Abstract
The selection and expansion of B cells undergoing affinity maturation in the germinal center is a hallmark of humoral immunity. A recent paper in Nature provides new insights into the relationships between the affinity of the immunoglobulin receptor for antigen, the ability of B cells to present antigen to T cells, and the processes of selection, mutation, and clonal expansion in the germinal center.
Keywords: germinal center, affinity maturation, antigen presentation, Tfh cells
A key feature of humoral immunity is the production of antibodies that develop increasing affinity for antigen over the course of an immune response [1]. This process is known as affinity maturation and occurs when low-affinity B cells undergo immunoglobulin (Ig) somatic hypermutation (SHM), clonal expansion, and affinity-based selection within the germinal center (GC), a specialized anatomical site that arises within lymphoid follicles following immunization [2,3]. Perturbations of the GC reaction impair the generation of protective high-affinity antibodies and cause the development of humoral autoimmunity. Thus, parsing out the mechanisms that regulate the GC response is critical to better understand both immunity to infections and autoimmune disorders.
It has long been known that GCs include two distinct regions known as dark zone (DZ) and light zone (LZ) [2,3]. The DZ is comprised of a dense network of B cells that display elevated proliferative activity. By contrast, the LZ has a lower density of B cells and contains T follicular helper (Tfh) cells along with follicular dendritic cells (FDCs) that bind antigen-containing immunocomplexes. Given these observations, Ian MacLennan proposed what is now considered the classical model of affinity maturation within the GC. In this model, B cells first undergo SHM and clonal expansion in the DZ and later proceed to the LZ, where they undergo selection based upon their competiveness for antigen binding [4].
Although this model has been proven to be largely correct, it does not satisfactorily explain observations indicating that high-affinity B cells develop through multiple rounds of mutation and selection. To accommodate these observations, it has been proposed that B cells undergo iterative rounds of mutation/expansion and affinity-based selection by moving back and forth between the DZ and LZ [5]. Recent technical innovations, including in vivo real-time imaging studies and use of a photoactivatable fluorescent GFP protein (PAGFP), confirm that GC B cells move bi-directionally between the DZ and LZ, with approximately 30% of B cells in the LZ migrating back to the DZ to undergo further mutation and expansion[6-9].
But how is affinity maturation regulated in the context of this cyclic re-entry model? An expanding body of evidence suggests that interaction with Tfh cells serves as limiting factor for the selection of high-affinity B cells in the LZ. In particular, the antigen presentation capacity of a B cell appears to dictate whether this B cell will re-enter the DZ or will rather differentiate into a memory B cell or plasma cell [2,3,7]. However, the regulatory mechanisms controlling B cell proliferation and SHM within the DZ and the role that these processes play in the accumulation of high-affinity B cell clones have remained poorly understood. A recent article in Nature by Gitlin et al. sheds new light on these mechanisms, providing fundamental new insights into the dynamics of affinity maturation within the GC [10].
The authors took advantage of an elegant model that permits antigen delivery directly to a small fraction of GC B cells, which thereby increase their ability to present antigen to Tfh cells in a manner independent of Ig affinity for antigen [7]. B cells targeted by antigen initially expanded in the DZ in proportion to the amount of antigen delivered. These data suggest that the amount of B cells proliferating within the DZ is a function of their ability to present antigen to Tfh cells within the LZ. To formally test this possibility, GC cell division was tracked in vivo using a suppressible fluorescence hematopoietic reporter mouse. These mice express both a tetracycline transactivator protein targeted to the hematopoietic lineage and an mCherry fusion protein driven by a doxycycline-regulated promoter. Thus, all hematopoietic cells are mCherry positive, unless exposed to doxycycline. This is a powerful tool, which mimics commonly used in vitro proliferation dyes. Indeed, after suppression of the fluorescent transgene, each cell division results in the dilution of the fluorescence signal from mCherry. Results demonstrate that increased antigen presentation to Tfh cells is associated with increased B cell division within the GC. Importantly, cell cycle analysis demonstrated that increased antigen presentation increases the frequency of B cells entering the S phase of the cell cycle within the DZ. In addition, the use of a PAGFP system demonstrated that the retention rate of B cells is increased in the DZ. Collectively, these findings imply that the number of divisions that a B cell undergoes in the DZ before returning to the LZ varies according to the ability of this B cell to interact with and present antigen to Tfh cells in the LZ.
Does differential proliferation contribute to affinity maturation during physiological immune responses? To address this issue, the authors examined the relation-ship between B cell division in the DZ and B cell affinity maturation under conditions where antigen presentation was not externally influenced. B cells undergoing the greatest number of divisions had approximately 6-fold higher affinity for antigen than B cells undergoing the least number of divisions. Moreover, highly dividing cells showed increased SHM. Thus, GCs are set up to allow B cells with the highest affinity for antigen to selectively expand and diversify through SHM, leading to a large net increase in the affinity of the overall response.
