Abstract
Objective
To evaluate the impact of smoking exposure on oncological outcomes in patients with upper tract urothelial carcinoma (UTUC) treated with radical nephroureterectomy (RNU).
Materials and Methods
Patient and disease characteristics from 288 patients with UTUC treated with RNU between 1995 and 2008 were collected from a prospectively maintained database at the Memorial Sloan-Kettering Cancer Center.
Disease recurrence was defined as distant metastases, or local failure in the operative site or regional nodes.
Factors associated with recurrence and death were determined.
Results
The prevalence of current, former and never smoking at diagnosis was 19.1%, 55.2%, and 25.7%, respectively.
71.0% of patients reported a ≥20 pack-year smoking history.
With a median follow-up of 4.02 years, disease recurrence occurred in 27% (n = 79) of patients and 41% (n = 117) died during follow-up.
While age at diagnosis, American Society of Anesthesiologists score, advanced stage, nodal involvement and high grade adversely affected recurrence-free survival, smoking status was not associated with risk of recurrence or death in multivariate analysis (P = 0.60).
Multivariate competing risks regression showed that current smokers faced a significantly higher risk of death than never smokers (hazard ratio 3.64, 95% confidence interval 1.59–8.34).
Conclusions
While smoking status at diagnosis and cumulative smoking exposure were not associated with UTUC recurrence, our findings highlight the substantial risk of death in patients with UTUC who are active smokers.
Treatment plans to promote smoking cessation are recommended for these patients.
Keywords: tobacco, smoking, urinary bladder neoplasms, outcome assessment, nephrectomy, prognosis
Introduction
Upper tract urothelial carcinoma (UTUC) is a rare cancer, accounting for 5–7% of all urothelial malignancies [1]. The 5-year survival rate is nearly 90% for patients with early-stage UTUC but decreases to <30% in patients with regional nodal metastases and <10% in patients with distant metastases [2]. Most strategies to reduce disease recurrence and progression have focused on optimizing diagnostic imaging and surgical techniques, with little attention given to addressing modifiable risk factors, such as cigarette smoking.
Cigarette smoking is the primary causative risk factor for both UTUC and lower tract urothelial carcinoma [3]. Cigarette smoking accounts for 50% of urothelial carcinoma cases in males and 30% in females [4,5], smokers are three times more likely to develop urothelial carcinoma than never smokers, and risk increases with lifetime duration of smoking and mean number of cigarettes smoked per day [5,6]. Smoking is also an important modifier of genetic risk, as smokers who carry variants in NAT2 and GSMT1 are at highest risk of developing urothelial carcinoma [7]. Although risk levels never return to those of never smokers [8], an immediate decrease in risk of ~40% is observed among those with high risk genetic polymorphisms who stop smoking, which implies that tobacco has a late-stage effect in urothelial carcinogenesis.
Despite evidence from animal models and cell lines suggesting that constituents in tobacco smoke may promote cancer growth by increasing cellular proliferation and decreasing apoptosis [9–13], previous clinical studies on the impact of smoking on the prognosis of lower tract urothelial carcinoma have yielded mixed results. In 2002, a systematic review of the effect of smoking on prognosis concluded that there was evidence to suggest that smoking cessation might favourably alter the course of bladder cancer [14]. Since then, one study reported that continued smoking after diagnosis was a significant predictor of shorter recurrence-free survival (RFS), while a second study found that smoking status at diagnosis was not associated with response to BCG therapy, disease recurrence, progression, all-cause mortality, or urothelial cancer-specific mortality [15,16]. For UTUC, only one study, based on 105 patients, has examined survival differences between ever and never smokers [17]. To inform clinical practice, more research is needed to elucidate the role of smoking on the prognosis of UTUC, particularly with respect to recency of smoking exposure.
In the present study, we examined the impact of smoking status and cumulative smoking exposure on the risk of recurrence and death among 288 patients with UTUC treated with radical nephroureterectomy (RNU) in a single high-volume referral center. We hypothesized that patients who were current smokers at the time of diagnosis would experience decreased RFS compared with patients who had stopped smoking before diagnosis (former smokers) or never smoked cigarettes.
