Abstract
Background
At our institution, incidental pancreatic cysts are frequently identified in asymptomatic patients undergoing routine imaging for staging of non-pancreatic malignancies. The management of these patients is unclear since a small but significant number of incidental pancreatic cysts are malignant.
Study Design
Our institutional database was reviewed for patients with ICD-9 codes for pancreatic cysts from 1980 to 2005. Clinicopathologic factors, including CT and endoscopic ultrasound (EUS) characteristics and management strategies were analyzed.
Results
Over 25 years, 942 patients were identified with pancreatic cysts. Excluding those with symptoms or pseudocysts, 350 patients remained with incidental pancreatic cysts. Mean overall survival was 41.4 months (mean follow-up 32.7 months). Forty-one patients underwent resection, of whom 38 (92.7%) had premalignant/malignant pathology. On univariate analysis, younger age, size >= 3 cm, nodularity, and presumptive diagnosis based on CT or EUS were all significant predictors of surgical resection. Only young age, EUS- and CT-based diagnoses and size >= 3 cm on CT were independent predictors of resection by multivariate analysis. Univariate analysis of variables predicting pathologic premalignant/malignant diagnosis identified pancreatic neck or body location as significant factors.
Conclusions
These data suggest that most incidental pancreatic cysts can be managed non-operatively using a selective strategy based on detailed review of CT imaging and EUS findings.
INTRODUCTION
Patients frequently undergo abdominal imaging for symptoms unrelated to their pancreas, or during staging for a primary cancer diagnosis. Occasionally, cystic pancreatic lesions are incidentally identified. While the incidence of malignant or premalignant findings within the identified pancreatic cystic lesions in these patients should be low1, there are currently no well-defined guidelines for the management of an incidentally identified pancreatic cyst. Without clear radiographic evidence of malignancy, it can be difficult to distinguish malignant or premalignant cystic lesions from benign cystic lesions. Furthermore, no consensus has been reached regarding the optimal imaging or endoscopic evaluation, surgical treatment and follow up of patients with asymptomatic cystic lesions of the pancreas. Khalid and Brugge2 reviewed the imaging, tumor marker analysis, and cytology findings for pancreatic cystic lesions and found that imaging modalities were generally less than 80% sensitive for establishing a diagnosis of malignancy, while the sensitivity for cytologic analysis tended to be less than 50%.
Pancreatic cystic lesions fall into two broad categories—neoplastic and nonneoplastic—as recently reviewed by Katz et al.3 Cystic variants of solid neoplasms, including adenocarcinoma, neuroendocrine tumors, acinar carcinoma, and mucinous cystadenocarcinoma, constitute the malignant subset of pancreatic cystic lesions. Neoplastic cystic lesions include mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN), which have some malignant potential and occasionally will harbor occult invasive or noninvasive cancer. Serous lesions include benign serous cystadenomas and, much less common, serous cystadenocarcinoma (SCA)4. Less common benign lesions include pseudopillary tumor of the pancreas, lymphoepithelial cysts, and congenital cysts (non-neoplastic).
Recently, the International Association of Pancreatology has proposed guidelines for the diagnosis and treatment of IPMN and MCN.5 These criteria established the current expectant management of small (<3 cm) side-branch IPMN and reinforced the need for resection of MCN, main-duct IPMN, and side-branch IPMN with high-risk features, such as size and nodularity. Unfortunately, these guidelines address IPMN and MCN only and are not informative for lesions that cannot be assigned to these categories.
The primary goal of this study was to test the hypothesis that most incidentally discovered cystic lesions are benign and can be managed non-operatively. To test this hypothesis, we reviewed the course of patients with incidental pancreatic cystic lesions identified at our institution. Specfically, we reviewed the records of patients with incidentally discovered pancreatic cystic lesions to determine the management approach taken with these patients and the utility of imaging studies and endoscopic ultrasonographic (EUS) findings in distinguishing benign cystic lesions from mucinous and malignant cystic lesions. Additionally, we reviewed the outcome of surgical treatment versus observation as applied to patients with incidentally discovered pancreatic cystic lesions to determine whether clinical factors were associated with the decision to perform surgical resection or predicted the presence of malignant findings within the resected cyst. In patients who were observed, we examined the natural history of incidental cystic lesions over time.
METHODS
Patient population
After approval by our Institutional Review Board, we performed a retrospective analysis of patients with pancreatic cystic lesions who were evaluated at The University of Texas M. D. Anderson Cancer Center between 1980 and 2005. We queried our institutional database and the prospective pancreatic cancer database maintained by the Department of Surgical Oncology for all patients with ICD9 codes for pancreatic cysts. A total of 942 patients were identified. We excluded patients with a diagnosis of pancreatic pseudocyst (diagnosed primarily by radiologic appearance) or patients with postoperative cystic fluid collections resulting from prior pancreatic surgery. In addition, we reviewed the clinical histories of all patients, and patients who presented with gastrointestinal symptoms (e.g., abdominal pain, weight loss) prior to abdominal imaging were excluded. After exclusion, a total of 350 patients with asymptomatic, incidental pancreatic cystic lesions remained and comprises the patient population for this study.
Radiologic Imaging
For most patients in this study, the cystic lesion of the pancreas was incidentally detected on computed tomography (CT) performed for staging evaluation after diagnosis of a nonpancreatic primary malignancy. For all patients in this study, a pancreas-protocol CT was performed. This protocol uses a multidetector CT to obtain images in two phases (dual-phase) at 33–46 seconds (pancreatic parenchymal phase) and 60–70 seconds (portal venous phase) after injection of 150 mL of Ioversol 300 mg/mL (Optiray; Mallinkrodt, St. Louis, MO, USA) at a rate of 5 mL/second, with water as an oral contrast agent. In both phases, images are obtained at 2.5mm, and then reconstructed to 1.25mm every 0.625mm for images obtained with the 16 MDCT, or 0.625mm every 0.625mm for images obtained with the 64 MDCT scanner.
