI] [PubMed] [Google Scholar]
- Nesbeth Y, Scarlett U, Cubillos-Ruiz J, Martinez D, Engle X, Turk MJ, Conejo-Garcia JR. CCL5-mediated endogenous antitumor immunity elicited by adoptively transferred lymphocytes and dendritic cell depletion. Cancer Res. 2009;69:6331–6338. doi: 10.1158/0008-5472.CAN-08-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbeth YC, Martinez DG, Toraya S, Scarlett UK, Cubillos-Ruiz JR, Rutkowski MR, Conejo-Garcia JR. CD4+ T cells elicit host immune responses to MHC class II- ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells. J Immunol. 2010;184:5654–5662. doi: 10.4049/jimmunol.0903247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med. 2012;209:201–209. doi: 10.1084/jem.20112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003;22:2453–2462. doi: 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209:495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-Regulation of PD-L1, IDO, and Tregs in the Melanoma Tumor Microenvironment Is Driven by CD8+ T Cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284:1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Epler J, Salazar LG, Riddell SR. Recognition of breast cancer cells by CD8+ cytotoxic T-cell clones specific for NY-BR-1. Cancer Res. 2006;66:6826–6833. doi: 10.1158/0008-5472.CAN-05-3529. [DOI] [PubMed] [Google Scholar]
- Watkins SK, Zhu Z, Riboldi E, Shafer-Weaver KA, Stagliano KE, Sklavos MM, Ambs S, Yagita H, Hurwitz AA. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121:1361–1372. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- Yagi K, Furuhashi M, Aoki H, Goto D, Kuwano H, Sugamura K, Miyazono K, Kato M. c-myc is a downstream target of the Smad pathway. J Biol Chem. 2002;277:854–861. doi: 10.1074/jbc.M104170200. [DOI] [PubMed] [Google Scholar]
- Yashiro-Ohtani Y, He Y, Ohtani T, Jones ME, Shestova O, Xu L, Fang TC, Chiang MY, Intlekofer AM, Blacklow SC, et al. Pre-TCR signaling inactivates Notch1 transcription by antagonizing E2A. Genes Dev. 2009;23:1665–1676. doi: 10.1101/gad.1793709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zha Y, Driessens G, Locke F, Gajewski TF. Transcriptional regulator early growth response gene 2 (Egr2) is required for T cell anergy in vitro and in vivo. J Exp Med. 2012;209:2157–2163. doi: 10.1084/jem.20120342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Singh V, Watkins SK, Bronte V, Shoe JL, Feigenbaum L, Hurwitz AA. High-avidity T cells are preferentially tolerized in the tumor microenvironment. Cancer Res. 2013;73:595–604. doi: 10.1158/0008-5472.CAN-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, related to Figure 1. Tumor microenvironment upregulate Foxp1 on infiltrating CD8+ T cells. (A) Densitometric normalization of Foxp1-expression on CD8+ T cells from tumors and peripheral bloods of human breast, ovarian cancer patients and healthy donors as described in Figure 1A&C. (B) Foxp1 mean fluorescent intensities of intracellular staining of CD3+CD8+ T cells from human breast tumors, tumor free tissues, and matching peripheral bloods of the same patients (p<0.05, Students t-test). (C) Human CD8+ T cells were CD3 and CD28-activated for 3 days, followed by incubation under normoxic or hypoxic (2% O2) conditions for 24 h and immunobloted for Foxp1. Representative of three independent experiments. (D) Mouse CD8+ T cells identically primed against ID8-Defb29-Vegf-a tumor antigens were incubated for 30 h with splenic MDSCs from advanced tumor-bearing mice, tumor ascities-derived CD11c+MHC-II+ DCs, PGE2 (1 μM), Estradiol (1 μM). (E–F) Foxp1-western blots of in vitro CD3 and CD28 stimulated mouse CD8+ T cells incubated with various cytokines as described in supplementary materials and methods. Representative data of 3 to 4 independent experiments. (G) Foxp1-expressions on CD3+CD8+PD1− T cells gated for CD69 from 4 independent human breast tumors and matching peripheral bloods. (H) Intracellular staining for Foxp1-expression in CD3+CD8+ T cells from human breast cancers gated for CD44 and CD69.