Materials and Methods
Patient Selection
Data from 324 consecutive patients with UTUC who underwent RNU with ipsilateral bladder cuff resection from 1995 to 2008 were obtained from a prospectively maintained clinical database. Genitourinary surgeons performed surgery according to standard protocol for RNU, which involves excision of the kidney with the entire length of the ipsilateral ureter and adjacent bladder cuff. The hilar and regional lymph nodes adjacent to the ipsilateral great vessels were resected as needed, along with enlarged lymph nodes that were either identified on preoperative CT scans or palpable intraoperatively. The institutional review board reviewed and approved the request for exemption of this retrospective chart review of clinical data and approved waivers for Health Insurance Portability and Accountability Act authorization and informed consent. Patients were excluded if they were treated with neoadjuvant chemotherapy (n = 30) or had missing information regarding smoking history (n = 6), resulting in a total sample size of 288.
Pathological Evaluation
Surgical specimens were processed using standard pathological techniques and reviewed by genitourinary pathologists. All specimens were histologically confirmed to be urothelial carcinoma. Tumours were staged according to the 2002 American Joint Committee on Cancer TNM classification [18]. Tumour grading was assessed according to the 1998 WHO consensus classification [19].
Follow-Up Regimen
Patients were seen every 3 months for the first year after RNU, every 4 months for the second year, every 6 months for the third, fourth and fifth years, and annually thereafter. Follow-up consisted of history, physical examination, serum chemistry studies, urinary cytology, chest radiography, cystoscopic evaluation and radiographic evaluation of the contralateral upper urinary tract. Disease recurrence was defined by pathologically proven tumour in the operative site, regional nodes or distant metastasis. The cause of death was determined by chart review and corroborated by death certificates.
Smoking Exposure
Self-reported smoking data were routinely collected at the time of diagnosis on all urology patients. Available variables for analysis included smoking status (current, former, or never smoker), duration of smoking (<20, 20–39, and ≥40 years), and quantity smoked (1–10, 11–20, 21–30, and >30 CPD – cigarettes per day). Patients were considered current smokers if they reported smoking at the time of diagnosis or stopped smoking within 1 year of diagnosis, and were considered former smokers if they had stopped smoking at least 1 year before diagnosis. Duration of smoking and CPD were used to construct pack-years smoked for all former and current smokers (<20 vs ≥20 pack-years) as a measure of cumulative smoking exposure.
Statistical Analyses
Descriptive statistics were calculated for demographic, disease and smoking characteristics. Associations between categorical variables were assessed using the chi-squared test and the Kruskal–Wallis test was used for continuous variables. Follow-up time was calculated from the date of diagnosis to the first of either recurrence or death. Patients alive and without disease recurrence were censored at the date of their last follow-up. RFS probabilities were estimated using the Kaplan–Meier method, in which recurrence and death were included as events. The log-rank test determined differences in survival function between groups. Because smoking is an established risk factor for common health problems that increase risk of death, we also conducted competing risks analyses. The cumulative incidence was estimated, where disease recurrence was treated as the primary event of interest, and death without recurrence as a competing event. Gray’s test was used to determine differences in cumulative incidence function between groups [20]. Multivariate Cox regression and competing risks regression models were adjusted for traditional prognostic factors that were identified a priori and included age at diagnosis, American Society of Anesthesiologists (ASA) score, pathological stage and grade, and nodal status. A P value <0.05 was considered to indicate statistical significance. Statistical analyses were conducted using SAS (version 9.2. Cary, NC: SAS Institute; 2008) and R (version 2.11.0. R Development Core Team; 2010), including the ‘SURVIVAL’ and ‘CMPRSK’ packages.
Results
Table 1 shows the associations between demographic and disease characteristics by smoking status. The patient population was 92% white and 65% male, with a median (range) age at diagnosis of 71 (37–90) years. Forty-seven percent of patients had ASA scores ≥3, 26% had advanced disease based on pathological tumour stage (pT ≤ 2), and 10% had lymph node involvement based on nodal stage. High grade disease was seen in 77% of patients. Nineteen percent of patients were current smokers at the time of diagnosis and 55% were former smokers. Of the ever smokers, 71% smoked for ≥20 pack-years. Compared with never and former smokers, current smokers were significantly more likely to be younger, and have localized disease, and low grade disease (all P < 0.05). Never smokers were more likely than current and former smokers to be female (P < 0.001).
Table 1.
