Abstract
While the mammalian brain is highly dependent on oxygen, and can withstand only a few minutes without air, there are both vertebrate and invertebrate examples of anoxia tolerance. One example is the freshwater turtle, which can withstand days without oxygen, thus providing a vertebrate model with which to examine the physiology of anoxia tolerance without the pathology seen in mammalian ischemia/reperfusion studies. Insect models such as Drosophila melanogaster have additional advantages, such as short lifespans, low cost and well-described genetics. These models of anoxia tolerance share two common themes that enable survival without oxygen: entrance into a state of deep hypometabolism, and the suppression of cellular injury during anoxia and upon restoration of oxygen. The study of such models of anoxia tolerance, adapted through millions of years of evolution, may thus suggest protective pathways that could serve as therapeutic targets for diseases characterized by oxygen deprivation and ischemic/reperfusion injuries.
Keywords: anoxia, brain, Drosophila melanogaster, heat shock protein, hypometabolism, neuroprotection, oxidative stress, PKG, potassium channel, Trachemys scripta
The mammalian brain without oxygen
The human brain is highly dependent on the presence of oxygen for survival, demanding up to 20% of systemic oxygen, despite comprising only 2% of the body mass. Owing to this high metabolic rate, an interruption in oxygen supply results in a cascade of pathological events that result in brain damage and death. ATP levels decline sharply upon disruption of oxidative phosphorylation, resulting in the cessation of ATP-dependent neuronal processes, including ion transport and neurotransmitter reuptake. Without pumping, ion gradients fail and neurons depolarize, releasing excessive levels of neurotransmitters, including excitotoxic compounds such as glutamate (Glu) and dopamine (DA). Both decreased reuptake, and an increase in vesicular and nonvesicular release contribute to elevated excitatory amino acid levels [1]. Overstimulation of Glu (NMDA and AMPA) receptors increases intracellular Ca2+ levels. This triggers multiple internal cascades that result in cell damage and death, including the activation of lipases, endonucleases and proteases [2], and mitochondrial-dependent apoptosis. Both hypoxia and reoxygenation (such as reperfusion following ischemia) can also result in oxidative stress, with the mitochondria as both the primary source of reactive oxygen species (ROS) and a significant target of ROS damage [3]. Following a hypoxic or ischemic event, ROS damage can continue to develop over a period of days. The brain is especially susceptible to oxidative stress due to its concentration of unsaturated fatty acids, high iron content and low antioxidant capacity [4].
Hypoxia/anoxia tolerant animal models
However, not all animals are equally susceptible to hypoxia, with some even able to tolerate complete anoxia for days to weeks. Animals known to tolerate extended periods of hypoxia without brain damage include hibernating mammals, such as the Arctic ground squirrel [5], diving animals, such as seals [6], the naked mole rat, which inhabits hypoxic underground burrows [7], and neonatal mammals; neonate rats may survive periods of anoxia as much as 25-times longer than adults [8]. More impressively, days to weeks of complete anoxia have been reported in various organisms including the crucian carp Carassius carassius, the fruit fly Drosophila melanogaster and several species of North American pond turtles, such as Chrysemys picta and Trachemys scripta [9]. The study of such animal models of anoxia tolerance, with brains adapted through millions of years of evolution, may thus suggest protective pathways that could serve as therapeutic targets for diseases characterized by oxygen deprivation and ischemic/reperfusion injuries.
The study of alternative animal models reveals survival without oxygen: a rapid suppression of overall metabolic rate, and the abrogation of cellular injury during the anoxic period, as well as upon restoration of oxygen. By entering a deep hypometabolic state, anoxia-tolerant organisms are able to reduce the energy demand to meet the reduced supply of anaerobic glycolysis, and thus prevent anoxic depolarization and the subsequent apoptotic cascade [9]. At the same time, protective pathways are strongly upregulated and potentially pathological mechanisms are simultaneously suppressed, protecting cellular integrity against the stressors of reduced energy supply, acidification and (upon reoxygenation) oxidative stress. A recent survey of mammalian studies suggests the benefits of defining these protective responses, as ischemic tolerance also appears to involve both increased neuroprotective signaling and decreased inflammatory or apoptotic pathway activation [10], along with gene reprogramming and metabolic downregulation.
Indeed, the therapeutic potential of rapid downregulation of basal metabolism has been widely investigated for ischemic events in mammals. Induction of hypometabolism lies behind the rapid cooling and induction of coma for perinatal asphyxiation and myocardial infarction, as well as pretreatment prior to cardiac surgery [11]. This is also the basis for the groundbreaking work of Blackstone et al. with H2S induction of hypometabolism in mice [12,13], as well as current work with injectable hydrogen saline, which acts to upregulate protective pathways and as an antioxidant [14]. However, in mammalian models, protective and pathological mechanisms are activated simultaneously, making it difficult to distinguish between them. On the other hand, in animals adapted for extended anoxia, pathological mechanisms are effectively suppressed, while protective pathways are greatly enhanced.
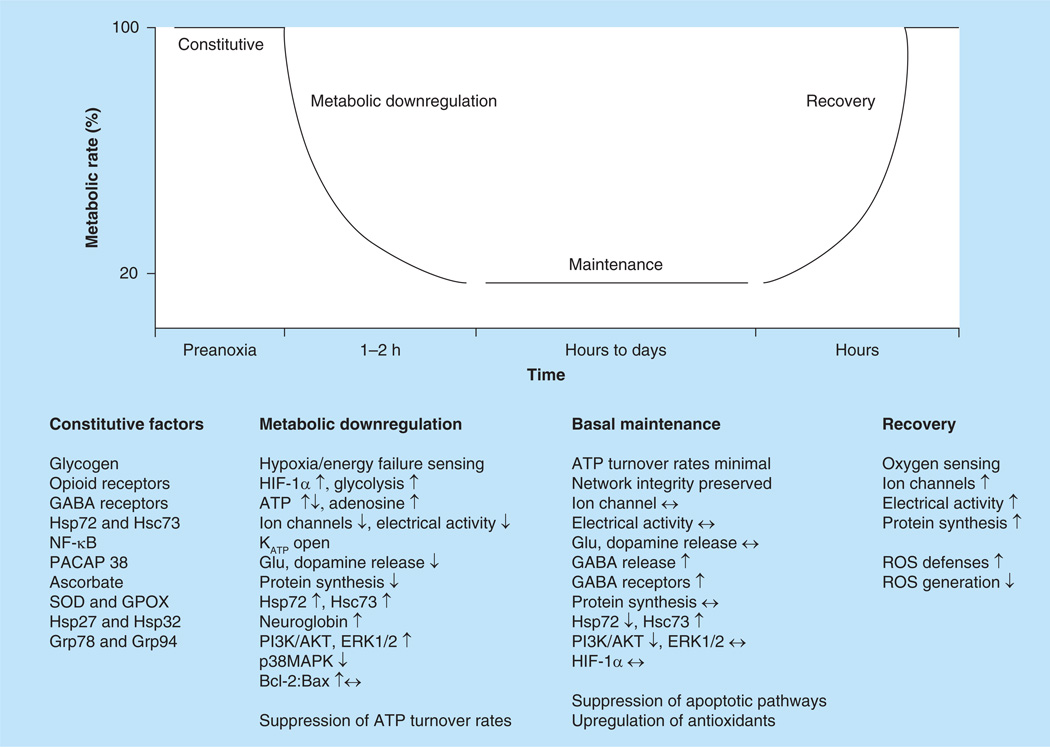
One model organism that is able to withstand days (at room temperature) to weeks (at 3°C) of complete anoxia is the pond turtle; both T. scripta (the red-eared pond slider) and C. picta (western painted turtle) have been widely studied. These turtles hibernate underwater in lakes that may freeze over for extended periods in the winter, and therefore the animals are unable to surface for air until the spring thaw. The physiology of anoxia tolerance in these organisms has been under investigation for over three decades, with recent work moving from neural physiology to molecular studies, genomics and epigenetics (summarized in Figure 1).
Figure 1. Updated overview of some of the factors involved in anoxic survival in the freshwater turtle.
Constitutive factors that predispose for anoxia tolerance include enhanced glycogen stores, constitutive production of heat shock proteins, increased densities of protective receptors and elevated antioxidant capacity. A coordinated downregulation of ATP demand to approximately 20% of basal involves NMDA, AMPA and ion channel arrest, a reduction in protein synthesis, alterations in neurotransmitter release and increases in cellular protective pathways including PI3K/AKT, ERK and heat shock proteins. Network integrity is preserved through the maintenance phase by the continued low level release and reuptake of neurotransmitters, protection against apoptosis and a further upregulation of some antioxidants. Upon reoxygenation, electrical activity and protein synthesis return over a period of hours, while certain antioxidants are further upregulated; excess ROS formation is simultaneously suppressed.
↑: Increase; ↓: Decrease; ↔: Remains the same; Glu: Glutamate; GPOX: Glutathione peroxidase; KATP: ATP-dependent K+ channels; ROS: Reactive oxygen species; SOD: Superoxide dismutase.
Adapted from [115].
Adaptations for anoxic survival in the brain of the turtle T. scripta
The most critical initial phase of anoxic survival is a rapid downregulation of energy demanding processes to match the reduced energy supply; this is achieved through decreased membrane ion permeability (channel arrest), decreased excitatory neurotransmitter release and increased inhibition through the release of GABA [15] and adenosine [16]. The result is a reversible ‘coma’ of curtailed electrical activity [17]. As more than 50% of the brain’s energy demand arises from ion pumping, significant energy savings are obtained by the suppression of both ion leak and through the reduction of action potentials, which in turn reduces the energy needed to maintain homeostasis. Such ‘channel arrest’ significantly decreases Ca2+ permeability [18] and the density of voltage-gated Na+ channels [19], and is seen in both reduced K+ flux [20] and the anoxia-linked decrease of voltage-dependent K+ channel transcription [21]. Ca2+ flux i s a lso r educed b y t he downregulation of glutamatergic NMDA receptors (NMDARs) and AMPA receptors [18,22]. The depression of glutamatergic signaling is coupled with a more than 80-fold increase in GABA release [15] and increased GABAA receptor density [23]. Reduced glutamatergic signaling and increased GABA-ergic responses together induce ‘spike arrest’, increasing the stimulation required to elicit an action potential in the turtle brain by more than 20-fold [24].
