Abstract
Fat, ethanol, and nicotine share a number of properties, including their ability to reinforce behavior and produce overconsumption. To test whether these substances act similarly on the same neuronal populations in specific brain areas mediating these behaviors, we administered the substances short-term, using the same methods and within the same experiment, and measured their effects, in areas of the hypothalamus (HYPO), amygdala (AMYG), and nucleus accumbens (NAc), on mRNA levels of the opioid peptide, enkephalin (ENK), using in situ hybridization and on c-Fos immunoreactivity (ir) to indicate neuronal activity, using immunofluorescence histochemistry. In addition, we examined for comparison another reinforcing substance, sucrose, and also took measurements of stress-related behaviors and circulating corticosterone (CORT) and triglycerides (TG), to determine if they contribute to these substances’ behavioral and physiological effects. Adult Sprague-Dawley rats were gavaged three times daily over five days with 3.5 ml of water, Intralipid (20% v/v), ethanol (12% v/v), nicotine (0.01% w/v) or sucrose (22% w/v) (approximately 7 kcal/dose), and tail vein blood was collected for measurements of circulating CORT and TG. On day five, animals were sacrificed, brains removed, and the HYPO, AMYG, and NAc processed for single- or double-labeling of ENK mRNA and c-Fos-ir. Fat, ethanol, and nicotine, but not sucrose, increased the single- and double-labeling of ENK and c-Fos-ir in precisely the same brain areas, the middle parvocellular but not lateral area of the paraventricular nucleus, central but not basolateral nucleus of the AMYG, and core but not shell of the NAc. While having little effect on stress-related behaviors or CORT levels, fat, ethanol, and nicotine all increased circulating levels of TG. These findings suggest that the overconsumption of these three substances and their potential for abuse are mediated by the same populations of ENK-expressing neurons in specific nuclei of the hypothalamus and limbic system.
Keywords: hypothalamus, amygdala, nucleus accumbens, rat, sucrose
1.1 INTRODUCTION
Recent evidence suggests that food rich in fat has a variety of common features with drugs of abuse, with animal studies showing dietary fat to have similar behavioral effects to those observed after acute or low doses of ethanol and nicotine. While the effects may vary depending on the specific experimental paradigm, these three substances can each cause overconsumption and signs of reward (Balfour, 1994; Lewis, 1996; Levin, 2005; Barrett and Bevins, 2012; Hilario et al., 2012) and also produce changes in emotional behaviors, such as arousal, anxiety, and impulsivity (Aragon et al., 1992; Prasad and Prasad, 1996; Langen et al., 2002; Balerio et al., 2005; Soulis et al., 2007; Chepulis et al., 2009), which themselves can contribute to the increase in intake (Koob and Volkow, 2010; Tsujino and Sakurai, 2013). These similar effects, along with clinical (Kesse et al., 2001) and animal (Olausson et al., 2001; Carrillo et al., 2004; Fornari et al., 2007) studies showing intake of one substance to stimulate or substitute for intake of another, may help to explain why these three substances are often simultaneously overconsumed (Barson et al., 2011b; Morganstern et al., 2011).
These common features of fat, ethanol, and nicotine lead one to ask whether these substances act through the same areas and neurochemical systems of the brain. While these three substances have not yet been directly compared within the same study, data collected in separate reports using different experimental paradigms show that fat (Rada et al., 2012), ethanol (Yoshimoto et al., 1992; Gonzales and Weiss, 1998), and nicotine (Nisell et al., 1994) each stimulate the release of accumbal dopamine, an effect that may underlie their common reinforcing properties. These substances also modulate endogenous expression of the opioid peptide, enkephalin (ENK), in brain structures involved in consummatory behavior, reward, and emotional aspects of eating and drug use (Chang et al., 2007; Chang et al., 2010a; Petruzziello et al., 2013). The changes observed in these studies, however, are not always consistent. For example, the expression of ENK is stimulated in the paraventricular nucleus of the hypothalamus (PVN) and central nucleus of the amygdala (CeA) by both fat and ethanol (Chang et al., 2007; Chang et al., 2010a) but only in the CeA by nicotine (Loughlin et al., 2006), and investigations of ENK in the nucleus accumbens (NAc) have yielded mixed results with these different substances (Mathieu et al., 1996; Kelley et al., 2003; Chang et al., 2010a). Studies of the immediate early gene, c-Fos, a transcription factor, have also yielded mixed findings in these brain areas. Whereas acute administration of fat, ethanol, or nicotine has a similar stimulatory effect in the PVN (Chang et al., 2004; Herring et al., 2004; Loughlin et al., 2006), and ethanol and nicotine both stimulate c-Fos in the CeA (Matta et al., 1993; Leriche et al., 2008), inconsistent effects have again been observed in the NAc (Bachtell et al., 1999; Shram et al., 2007). Further, studies involving chronic administration show c-Fos immunoreactivity (ir) to be increased in the CeA and NAc core (NAcC) after ethanol (Bachtell et al., 1999) and in the NAcC after nicotine (Pich et al., 1997), but they have failed to reveal any effect of ethanol on c-fos in the PVN or NAc shell (NAcSh) (Hansson et al., 2008). Whereas the neurochemical phenotype of the activated neurons is generally unknown, there are two studies focusing on ENK which show the density of neurons that double-label with c-Fos to be increased in the PVN after acute ethanol (Criado and Morales, 2000) and in the PVN and CeA after acute nicotine (Loughlin et al., 2006).