Characteristics of 288 patients with UTUC by smoking status.
| Characteristic | Smoking status
|
P* | |||
|---|---|---|---|---|---|
| Overall | Never 74 (25.7) | Former 159 (55.2) | Current 55 (19.1) | ||
| Median (range) age at diagnosis | 71.0 (37.0–90.0) | 73.0 (43.0–88.0) | 72.0 (38.0–90.0) | 64.0 (37.0–82.0) | <0.001 |
| Sex, n (%) | <0.001 | ||||
| Male | 187 (64.9) | 35 (47.3) | 117 (73.6) | 35 (63.6) | |
| Female | 101 (35.1) | 39 (52.7) | 42 (26.4) | 20 (36.4) | |
| Median (range) body mass index | 26.9 (15.6–42.7) | 26.6 (17.1–42.2) | 27.3 (17.8–42.7) | 26.9 (15.6–42.4) | 0.318 |
| Race, n (%) | 0.795 | ||||
| White | 266 (92.4) | 68 (91.9) | 146 (91.8) | 52 (94.5) | |
| Other | 22 (7.6) | 6 (8.1) | 13 (8.2) | 3 (5.5) | |
| ASA score†, n (%) | 0.167 | ||||
| Healthy | 152 (52.8) | 46 (62.2) | 78 (49.1) | 28 (50.9) | |
| Not healthy | 136 (47.2) | 28 (37.8) | 81 (50.9) | 27 (49.1) | |
| pT stage‡, n (%) | 0.031 | ||||
| Localized | 213 (74.0) | 55 (74.3) | 110 (69.2) | 48 (87.3) | |
| Advanced | 75 (26.0) | 19 (25.7) | 49 (30.8) | 7 (12.7) | |
| N stage, n (%) | 0.071 | ||||
| Nx or N0 | 260 (90.3) | 64 (86.5) | 142 (89.3) | 54 (98.2) | |
| N1 or N2 | 28 (9.7) | 10 (13.5) | 17 (10.7) | 1 (1.8) | |
| Grade, n(%) | 0.025 | ||||
| Low | 65 (22.6) | 14 (18.9) | 31 (19.5) | 20 (36.4) | |
| High | 223 (77.4) | 60 (81.1) | 128 (80.5) | 35 (63.6) | |
| Pack-years, n (%) | <0.001 | ||||
| <20 | 62 (29.0) | NA | 55 (34.6) | 7 (12.7) | |
| ≥20 | 152 (71.0) | NA | 104 (65.4) | 48 (87.3) | |
Chi-squared test when categorical and Kruskal–Wallis test when continuous.
Healthy = healthy or mild systemic disease; Not healthy = severe systemic disease.
Localized = Ta, T1, T2; Advanced = T3 or T4.
The median (range) follow-up for patients was 4.02 (0.03–14.65) years. During the follow-up period, 41% (n = 117) of the study population died. Of the 117 deaths, 56% (n = 66) were attributable to disease, 28% (n = 33) were attributable to other causes, and 15% (n = 18) resulted from an unknown cause. Disease recurrence occurred in 27% (n = 79) of patients, and 80% (n = 63) of the patients with disease recurrence ultimately died from disease.
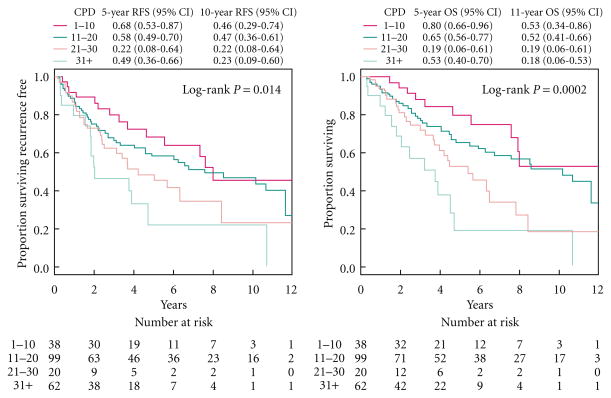
Figure 1 presents survival probabilities by smoking status. Smoking status was not significantly associated with RFS in univariate analysis (P = 0.684, Fig. 1A), nor was pack-years (P = 0.622; data not shown). In multivariate analysis, smoking status was not associated with risk of recurrence or death (P = 0.600, Table 2) after adjusting for traditional prognostic factors. Increased age at diagnosis, ASA score ≥3, advanced pT stage, positive nodal status, and high grade disease were all associated with decreased RFS probability in univariate analysis and remained associated with increased risk of recurrence or death in multivariate analysis.
Fig. 1.
Kaplan-Meier estimates of recurrence-free survival probabilities (left) and overall free survival probabilities (right) stratified by cigarettes per day.
Table 2.