Initial decreases in both Glu and excitotoxic DA release, which occur during the first 1–2 h of anoxia, are linked to increases in adenosine and the activation of ATP-dependent K+ channels (KATP) [25–27]. Inhibition of excitatory neurotransmitter release both reduces the likelihood of anoxic depolarization and reduces the expense of neurotransmitter reuptake, estimated to be as high as 1.5 ATP/molecule [28]. The temporary increases reported in adenosine release and the opening of KATP in the turtle brain are probably due to an initial relatively small, but significant, 20% decrease in brain ATP [29]. However, maintenance of normal brain ATP levels appears critical to brain survival over the long term. Interestingly, long-term anoxia in the turtle brain is marked by both high ATP levels and the continued release and reuptake of Glu and DA (albeit at reduced levels), which keep extracellular levels constant. The continued release and reuptake of excitatory neurotransmitters suggests that maintaining normal synaptic levels, despite an overall suppression of brain function, is a key factor of anoxic and perhaps postanoxic survival, and could explain the repeated failure of the numerous clinical interventions for ischemia that interfere primarily with Glu receptors. In a mammalian cell culture model, experiments with antagonists, as well as low doses of NMDA, in fact suggest a role for Glu in the development of ischemic tolerance [30]. It has also been recently hypothesized that the differences between ischemic tolerance and cell death may be related to NMDAR subtypes, with synaptic NR2A receptor stimulation increasing survival, while extrasynaptic (primarily NR2B) stimulation leads to cell death [10]. In severe ischemia, both receptor subtypes would be activated, and pathological effects would overwhelm any protective mechanisms, such as the activation of the protective transcription factor CREB by Glu. NMDAR subtypes have not been investigated in the turtle.
KATP channel activation appears to play a critical role in anoxic survival, both in channel arrest and in the modulation of neurotransmitter release. The 52% decrease in anoxic AMPA receptor currents, for example, is abolished by mitochondial KATP (mKATP) antagonists [31], as is the 50% decrease in anoxic NMDAR currents [32]. The reduced efflux of K+ in early anoxia is also mediated in part by the opening of KATP channels [33], and blocking KATP together with adenosine receptor blockade increases both Glu and DA release in the early anoxic turtle brain [26,27]. As ATP demand is suppressed to meet reduced energy delivery, however, ATP levels return to basal, KATP channels close and other mechanisms function to maintain reduced brain function, including the aforementioned GABA increases [34] and an upregulation of protective pathways.
In mammals, KATP channel activation results in an initial protective hyperpolarization [35], but eventually leads to anoxic depolarization. By contrast, a combination of protective strategies in the turtle brain allows the initial opening of KATP channels to be translated into longer-term protection. Zivkovic and Buck suggest that the increased K+ conductance [31], which mildly uncouples mitochondria [32], slightly increases cytoplasmic Ca2+ levels due to the decreased driving force for Ca2+ uptake into the mitochondria. This cytoplasmic Ca2+ increase, in turn, reduces AMPA receptor and NMDAR currents. Further Ca2+ increases would thus be prevented, blocking excitatory neurotransmitter release, as seen with DA and Glu. In mammals, it has been similarly suggested that mKATP channels decrease mitochondrial Ca2+ overload, thus preventing the formation of the mitochondrial pore and induction of apoptosis [36]. Thus, a temporary activation of KATP channels, particularly of mitochondrial KATP channels, would appear to be an area of interest in the development of potential therapeutic targets. Indeed, mKATP channel activation has been widely investigated in the development of neuroprotective preconditioning (PC) responses.
Anoxia tolerance & ischemic PC share common mechanisms
PC is a well-known phenomenon in mammals, described as “an innate protective and adaptive mechanism, whereby a sublethal insult protects against a subsequent lethal insult” [36]. Often investigated with a sublethal ischemic event inducing a later, longer tolerance, PC has multiple, diverse stimuli and effector mechanisms, resulting in cross-tolerance to other stressors as well. In both the heart and brain, PC induces a two-stage protection; initial brief (minutes to hours) changes are wrought by alterations in the activity and post-translational modifications of existing proteins. A ‘second window of protection’ occurs hours to several days later as a result of gene induction and de novo protein synthesis. While the multifactorial aspects of mammalian PC are beyond the scope of this review [36,37], much work suggests the mitochondria as key regulators of endogenous neuroprotection owing to their roles in energy generation, Ca2+ regulation, redox signaling and as arbiters of apoptosis or cell survival [38]. In this scenario, mitochondrially generated ROS and mKATP play key roles in PC. Pharmacological stimulation of mKATP depolarizes mitochondria, increases ROS and activates the protein kinase C pathway, inducing the PC phenotypes [39]. Low ROS levels themselves are able to induce protection, possibly through the activation of hypoxia-inducible factor (HIFs) and subsequently erythropoietin [40], while ROS scavengers abolish protection [41].
The role of HIF in PC is the subject of a great deal of research, and has been reviewed elsewhere [42,43]. HIF is considered to be a master regulator of hypoxia responses; when oxygen levels are low, HIF-1α is stabilized and translocates to the mitochondria, where it heterodimerizes with HIF-1β to become HIF. The binding of HIF to hypoxia response elements on numerous genes stimulates responses concerned with oxygen delivery and energy metabolism. These responses involve up to 200 genes for angiogenesis, erythropoiesis, antiapoptosis, free radical production/scavenging, necrosis and stem cell differentiation [44]. Cellular PC through HIF upregulation has been extensively investigated in the neonate model of hypoxia/ ischemia [42], but is also being examined in the heart [45,46], brain [47], kidney [48] and liver [49].
HIF activation has also been investigated in mammalian models of hypoxic PC (HPC), as it relates to the activation of δ-opioid receptors (DORs). The δ-opioid (d-Ala 2 and d-Leu 5) enkephalin has been implicated in hibernation and neuronal survival, and reduces the neuronal damage induced by ischemia/reperfusion [50]. DORs are known to play a role in the resistance to Glu and hypoxic stress in mammals [51,52], with a recent study by Gao et al. reporting that the improved neurological outcome and decreased apoptosis of HPC in the rat were attenuated by DOR antagonists [53]. Both DOR activation and the HPC induction of HIF were decreased when HIF was knocked down with siRNA, suggesting a link between the two responses. In turtles, the potential for DOR effects was first suggested by Xia and Haddad, who reported that receptor density was more than four-times higher in the turtle cortex compared with the rat, despite the significantly lower basal metabolic rate of reptiles [54]. Furthermore, as has been shown in mammalian HPC, Glu excitotoxicity in the anoxic turtle is also suppressed by DOR activation, as DOR blockade potentiates NMDAR currents, increases cytosolic Ca2+ levels and induces anoxic depolarization [55].
However, HIF activation in the brain may be a double-edged sword, in that HIF activation also leads to edema, due to the increased permeability of the blood–brain barrier [42]. Thus, while HIF in general is strongly upregulated by hypoxia, hypoxia tolerance is maintained in HIF-1α-deficient mice, and mice with a neuron-specific HIF-1α deficiency had decreased ischemic injury after transient global ischemia, rather than increased damage [56]. Recent work in the authors’ laboratory, in fact, has demonstrated that while HIF-1α protein levels were readily detected in the normoxic turtle brain, no further increase occurred during anoxia [Milton SL, Unpublished Data]. Nuclear levels of the HIF-1α protein decreased significantly in anoxia, which was reflected in the decreased binding activity in anoxia evidenced by an eletrophoretic mobility shift assay. Furthermore, induction of HIF-1 through a small molecule activator, tilorone, signif icantly increased cell death during anoxia in cultured turtle neurons. This anoxic suppression of HIF-1α nuclear translocation may be a key factor allowing metabolic downregulation and anoxic survival in the freshwater turtle, since many of the genes upregulated by HIF increase energy flux and delivery, in marked contrast to the adaptations of true anoxia tolerance, which result in profound hypometabolism. Even glycolytic enzymes are downregulated in T. scripta, for example, as part of the general metabolic depression [57], with recent work suggesting that phosphorylation and acetylation result in less active enzyme forms [58]. DNA microarray studies have shown that while mammalian PC is associated with an upregulation of certain genes, including heat shock proteins (HSPs), the antiapoptotic Bcl-2 and others that increase survival [59], nearly as many genes were likewise downregulated, including those associated with metabolic pathways, iron transport and immune responses, leading Yenari et al. to suggest that metabolic downregulation is the common pathway of neuroprotection seen in PC, hibernation and hypothermia [60].
Key survival genes upregulated in the turtle include HSPs, MAPKs, the antiapoptotic Bcl-2 and a variety of antioxidants, including neuroglobin. Since many of these compounds are present in the turtle brain even under normoxic conditions, it has been suggested that the animals are in a state of ‘constitutive PC’ [61], with detectable levels of Hsp72, Hsp27 and Hsp32 (HO-1), and Hsp60, as well as the glucose-regulated proteins Grp78 and Grp94 [62]. Unlike the delayed response seen in mammalian ischemic models, Hsp27, Hsp72 and HO-1 all increase rapidly in anoxia, with significant changes by 1 h, and further increases over 24 h of anoxia. Interestingly, the stress proteins that are most enhanced are those associated in mammals with glia (although the cellular location of the HSPs in turtles has not yet been investigated), suggesting a larger role for glia in the maintenance of neuronal integrity than has been previously noted [62]. Reoxygenation did not further increase the HSPs, adding further support to the idea that the turtles do not undergo significant oxidative stress following anoxia/reoxygenation (see below). Mammalian work with hsp70 knockout mice [63] and gene transfer of hsp70 [60] has shown a role for HSPs in ischemic protection, although there appears to be no direct evidence for the involvement of Hsp70 in ischemic tolerance [10].