A team of four faculty radiologists (E.T., L.M., A.B., and P.B.) with expertise in pancreatic imaging re-reviewed the cross-sectional imaging studies for 286 of the 350 study patients (electronic imaging records were not available for the remainder of the patients). This CT assessment was the basis of the initial diagnosis for each patient. When re-review of the CT scans was not possible, the cysts were classified on the basis of the initial radiology report. This detailed re-review of CT scans by our radiology team of the majority of patients in this study was used to classify the cystic lesions into three broad radiographic diagnostic categories: benign, malignant, or mucinous (Table 1). Benign cystic lesions included serous cystadenomas, cystic lesions that did not have sufficient characteristics to be classified more specifically and were thus defined as “not otherwise specified” (NOS), and other benign lesions. Malignant cystic lesions included adenocarcinomas, mucinous cystadenocarcinomas, neuroendocrine lesions, and metastatic lesions from nonpancreatic primary malignancies. Finally, mucinous cystic lesions included IPMNs and mucinous cystadenomas.
Table 1.
Computed tomography–based classification of cystic lesions of the pancreas
| Benign | Malignant | Mucinous |
|---|---|---|
| Serous cystadenoma | Adenocarcinoma | IPMN |
| Cyst (not otherwise specified) | Mucinous cystadenocarcinoma | Mucinous cystadenoma |
| Other (dilated PD side branch, fat, adenoma) | Neuroendocrine | |
| Metastasis | ||
IPMN. intraductal papillary mucinous neoplasm; PD, pancreatic duct.
Endoscopic Ultrasonography
EUS images were obtained at the discretion of the primary attending physician to further evaluate cystic lesions suspected to be malignant or mucinous. On an individual basis, the gastroenterologist who performed EUS (in consultation with the primary attending physician) decided to perform fine-needle aspiration (FNA) or evaluate the levels of CEA or CA19-9 in the cystic fluid. Data regarding sonographic studies was obtained from patient records. For this study, we recorded the characteristics of the cyst seen on EUS/FNA, including size, presence of septations, calcifications, communication with the main pancreatic duct, dilatation of the main pancreatic duct, presence of a solid component within the cyst, and the presence of mucin.
Surgical Resection
Although not all patients were evaluated by a surgeon, our surgical group employed a previously described selective approach to surgical resection.3 Briefly, the risk of malignancy, the need for EUS/FNA, and surgical resectability are principally determined radiographically. When radiologic evaluation raised suspicion for malignancy, patients were offered resection. In cases of suspected IPMN or MCN, International Consensus Guidelines were observed5. Finally, when the radiologic appearance of the cystic lesion suggested a low risk of malignancy, patients were observed with serial CT imaging.
Classification of Cystic Lesions
Pancreatic cystic lesions were initially classified as benign, malignant, or mucinous based on radiologic imaging (initial diagnosis). For those cysts that were subjected to EUS/FNA, some were reclassified to another category based on the EUS/FNA findings, and we defined this as the intermediate diagnosis. Finally, a proportion of patients underwent surgical resection, and the surgical pathology report was used to reclassify the lesion to the most appropriate category. The final diagnosis was determined by surgical pathology if resection was performed or, alternatively, the clinical impression based on imaging and EUS, if surgery was not performed. In addition, for those patients who did not undergo biopsy or surgical resection to confirm the benign nature of their cyst, we classified the cyst as benign if the patient had a follow-up greater than 2 years with no evidence of cancer. If follow-up was less than 2 years, the patient’s final clinical status was considered “unknown.” All patients were thus assigned a final diagnosis of (1) benign, (2) malignant, (3) mucinous, or (4) unknown. Patients who had metastasis to the pancreas from a non-pancreatic primary tumor were classified as having a malignant lesion.
Statistical Methods
We analyzed clinical, radiologic, and pathologic information from the patients’ electronic medical records to determine if there was a relationship between this information and the patients’ management and final diagnoses. The primary end points analyzed were whether surgical resection was performed and, if so, the results of surgical pathology. We performed chi-square tests to analyze the relationship between the categorical factors and the patients’ management and final diagnoses. Univariate and multivariate logistic regression models were used to assess the relationship between the clinical, radiologic, and pathologic factors and the likelihood of a patient undergoing resection and having malignant/premalignant surgical pathology. The backward selection method was applied to fit the multivariate model. In all our analyses, a P value of ≤0.05 was considered to be significant. We used the SAS 9.1 software package (SAS Institute Inc., Cary, NC, USA) to perform all statistical analyses.
RESULTS
Primary Diagnoses of Patients with Cystic lesions
The majority of pancreatic cystic lesions in this study (88%) were identified during interpretation of a CT scan performed for staging of a non-pancreatic malignancy (Table 2). The most common primary cancer diagnoses in our study population were lymphoma/leukemia and genitourinary cancer. Several patients possessed multiple primary non-pancreatic malignancies and were included in each relevant diagnostic category. Seventy-eight patients (22%) did not have a primary diagnosis of cancer; these patients had been either assessed for nonmalignant conditions or referred after incidental identification of a pancreatic cystic lesion at an outside facility. We reviewed the available records from the outside facilities to confirm that patients were asymptomatic at the time of referral for their pancreatic cyst. These patients are classified as “none” for initial cancer diagnosis.
Table 2.
Diagnostic and pathologic categories in patients with pancreatic lesions and comorbid cancer diagnoses
| Initial cancer diagnosis* | Number of patients (%) (n = 350) |
|---|---|
| Lymphoma/leukemia | 42 (12%) |
| Genitourinary | 41 (12%) |
| Prostate | 37 (11%) |
| Lower gastrointestinal | 37 (11%) |
| Breast | 32 (9%) |
| Gynecologic | 28 (8%) |
| Lung | 21 (6%) |
| Melanoma | 15 (4%) |
| Upper gastrointestinal | 13 (4%) |
| Hepatobiliary | 11 (3%) |
| Endocrine | 6 (2%) |
| Other | 25 (7%) |
| None† | 78 (22%) |
Refers to primary malignancy
These patients were assessed at The University of Texas M. D. Anderson Cancer Center for nonmalignant conditions or were referred after incidental identification of a pancreatic cystic lesion with no diagnosis of cancer otherwise.
Percentages are based on total number in each primary category.