It remains unclear which specific features shared by fat, ethanol, and nicotine may contribute to their similar effects on ENK in hypothalamic and limbic brain regions. While some reports show these substances to induce psychological stress (Scheufele et al., 2000; Walker et al., 2010; Can et al., 2012), which itself may stimulate ENK and c-Fos (Shoji and Mizoguchi, 2010; Christiansen et al., 2011; Noh et al., 2012), there are others using low doses or short-term administration showing no such effects (Pohorecky, 1990; Villegier et al., 2010; Morganstern et al., 2012). These three substances also have reinforcing properties in common (Corrigall et al., 1994; Czachowski and Samson, 1999; Ackroff and Sclafani, 2014). While another substance, sucrose, is also reinforcing (Czachowski et al., 2003) and its intake similarly stimulates accumbal dopamine release (Hajnal and Norgren, 2001; Rada et al., 2005), its consumption is actually found to suppress ENK expression in the NAc (Kelley et al., 2003) and to have little impact on c-Fos-ir in the PVN, CeA, or NAc (Bachtell et al., 1999; Pomonis et al., 2000; Ulrich-Lai et al., 2007; Mitra et al., 2010). Thus, neither psychological stress nor reinforcement appears to be a primary factor involved in any similar neurochemical effects induced by fat, ethanol, and nicotine.
To clarify these issues, the present report provides a systematic analysis of the specific neuronal populations stimulated by dietary fat and drugs of abuse, to determine whether they are similar in their sites of action and neurochemical changes in the brain and, if so, whether these changes are accompanied by similar behavioral or physiological responses that may contribute to or explain their actions. The first goal was to examine fat, ethanol, and nicotine, as well as sucrose for comparison, within the same experiment and using the same mode and short period of administration. The second goal was to provide a more precise anatomical analysis of changes in three main brain structures, hypothalamus (HYPO), amygdala (AMYG), and NAc, with measurements of both single- and double-labeling of ENK mRNA and c-Fos-ir. The final goal was to take steps towards determining whether fat, ethanol, and nicotine have specific behavioral and physiological effects in common, which may be related to their similar actions in the brain. These analyses involved measurements of stress-related behaviors and blood levels of the stress hormone corticosterone (CORT) and also the lipids, triglycerides (TG), which under some conditions are increased by these substances (Balfour et al., 1975; Widmaier et al., 1992; Scheufele et al., 2000; Chattopadhyay and Chattopadhyay, 2008; Barson et al., 2009; Cippitelli et al., 2014) and can themselves stimulate expression of ENK (Ahima et al., 1992; Chang et al., 2004) and c-Fos (Chang et al., 2004; Herring et al., 2004; Loughlin et al., 2006). A 5-day exposure was used in order to examine animals beyond their first encounter with the substances, which generally stimulates c-Fos expression in the brain (Ryabinin et al., 1997; Salminen et al., 2000; Chang et al., 2004), but before they become dependent or obese, which can decrease basal levels of ENK (McLaughlin et al., 1986) and greatly reduce a c-Fos response (Ryabinin et al., 1997; Salminen et al., 2000; Chang et al., 2004), therefore masking direct effects of the substances themselves. By directly comparing fat, ethanol, and nicotine, the results of this study should allow a more definitive answer as to whether and why these substances are similar in their effects on the activity of ENK-expressing neurons in specific brain sites.