Another comparable response between anoxia in the turtle and ischemia in the mammalian brain is activation of the MAPK and the serine–threonine kinase (PI3K/AKT) signaling pathways. Both proapoptotic pathways (JNK and p38MAPK) and ostensible survival pathways (ERK and AKT) are activated simultaneously in response to cellular stress in mammalian cells [32]. The turtle brain, however, again shows a robust protective response; ERK and AKT are strongly upregulated in the anoxic brain, but with a marked suppression of p38MAPK [64]. In mammalian models, DOR-mediated HPC protection has also been related to increases in ERK and Bcl-2 activity, thus counteracting detrimental hypoxic increases in p38MAPK activity and cytochrome C release [65,66]. Links between DOR and the MAPKs have not been investigated in the turtle, although MAPK responses have been linked in vivo and in vitro to adenosine receptor stimulation [64,67], and adenosine A1 receptor (ADR) blockade increases cell death [67]. While ERK activation has been linked to both neuroprotection and cell death in mammals, related possibly to the extent and timing of ERK activation, the turtle in vitro shows a strong, long-term upregulation of ERK. This suggests that it may be other factors that trigger cell death in mammalian models over the long term, possibly overwhelming any protective effects of ERK [67].
Protective signaling through ERK and AKT is thought to occur by altering apoptotic signaling, decreasing Bad and increasing CREB phosphorylation and activation of Bcl-2, and thus inducing a protected phenotype. Furthermore, robust cellular protection can be seen in the anoxic turtle, with little change or a slight increase in Bcl-2–Bax ratios [62,67]. In addition to its direct antiapoptotic role, Bcl-2 has been demonstrated to have a major role in suppressing cell death during times of oxidative stress by enhancing levels of antioxidants and suppressing the generation of free radicals [68]. In the turtle, a greater induction of Bax over Bcl-2 occurred when ADR were blocked [67]; and ADR blockade also increases ROS production and cell death [69], thus implying that Bcl-2 and ADR activation are key elements of ROS suppression.
Adaptations for reoxygenation
As a significant portion of ischemic brain damage occurs from ROS damage upon reperfusion, preventing ROS increases is a key factor in decreasing stroke morbidity. The freshwater turtle model has been shown to have little accumulation of lipid peroxidation damage products such as thiobarbuturic acid reactive substances following anoxic submergence and recovery [70], due in part to surprisingly high constitutive levels of antioxidants in some cases. In the case of superoxide dismutase and glutathione peroxidase, enzyme activities in the turtle liver are comparable with mammalian levels [71], while ascorbate levels in the Trachemys brain are equal to that of mammals, despite much lower overall metabolic rates in the turtle [72]. Interestingly, increases in antioxidants in mammalian ischemia/reperfusion studies have been linked to DOR activation [73], although links in the turtle between surprisingly high DOR levels and antioxidants have not been investigated.
While its role is not yet understood, the recently discovered heme protein Ngb is also constitutively present in T. scripta [74], where mRNA and protein levels increase significantly in hypoxia [75,76]. Ngb has potential roles as an oxygen storage protein or as an antioxidant, and increasing evidence suggests it could be an endogenous neuroprotective molecule against hypoxic/ischemic and oxidative stress-related insults in cultured neurons and animal models, as well as in neurodegenerative disorders such as Alzheimer’s disease [77].
Along with high levels and further upregulation of antioxidant systems both during anoxia and upon reoxygenation, the freshwater turtle has an apparently inherent ability to suppress ROS formation upon the reintroduction of systemic oxygen [69,78]. Work in the author’s laboratory suggests that this suppression is related to elevated Hsp72 levels directly, as siRNA knockdown of Hsp72 significantly increases H2O2 release in cell cultures undergoing anoxia/reoxygenation [Milton SL, Unpublished Data]. Similarly, Ma et al. recently demonstrated that overexpression of the recently described Hsp70 member HSPA12B decreased infarct volume and improved outcomes following focal ischemia in mice [79], while targeted overexpression of Hsp72 or SOD2 in rat brain astrocytes reduced oxidative stress [80]. Together, the strong upregulation of antioxidants and the suppression of excess ROS formation suggest that surviving reoxygenation is a critical stage of anoxia tolerance, even in these highly adapted animals.
Invertebrate models of anoxia tolerance
Invertebrates, such as insects, have also evolved a number of adaptations to deal with unpredictable and extreme environments, including the lack of oxygen. Some insects thrive at extreme elevations (~6800 m) where atmospheric oxygen is below 10%. Even in acute or unpredictable circumstances such as flooding and rainfall, numerous insect species can survive 0% oxygen for several hours, and, like the turtle, can survive anoxia for days to weeks at colder temperatures [81]. For example, larvae of the tiger beetle, Cicindela togata, survive anoxia due to flooding for over 6 days at 25°C through metabolic depression [82]. Furthermore, the adult fruit fly, D. melanogaster, can survive 4 h of complete anoxia with no mortality [83]. Owing to the ability of these insects to adapt to such environments and their genetic and neurophysiological homology to humans, these model organisms can be used to discover novel genes and pharmacological agents for anoxic neuropathologies, such as spreading depression, traumatic brain injury or stroke.
D. melanogaster have been used as a model organism in the scientific community for over a century. They are inexpensive to maintain, have an extremely short life cycle from embryo to adult (10 days at 25°C) and have a very accessible genome that is highly homologous to humans both genetically and physiologically at the cellular level. For instance, approximately 70% of all genetic diseases in humans have a homologous gene in D. melanogaster [84], and numerous genetic tools have been developed to allow for the enhanced stability and easy manipulation of the genome in any cell and at any given time [85]. Owing to the ease and ability to genetically manipulate these animals, they have become ideal models for examining the roles of specific genes, gene families and pharmaceuticals (in a genome-dependent manner) in disease, including anoxia. Experimenters also have the ability to control for life history of the animal (environmental factors e.g., rearing density and circadian environment), and even more importantly, age. Since a fruit fly’s lifespan is approximately 70 days (maximum 120 days under specific genotypes), one can examine the development of age-related diseases, such as peri-infarct depolarizations and spreading depression in the nervous system [86]. Unlike mammalian model systems, fruit flies can be used to screen for genes and/or pharmacological compounds resulting in neuroprotection from anoxic stress [87–89].
A number of factors can be examined in these animals that relate to hypoxic stress, including development [89], genetic adaptations [89,90], behavior and locomotion [87,90,91], synaptic physiology [69], cellular homeostasis [86,92,93] and survival [83,87,90]. Such changes can be easily observed and screened under varying genetic and pharmacological treatments to uncover novel molecules that may enhance or arrest such phenotypic changes due to hypoxia relatively rapidly and inexpensively. Such is the case with D. melanogaster that were evolved in the laboratory over 32 generations to survive at very low levels of oxygen; these hypoxia-selected lines behave normally at 4% oxygen, but also exhibit decreased body size of approximately 50% from their counterparts reared at 21% oxygen [69]. Interestingly, these evolved lines also behave normally when brought back to normoxic conditions, and their body size after a single generation returns to that of naive flies. It was discovered through the use of microarrays that the genome of these laboratory lines evolved at 4% oxygen had changed significantly, thus giving insight into genes that may enhance an organism’s ability to survive extremely low oxygen environments [69]. For example, upregulated genes in the tolerant flies included signal transduction pathways (e.g., Notch and Toll/Imd pathways), while some metabolic genes were downregulated.
In addition to the ease of laboratory manipulation, populations of D. melanogaster vary in their natural ability to tolerate environmental stressors such as high temperature [94–96] and low oxygen levels [87,90,91]. Owing to this, researchers can exploit such variation to discover molecular pathways responsible for hypoxia tolerance. For instance, natural polymorphisms of specific genes conferring hypoxia tolerance on flies exist within the same population. Wingrove and O’Farrel [90] first demonstrated that the for gene encodes a protein kinase G (PKG) and has two natural alleles; high PKG (named rover) and low PKG (sitter), which differ in their hypoxia tolerance. It has been demonstrated that these alleles exist in populations generally at 70% rover and 30% sitter [96–98]. In the D. melanogaster larval stage under acute hypoxia, rovers survived significantly longer under hypoxic conditions than sitters [90]. This pattern of survival was also demonstrated in the adult stage where high PKG animals survived significantly longer than low PKG animals under acute hypoxia [87]. Knowing that natural variation exists for hypoxia tolerance in single populations, techniques such as quantitative trait loci mapping have the potential to allow for efficient hypoxia tolerance screens [99].