The proportion of patients with benign and malignant cystic lesions did not differ significantly by primary cancer diagnosis; in each group, the majority of cysts (78–100%) carried a final diagnosis of benign or unknown (data not shown). In the group of 78 patients with no cancer diagnosis, 42 (54%) were classified as having benign cysts, and 36 (46%) were classified as having malignant or mucinous cysts, likely reflecting a higher proportion of patients with cystic lesions suspicious for malignancy among those referred from outside institutions.
Demographics and Follow-up
Table 3 summarizes the demographic, follow-up, and survival data for our study group according to each final diagnosis category. The mean age for the entire study population was 65 years, and the mean age did not differ significantly between patients with benign, malignant, mucinous, or unknown cystic lesions. The median length of follow-up was 32.7 months, and overall survival was 41.4 months. Of 350 patients with asymptomatic pancreatic cystic neoplasms, the final diagnostic categories were: 288 benign or presumed benign (“unknown”) (210 and 78 respectively, 82% total), 28 malignant (8%) and 34 mucinous (10%).
Table 3.
Patient demographics
| Clinical and Pathologic Final Diagnosis | |||||
|---|---|---|---|---|---|
| Total | Benign | Malignant | Mucinous | Unknown | |
| No. of patients with an incidental diagnosis (%) | 350 | 210 (60) | 28 (8) | 34 (10) | 78 (22) |
| Mean age at diagnosis, years (range) | 65 (14–97) | 65.5 (14–91) | 54.1 (22–87) | 66.8 (43–90) | 66.6 (23–97) |
| Sex | |||||
| Male (%) | 153 (44)* | 86 (56) | 14 (9) | 16 (10) | 37 (24) |
| Female (%) | 197 (56)* | 125 (63) | 13 (7) | 18 (9) | 41 (21) |
| Median follow-up, months (range) | 32.7 (0.1–160) | 41.6 (0.3– 45) | 18.8 (0.4 – 99) | 30.3 (0.3 – 160) | 11.8 (0–24) |
| Median overall survival, months (range) | 41.4 (0.1–182) | 49.7 (0.4–162) | 20.6 (0.4–123) | 47.0 (2.0–182) | 23.7 (0.1–159) |
| No. of patients who had surgical consultation (%) | 118 (34)* | 54 (46) | 17 (14) | 30 (25) | 17 (14) |
Percentages listed are based on the total for each category listed in the first column, except for those marked with an asterisk (*), which represent the percentage of the total study population.
Outcomes in Patients Referred for Surgical Consultation
One hundred eighteen patients (34%) were referred to a member of the Section of Pancreas Surgery at our institution for consultation. Most of these patients (60%) were assigned a final diagnosis of benign or unknown, whereas 30 (25%) patients had a final diagnosis of a mucinous lesion and 17 (14%) a malignant lesion. Thus, 47 (39.8%) of the 118 patients referred for surgical consultation had a final diagnosis other than benign or presumed benign (unknown), a rate significantly higher than the study population as a whole (see above). Finally, for those patients who underwent resection (n = 41), the final diagnostic categories were almost equally represented (34% benign, 27% malignant, and 39% mucinous).
Management of Cystic lesions
Figure 1 is a flow diagram of how the patients’ pancreatic cystic lesions were classified at each diagnostic step in their management: radiologic imaging, EUS (with or without FNA), and surgical resection. After radiologic imaging, 222 patients (63%) had an initial diagnosis of benign cystic lesions, 20 (6%) had an initial diagnosis of malignant cystic lesions, and 108 (31%) had an initial diagnosis of mucinous cystic lesions. The management of each of these groups of patients is described below and depicted in Figure 1.
Figure 1.
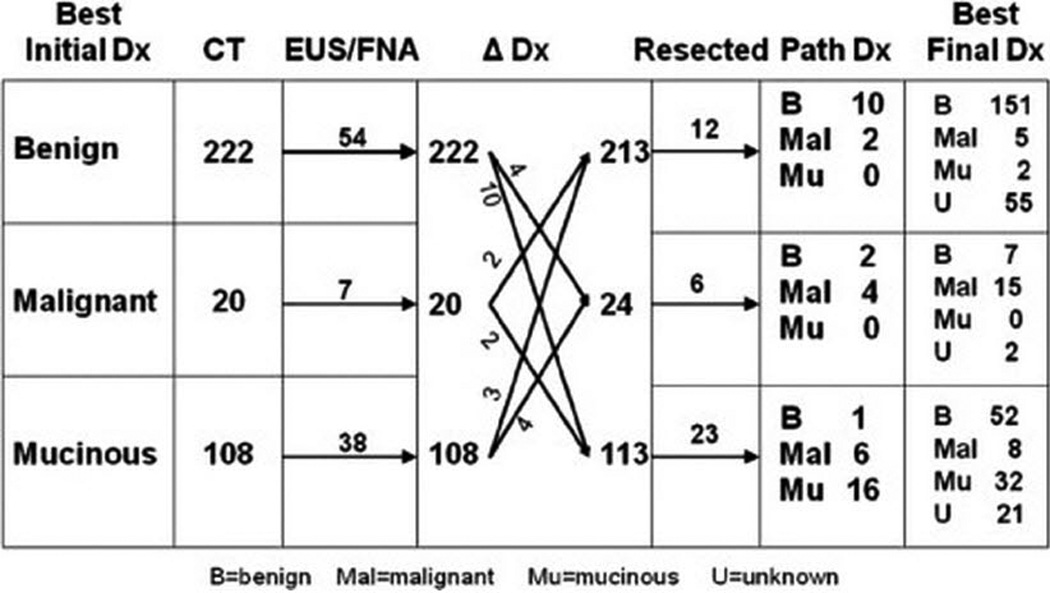
Flow diagram of diagnosis and management of 350 incidental cystic lesions. Based on initial radiologic diagnosis (by re-evaluation of imaging, if available, or radiologic diagnosis at the time of initial study), an initial diagnosis was assigned to each patient in terms of 3 diagnostic categories: benign, malignant, and mucinous. Ninety-nine of these patients underwent endoscopic evaluation (“EUS/FNA” column). EUS/FNA changed the working diagnosis in 25 patients (“Δ Dx” column), with the resulting changes in the number of patients in each category indicated. Forty-one patients underwent resection (“Resected” column), with the pathologic results shown (“Path Dx” column). Finally, a final diagnosis was assigned to each patient based on the pathology results, when available, or the clinical impression based on radiologic, EUS, and clinical factors.