1.2 EXPERIMENTAL PROCEDURES
1.2.1 Subjects
Adult, Sprague-Dawley rats (N=84, Charles River Laboratories International, Inc., Wilmington, MA), weighing approximately 350–400 g at the onset of experiments, were individually housed (22°C, 12:12-hr light-dark cycle with lights off at 11:00 a.m.) in a fully accredited American Association for the Accreditation of Laboratory Animal Care facility. All animals were given one week to acclimate to laboratory conditions, during which time they were maintained ad libitum on laboratory chow (LabDiet Rodent Chow 5001) and water. All procedures were approved by the Rockefeller University Animal Care and Use Committee and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Only male rats were used, as the greater preference of females for fat, ethanol, and nicotine (Leibowitz et al., 1991; Torres et al., 2009; Torres et al., 2014) can fluctuate significantly across the estrus cycle (Forger and Morin, 1982; Leibowitz et al., 1998), which could introduce variability in the neurochemical responses examined in this study.
1.2.2 Administration of fat, ethanol, nicotine, and sucrose
Rats were separated into four groups (N=64; n=16/group) of equal body weight, handled daily and given 5 days of mock gavage to adapt them to this test procedure in order to minimize stress. Equal volumes (3.5 mL) of Intralipid (20% v/v), ethanol (12% v/v), nicotine (0.01% w/v), or water were administered three times a day (at 10 a.m., 2 p.m., and 6 p.m.) for 5 days. An additional set of rats was divided into two groups (N=20; n=10/group) and was treated in the same manner with sucrose solution (22% w/v) or water. The concentrations of ethanol and sucrose were chosen to match the calorie content of Intralipid (7 kcal). On the day of the sacrifice, after a total of 13 treatments, animals were food deprived for one hour, gavaged one hour prior to dark onset, and sacrificed at dark onset. The experimental design is similar to our previously published studies that show a 5-day exposure to have profound effects on neurochemical and behavioral parameters with little effect on body weight (Chang et al., 2010b; Barson et al., 2011a).
1.2.3 Digoxigenin-labeled in situ hybridization for ENK
One hour after the final substance administration, animals were sacrificed by rapid decapitation. Brains were immediately removed and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) for 72 hr and then transferred to 25% sucrose in PB for 28 hr prior to freezing at − 80°C. Thirty µm free-floating serial coronal sections (every third section) were cut with a cryostat and used for digoxigenin-labeled in situ hybridization (DIG) to label ENK. DIG-labeled cRNA probes were synthesized as previously described (Wortley et al., 2003). As described (Leibowitz et al., 2007; Chang et al., 2010a), sections were consecutively processed as follows: 10 min in 0.001% proteinase K, 5 min in 4% paraformaldehyde, 8 min in 0.2 N HCl, and 10 min in acetylation solution, with a 10-min wash in PB between each step. After the wash, the sections were hybridized with a DIG-labeled probe at 55 °C for 18 hr. Post-hybridization, the sections were washed in 5× sodium chloride and sodium citrate (SSC), and the nonspecifically bound probe was removed by RNase (Sigma-Aldrich, St. Louis, MO) treatment for 30 min at 37 °C. Sections were then run through a further stringency wash with formamide (Invitrogen, Carlsbad, CA) in 2× SSC at 55 °C and then were blocked and incubated in AP-conjugated sheep anti-digoxigenin antibody (Sheep Anti-DIG-AP, Fab fragments, 1:1000; Roche Diagnostics, Indianapolis, IN) overnight. After washing in Tris buffer (0.1 M, pH 9.5), the signal was revealed with NBT/BCIP, and the sections were mounted, dehydrated, cleared, and coverslipped.
1.2.4 Immunofluorescent histochemistry for c-Fos expression
One hour after the final substance administration, animals were deeply anesthetized with Nembutal (Henry Schein, Inc., Melville, NY; 50 mg/kg, i.p.) and transcardially perfused with 300 ml of 0.9% normal saline followed by 200 ml of 4% paraformaldehyde in 0.1 M PB (pH 7.4). Brains were immediately removed and post-fixed for 4 hr in the same fixative at 4°C, then transferred in 25% sucrose in PB (0.1M, pH 7.4) at 4°C for 48 hr. Thirty µm free-floating serial coronal sections (every third section) were cut with a cryostat and used for c-Fos immunofluorescent histochemistry. Sections were sequentially incubated as follows: 3 hr in 0.5% Triton X-100, 1% normal donkey serum, 0.5% TNB buffer in PBS, 60–72 hr at 4°C in rabbit anti-c-Fos serum (1:50, Santa Cruz Biotechnology, CA), 6 × 10 min wash in PBS, 1 hr Cy3-conjugated donkey anti-rabbit (1:200, JacksonImmunoResearch Inc., West Grove, PA). Sections were then rinsed 6 × 10 min in PBS (0.01M, pH 7.4), mounted, dehydrated, cleared, and coverslipped with VECTASHIELD mounting medium (Vector Laboratories Inc., Burlingame, CA).