Neuronal depression as a mechanism of anoxic survival
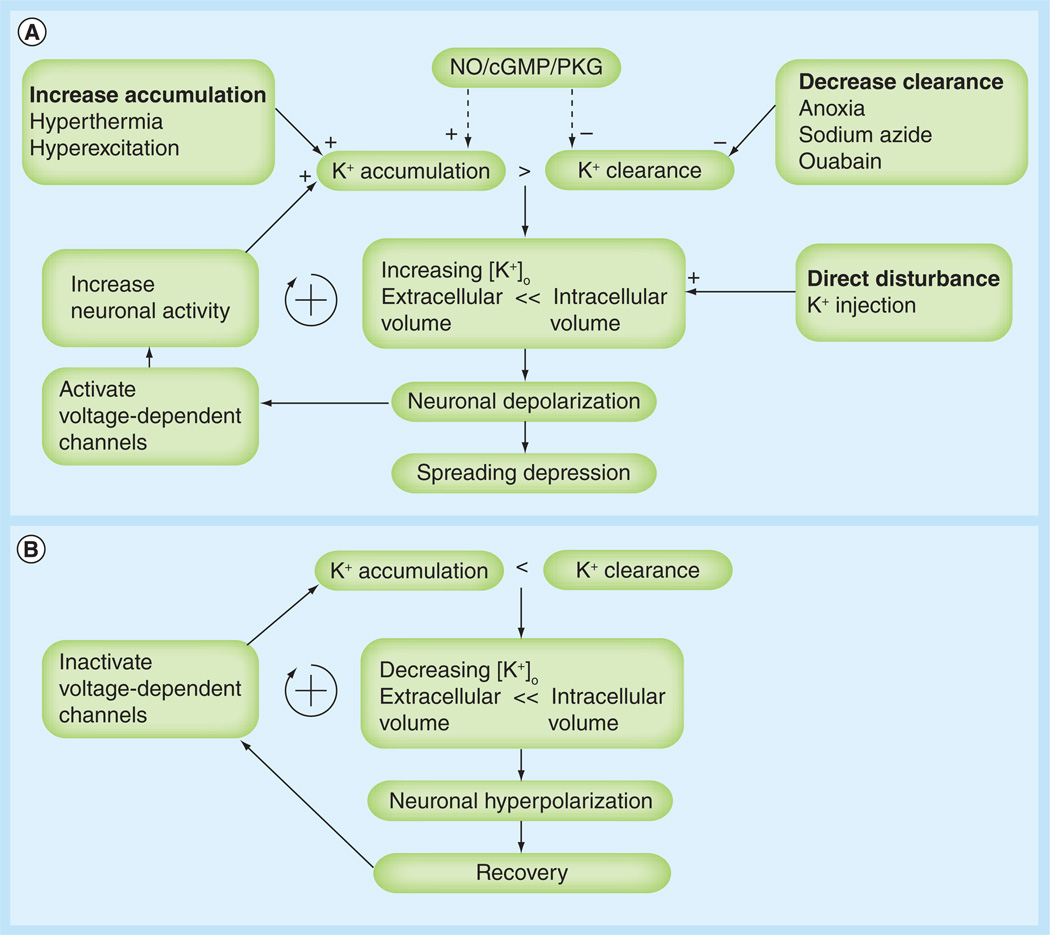
Similar to the suppressed electrical activity seen in anoxic turtles, insects including D. melanogaster lapse into a quiescent state resembling a coma during extreme oxygen deprivation, which is easily measured visually or through automation [83,87,90,91]. Losing function quickly to enter the coma-like state is reflected in greater survival over long-term anoxia [83,87,100]. This behavioral observation in the fruit fly reflects that of turtle neurons exposed to acute anoxia, which also become reversibly unresponsive [17]. This correlation between reversible neuronal hypoactivity and survival is probably indicative of the high-energy dependence of neuronal activity, and the need for aerobic respiration for normal function. Investigation into the physiological processes that result in the inactivity observed in both behavior and neural function in the fly revealed that during anoxia a large increase in extracellular K+ (from 10 to 60 mM) occurs in the neuropil of adult Drosophila [93]. When the animal or tissue is returned to normoxia, this alteration in ion balance recovers back to basal conditions, and both neuronal signaling and behavior resumes [92,93]. Similarly, spreading depression-like events occur in the locust in response to heat or anoxic stress, and these are characterized by an abrupt increase in extracellular K+ concentrations [101]. Unlike mammalian systems, however, neurons were only partly depolarized. This raises the question of why these insects, and potentially animals in general, adapted to destabilize cellular homeostasis in response to anoxic stress. It is worth noting that this physiological response to anoxia similarly occurs in insects exposed to hypothermic and hyperthermic stress [92,93,102]. When examining the common links between these stressors in insects and in the anoxic turtle brain, it is clear that a key factor is maintaining the balance between ATP supply and demand [9], as the Na+/K+ ATPase in the human nervous system consumes approximately two-thirds of all ATP produced [103]. It has been shown that the loss of this pump results in a similar increase in extracellular K+, and therefore may trigger this hypometabolism [86]. Understanding the physiological processes involved in anoxic stress and tolerance allows for potential screens that could delay K+ imbalances and, hence, functional loss during anoxic stress at the level of single cells (Figure 2). Conversely, a rapid, but milder, depolarization may lower cellular energy demand and, thus, increase the likelihood of long-term survival.
Figure 2. Spreading depression-like events in the locust metathoracic ganglion.
(A) Onset: spreading depression-like event is evoked when extracellular K+ accumulation exceeds clearance under conditions when [K+]o can appreciably affect the K+ equilibrium potential and cause neuronal depolarization, thus entering a positive feedback cycle (+). This can be induced by increasing accumulation, decreasing clearance or directly manipulating [K+]o. (B) Recovery: if cessation of neural activity is sufficient to enable K+ clearance to predominate, then the system enters a positive feedback cycle that restores [K+]o and membrane potentials to prespreading depression-like event levels.
Dotted arrows denote a pharmacological effect.
[K+]o: Extracellular K+ concentration; cGMP: Cyclic GMP; NO: Nitric oxide; PKG: Protein kinase G.
Reproduced with permission from [101].
As in mammals, neuronal protection in insects results from PC, although in such cases both function and long-term survival may be protected. Heat shock (HS) is one such stressor that results in the PC phenotype, although the neurophysiological consequences resulting from the upregulation of HSPs are unclear. One mechanism that has been described occurs in neurons of the insect Locusta migratoria, where a prior HS of 45°C for 3 h and a 1-h recovery leads to the transcription and translation of a number of HSPs with a concomitant reduction in whole-cell K+ conductance in the brain [102,104]. The whole-cell peak current (A current) of the K+ channels is not disturbed, while the prolonged transient current (B current) is significantly reduced [105]. In D. melanogaster, similar PC results in both prolonged behavior (delayed coma) [95] and continued synaptic transmission [86,95] under acute hyperthermia.
The problem, of course, is that utilizing HS or HSPs for human therapeutics is not a tenable option, since the HS response is required prior to the time of insult, while in humans a strokelike injury, traumatic brain injury and cortical spreading depression are generally not predictable. Since K+ channel modulation correlates with neuroprotection due to anoxic insult, other molecules that can alter K+ channels may have therapeutic potential. The direct modulation of K+ channels for use in human therapeutics has been a goal of medical research for years, since a number of pathologies are associated with the ion channels. However, as these proteins cannot be altered significantly without detrimental changes in normal physiological functioning, this approach has not proved an appropriate pharmacological target. There are a number of molecules that operate upstream from the K+ channel axis, however, that would allow for larger amounts of variability with a strong impact on K+ channel function. One such protein under investigation is the previously mentioned PKG, encoded in flies by the for gene.
Manipulation of the PKG pathway alters anoxia tolerance
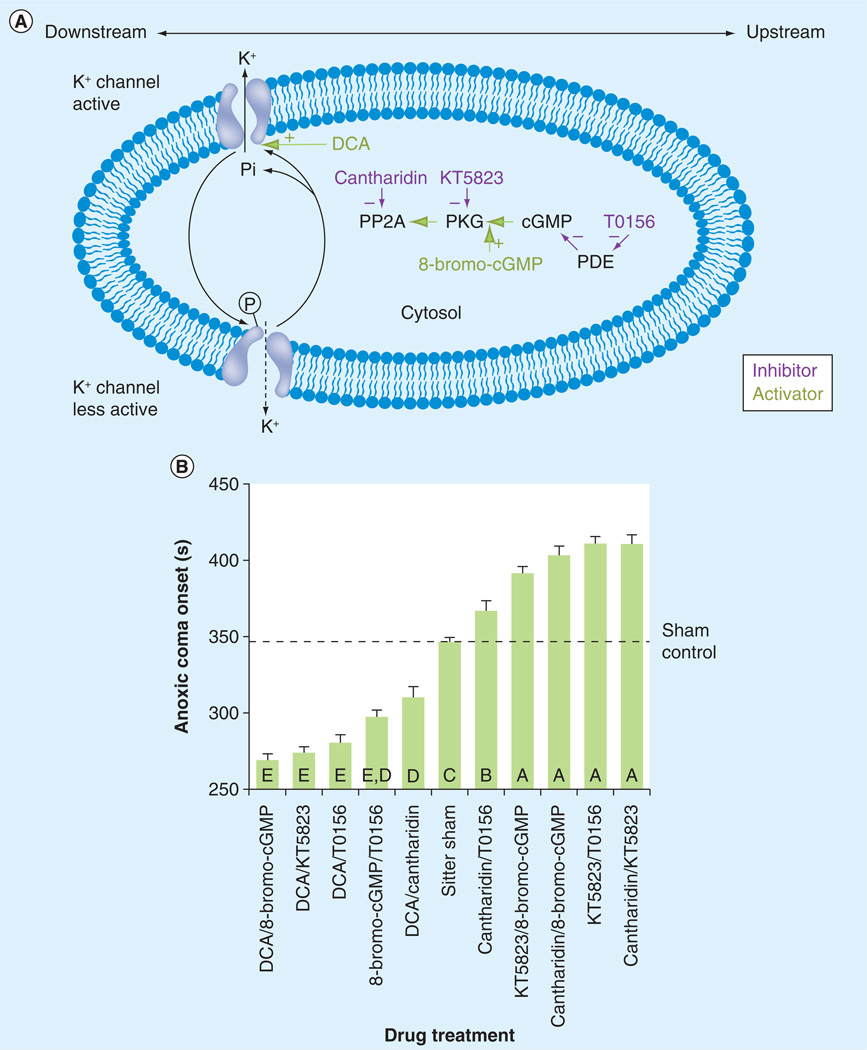
Cyclic GMP-dependent PKG plays a significant role in a number of organisms to control feeding behavior, metabolism, learning and memory, and stress tolerance [106,107], and the for gene in flies has significant homology to the human PKG gene PRKG1 [108]. PKG participates in a molecular pathway that modulates K+ channel conductance, where increased PKG activation or expression increases K+ channel conductance, and the inhibition of PKG activity or expression results in a reduced conductance (Figure 3) [109]. Interestingly, the reduction in whole-cell K+ channel conductance from PKG inhibition mirrors the K+ conductance reduction observed in insects that have undergone a protective HS [105,109]. For example, the spreading depression-like events of HS or anoxic stress in locusts are suppressed by pharmacological inhibition of the nitric oxide (NO)/cyclic GMP/PKG pathway [101]. Fruit flies that have lower PKG expression due to genetic variation or by modulating activity through pharmacological treatment show prolonged activity in response to acute anoxia [87]. Similarly, when PKG is pharmacologically inhibited by PKG pathway blockers, the locust nervous systems exposed to acute anoxia exhibit neural protection through extended function and a delay in the increase in extracellular K+; modulating ion channels in this fashion strengthens the membrane’s ability to maintain homeostasis [102,110]. This ‘ionic fortification’ via K+ channel conductance inhibition is of importance since it is now possible to mimic the K+ channel conductance dynamics of a heat shocked animal (upregulated HSPs) via an acute pharmacological treatment in only seconds, whereas natural induction after HS takes hours [105,109].