Initial Diagnosis of Benign Cystic Lesion
Of the 222 patients who had an initial diagnosis of benign cystic lesion, 54 underwent EUS/FNA, which resulted in four patients being reclassified as a malignant cystic lesion and 10 as a mucinous cystic lesion (intermediate diagnosis). Two patients with an initial diagnosis of malignant cystic lesion and three with an initial diagnosis of mucinous cystic lesion were reclassified as having benign cystic lesion on the basis of EUS/FNA. Thus, 213 patients were in the benign category after CT and EUS/FNA.
Of these 213 patients with an intermediate diagnosis of benign cystic lesion, 12 underwent surgical resection for an increase in the size of the cystic lesion and/or the development of symptoms. Ten of these patients were found to have benign cystic lesions—nine had SCAs, and one had a pseudocyst. Two patients with an initial radiographic diagnosis of benign cystic lesions were diagnosed with malignant lesions based on surgical resection (prompted by symptom progression) that confirmed metastatic disease from their underlying cancers. None of the resected patients in this sub-group were found to have mucinous lesions on surgical pathology, and none had a primary pancreatic cancer.
Of the 222 patients initially diagnosed with benign cystic lesions, 206 (94%) were classified as having benign (151) or unknown (55) cystic lesions on the final diagnosis. The five patients who were classified as having malignant cystic lesions on the final diagnosis were all found to have metastasis to the pancreas from a nonpancreatic malignancy; none had a primary pancreatic cancer. The two patients with a final diagnosis of mucinous lesions had an original diagnosis of IPMN at the time of their presentation; thus, for these patients, there was inconsistency between the radiologic diagnoses provided at the time of staging evaluation and those provided following re-review of radiologic imaging as part of this study.
We assessed size and change in size as criteria for resection of lesions otherwise felt to be benign. In the 222 patients who were initially diagnosed with benign cystic lesions, the overall median follow-up time was 35 months, and the median radiographic follow-up time was 22 months. Fifty-two of these patients (23%) had a change in the size of their cystic lesions during the course of follow-up. Sixteen of these patients (7%) experienced a decrease in the size of their cystic lesions, and all of these patients had a final diagnosis of benign (n = 15) or unknown (n = 1); none of these patients underwent resection. Thirty-six patients had cystic lesions that grew by an average of 0.62 cm (range, 0.1–2.4 cm) over a median follow-up time of 28 months. Overall, 35 of these patients (97%) had benign cystic lesions on final diagnosis. No malignant or mucinous cystic lesions were identified based on a size > 4 cm (seven cystic lesions were all consistent with SCA pathology on radiologic imaging). Of 131 patients with cystic lesions that maintained a stable size, nine had cystic lesions > 4 cm. Eight of these patients were referred to our surgical group for consultation, and only one of these patients had a malignant cystic lesion (in the form of a metastasis from a nonpancreatic primary malignancy). This patient ultimately underwent resection for palliation of symptoms.
Initial Diagnosis of Malignant Cystic Lesion
Twenty patients had an initial diagnosis of malignant cystic lesion (Figure 1). After EUS/FNA, four patients were re-classified as having either benign or mucinous cystic lesions. In addition, four patients from the initial benign category and four from the initial mucinous category were re-classified as having malignant lesions, for a total of 24 patients with an intermediate diagnosis of malignant cystic lesion. Seven of these patients were referred for surgical evaluation, and six patients ultimately underwent resection. Surgical pathology revealed two benign cystic lesions (one SCA and one serous pseudopapillary tumor) and four malignant lesions (one pancreatic neuroendocrine tumor, one mucinous cystadenocarcinoma, and two metastatic lesions from non-pancreatic primary tumors).
Of the 20 patients with a malignant lesion on initial diagnosis, nine had a final diagnosis of benign or unknown, including two patients who had an SCA or serous pseudopapillary tumor on surgical pathology and five patients who had an SCA or cysts that were originally believed to represent simple cysts based on CT appearance. These latter patients represent inconsistency between the original radiographic evaluation and the review performed for this study.
Initial Diagnosis of Mucinous Cystic Lesion
Of the 108 patients with an initial diagnosis of mucinous lesion, 38 underwent EUS/FNA and 23 underwent resection. Among the patients who underwent resection, one had a benign cystic lesion that was found to be an SCA and six had malignant lesions: —three had neuroendocrine tumors, one had adenocarcinoma, and two had metastases from underlying disease that were re-read as mucinous in the course of the radiologic review for this study. Two patients with malignant disease did not undergo resection; one patient had an adenocarcinoma with necrosis (unresectable due to the presence of metastases), and one patient had a mucinous cystadenocarcinoma. Based on the original radiologic evaluations of benign lesions at the time of presentation, 52 patients were finally classified as having benign cystic lesions and 21 patients having unknown cystic lesions (based on follow-up time). Thus, a total of 32 of 108 patients (30%) were correctly classified as having mucinous lesions on initial diagnosis.
Malignant Cystic Lesions on Final Diagnosis
We were most concerned about the possibility that a patient with a malignant or premalignant cystic lesion could be assigned to the benign category, representing a false-negative diagnosis. Of 213 patients diagnosed with benign cystic lesions based on radiologic imaging with or without EUS/FNA (i.e., an intermediate diagnosis of benign), five had a final diagnosis of malignant disease (all with metastasis to the pancreas from a non-pancreas tumor), and two had mucinous neoplasms. Thus, the false-negative rate was 3.3%. In the malignant group the true-positive rate was 62.5%. Among the 113 patients assigned to the mucinous group based on CT with or without EUS/FNA, eight patients (7%) were ultimately found to have invasive cancer and 32 (28%) had a mucinous lesion.