Multivariate Cox regression for the association between smoking status and risk of recurrence or death.
| HR (95% CI) | P | |
|---|---|---|
| Smoking status | 0.600 | |
| Never | Ref | |
| Former | 0.90 (0.59–1.37) | |
| Current | 1.15 (0.66–1.99) | |
| Age at diagnosis | 1.04 (1.01–1.06) | 0.001 |
| ASA score | 0.023 | |
| Healthy | Ref | |
| Unhealthy | 1.54 (1.06–2.24) | |
| pT stage | <0.001 | |
| Localized | Ref | |
| Advanced | 2.24 (1.52–3.30) | |
| N stage | <0.001 | |
| Nx or N0 | Ref | |
| N1 or N2 | 3.18 (1.87–5.39) | |
| Grade | 0.048 | |
| Low | Ref | |
| High | 1.62 (1.00–2.61) |
Since smoking is associated with increased risk of death from other health problems such as heart disease, we used competing risks analyses to examine the cumulative incidence of recurrence where death without recurrence was treated as a competing event. Smoking status was significantly associated with cumulative incidence of recurrence in univariate analysis (P = 0.009), where never smokers had the highest cumulative incidence of recurrence and current smokers had the lowest. Smoking status was also significantly associated with the competing event of death without recurrence (P = 0.014), but the direction was reversed so that current smokers had the highest cumulative incidence of death without recurrence (Fig. 1B). Pack-years was not significantly associated with cumulative incidence of recurrence or death without recurrence (P = 0.844 and P = 0.202, respectively; data not shown). After adjusting for traditional prognostic factors in multivariate competing risks regression, smoking status was no longer associated with recurrence (P = 0.180, Table 3), but a strong association remained between smoking status and death without recurrence (P < 0.001), where current smokers had increased risk of death without recurrence compared with never smokers. Advanced pT stage, positive nodal status, and high grade disease were all associated with increased cumulative incidence of recurrence in univariate analysis and remained associated with increased risk of recurrence in multivariate analysis.
Table 3.
Multivariate competing risks regression for the association between smoking status and risk of recurrence, with death as a competing event.
| Recurrence
|
Death without recurrence
|
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Smoking status | 0.180 | <0.001 | ||
| Never | Ref | Ref | ||
| Former | 0.82 (0.47,1.42) | 0.94 (0.48,1.85) | ||
| Current | 0.44 (0.19,1.06) | 3.64 (1.59,8.34) | ||
| Age at diagnosis | 1.01 (0.99,1.03) | 0.460 | 1.09 (1.05,1.13) | <0.001 |
| ASA score | 0.170 | 0.740 | ||
| Healthy | Ref | Ref | ||
| Unhealthy | 1.44 (0.85,2.45) | 1.11 (0.61,2.03) | ||
| pT stage | <0.001 | 0.780 | ||
| Localized | Ref | Ref | ||
| Advanced | 3.27 (1.92,5.59) | 0.9 (0.44,1.85) | ||
| N stage | 0.005 | 0.320 | ||
| Nx or N0 | Ref | Ref | ||
| N1 or N2 | 2.55 (1.34,4.86) | 0.45 (0.09,2.19) | ||
| Grade | 0.009 | 0.410 | ||
| Low | Ref | Ref | ||
| High | 3.57 (1.37,9.31) | 0.77 (0.41,1.44) | ||
Discussion
The present findings suggest that smoking status at diagnosis and cumulative smoking exposure do not significantly increase risk of UTUC recurrence; however, these findings also highlight the substantial risk of death faced by patients with UTUC who are current smokers, and underscore the importance of promoting smoking cessation among patients with UTUC.
The only previous report to consider smoking exposure in the context of UTUC prognosis was conducted among 105 patients. In univariate analyses, Simsir et al. [17] found that ever smokers experienced shorter time to bladder recurrence than never smokers, but did not differ with respect to time to pelvic recurrence or distant metastasis. Importantly, recurrence of upper tract tumours in the bladder was not an endpoint in the present study, and we believe this may represent tumour seeding or multifocal disease as opposed to new tumour growth. Consistent with the present findings, Simsir et al. did not find that pack-years of smoking affected UTUC prognosis. The present study extends their report in several ways. First, we subdivided ever smokers into current and former smokers to better understand whether recency of smoking influences UTUC outcome. Second, we conducted multivariate analyses that showed that smoking status was not an independent predictor of recurrence when traditional prognostic factors were taken into account. Most notably, our competing risks analyses showed that, although current smoking did not increase the risk of recurrence, it imparted a significant risk of dying compared with former and never smoking. Without the latter analyses, we would have erroneously concluded that smoking status is not relevant in UTUC survival.