Figure 3. Combinations of pharmacological agents that modulate time to anoxic coma onset during acute hypoxia reveal downstream and upstream molecular targets in the cyclic GMP-dependent protein kinase G pathway.
(A) This diagram and experimental design represent upstream and downstream intracellular targets for manipulating the PKG pathway partially implicated in the modulation of hyperthermic stress [104,116]. PP2A, PKG, cGMP, PDE and K+ channels are shown as potential targets for pharmacological manipulation. Inhibitory compounds are shown with a minus (−) sign, while activators are shown with a plus (+) sign. Molecular targets and pharmacological compounds to the left are downstream of those on the right, as shown by the large double arrow at the top of the diagram. The hypothesis states that inhibition of this pathway would result in a decrease in whole-cell K+ channel conductance, thereby leading to increased resilience to anoxic coma onset. The dotted arrow shows decreased K+ conductance. (B) Combinations of the pharmacological agents used in vivo, shown in Figure 2, were administered to adult Drosophila melanogaster, and then the animals were tested for resilience to anoxic coma onset during acute hypoxia. The vertical bar chart is shown as mean ± standard error. Letters within the bars indicate statistical groupings. Letters that differ indicate a significant difference between those groups (p < 0.05). The horizontal dotted line represents the mean of the sham control for ease of comparison between treatments.
cGMP: Cyclic GMP; DCA: Dichloroacetate; P: Phosphorylated; PDE: Phosphodiesterase; Pi: Dephosphorylation; PKG: Protein kinase G.
Adapted from [87].
However, examination of anoxic survival, as opposed to protection of neuronal function, leads to the surprising conclusion that inhibition of the K+ channel axis via fast pharmacological manipulation of the PKG pathway may not necessarily be the ‘holy grail’ of neuroprotection. Under acute conditions, significant neuroprotection is afforded by this approach, contributing to the continued operation of neural circuits and behavior under anoxic stress [87,92]. However, when fruit flies are deprived of oxygen for hours instead of minutes, a very different story emerges, more similar to what is observed in anoxic turtles who survive anoxia for days to weeks. Unlike what is observed with the induction of a HS PC, chronic anoxic stress over 6 h in animals with low PKG resulted in significantly lower survival than those expressing high PKG in both the larval [90] and adult [87] flies. This effect was further confirmed using pharmacological compounds that inhibited or activated the PKG pathway in adult animals [87]. In these experiments, inhibition of the PKG pathway, and hence the K+ channel axis, led to acute (less than 1 h) neuroprotection of neural and behavioral function at the cost of survival of the animal over extended anoxia. By contrast, increased PKG activity has a significant negative impact on function, causing a faster onset of the anoxic coma than in nontreated animals, while activation of the PKG pathway results in increased fruit fly survival [87,90]. Furthermore, upstream activators of the PKG pathway, such as phosphodiesterase-5 inhibitors, result in significant protection of the brain in mice as shown by Caretti et al., where phosphodiesterase-5 inhibition abolishes neuron apoptosis induced by chronic hypoxia [100].
These differences between acute and long-term neuroprotection may also help explain the conflicting results found in the literature concerning the complex responses of mammalian systems to NO, which works in part through PKG [111]. NO appears to influence the pathophysiology of the brain in complex ways depending on the source of production (endogenous or exogenous) and whether NO activation is acute or chronic. Thus, while some protective pathways appear to be mediated by exogenous NO, including inhibition of the proapoptotic p38MAPK [112] and JNK pathways [113], the overproduction of NO can increase cerebral damage and increase susceptibility to NMDAR excitotoxicity [114]. Thus, genetically and pharmacologically tractable animal models such as the anoxia-tolerant fruit fly, or the turtle with its robust adaptive responses, may help to further decipher the complexities of survival without oxygen.
Conclusion
From this overview of both a vertebrate and an invertebrate model of survival without oxygen, a number of adaptations in common suggest profitable avenues of investigation for therapeutic interventions in diseases involving hypoxia or ischemia. Key mechanisms include a rapid metabolic suppression that preserves energy charge via changes in ion flow and neurotransmitter balance; the maintenance of neural networks, ion balance, and neurotransmitter levels during extended anoxia; and the abrogation of oxidative damage. Survival depends on the suppression of pathological mechanisms and apoptotic pathways, with a simultaneous upregulation of survival pathways. Control of ion flux is critical, with K+ channels in particular a likely point of convergence for several protective mechanisms. These mechanisms do not appear unique to these anoxia tolerant models, but are more robust expressions of conserved pathways also reported in mammals.
Future perspective
Understanding the mechanisms responsible for both neurodegeneration and hypoxia resistance through the use of anoxia-tolerant organisms will assist in the identification of novel therapeutic targets. Thus, the anoxia-tolerant turtle provides insight into survival without oxygen, as well as during reoxygenation, with the robust upregulation of key protective pathways and the suppression of pathological mechanisms. Many of these protective pathways are clearly intertwined, possibly providing redundant protections against hypoxic stress, and these links, such as the crosstalk between DOR, the MAPKs and antioxidants, should be further investigated in robust models of anoxia tolerance, such as the turtle. While the molecular mechanisms behind survival are increasingly well described, genomic or proteomic screening could provide a more comprehensive profile of anoxia tolerance, and thus provide a basis for drug screening of potential therapeutic agents. Drugs that induce the ‘protected’ phenotype for up- and down-regulated pathways simultaneously could be identified and targeted for further research. The potential for drug screening is also a significant advantage to working with the more easily manipulated and short-lived anoxia-tolerant fruit fly. Findings from inexpensive and fast invertebrate genetic and pharmacological screens could be translated to mammalian models and then humans to address a number of pathologies for which neuroprotection is required.
However, here we describe two phenotypes for neuroprotection to be considered when such screens are performed. Classic neuroprotection, as seen in the anoxia-tolerant turtle, involves suppression of function and increases in protective pathways. However, in the fruit fly, manipulation of the PKG pathway protects cells by different mechanisms and with mutually exclusive outcomes. Ionic fortification leads to the protection of function under acute stress, but at the cost of long-term cell survival, while conversely, profound increases in survival under stress result from knocking out function early on. Thus, cessation of locomotion in response to anoxia in insects, for example, may result from either increased susceptibility to anoxic stress or quick induction of hypometabolism resulting in long-term protection from anoxic stress. Secondary screens, such as animal or cell survival, thus need to be considered within this paradigm.
Executive summary.
The mammalian brain without oxygen
-
▪
The human brain is highly dependent on oxygen; energy failure results in a cascade of pathological events ending in cellular damage and death.
-
▪
The brain is also highly susceptible to oxidative stress in the face of ischemia/reperfusion events.
Hypoxia/anoxia-tolerant animal models
-
▪
Some animals are able to withstand extended periods of low or no oxygen without pathology, including certain freshwater turtle species and the fruit fly Drosophila melanogaster.
Adaptations for anoxic survival in the brain of the turtle Trachemys scripta
-
▪
Anoxic survival depends on three key adaptations: the rapid profound suppression of basal metabolic rate; an upregulation of protective cellular mechanisms and the depression of proapoptotic pathways; and significant defenses against oxidative stress.
-
▪
In the hypometabolic state, ATP-dependent processes are downregulated to match the reduced energy supply provided by anaerobic processes; neurotransmitter release, ion leak and the generation of action potentials are all suppressed.
-
▪
ATP-dependent K+ channels play a critical early role in establishing hypometabolism; adenosine and GABA play important roles in long-term survival.
Anoxia tolerance & ischemic preconditioning share common mechanisms
-
▪
Molecular defenses against anoxia include the upregulation of MAPK pathways and heat shock proteins, as well as the constitutive presence of δ-opioid receptors, heat shock proteins, antiapoptotic mechanisms and antioxidants.
-
▪
Protective pathways are similar in the anoxia-tolerant turtle and the preconditioned mammal, but protection is more rapid and robust in the turtle, and mechanisms that would destroy cellular integrity are suppressed.
Adaptations for reoxygenation
-
▪
Antioxidant levels are high in the turtle, which coupled with a suppression of reactive oxygen species generation, allow the turtle brain to survive reoxygenation without oxidative damage.
Invertebrate models of anoxia tolerance
-
▪
The low cost, ease of genetic manipulation and genetic homology to humans make insects useful model organisms in which to look for novel genes and pharmacological agents for anoxic neuropathologies.
Neuronal depression as a mechanism of anoxic survival
-
▪
As in vertebrate models, anoxia tolerance in D. melanogaster is afforded in part by a rapid suppression of neuronal activity.
-
▪
Changes in neural activity are seen as alterations in potassium currents, which can be modulated by the cyclic GMP-dependent protein kinase G (PKG) pathway.
Manipulation of the PKG pathway alters anoxia tolerance
-
▪
Levels of PKG affect anoxia tolerance; fruit flies that have lower PKG activity due to genetic variation or through pharmacological treatment demonstrate prolonged activity in response to acute anoxia, but lower rates of survival.
-
▪
Flies with high PKG activity more rapidly enter a coma-like state in which neuronal activity is curtailed, but anoxic survival is higher.
-
▪
Thus, there appears to be an inverse relationship between neuronal activity and anoxic survival, and either continued activity or increased survival could be viewed as neuroprotection.
-
▪
Manipulation of the PKG pathway may provide a rapid means to affect neuronal activity in cases of low oxygen pathologies.
Future perspective
-
▪
Understanding the mechanisms responsible for both neurodegeneration and hypoxia resistance through the use of anoxia-tolerant organisms will help identify novel therapeutic targets for the treatment of low oxygen pathologies.
-
▪
Neuroprotection has two potential phenotypes that need to be considered when such screens are performed, where either protection of function or survival may be induced. Secondary screens, such as animal or cell survival, thus need to be considered within this paradigm.