1.2.5 Fluorescent double-labeling of c-Fos with ENK
Alternate slices from the brains of animals from section 1.2.4 were processed for fluorescence in situ hybridization (FISH) to label ENK mRNA. Antisense RNA probe was labeled with Fluorescein-12-UTP (Roche), using the same methods as in section 1.2.3 for DIG until the post-hybridization wash. After this wash, sections were blocked for 2 hr in 0.5% TNB blocking buffer (TSA Fluorescein Kit, Perkin Elmer, Inc., Boston MA) and then incubated overnight at 4°C in sheep anti-fluorescein-POD (1:100, Roche) in 0.3% TritonX-100, 0.5% TNB buffer. After rinsing 3 × 15 min each in TNT wash buffer (TSA Fluorescein Kit, Perkin Elmer, Inc., Melville, NY), sections were treated for 5 min with fluorescein-tyramide (1:50, Perkin Elmer) and then washed 3 × 10 min in TNT buffer. Sections were then processed for fluorescently-labeled c-Fos. First, they were blocked in 0.5% TritonX-100, 1% normal donkey serum, 0.5% TNB buffer for 3 hr, then incubated in rabbit anti-c-Fos (1:50) for 60–72 hr at 4°C. After washing in PBS 6 × 10 min, sections were incubated for 1 hr in Cy3-conjugated donkey anti-rabbit (1:100, JacksonImmunoResearch Inc., West Grove, PA). Finally, sections were washed 6 × 10 min in PBS, mounted, and coverslipped with VECTASHIELD mounting medium (Vector Laboratories Inc., Burlingame, CA).
1.2.6 Quantitation
For quantitation of DIG, sections were viewed using a light microscope (Leitz Dialux 20, Leica Microsystems, Buffalo Grove, IL) with 10× illumination objective, and images were captured with a Nikon DXM 1200 digital camera (Melville, NY). For quantitation of c-Fos, fluorescent images were captured using a fluorescence microscope (Zeiss Axioplan 2; Thornwood, NY) with MetaVue acquisition software (Molecular Devices, Downington, PA). Images were analyzed using Image-Pro Plus software (Version 4.5, Media Cybernetics Inc., MD) on a grey-value scale from 1 to 255. Sections containing the parvocellular PVN were defined as anterior (−0.8 to −1.3 mm), middle (−1.4 to −1.9 mm), or posterior (−2.00 to −2.3 mm), depending on their relative position from bregma, as defined by Paxinos and Watson (2005). Magnocellular cells of the lateral PVN were also examined (−1.4 to −2.3 mm). The CeA and basolateral nucleus (BLA) within the AMYG and the NAcC and NAcSh regions of the NAc were defined by their relative positions according to Paxinos and Watson (2005). For each animal, 8–12 sections at the same level were used to examine each nucleus of interest, and the entire nucleus was outlined and analyzed. The number of DIG or c-Fos-labeled cells in the area was counted and expressed as density of ENK mRNA-containing cells (number of cells/µm2) or as density of c-Fos-ir (cells/µm2). Before measurements, a threshold for each nucleus was established. Using the selected sections, this threshold was set by matching the number of cells counted by the software in a defined area with the number of cells counted manually in that same area. This method, which was the same for all brain areas, yielded different threshold values (average of thresholds obtained within the same area in the 8–12 sections) for the different brain areas. This semi-quantitative procedure allowed us to count the number of neurons in a specific area, which was then expressed as the cell density. In all analyses, the cell number was counted only on one plane in each section, and only those cells containing a nucleus in the plane (>10 µm2) were counted, thereby excluding fractions of cells. The average cell density for the different groups was then compared and statistically analyzed. Analyses were performed by an observer blind to the identity of the animals.
For quantitation of FISH and double-labeling of FISH with c-Fos-ir, 8–12 sections were collected from the middle PVN, CeA, and NAcC of each animal, and the fluorescent images were captured using a fluorescence microscope (Zeiss Axioplan 2) with MetaVue acquisition software, with exposure conditions kept the same for each antibody. The density of immunofluorescent signal was quantified with Image-Pro Plus software, as described above for c-Fos-ir and DIG, and is reported as cell density (cells/µm2). Images were captured with a 20× objective, and the cells double-labeled for ENK with c-Fos were confirmed with a 40× objective and further validated by confocal Z-sectioning with a 40× water-immersion lens on a Zeiss LSM 510 META confocal microscope (Melville, NY). Double-labeled cells were counted and reported as percentage of total single-labeled cells.