Our hypothesis that current smoking adversely impacts recurrence risk was not supported. In fact, compared with never and former smokers, current smokers had lower proportions of advanced stage disease, nodal involvement, and high grade disease. It is possible that selection bias influenced our results since only patients who were healthy enough to undergo RNU were included in the present analysis. Current smokers with advanced disease may be less likely to be eligible for surgery and therefore be underrepresented in the study. This is indirectly supported by the significantly younger age of the current smokers compared with former and never smokers. Remarkably, current smokers selected for surgery and representing a younger and potentially healthier population, still died at a greater rate than former and never smokers during follow-up. Since effective smoking cessation treatments exist for patients with cancer [21], the promotion of smoking cessation among patients with UTUC who are current smokers at diagnosis is warranted.
Strengths of the present study include the multiple aspects of smoking status evaluated and an analytical approach that considered competing risks. Limitations are acknowledged. As prospective data on changes in smoking behaviour after diagnosis were not available, we considered only patients’ smoking status at diagnosis. Recent findings from other cancers suggest that post-diagnosis smoking is an important factor to consider in prognostic studies [22,23]. In the present study, 20% of patients with UTUC reported being current smokers at diagnosis. This may be an underestimate since it was based on self-reporting, which is prone to recall bias and intentional false reporting [24]. Future studies should consider pre- and post-diagnosis smoking exposure and should biochemically verify smoking status among larger groups of patients with UTUC.
Cancer diagnosis and treatment present an excellent opportunity to encourage smoking cessation among patients [25–27]; however, a recent survey of 1821 American urologists found that more than half of them never discuss smoking cessation with their patients [28]. Given the frequency of contact between urologists and patients with bladder cancer, urologists are in a unique position to promote smoking cessation as part of comprehensive clinical care.
In conclusion, the present study of patients with UTUC treated with RNU found that the majority of patients had been exposed to cigarette smoking and 71% of these patients report smoking heavily (≥20 pack-years). While smoking status did not adversely affect UTUC recurrence, current smokers had a significantly higher risk of death without recurrence than never smokers. Given that cigarette smoking is a modifiable risk factor and effective smoking cessation treatments exist [29], physicians treating patients with cancer should provide smoking cessation advice and counselling as part of the comprehensive management of their disease [30].
What’s known on the subject? and What does the study add?
Cigarette smoking is the leading cause of urothelial carcinoma; however, the impact of smoking on outcomes after surgery for upper tract urothelial carcinoma is unknown. One study suggests that patients with a smoking history have an increased risk of recurrence in the bladder compared with never smokers but these patients did not differ with respect to time to pelvic recurrence or distant metastasis.
We subdivided smokers into current and former smokers and performed multivariate analyses that showed that smoking status was not an independent predictor of recurrence when traditional prognostic factors were taken into account. In addition, competing risks analyses showed that although current smoking did not increase the risk of recurrence, it imparted a significant risk of dying compared with former and never smoking.
Acknowledgments
Funding: this study was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers.
Abbreviations
- CPD
cigarettes per day
- UTUC
upper tract urothelial carcinoma
- RNU
radical nephroureterectomy
- RFS
recurrence-free survival
- ASA
American Society of Anesthesiologists
- HR
hazard ratio
Footnotes
Conflict of Interest
None declared.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224–33. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 3.Hartge P, Silverman D, Hoover R, et al. Changing cigarette habits and bladder cancer risk: a case-control study. J Natl Cancer Inst. 1987;78:1119–25. [PubMed] [Google Scholar]