Acknowledgments
The authors’ work included in this paper was supported by grants from the NIH, the American Federation of Aging Research and the Florida Atlantic University Foundation.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Dawson LA, Djali S, Gonzales C, Vinegra MA, Zaleska MM. Characterization of transient focal ischemia-induced increases in extracellular glutamate and aspartate in spontaneously hypertensive rats. Brain Res. Bull. 2000;53(6):767–776. doi: 10.1016/s0361-9230(00)00363-4. [DOI] [PubMed] [Google Scholar]
- 2.Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79(4):1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 3.Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2012;47(1):9–23. doi: 10.1007/s12035-012-8344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solberg R, Perrone S, Saugstad OD, Buonocore G. Risks and benefits of oxygen in the delivery room. J. Matern. Fetal Neonatal Med. 2012;25(Suppl. 1):41–44. doi: 10.3109/14767058.2012.665236. [DOI] [PubMed] [Google Scholar]
- 5.Drew KL, Harris MB, LaManna JC, Smith MA, Zhu XW, Ma YL. Hypoxia tolerance in mammalian heterotherms. J. Exp. Biol. 2004;207(Pt 18):3155–3162. doi: 10.1242/jeb.01114. [DOI] [PubMed] [Google Scholar]
- 6.Folkow LP, Ramirez JM, Ludvigsen S, Ramirez N, Blix AS. Remarkable neuronal hypoxia tolerance in the deep-diving adult hooded seal (Cystophora cristata) Neurosci. Lett. 2008;446(2–3):147–150. doi: 10.1016/j.neulet.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 7.Peterson BL, Larson J, Buffenstein R, Park TJ, Fall CP. Blunted neuronal calcium response to hypoxia in naked mole-rat hippocampus. PloS ONE. 2012;7(2):e31568. doi: 10.1371/journal.pone.0031568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bickler PE, Fahlman CS, Taylor DM. Oxygen sensitivity of NMDA receptors: relationship to NR2 subunit composition and hypoxia tolerance of neonatal neurons. Neuroscience. 2003;118(1):25–35. doi: 10.1016/s0306-4522(02)00763-7. [DOI] [PubMed] [Google Scholar]
- 9.Lutz PL, Nilsson GE, Prentice HM. The Brain Without Oxygen. 3rd Edition. USA: Kluwer Academic Publishers, MA; 2003. [Google Scholar]
- 10.Kitagawa K. Ischemic tolerance in the brain: endogenous adaptive machinery against ischemic stress. J. Neurosci. Res. 2012;90(5):1043–1054. doi: 10.1002/jnr.23005. [DOI] [PubMed] [Google Scholar]
- 11.Faridar A, Bershad EM, Emiru T, Iaizzo PA, Suarez JI, Divani AA. Therapeutic hypothermia in stroke and traumatic brain injury. Front. Neurol. 2011;2:80. doi: 10.3389/fneur.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308(5721):518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 13.Blackstone E, Roth MB. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;27(4):370–372. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Dong Y, Chen H, et al. Protective effects of hydrogen-rich saline in a rat model of permanent focal cerebral ischemia via reducing oxidative stress and inflammatory cytokines. Brain Res. 2012;1486:103–111. doi: 10.1016/j.brainres.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson GE, Lutz PL. Release of inhibitory neurotransmitters in response to anoxia in turtle brain. Am. J. Physiol. 1991;261(1 Pt 2):R32–R37. doi: 10.1152/ajpregu.1991.261.1.R32. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson G, Lutz PL. Adenosine release in the anoxic turtle brain as a mechanism for anoxic survival. J. Exp. Biol. 1992;162:345–351. [Google Scholar]
- 17. Fernandes JA, Lutz PL, Tannenbaum A, Todorov AT, Liebovitch L, Vertes R. Electroencephalogram activity in the anoxic turtle brain. Am. J. Physiol. 1997;273(3 Pt 2):R911–R919. doi: 10.1152/ajpregu.1997.273.3.R911. ▪ Shows whole-brain reduction in electrical activity, which is the hallmark of hypometabolic coma.
- 18.Bickler PE, Donohoe PH, Buck LT. Hypoxia-induced silencing of NMDA receptors in turtle neurons. J. Neurosci. 2000;20(10):3522–3528. doi: 10.1523/JNEUROSCI.20-10-03522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Pinzon MA, Chan CY, Rosenthal M, Sick TJ. Membrane and synaptic activity during anoxia in the isolated turtle cerebellum. Am. J. Physiol. 1992;263(5 Pt 2):R1057–R1063. doi: 10.1152/ajpregu.1992.263.5.R1057. [DOI] [PubMed] [Google Scholar]
- 20.Pék M, Lutz PL. Role for adenosine in channel arrest in the anoxic turtle brain. J. Exp. Biol. 1997;200(Pt 13):1913–1917. doi: 10.1242/jeb.200.13.1913. [DOI] [PubMed] [Google Scholar]
- 21.Prentice HM, Milton SL, Scheurle D, Lutz PL. Gene transcription of brain voltage-gated potassium channels is reversibly regulated by oxygen supply. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285(6):R1317–R1321. doi: 10.1152/ajpregu.00261.2003. [DOI] [PubMed] [Google Scholar]
- 22.Pamenter ME, Shin DS, Buck LT. AMPA receptors undergo channel arrest in the anoxic turtle cortex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294(2):R606–R613. doi: 10.1152/ajpregu.00433.2007. [DOI] [PubMed] [Google Scholar]
- 23.Lutz PL, Leone-Kabler SL. Upregulation of the GABAA/benzodiazepine receptor during anoxia in the freshwater turtle brain. Am. J. Physiol. 1995;268(5 Pt 2):R1332–R1335. doi: 10.1152/ajpregu.1995.268.5.R1332. [DOI] [PubMed] [Google Scholar]
- 24.Pamenter ME, Hogg DW, Ormond J, Shin DS, Woodin MA, Buck LT. Endogenous GABA(A) and GABA(B) receptor-mediated electrical suppression is critical to neuronal anoxia tolerance. Proc. Natl Acad. Sci. USA. 2011;108(27):11274–11279. doi: 10.1073/pnas.1102429108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milton SL, Lutz PL. Low extracellular dopamine levels are maintained in the anoxic turtle (Trachemys scripta) striatum. J. Cereb. Blood Flow Metab. 1998;18(7):803–807. doi: 10.1097/00004647-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Milton SL, Lutz PL. Adenosine and ATP-sensitive potassium channels modulate dopamine release in the anoxic turtle (Trachemys scripta) striatum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289(1):R77–R83. doi: 10.1152/ajpregu.00647.2004. [DOI] [PubMed] [Google Scholar]
- 27.Milton SL, Thompson JW, Lutz PL. Mechanisms for maintaining extracellular glutamate levels in the anoxic turtle striatum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282(5):R1317–R1323. doi: 10.1152/ajpregu.00484.2001. [DOI] [PubMed] [Google Scholar]
- 28.Swanson R, Duan S. Regulation of glutamate transporter function. Neuroscience. 1999;5:280–282. [Google Scholar]
- 29.Sick TJ, Rosenthal M, LaManna JC, Lutz PL. Brain potassium ion homeostasis, anoxia, and metabolic inhibition in turtles and rats. Am. J. Physiol. 1982;243(3):R281–R288. doi: 10.1152/ajpregu.1982.243.3.R281. [DOI] [PubMed] [Google Scholar]
- 30.Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors. J. Neurosci. 1999;19(5):1657–1662. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zivkovic G, Buck LT. Regulation of AMPA receptor currents by mitochondrial ATP-sensitive K+ channels in anoxic turtle neurons. J. Neurophysiol. 2010;104(4):1913–1922. doi: 10.1152/jn.00506.2010. [DOI] [PubMed] [Google Scholar]
- 32.Pamenter ME, Hogg DW, Buck LT. Endogenous reductions in N-methyl-daspartate receptor activity inhibit nitric oxide production in the anoxic freshwater turtle cortex. FEBS Lett. 2008;582(12):1738–1742. doi: 10.1016/j.febslet.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 33.Pék-Scott M, Lutz PL. ATP-sensitive K+ channel activation provides transient protection to the anoxic turtle brain. Am. J. Physiol. 1998;275(6 Pt 2):R2023–R2027. doi: 10.1152/ajpregu.1998.275.6.R2023. [DOI] [PubMed] [Google Scholar]
- 34.Thompson JW, Prentice HM, Lutz PL. Regulation of extracellular glutamate levels in the long-term anoxic turtle striatum: coordinated activity of glutamate transporters, adenosine, K (ATP) (+) channels and GABA. J. Biomed. Sci. 2007;14(6):809–817. doi: 10.1007/s11373-007-9190-2. [DOI] [PubMed] [Google Scholar]
- 35.Krnjević K. Early effects of hypoxia on brain cell function. Croat. Med. J. 1999;40(3):375–380. [PubMed] [Google Scholar]