Figure S2, related to Figure 2. In vitro and in vivo survival and proliferation of Foxp1-deficient CD8+ T cells. (A) Representative data of Figure 2A shows CD4+ and CD8+ proportions among in vitro tumor antigen primed Foxp1-deficient and WT T cells before (day 0) and after adoptive transfer to ID8-Defb29-Vegf-a tumor (day 7). (B) Representative data for Figure 2C shows enhanced proliferation of tumor antigen primed, Cell Trace Violet labeled, Foxp1-deficient, but not WT CD8+ T cells in the tumor microenvironment. (C) Additional data for Figure 2D showing identical levels apoptosis and cell deaths of in vitro tumor antigen primed Foxp1-deficient and WT CD8+ T cells before transfer (day 0) and 7 days after transfer to the tumor microenvironment. (D) Annexin V and 7AAD staining of tumor antigen primed WT and Foxp1-deficient CD4+ T cells 3 and 7 days after adoptive transfer into ID8-Defb29-Vegf-a tumor bearing mice (E) Data in duplicates showing adoptively transferred, tumor antigen primed WT CD4+ T cells not proliferating in the ID8-Defb29-Vegf-a tumors. (F) Data in duplicate showing tumor antigen dependent proliferation of Foxp1-deficient but not WT CD8+ T cells. ID8-Defb29-Vegf-a tumor or NIH-3T3 fibroblast-derived antigen primed CD8+ T cells on day 7 were labeled with Cell Trace Violet and adoptively transferred into day 24 syngeneic tumor bearing CD45.1+ mice (left) or into the peritoneal cavity of healthy tumor free congenic mice (right). Cells were recovered on day 4 of transfer and analyzed for proliferation. Data representative of three independent experiments. (G) Intracellular IL-2 staining of tumor antigen primed Foxp1-deficient and WT CD8+ T cells 7 days after adoptive transfer into tumor ascities. Representative data of two independent experiments. (H) CD69 expression on tumor antigen primed WT CD8+ T cells 3 days after transfer into ID8-Defb29-Vegf-a tumors. Data representative of three independent experiments.
Figure S3, related to Figure 3. Foxp1 impairs T cell anti-tumor responses. (A) Foxp1f/f Cd4 Cre (n=6) and control Foxp1f/f WT mice (n=6) were challenged with 2×106 ID8-Defb29-Vegf-a tumor cells intraperitoneally followed by observing survival. Data pooled from two independent experiments, P<0.01, Mantel-Cox test. (B) Additional data showing massive necrosis induced by intratumoral administration of Foxp1−/−, but not wild-type tumor-reactive T cells in Trp53-Kras mice challenged with s.c. adenovirus-Cre to induce flank sarcomas as described in Figure 3D. Scale bars 200 μM.
Figure S4, related to Figure 4. Foxp1-enhances CD8+ T cell susceptibility to TGF-β1. (A) Proliferation of Foxp1-deficient or WT T cells in vitro primed with ID8-Defb29-Vegf-a tumor antigens for 6 days, then treated with TGF-β1 (5 ng/ml) for 5 hours. Cells were then labeled with Cell Trace Violet and adoptively transferred into mice bearing day 24 syngeneic tumors. Cells were recovered after 4 days and analyzed for proliferation using flowcytometry. Reprentative data of three independent experiments. (B) Foxp1−/− or WT T cells were labeled with Cell Trace Violet and stimulated in vitro with CD3 and CD28 microbeads (+/− 5ng/ml TGF-β1) for 5 days as described in Figure 4A, surface stained for CD8+ and analyzed for proliferation using flow cytometry. (C) Response of Foxp1f/f CD8+ T cells transduced with MigR1GFP-Cre (Foxp1-deficient CD8+) or control MigR1GFP (Foxp1 WT CD8+) to TGF-β1 treatement as described in Figure 4B. (D) Orthotopic ID8-Defb29-Vegf-a tumor-bearing mice received tumor antigen-primed dnTGF-bRII (n=15) or wild-type T cells (n=15) on day 24 post-tumor challenge. Fifteen additional control tumor-bearing mice received PBS. Data pooled from three independent experiments. P<0.0001, Mantel-Cox test. (E) Foxp1 expression on dnTGFb-RII CD8+ T cells primed in vitro with ID8-Defb29-Vegf-a tumor antigens, recovered from peritoneal wash 3 days after intraperitoneal adoptive transfer into congenic tumor-bearing mice. Data representative of two independent experiments. (F) Foxp1 expression on MPKAS tumor antigen primed CD45.2+ dnTGFb-RII CD8+ T cells treated on day 6 of in vitro priming with 5 ug/ml anti mouse-CXCR-4 or Rat IgG and injected to intratumorally into congenic mice bearing day 10 orthotopic tumors. Drayining lymph nodes were collected three days after T cell injection, stained for intracellular Foxp1 and analyzed by flow cytometry. Data representative of two independent analysis. (G) Survival curves of MPKAS sarcoma-bearing mice receiving tumor antigen-primed dnTGFb-RII T cells pre-treated with neutralizing anti-mouse CXCR4 or control Rat IgG, Foxp1−/−, WT T cells, or PBS (n=6 mice per group).
Figure S5, related to Figure 7. Foxp1-regulation of c-Myc expression in CD8+ T cells. (A) Foxp1-deficient and WT CD8+ T cells were stimulated with CD3 and CD28 microbeads (+/− 5 ng/ml TGF-β1) in the presence or absence of IL-7 (5 ng/ml). For inhibition of ERK activation, MEK/Erk inhibitor U0126 (100 μM) was added to CD8+ T cells after 5 hours of CD3 and CD28 stimulation. Cells were lyzed after 24 hours and analyzed for c-Myc expression by western blotting. Data representative of 2 independent experiments (B) Meassurement of IL-7 amounts in ID8-Defb29-Vegf-a induced tumor ascites (total of 11 ascities samples).