- 4.Strope SA, Montie JE. The causal role of cigarette smoking in bladder cancer initiation and progression, and the role of urologists in smoking cessation. J Urol. 2008;180:31–7. doi: 10.1016/j.juro.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 5.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA : the Journal of the American Medical Association. 2011;306:737–45. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89:630–9. doi: 10.1002/1097-0142(20000801)89:3<630::aid-cncr19>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Rothman N, Garcia-Closas M, Chatterjee N, et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet. 2010;42:978–84. doi: 10.1038/ng.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scelo G, Brennan P. The epidemiology of bladder and kidney cancer. Nature clinical practice. Urology. 2007;4:205–17. doi: 10.1038/ncpuro0760. [DOI] [PubMed] [Google Scholar]
- 9.Chen CS, Lee CH, Hsieh CD, et al. Nicotine-induced human breast cancer cell proliferation attenuated by garcinol through down-regulation of the nicotinic receptor and cyclin D3 proteins. Breast Cancer Res Treat. 2011;125:73–87. doi: 10.1007/s10549-010-0821-3. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta P, Chellappan SP. Nicotine-mediated cell proliferation and angiogenesis: new twists to an old story. Cell Cycle. 2006;5:2324–8. doi: 10.4161/cc.5.20.3366. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta P, Rizwani W, Pillai S, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catassi A, Servent D, Paleari L, Cesario A, Russo P. Multiple roles of nicotine on cell proliferation and inhibition of apoptosis: implications on lung carcinogenesis. Mutation Research. 2008;659:221–31. doi: 10.1016/j.mrrev.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Mousa S, Mousa SA. Cellular and molecular mechanisms of nicotine’s pro-angiogenesis activity and its potential impact on cancer. Journal of Cellular Biochemistry. 2006;97:1370–8. doi: 10.1002/jcb.20741. [DOI] [PubMed] [Google Scholar]
- 14.Aveyard P, Adab P, Cheng KK, Wallace DM, Hey K, Murphy MF. Does smoking status influence the prognosis of bladder cancer? A systematic review. BJU Int. 2002;90:228–39. doi: 10.1046/j.1464-410x.2002.02880.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen CH, Shun CT, Huang KH, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer. BJU Int. 2007;100:281–6. doi: 10.1111/j.1464-410X.2007.06873.x. [DOI] [PubMed] [Google Scholar]
- 16.Sfakianos J, Shariat SF, Favaretto RL, Rioja J, Herr HW. Impact of smoking on outcomes after intravesical bacillus Calmette-Guerin therapy for urothelial carcinoma not invading muscle of the bladder. BJU Int. 2010;108:526–30. doi: 10.1111/j.1464-410X.2010.09874.x. [DOI] [PubMed] [Google Scholar]
- 17.Simsir A, Sarsik B, Cureklibatir I, Sen S, Gunaydin G, Cal C. Prognostic factors for upper urinary tract urothelial carcinomas: stage, grade, and smoking status. Int Urol Nephrol. 2011;43:1039–45. doi: 10.1007/s11255-011-9915-z. [DOI] [PubMed] [Google Scholar]
- 18.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6. New York: Springer; 2002. [Google Scholar]
- 19.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–48. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 21.Treating tobacco use and dependence: 2008 update U S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53:1217–22. [PubMed] [Google Scholar]
- 22.Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA : the Journal of the American Medical Association. 2011;305:2548–55. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshu CE, Mondul AM, Meinhold CL, et al. Cigarette smoking and prostate cancer recurrence after prostatectomy. J Natl Cancer Inst. 2011;103:835–8. doi: 10.1093/jnci/djr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benowitz NL, Schultz KE, Haller CA, Wu AH, Dains KM, Jacob P., 3rd Prevalence of smoking assessed biochemically in an urban public hospital: a rationale for routine cotinine screening. Am J Epidemiol. 2009;170:885–91. doi: 10.1093/aje/kwp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBride CM, Ostro3 JS. Teachable moments for promoting smoking cessation: the context of cancer care and survivorship. Cancer Control: Journal of the Moffitt Cancer Center. 2003;10:325–33. doi: 10.1177/107327480301000407. [DOI] [PubMed] [Google Scholar]
- 26.Gritz ER, Fingeret MC, Vidrine DJ, Lazev AB, Mehta NV, Reece GP. Successes and failures of the teachable moment: smoking cessation in cancer patients. Cancer. 2006;106:17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- 27.Dearing J. Disease-centred advice for patients with superficial transitional cell carcinoma of the bladder. Ann R Coll Surg Engl. 2005;87:85–7. doi: 10.1308/147870805X28109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjurlin MA, Goble SM, Hollowell CM. Smoking cessation assistance for patients with bladder cancer: a national survey of American urologists. J Urol. 2010;184:1901–6. doi: 10.1016/j.juro.2010.06.140. [DOI] [PubMed] [Google Scholar]
- 29.Curry SJ, Keller PA, Orleans CT, Fiore MC. The role of health care systems in increased tobacco cessation. Annu Rev Public Health. 2008;29:411–28. doi: 10.1146/annurev.publhealth.29.020907.090934. [DOI] [PubMed] [Google Scholar]
- 30.Tobacco cessation and quality cancer care. J Oncol Pract. 2009;5:2–5. doi: 10.1200/JOP.0913501. [DOI] [PMC free article] [PubMed] [Google Scholar]