Figure 2 is a flow diagram of the patients with a final diagnosis of malignant lesions. Of 28 patients with a final diagnosis of malignant lesions (8% of the total study population), 15 had an initial diagnosis of malignant lesions, for an accuracy rate of 53.6% The malignant lesions were primary pancreatic cancer in 18 patients and metastasis to the pancreas in 10 patients, reflecting our cancer center patient population.
Figure 2.
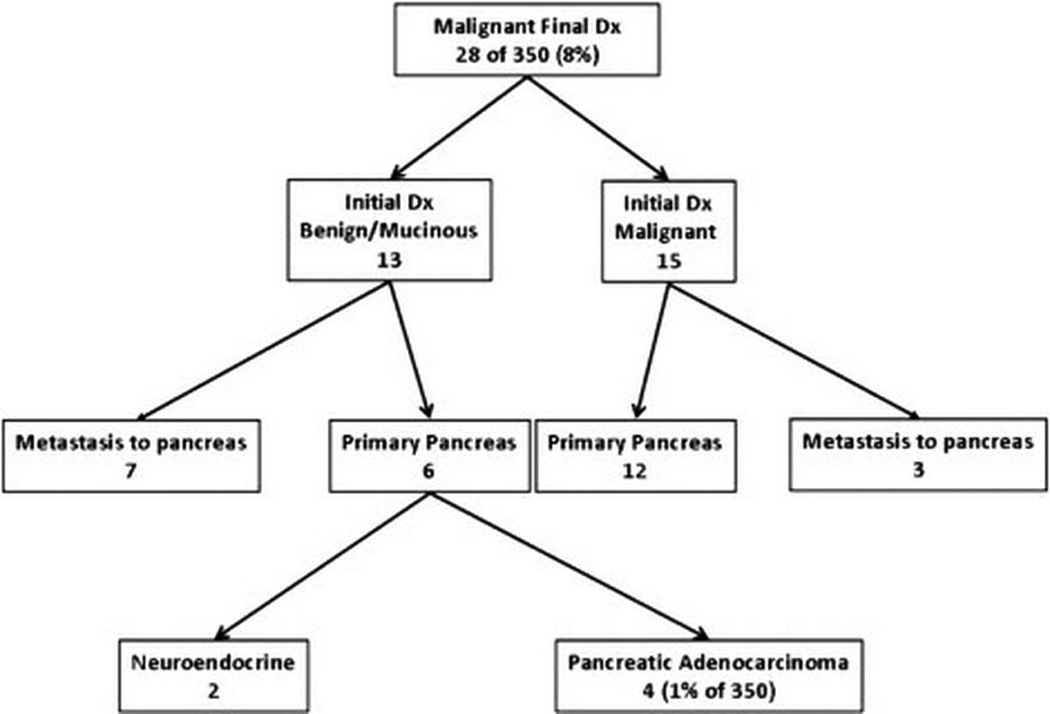
Patients with a final diagnosis of malignant cystic lesions. Patients are presented in terms of their initial diagnosis and the specific clinical entities with which they were diagnosed.
Thirteen patients with a final diagnosis of malignant cystic lesions had an initial diagnosis of benign or mucinous cystic lesion. Seven of these patients had metastatic lesions to the pancreas, while six had primary pancreatic cancer. Only four of these patients had a pancreatic adenocarcinoma, representing 1% of the 350 patients in this study population with an incidental pancreatic cystic lesion. In theory, these represent possible missed malignancies, but no cases of resectable adenocarcinoma were actually missed in our series.
Factors Associated with Resection and Malignant or Mucinous Pathology
A selective strategy for surgical management was employed for asymptomatic pancreatic cystic lesions, as previously described3. We analyzed the clinical, radiologic, and pathologic factors associated with whether patients underwent resection. On univariate analysis (Table 4), younger patient age, referral to our institution for management of pancreatic lesion without another diagnosis, lesion size > 3 cm on CT, nodular component on CT, malignant diagnosis on radiologic imaging, and performance of EUS, were significantly associated with surgical resection. On multivariate analysis (Table 5), only younger patient age, EUS diagnosis of malignant or mucinous lesion, lesion size > 3 cm on CT, and primary radiologic diagnosis of malignant or mucinous lesion were significantly associated with undergoing resection.
Table 4.
Univariate analysis of clinical, radiologic, and pathologic features associated with resection
| Clinicopathologic Features | OR | 95% CI | P value |
|---|---|---|---|
| Demographic and clinical features | |||
| Age | 0.95 | 0.93–0.97 | < 0.0001 |
| Initial referral for pancreatic lesion | 9.30 | 4.20–20.6 | < 0.0001 |
| Radiologic features | |||
| Size > 1 cm | 0.63 | 0.32–1.22 | 0.17 |
| Size > 3 cm | 4.20 | 2.01–8.77 | < 0.0001 |
| Change in size | 0.55 | 0.22–1.40 | 0.21 |
| Septations | 1.49 | 0.71–3.11 | 0.29 |
| Calcifications | 0.82 | 0.39–1.71 | 0.59 |
| Solid component | 2.08 | 0.66–6.63 | 0.21 |
| Nodular component | 5.03 | 2.18–11.60 | < 0.0001 |
| Wall thickening | 2.93 | 0.75–11.4 | 0.12 |
| 2–5 cysts vs unilocular | 0.63 | 0.21–1.87 | 0.40 |
| > 6 cysts vs unilocular | 1.85 | 0.84–4.11 | 0.13 |
| Lobular wall vs smooth | 1.35 | 0.64–2.84 | 0.42 |
| Irregular wall vs smooth | 0.57 | 0.19–1.71 | 0.32 |
| Primary radiologic diagnosis | 4.18 | 1.90–9.21 | 0.0001 |
| Endoscopic features | |||
| EUS performed | 6.35 | 3.16–12.7 | < 0.0001 |
| EUS diagnosis of malignant lesions | 4.26 | 1.70–10.70 | 0.002 |
| Size on EUS (as continuous variable) | 1.01 | 0.98–1.03 | 0.65 |
| Septations | 2.56 | 0.63–10.4 | 0.19 |
| Calcifications | 2.13 | 0.33–13.7 | 0.42 |
| Communication with pancreatic duct | 3.14 | 0.51–19.2 | 0.22 |
| Pancreatic duct dilatation | 2.69 | 0.62–11.8 | 0.19 |
| Solid component | 5.20 | 0.97–28.0 | 0.06 |
| Mucin present | 3.54 | 1.91–13.70 | 0.07 |
CI, confidence interval; EUS, endoscopic ultrasonography; OR, odds ratio.