- 36.Correia SC, Cardoso S, Santos RX, et al. New insights into the mechanisms of mitochondrial preconditioning-triggered neuroprotection. Curr. Pharm. Des. 2011;17(31):3381–3389. doi: 10.2174/138161211798072490. [DOI] [PubMed] [Google Scholar]
- 37.Stenzel-Poore MP, Stevens SL, King JS, Simon RP. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke. 2007;38(Suppl. 2):680–685. doi: 10.1161/01.STR.0000251444.56487.4c. [DOI] [PubMed] [Google Scholar]
- 38.Miyawaki T, Mashiko T, Ofengeim D, et al. Ischemic preconditioning blocks BAD translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc. Natl Acad. Sci. USA. 2008;105(12):4892–4897. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kis B, Nagy K, Snipes JA, et al. The mitochondrial K(ATP) channel opener BMS-191095 induces neuronal preconditioning. Neuroreport. 2004;15(2):345–349. doi: 10.1097/00001756-200402090-00027. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Cotten JF, Schuyler JA, et al. Protective effects of TASK-3 (KCNK9) and related 2P K channels during cellular stress. Brain Res. 2005;1031(2):164–173. doi: 10.1016/j.brainres.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 41.Correia SC, Santos RX, Cardoso SM, Santos MS, Oliveira CR, Moreira PI. Cyanide preconditioning protects brain endothelial and NT2 neuron-like cells against glucotoxicity: role of mitochondrial reactive oxygen species and HIF-1α. Neurobiol. Dis. 2012;45(1):206–218. doi: 10.1016/j.nbd.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res. Rev. 2009;62(1):99–108. doi: 10.1016/j.brainresrev.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Harten SK, Ashcroft M, Maxwell PH. Prolyl hydroxylase domain inhibitors: a route to HIF activation and neuroprotection. Antiox. Redox Signal. 2010;12(4):459–480. doi: 10.1089/ars.2009.2870. [DOI] [PubMed] [Google Scholar]
- 44.Ong SG, Hausenloy DJ. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol. Ther. 2012;136(1):69–81. doi: 10.1016/j.pharmthera.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Xue W, Cai L, Tan Y, et al. Cardiac-specific overexpression of HIF-1{alpha} prevents deterioration of glycolytic pathway and cardiac remodeling in streptozotocin-induced diabetic mice. Am. J. Pathol. 2010;177(1):97–105. doi: 10.2353/ajpath.2010.091091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao HX, Wang XL, Wang YH, et al. Attenuation of myocardial injury by postconditioning: role of hypoxia inducible factor-1alpha. Basic Res. Cardiol. 2010;105(1):109–118. doi: 10.1007/s00395-009-0044-0. [DOI] [PubMed] [Google Scholar]
- 47.Wacker BK, Perfater JL, Gidday JM. Hypoxic preconditioning induces stroke tolerance in mice via a cascading HIF, sphingosine kinase, and CCL2 signaling pathway. J. Neurochem. 2012;123(6):954–962. doi: 10.1111/jnc.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang CC, Lin LC, Wu MS, Chien CT, Lai MK. Repetitive hypoxic preconditioning attenuates renal ischemia/reperfusion induced oxidative injury via upregulating HIF-1 alpha-dependent bcl-2 signaling. Transplantation. 2009;88(11):1251–1260. doi: 10.1097/TP.0b013e3181bb4a07. [DOI] [PubMed] [Google Scholar]
- 49.Song X, Zhang N, Xu H, Cao L, Zhang H. Combined preconditioning and postconditioning provides synergistic protection against liver ischemic reperfusion injury. Int. J. Biol. Sci. 2012;8(5):707–718. doi: 10.7150/ijbs.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borlongan CV, Wang Y, Su TP. Delta opioid peptide (D-Ala 2, D-Leu 5) enkephalin: linking hibernation and neuroprotection. Front. Biosci. 2004;9:3392–3398. doi: 10.2741/1490. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Gibney GT, Zhao P, Xia Y. Neuroprotective role of delta-opioid receptors in cortical neurons. Am. J. Physiol. Cell Physiol. 2002;282(6):C1225–C1234. doi: 10.1152/ajpcell.00226.2001. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Haddad GG, Xia Y. Delta-, but not mu- and kappa-, opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res. 2000;885(2):143–153. doi: 10.1016/s0006-8993(00)02906-1. [DOI] [PubMed] [Google Scholar]
- 53.Gao CJ, Niu L, Ren PC, et al. Hypoxic preconditioning attenuates global cerebral ischemic injury following asphyxial cardiac arrest through regulation of delta opioid receptor system. Neuroscience. 2012;202:352–362. doi: 10.1016/j.neuroscience.2011.11.060. [DOI] [PubMed] [Google Scholar]
- 54.Xia Y, Haddad GG. Major difference in the expression of delta- and mu-opioid receptors between turtle and rat brain. J. Comp. Neurol. 2001;436(2):202–210. [PubMed] [Google Scholar]
- 55.Pamenter ME, Buck LT. Delta-opioid receptor antagonism induces NMDA receptor-dependent excitotoxicity in anoxic turtle cortex. J. Exp. Biol. 2008;211(Pt 21):3512–3517. doi: 10.1242/jeb.021949. [DOI] [PubMed] [Google Scholar]
- 56.Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J. Neurosci. 2007;27(23):6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelly DA, Storey KB. Organ-specific control of glycolysis in anoxic turtles. Am. J. Physiol. 1988;255(5 Pt 2):R774–R779. doi: 10.1152/ajpregu.1988.255.5.R774. [DOI] [PubMed] [Google Scholar]
- 58.Xiong ZJ, Storey KB. Regulation of liver lactate dehydrogenase by reversible phosphorylation in response to anoxia in a freshwater turtle. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012;163(2):221–228. doi: 10.1016/j.cbpb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J. Neurochem. 2004;89(1):73–89. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- 60.Yenari M, Kitagawa K, Lyden P, Perez-Pinzon M. Metabolic downregulation: a key to successful neuroprotection? Stroke. 2008;39(10):2910–2917. doi: 10.1161/STROKEAHA.108.514471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milton SL, Prentice HM. Beyond anoxia: the physiology of metabolic downregulation and recovery in the anoxia-tolerant turtle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007;147(2):277–290. doi: 10.1016/j.cbpa.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kesaraju S, Schmidt-Kastner R, Prentice HM, Milton SL. Modulation of stress proteins and apoptotic regulators in the anoxia tolerant turtle brain. J. Neurochem. 2009;109(5):1413–1426. doi: 10.1111/j.1471-4159.2009.06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee SH, Kwon HM, Kim YJ, Lee KM, Kim M, Yoon BW. Effects of hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke. 2004;35(9):2195–2199. doi: 10.1161/01.STR.0000136150.73891.14. [DOI] [PubMed] [Google Scholar]
- 64.Milton SL, Dirk LJ, Kara LF, Prentice HM. Adenosine modulates ERK1/2, PI3K/Akt, and p38MAPK activation in the brain of the anoxia-tolerant turtle Trachemys scripta. J. Cereb. Blood Flow Metab. 2008;28(8):1469–1477. doi: 10.1038/jcbfm.2008.45. [DOI] [PubMed] [Google Scholar]
- 65.Ke S, Dian-san S, Xiang-rui W. Delta opioid agonist [D-Ala2, D-Leu5] enkephalin (DADLE) reduced oxygen-glucose deprivation caused neuronal injury through the MAPK pathway. Brain Res. 2009;1292:100–106. doi: 10.1016/j.brainres.2009.06.104. [DOI] [PubMed] [Google Scholar]
- 66.Ma MC, Qian H, Ghassemi F, Zhao P, Xia Y. Oxygen-sensitive {delta}-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J. Biol. Chem. 2005;280(16):16208–16218. doi: 10.1074/jbc.M408055200. [DOI] [PubMed] [Google Scholar]
- 67.Nayak GH, Prentice HM, Milton SL. Neuroprotective signaling pathways are modulated by adenosine in the anoxia tolerant turtle. J. Cereb. Blood Flow Metab. 2011;31(2):467–475. doi: 10.1038/jcbfm.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee YJ, Chen JC, Amoscato AA, et al. Protective role of Bcl2 in metabolic oxidative stress-induced cell death. J. Cell Sci. 2001;114(Pt 4):677–684. doi: 10.1242/jcs.114.4.677. [DOI] [PubMed] [Google Scholar]
- 69. Milton SL, Nayak G, Kesaraju S, Kara L, Prentice HM. Suppression of reactive oxygen species production enhances neuronal survival in vitro and in vivo in the anoxia-tolerant turtle Trachemys scripta. J. Neurochem. 2007;101(4):993–1001. doi: 10.1111/j.1471-4159.2007.04466.x. ▪▪ First evidence of reactive oxygen species suppression as a mechanism of anoxia/reoxygenation survival in the turtle brain.