This work marks a major advancement in our understanding of GC dynamics (Figure 1). By outcompeting lower affinity B cells for both antigen binding and interaction with Tfh cells, higher affinity B cells undergo enhanced clonal expansion and Ig gene diversification via SHM. This process results in a feed-forward loop, where each interzonal cycle of a B cell within the GC produces incremental affinity for antigen at an increasing magnitude. Overall, these new findings provide a mechanistic framework that helps to conceptualize how affinity maturation develops over the course of an immune response.
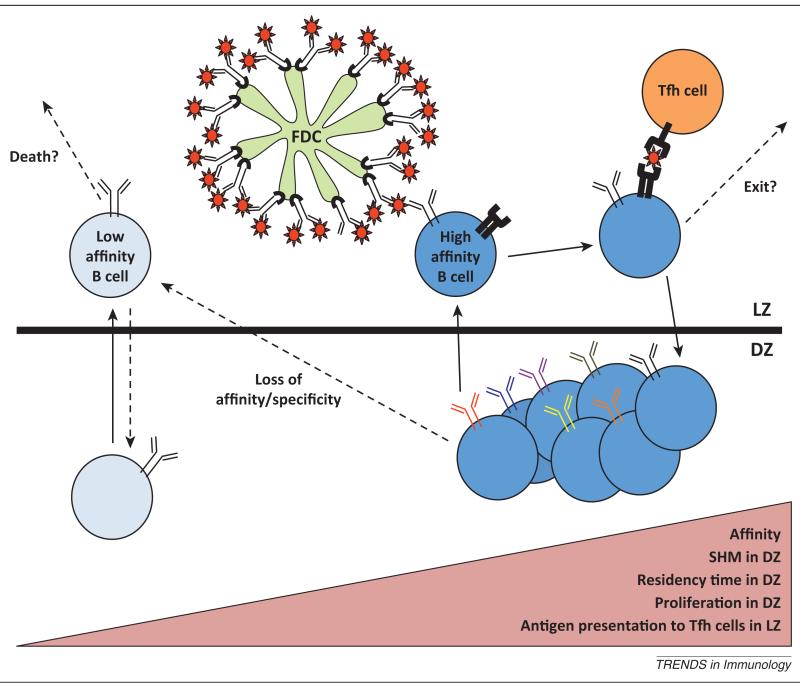
Figure 1.
Affinity maturation in the GC. During a T cell-dependent antibody response to protein antigens, B cells undergo expansion and somatic hypermutation (SHM) in the dark zone (DZ) of the germinal center. They then migrate to the light zone (LZ), where the highest affinity cells are selected through their ability to acquire antigen from the surface of follicular dendritic cells (FDCs) and present this antigen to T follicular helper (Tfh) cells. By contrast, B cells that cannot compete for antigen binding are eliminated. Antigen presentation to Tfh cells directs B cells to reenter the DZ, where they undergo additional rounds of division and SHM. The amount of proliferation and mutation B cells undergo during each interzonal GC cycle is proportional to their affinity for antigen and the amount of antigen they present to T cells. This creates a feed-forward loop, where B cells with the highest affinity are continuously selected, expanded and diversified. This model explains how affinity maturation can occur in the course of an immune response.
Though this work provides one of the clearest pictures to date of GC B cell regulation, many questions remain unanswered. What is the mechanism by which increased antigen presentation to Tfh cells in the LZ translates into an enhanced proliferative capacity in the DZ? What signals determine when a high-affinity B cell should stop cycling and exit the GC reaction? Similarly, how are B cells selected to differentiate into either long-lived plasma cells or memory B cells? Finally, how do negative signals from T follicular regulatory cells regulate antigen presentation to Tfh cells and affinity maturation? Answering these questions will help to further decipher the complex mechanisms governing the GC reaction and provide important insights into the generation of protective and pathogenic antibodies.
References
- 1.Eisen HN, Siskind GW. Variations in affinities of antibodies during the immune response. Biochemistry. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- 2.Victora GD, Nussenzweig M. Germinal centers. Annu. Rev. Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 3.Allen CD, et al. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLennan IC. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 5.Oprea M, Perelson AS. Somatic mutation leads to efficient affinity maturation when centrocytes recycle back to centroblasts. J. Immunol. 1997;158:5155–5162. [PubMed] [Google Scholar]
- 6.Hauser AE, et al. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity. 2007;26:655–667. doi: 10.1016/j.immuni.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen CD, et al. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 9.Schwickert TA, et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 10.Gitlin AD, et al. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509:637–640. doi: 10.1038/nature13300. [DOI] [PMC free article] [PubMed] [Google Scholar]