Table 5.
Multivariate analysis of clinicopathologic features associated with resection
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Age | 0.93 | 0.89 – 0.96 | < 0.0001 |
| EUS diagnosis of malignant lesions | 11.04 | 3.97–30.64 | < 0.0001 |
| Size on CT > 3 cm | 4.64 | 1.75–12.33 | 0.002 |
| Primary radiologic diagnosis | 4.15 | 1.61– 10.68 | 0.003 |
CI, confidence interval; CT, computed tomography; EUS, endoscopic ultrasonography; OR, odds ratio.
A total of 41 patients underwent resection; in these patients, univariate analysis demonstrated a significant association between malignant or mucinous surgical pathology and a primary radiologic diagnosis of malignant or mucinous lesion (Table 6). No factors could be associated with malignant/mucinous surgical pathology on multivariate analysis.
Table 6.
Univariate analysis of factors associated with malignant or mucinous surgical pathology
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Demographic and clinical Features | |||
| Age (continuous) | 1.04 | 1.00–1.09 | 0.064 |
| Initial referral for pancreatic lesion | 6.22 | 0.69–55.8 | 0.102 |
| Initial surgical management | 0.28 | 0.03–2.50 | 0.252 |
| Size of cyst on CT | |||
| > 3 cm | 2.10 | 0.37–11.8 | 0.40 |
| Location of cyst on CT | |||
| Head | 1.42 | 0.13–15.6 | 0.77 |
| Uncinate | 0.36 | 0.03–4.66 | 0.43 |
| Neck | 0.08 | 0.01–0.87 | 0.04 |
| Body | 0.09 | 0.01–1.04 | 0.05 |
| Tail | 4.00 | 0.37–43.4 | 0.25 |
| Primary radiologic diagnosis | >999.999 | NA | NA |
| Endoscopic features | |||
| EUS performed | 6.00 | 1.21–29.7 | 0.03 |
| Diameter of cyst | 0.99 | 0.94–1.05 | 0.7656 |
CI, confidence interval; CT, computed tomography; EUS, endoscopic ultrasonography; NA, not applicable; OR, odds ratio.
The effect of various factors on survival could not be meaningfully tested in our cancer patient population, but we did note that few patients died of pancreatic disease during the follow-up period of this study. During the follow-up period, 30% of the patients died, with 11 deaths (3%) attributed to pancreatic cancer. Ten of these 11 patients died of metastatic disease, and one patient died perioperatively. No patients missed an appropriate opportunity for resection. In the remaining 94 patients who died of other causes, one possible opportunity for resection was missed because the patient was lost to follow-up.
DISCUSSION
In general, our data indicate that asymptomatic incidental cystic lesions of the pancreas are usually benign, and that a reliable diagnosis can usually be determined by adequate imaging, preferably a pancreatic-protocol CT scan, when interpreted by an experienced radiologist. Patients with cystic lesions that are identified as likely malignant or mucinous based on pancreas-protocol CT should generally be evaluated to determine if surgical resection is appropriate. In lesions that are followed nonoperatively, a minority will increase in size; while an increase in size is often taken as evidence that surgical resection is indicated, we did not identify an association between growth and malignant or mucinous pathology. In our study, radiographic evaluation alone was less reliable for ruling out invasive cancer in patients with suspected mucinous lesions. EUS/FNA changed the diagnosis in some patients and tended to change benign diagnoses to malignant and mucinous diagnoses—confirming the ability of EUS/FNA to raise suspicions or establish an indication for surgical resection. EUS/FNA was not used to rule out the need for surgical resection. Our selective operative approach for patients with incidentally identified pancreatic cystic lesions did not miss any “windows of opportunity” to appropriately treat patients with resectable pancreatic cancer, supporting the hypothesis that most asymptomatic pancreatic cystic lesions can be managed by selective serial observation rather than immediate surgical resection.
The overall median follow-up time in our study was 32 months. We were able to identify 151 patients with an initial diagnosis of benign cystic lesion who had follow-up greater than 2 years whom we designated benign by final diagnosis. We also identified 55 patients who were designated as “unknown” because their follow-up time was less than 2 years. This suggests that the evaluation and management of patients with incidentally identified cystic pancreatic lesions is analogous to the evaluation and management of patients with small pulmonary nodules, which are generally serially imaged for 2 years; surveillance beyond 2 years is generally not recommended when pulmonary nodules are stable for this period of time.6 In our study, a large group of patients had stable disease over a 2-year follow-up, and in these patients it appears unlikely that their cystic lesions will ever become a clinically significant management problem requiring surgical intervention. Our experience suggests, then, that patients may be safely transitioned to a less-intensive surveillance strategy after an initial intensive follow-up period of approximately 2 years’ time.
With the more frequent use of abdominal imaging during the initial staging work-up for cancer patients, identification of incidental cystic lesions of the pancreas is becoming more common. The incidence of cystic lesions ranged from 0.2–1.2% in two radiologic imaging series7–8 to 24.3% in one autopsy series.9 Most cystic lesions are benign and do not require resection. However, in a recent series of 539 consecutive patients with cystic lesions, 170 patients had surgical pathology review of the cyst, and 18% were found to have a malignant cystic lesion.10 Other studies have reported similar rates of malignant pathology in patients with pancreatic cystic lesions.7, 11
Unlike symptomatic cystic lesions, data on asymptomatic cystic lesions have indicated a relatively low risk of malignancy, but the natural history of such lesions, especially smaller lesions, remains unknown.5 Though an aggressive approach has been advocated for patients with asymptomatic pancreatic cystic lesions regardless of imaging characteristics, the low risk of malignancy makes a selective approach attractive, thus sparing most patients the risks of mortality and morbidity associated with pancreatic resection. Fernandez-Castillo et al. reported that 2.5% of asymptomatic patients with a pancreatic cyst had a pathologic diagnosis of invasive ductal adenocarcinoma versus 9% in symptomatic patients.1 In that series of mostly resected patients, 17% percent of asymptomatic patients had invasive or in situ carcinoma, and 42% had premalignant lesions. In contrast, Walsh et al.12 performed a prospective study in which patients with asymptomatic pancreatic cystic lesions for whom no evidence of either cancer or premalignant lesions was initially found were observed. In that study, one of 141 patients initially managed with observation ultimately underwent resection (for a mucinous neoplasm) during a 24-month follow-up period.12 Such reports reveal an unexplained inconsistency in the observed rates of malignancy and premalignancy between asymptomatic patients who are treated operatively and patients whom specialists are willing to selectively treat nonoperatively. The “true” risk that cystic lesions are malignant in the latter group of patients remains unknown, largely because data on these patients are absent from exclusively surgical series.