- 70.Willmore WG, Storey KB. Antioxidant systems and anoxia tolerance in a freshwater turtle Trachemys scripta elegans. Mol. Cell. Biochem. 1997;170(1–2):177–185. doi: 10.1023/a:1006817806010. [DOI] [PubMed] [Google Scholar]
- 71.Willmore WG, Storey KB. Glutathione systems and anoxia tolerance in turtles. Am. J. Physiol. 1997;273(1 Pt 2):R219–R225. doi: 10.1152/ajpregu.1997.273.1.R219. [DOI] [PubMed] [Google Scholar]
- 72.Rice ME, Lee EJ, Choy Y. High levels of ascorbic acid, not glutathione, in the CNS of anoxia-tolerant reptiles contrasted with levels in anoxia-intolerant species. J. Neurochem. 1995;64(4):1790–1799. doi: 10.1046/j.1471-4159.1995.64041790.x. [DOI] [PubMed] [Google Scholar]
- 73.Yang Y, Xia X, Zhang Y, et al. Delta-opioid receptor activation attenuates oxidative injury in the ischemic rat brain. BMC Biol. 2009;7:55. doi: 10.1186/1741-7007-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407(6803):520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 75.Milton SL, Nayak G, Lutz PL, Prentice HM. Gene transcription of neuroglobin is upregulated by hypoxia and anoxia in the brain of the anoxia-tolerant turtle Trachemys scripta. J. Biomed. Sci. 2006;13(4):509–514. doi: 10.1007/s11373-006-9084-8. [DOI] [PubMed] [Google Scholar]
- 76.Nayak G, Prentice HM, Milton SL. Role of neuroglobin in regulating reactive oxygen species in the brain of the anoxia-tolerant turtle Trachemys scripta. J. Neurochem. 2009;110(2):603–612. doi: 10.1111/j.1471-4159.2009.06157.x. [DOI] [PubMed] [Google Scholar]
- 77.Yu Z, Liu N, Liu J, Yang K, Wang X. Neuroglobin, a novel target for endogenous neuroprotection against stroke and neurodegenerative disorders. Int. J. Mol. Sci. 2012;13(6):6995–7014. doi: 10.3390/ijms13066995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pamenter ME, Richards MD, Buck LT. Anoxia-induced changes in reactive oxygen species and cyclic nucleotides in the painted turtle. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2007;177(4):473–481. doi: 10.1007/s00360-007-0145-8. [DOI] [PubMed] [Google Scholar]
- 79.Ma Y, Lu C, Li C, et al. Overexpression of HSPA12B protects against cerebral ischemia/ reperfusion injury via a PI3K/Akt-dependent mechanism. Biochim. Biophys. Acta. 2012;1832(1):57–66. doi: 10.1016/j.bbadis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 80.Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia. 2010;58(9):1042–1049. doi: 10.1002/glia.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lighton JR, Schilman PE. Oxygen reperfusion damage in an insect. PLoS ONE. 2007;2(12):e1267. doi: 10.1371/journal.pone.0001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoback WW, Podrabsky JE, Higley LG, Stanley DW, Hand SC. Anoxia tolerance of con-familial tiger beetle larvae is associated with differences in energy flow and anaerobiosis. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2000;170(4):307–314. doi: 10.1007/s003600000104. [DOI] [PubMed] [Google Scholar]
- 83.Krishnan SN, Sun YA, Mohsenin A, Wyman RJ, Haddad GG. Behavioral and electrophysiologic responses of Drosophila melanogaster to prolonged periods of anoxia. J. Insect Physiol. 1997;43(3):203–210. doi: 10.1016/s0022-1910(96)00084-4. [DOI] [PubMed] [Google Scholar]
- 84.Chien S, Reiter LT, Bier E, Gribskov M. Homophila: human disease gene cognates in Drosophila. Nucleic Acids Res. 2002;30(1):149–151. doi: 10.1093/nar/30.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duffy JB. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34(1–2):1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 86.Armstrong GA, Xiao C, Krill JL, Seroude L, Dawson-Scully K, Robertson RM. Glial Hsp70 protects K+ homeostasis in the Drosophila brain during repetitive anoxic depolarization. PLoS ONE. 2011;6(12):e28994. doi: 10.1371/journal.pone.0028994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dawson-Scully K, Bukvic D, Chakaborty-Chatterjee M, Ferreira R, Milton SL, Sokolowski MB. Controlling anoxic tolerance in adult Drosophila via the cGMP-PKG pathway. J. Exp. Biol. 2010;213(Pt 14):2410–2416. doi: 10.1242/jeb.041319. ▪▪ Determines that the fast onset of hypometabolism results in increased survival during long-term periods of anoxia during protein kinase G activation.
- 88.Haddad GG. Tolerance to low O2: lessons from invertebrate genetic models. Exp. Physiol. 2006;91(2):277–282. doi: 10.1113/expphysiol.2005.030767. [DOI] [PubMed] [Google Scholar]
- 89.Zhou D, Xue J, Lai JC, Schork NJ, White KP, Haddad GG. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet. 2008;4(10):e1000221. doi: 10.1371/journal.pgen.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wingrove JA, O’Farrell PH. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell. 1999;98(1):105–114. doi: 10.1016/S0092-8674(00)80610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Charette M, Darveau CA, Perry SF, Rundle HD. Evolutionary consequences of altered atmospheric oxygen in Drosophila melanogaster. PLoS ONE. 2011;6(10):e26876. doi: 10.1371/journal.pone.0026876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rodgers CI, Armstrong GA, Shoemaker KL, LaBrie JD, Moyes CD, Robertson RM. Stress preconditioning of spreading depression in the locust CNS. PLoS ONE. 2007;2(12):e1366. doi: 10.1371/journal.pone.0001366. ▪ Determines that spreading depression-like events in locusts are adaptive to induce hypometabolism and conserve energy during stress and that they can be preconditioned by experience.
- 93.Rodriguez EC, Robertson RM. Protective effect of hypothermia on brain potassium homeostasis during repetitive anoxia in Drosophila melanogaster. J. Exp. Biol. 2012;215(Pt 23):4157–4165. doi: 10.1242/jeb.074468. [DOI] [PubMed] [Google Scholar]
- 94.Feder ME, Krebs RA. Ecological and evolutionary physiology of heat shock proteins and the stress response in Drosophila: complementary insights from genetic engineering and natural variation. EXS. 1997;83:155–173. doi: 10.1007/978-3-0348-8882-0_9. [DOI] [PubMed] [Google Scholar]
- 95.Dawson-Scully K, Armstrong GA, Kent C, Robertson RM, Sokolowski MB. Natural variation in the thermotolerance of neural function and behavior due to a cGMP-dependent protein kinase. PLoS ONE. 2007;2(1):e773. doi: 10.1371/journal.pone.0000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Belle JS, Hilliker AJ, Sokolowski MB. Genetic localization of foraging (for): a major gene for larval behavior in Drosophila melanogaster. Genetics. 1989;123(1):157–163. doi: 10.1093/genetics/123.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osborne KA, Robichon A, Burgess E, et al. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science. 1997;277(5327):834–836. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- 98.Sokolowski MB. Genetic-aspects to differences in foraging behavior. Behav. Brain Sci. 1985;8(2):348–349. [Google Scholar]
- 99.Mackay TF. Quantitative trait loci in Drosophila. Nat. Rev. Genet. 2001;2(1):11–20. doi: 10.1038/35047544. [DOI] [PubMed] [Google Scholar]
- 100.Caretti A, Bianciardi P, Ronchi R, Fantacci M, Guazzi M, Samaja M. Phosphodiesterase-5 inhibition abolishes neuron apoptosis induced by chronic hypoxia independently of hypoxiainducible factor-1alpha signaling. Exp. Biol. Med. (Maywood) 2008;233(10):1222–1230. doi: 10.3181/0802-RM-73. [DOI] [PubMed] [Google Scholar]
- 101.Armstrong GA, Rodgers CI, Money TG, Robertson RM. Suppression of spreading depression-like events in locusts by inhibition of the NO/cGMP/PKG pathway. J. Neurosci. 2009;29(25):8225–8235. doi: 10.1523/JNEUROSCI.1652-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Money TG, Rodgers CI, McGregor SM, Robertson RM. Loss of potassium homeostasis underlies hyperthermic conduction failure in control and preconditioned locusts. J. Neurophysiol. 2009;102(1):285–293. doi: 10.1152/jn.91174.2008. [DOI] [PubMed] [Google Scholar]
- 103.Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 2012;32(7):1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dawson-Scully K, Meldrum Robertson R. Heat shock protects synaptic transmission in flight motor circuitry of locusts. Neuroreport. 1998;9(11):2589–2593. doi: 10.1097/00001756-199808030-00030. [DOI] [PubMed] [Google Scholar]
- 105.Ramirez JM, Elsen FP, Robertson RM. Long-term effects of prior heat shock on neuronal potassium currents recorded in a novel insect ganglion slice preparation. J. Neurophysiol. 1999;81(2):795–802. doi: 10.1152/jn.1999.81.2.795. [DOI] [PubMed] [Google Scholar]
- 106.Kaun KR, Chakaborty-Chatterjee M, Sokolowski MB. Natural variation in plasticity of glucose homeostasis and food intake. J. Exp. Biol. 2008;211(Pt 19):3160–3166. doi: 10.1242/jeb.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaun KR, Hendel T, Gerber B, Sokolowski MB. Natural variation in Drosophila larval reward learning and memory due to a cGMP-dependent protein kinase. Learn. Mem. 2007;14(5):342–349. doi: 10.1101/lm.505807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Orstavik S, Natarajan V, Tasken K, Jahnsen T, Sandberg M. Characterization of the human gene encoding the type I alpha and type I beta cGMP-dependent protein kinase (PRKG1) Genomics. 1997;42(2):311–318. doi: 10.1006/geno.1997.4743. [DOI] [PubMed] [Google Scholar]
- 109.Renger JJ, Yao WD, Sokolowski MB, Wu CF. Neuronal polymorphism among natural alleles of a cGMP-dependent kinase gene, foraging, in Drosophila. J. Neurosci. 1999;19(19):RC28. doi: 10.1523/JNEUROSCI.19-19-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodgers CI, Labrie JD, Robertson RM. K+ homeostasis and central pattern generation in the metathoracic ganglion of the locust. J. Insect Physiol. 2009;55(7):599–607. doi: 10.1016/j.jinsphys.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 111.Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischaemia–reperfusion: opportunities and obstacles for survival signaling. Br. J. Pharmacol. 2007;152(6):855–869. doi: 10.1038/sj.bjp.0707409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qi SH, Hao LY, Yue J, Zong YY, Zhang GY. Exogenous nitric oxide negatively regulates the S-nitrosylation p38 mitogen-activated protein kinase activation during cerebral ischaemia and reperfusion. Neuropathol. Appl. Neurobiol. 2013;39(3):284–297. doi: 10.1111/j.1365-2990.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- 113.Pei DS, Song YJ, Yu HM, Hu WW, Du Y, Zhang GY. Exogenous nitric oxide negatively regulates c-Jun N-terminal kinase activation via inhibiting endogenous NO-induced S-nitrosylation during cerebral ischemia and reperfusion in rat hippocampus. J. Neurochem. 2008;106(4):1952–1963. doi: 10.1111/j.1471-4159.2008.05531.x. [DOI] [PubMed] [Google Scholar]
- 114.Shi ZQ, Sunico CR, McKercher SR, et al. S-nitrosylated SHP-2 contributes to NMDA receptor-mediated excitotoxicity in acute ischemic stroke. Proc. Natl Acad. Sci. USA. 2013;110(8):3137–3142. doi: 10.1073/pnas.1215501110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lutz PL, Milton SL. Negotiating brain anoxia survival in the turtle. J. Exp. Biol. 2004;207(Pt 18):3141–3147. doi: 10.1242/jeb.01056. [DOI] [PubMed] [Google Scholar]
- 116.Zhou XB, Ruth P, Schlossmann J, Hofmann F, Korth M. Protein phosphatase 2A is essential for the activation of Ca2+-activated K+ currents by cGMP-dependent protein kinase in tracheal smooth muscle and Chinese hamster ovary cells. J. Biol. Chem. 1996;271(33):19760–19767. doi: 10.1074/jbc.271.33.19760. [DOI] [PubMed] [Google Scholar]