Das et al. have suggested that most MCNs and branch IPMNs that are initially < 3 cm are not likely to grow prior to 2 years from baseline evaluation, and patients can therefore begin surveillance at 2 years.13 We do not currently advocate observation of most lesions that are initially identified as MCNs. In our study, only 18 of 34 patients did not undergo resection, and nine of these patients were followed by surgeons; one patient had metastatic disease at work-up, and the remaining eight had stable disease with cystic lesions < 3 cm and were usually observed due to the presence of significant comorbidities or advanced age. Therefore, we can provide no data relevant to observing MCNs in this study, except that no patient who underwent resection for suspicion of an MCN had benign pathology as defined in our study.
An important issue our study does not address specifically is the question of how to manage lesions believed to be side-branch IPMN. Our study did not capture enough data on patients with small side-branch IPMNs to identify a high-risk subgroup. The current strategy at our institution is to follow the Consensus guidelines. Specifically, we perform serial reimaging without surgery for patients with lesions < 3 cm in size. Pelaez-Luna et al. have recently suggested that the absence of any consensus indications for resection in a given patient (size > 3 cm, symptoms, main duct size > 10 mm, intramural nodules, and suspicious/malignant cystic fluid cytology) allows the safe observation of branch IPMN.14 Likewise, Schmidt et al. have suggested that IPMN type, main duct diameter, number of branch IPMN lesions, and cyst cytology are predictive of invasive and malignant pathology but that size is not.15
Most large surgical series of patients with pancreatic cystic lesions have reported data for patients who undergo resection and have provided the statistical basis of our current understanding of the risk of malignancy associated with pancreatic cystic lesions. Fernandez-del Castillo et al. reported that of asymptomatic patients with pancreatic cystic lesions who underwent resection, 17% had invasive or in situ cancer and 42% had premalignant lesions1. In that study, 78% of asymptomatic patients who were referred for surgical consultation underwent resection. In calculating the relative incidence of cancer or premalignant disease, Fernandez-del Castillo et al. included patients in whom radiologic diagnoses of malignant and premalignant disease could be made prior to surgery. In our study, when we excluded patients in whom a clear surgical indication was present at initial work-up, we found that very few patients could be classified as having malignant or premalignant disease (7 of 213 patients [3%]). When patients were referred for surgical evaluation that proportion rose to 13% (7 of 54 patients). Additional evaluation changed the intermediate diagnosis such that among the 16 resected patients who had an initial diagnosis of benign cystic lesions, 12 underwent resection. Two of these patients were found to have malignant cystic lesions (17%), and no patients had premalignant disease. Thus, our study is consistent with the findings presented by Fernandez-del Castillo et al. in terms of the rate of malignant disease and illustrates very clearly that the clinical concern that prompts surgical evaluation and resection progressively enriches the proportional incidence of malignant and premalignant disease in the patient group studied.1
Our results are most relevant for patients in whom uncharacterized cystic lesions or serous cystic lesions are identified incidentally, as management algorithms for suspected malignant and mucinous cystic lesions have already been established. In the largest single-institution series on serous cystic lesions reported thus far, Galanis et al. focused their attention on serous cystic neoplasms and found an exceedingly low rate of malignancy regardless of symptom status,4 which is in keeping with our results. Likewise, Lee et al. reported a 3.3% incidence of malignancy in asymptomatic patients with no initial features of malignancy.17 In another study, Tseng et al. analyzed the growth rate of serous cystic adenomas and found that lesions > 4 cm tended to grow faster and be more symptomatic.18 However, in that study, as in our study, a relationship between tumor size and malignancy could not be demonstrated. Thus, our experience is in agreement with prior studies demonstrating that patients with pancreatic cystic lesions that are asymptomatic and have no features of malignant or premalignant disease on initial work-up can be safely observed. Moreover, surgical evaluation for small asymptomatic cystic lesions that are otherwise bland may not be mandatory.
In this study, we have demonstrated the success of a selective management approach for asymptomatic pancreatic cystic lesions. Walsh et al. prospectively followed a group of patients and found no cases of malignancy in the few patients who required resection over a short follow-up period.12 Similarly, the experience reported by surgeons at Memorial Sloan-Kettering Cancer Center10 supports a selective management approach. Our study expands knowledge of the natural history of asymptomatic pancreatic cystic lesions because we included patients who did not come to surgical attention. In this group, pancreas-related morbidity and mortality is generally limited to those few patients with known malignant diagnosis who have unresectable and/or metastatic disease. In our study, most asymptomatic patients with pancreatic cystic lesions lacking malignant or mucinous features exhibited no sequelae of their lesions, and few developed symptoms.
Our study population is distinct in several ways from those in other studies of pancreatic cystic lesions. First, by focusing on asymptomatic patients, we were specifically focused on and confirmed the presence of a low-risk group of patients with pancreatic cystic lesions. Second, we included patients who were seen by specialist surgeons as well as patients who were not referred for surgical consultation. The relative proportion of patients who had malignant or mucinous lesions in the overall group was lower than in the group of patients who came to our institution for a consultation, with the highest proportion of malignant or mucinous pathology in the group that underwent resection. This finding illustrates how surgical series of patients with pancreatic cystic lesions may generally be biased in terms of reporting the incidence of invasive cancer, suggesting that the higher rates of malignancy reported in these studies could be explained by the fact that the group of patients sent for surgical consultation is not representative of all patients with pancreatic cystic lesions. This may be a result of higher-resolution imaging, which detects small cystic lesions of little clinical significance with greater frequency. Finally, our study population is distinct from other reports since the majority of our patients had a primary nonpancreatic malignancy, therefore we were unable to perform a meaningful survival analysis.
As described previously by Katz et al., the most important component of the management approach was the initial radiographic evaluation. This was validated in our current study, in which the primary radiologic diagnosis was the best predictor of malignant or premalignant disease.3 Our approach can be summarized as follows. After ruling out the possibility of an inflammatory pseudocyst, a high-quality imaging study should be obtained. At that point, an initial diagnosis of a serous cystadenoma or a mucinous lesion may be possible, but a number of lesions will remain indeterminate. For such lesions, if clinical concern is high enough, we recommend further evaluation by EUS/FNA. If the lesion remains indeterminate and if the patient does not have any contraindications to surgery, we would offer surgical resection. For patients with SCA, we consider resection for all lesions > 4 cm based on these lesions’ proclivity to grow and become symptomatic, but not on the basis of an increased risk of malignancy. For lesions < 4 cm, we obtain serial imaging to determine the stability or growth rate of the lesion, usually at 6 month intervals initially (up to 2 years) followed by yearly studies. In all cases, the development of symptoms serves as an indication for resection. In cases thought to represent mucinous lesions, those believed to be IPMN are observed if they are side-branch lesions that are < 3 cm, but these lesions require serial imaging to establish stability. Otherwise, we recommend that any suspected MCN, all main-branch IPMNs, and branch IPMN > 3 cm should be resected.
To summarize, we have presented data on patients with asymptomatic cystic lesions of the pancreas to identify those patients who can be safely followed without immediate surgical intervention. We conducted a radiographic review and chart review to group patients into diagnostic categories at different points during their evaluation and disease course. We found that benign lesions could be reliably identified at the outset, with a very low risk of missed malignancy or progression to primary pancreatic cancer. We documented that mucinous lesions could harbor occult malignancy, thus warranting an aggressive approach. Finally, resectable primary pancreatic cancer was rarely identified among patients with asymptomatic, incidentally identified pancreatic cysts. In these cases, evaluation should be thorough and take the patient’s underlying disease, if any, into account.
Abbreviations
- MCN
mucinous cystic neoplasm
- IPMN
intraductal papillary mucinous neoplasm
- EUS
endoscopic ultrasonography
- FNA
fine-needle aspiration
- CT
computed tomography
- SCA
serous cystadenocarcinoma
- NOS
not otherwise specified
- CI
confidence interval
- OR
odds ratio
- CEA
carcinoembryonic antigen
- CA19-9
carbohydrate antigen 19-9
REFERENCES
- 1.Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003 Apr;138(4):427–423. doi: 10.1001/archsurg.138.4.427. discussion 433-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol. 2007 Oct;102(10):2339–2349. doi: 10.1111/j.1572-0241.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- 3.Katz MH, Mortenson MM, Wang H, et al. Diagnosis and management of cystic neoplasms of the pancreas: an evidence-based approach. J Am Coll Surg. 2008 Jul;207(1):106–120. doi: 10.1016/j.jamcollsurg.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 4.Galanis C, Zamani A, Cameron J, et al. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg. 2007;11(7):820–826. doi: 10.1007/s11605-007-0157-4. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Chari ST, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2005;6(1–2):17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 6.Gould MK, Ananth L, Barnett PG. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007 Feb;131(2):383–388. doi: 10.1378/chest.06-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spinelli K, Fromwiller T, Daniel R, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239(5):651–657. doi: 10.1097/01.sla.0000124299.57430.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SH, Shin CM, Park JK, et al. Outcomes of cystic lesions in the pancreas after extended follow-up. Dig Dis Sci. 2007 Oct;52(10):2653–2659. doi: 10.1007/s10620-006-9634-y. [DOI] [PubMed] [Google Scholar]
- 9.Edirimanne S, Connor SJ. Incidental pancreatic cystic lesions. World J Surg. 2008 Sep;32(9):2028–2037. doi: 10.1007/s00268-008-9633-6. [DOI] [PubMed] [Google Scholar]
- 10.Allen PJ, D'Angelica M, Gonen M, et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006 Oct;244(4):572–582. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goh BK, Tan YM, Cheow PC, et al. Cystic lesions of the pancreas: an appraisal of an aggressive resectional policy adopted at a single institution during 15 years. Am J Surg. 2006 Aug;192(2):148–154. doi: 10.1016/j.amjsurg.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Walsh RM, Vogt DP, Henderson JM, et al. Natural history of indeterminate pancreatic cysts. Surgery. 2005 Oct;138(4):665–670. doi: 10.1016/j.surg.2005.07.019. discussion 670-661. [DOI] [PubMed] [Google Scholar]
- 13.Das A, Wells CD, Nguyen CC. Incidental cystic neoplasms of pancreas: what is the optimal interval of imaging surveillance? Am J Gastroenterol. 2008 Jul;103(7):1657–1662. doi: 10.1111/j.1572-0241.2008.01893.x. [DOI] [PubMed] [Google Scholar]
- 14.Pelaez-Luna M, Chari ST, Smyrk TC, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007 Aug;102(8):1759–1764. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt C, White P, Waters J, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246(4):644–651. doi: 10.1097/SLA.0b013e318155a9e5. [DOI] [PubMed] [Google Scholar]
- 16.Farnell MB. Surgical management of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. J Gastrointest Surg. 2008 Mar;12(3):414–416. doi: 10.1007/s11605-007-0349-y. [DOI] [PubMed] [Google Scholar]
- 17.Lee CJ, Scheiman J, Anderson MA, et al. Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008 Feb;12(2):234–242. doi: 10.1007/s11605-007-0381-y. [DOI] [PubMed] [Google Scholar]
- 18.Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandez-del Castillo C. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005 Sep;242(3):413–419. doi: 10.1097/01.sla.0000179651.21193.2c. discussion 419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]