1.2.7 Behavioral testing
On the third day of gavage, one hour following the second daily administration, a separate group of rats receiving Intralipid, ethanol, nicotine, or water was tested for locomotor activity, rearing, and grooming in a novel open field. This time-point relative to administration was chosen to match the time of blood collection (see Section 1.2.8) and of sacrifice for ENK and c-Fos analysis (see Sections 1.2.3 and 1.2.4), to allow the animals to recover from any acute stress from the administration procedure itself, and to examine the animals while levels of fatty acids, ethanol, and nicotine in the brain should still be in their rising or plateau phase (Ferraro et al., 1991; Crooks et al., 1997; Adalsteinsson et al., 2006). Rats were moved to a 60 cm × 60 cm × 60 cm wooden box, with its plexiglass floor divided into 16 equal-sized squares, located in a sound-attenuated room under red light. Similar to published studies (Takeda et al., 2007; Kucuk et al., 2008; Ishikawa et al., 2014), rats were placed in the center of the open field and left to explore the field for five minutes while being videotaped with an overhead camera. The videotapes were later scored, by an observer blind to the treatment condition, for lines crossed (number of squares crossed with four paws), number of rearing episodes (posture sustained with hind-paws on the floor), and number of grooming episodes (washing of limbs, face, and body). Line crossing was used to determine locomotor activity, rearing to determine exploration, and grooming to determine arousal.
On the fourth day of gavage, one hour following the second daily administration, the same group of rats was tested for anxiety in an elevated plus maze (EPM). Rats were placed on the maze, which was located in a sound-attenuated room under red light and consisted of four arms (10 × 50 cm each) elevated 55 cm above the floor. Two opposite arms were enclosed by 30 cm-high opaque walls, while the two other arms were not enclosed. Rats were placed in the center of the maze, alternated between facing an open or closed arm, and were left to explore the maze for 5 minutes while being videotaped. The videotapes were later scored, by an observer blind to the treatment condition, for the amount of time in open arms and the number of entries into open and closed arms. The criterion used to determine arm entry was both forepaws into an arm. Time and entries into the open arm was used to determine anti-anxiety behavior, while closed and total arm entries were used to determine locomotor activity.
1.2.8 Corticosterone, triglycerides, blood ethanol, and nicotine concentration
In the rats given behavioral testing, on the fifth day of gavage, tail vein blood was collected one hour following administration of Intralipid, ethanol, or nicotine at dark onset. Serum from blood was used to measure levels of corticosterone (CORT) using a Corticosterone 3H RIA kit (MP Biomedicals, Orangeburg, NY) and levels of triglycerides (TG) using a Triglyceride Assay kit (Sigma-Aldrich Co.), with values measured on an E-Max Microplate Reader (Sunnyvale, CA). Blood ethanol concentration was measured in ethanol-treated animals using the Analox GM7 Fast Enzymatic Metabolic Analyser (Lunenburg, MA). Plasma nicotine analysis by HPLC was measured in nicotine treated animals and carried out at the Proteomics Resource Center of The Rockefeller University.
1.2.9 Statistical analysis
Comparisons between the four groups, water, Intralipid, ethanol, and nicotine, were made using a univariate analysis of variance, followed-up by Tukey’s post-hoc tests when appropriate. Comparisons between the two groups, water and sucrose, were performed using unpaired two-tailed Student’s t-tests. The criterion for significance was p < 0.05, and all values greater than this are reported as ns (not significant). Data are reported as mean ± standard error of the mean (S.E.M.).
1.3 RESULTS
1.3.1 Experiment 1: Effect of fat, ethanol, and nicotine on neurons expressing ENK mRNA
This experiment used in situ hybridization to examine the effects of short-term administration of fat, ethanol, and nicotine, compared to water (n=5/group), on the gene expression of ENK in different brain areas. In the water control group, consistent with our prior findings (Barson et al., 2011a), the ENK-expressing neurons in the HYPO, almost exclusively of the parvocelluar type that were small (4–6 µm) and medium (10–18 µm) in size, were densest in the medial region of the PVN at the level of the middle and posterior PVN and less concentrated in the anterior and lateral PVN. In the AMYG, they were denser in the CeA, specifically its lateral subregion and lateral capsular area, than in the BLA where a small number could be seen in the ventral part of this nucleus, and in the NAc, they were dense throughout the NAcC and also evident in the ventral part of the NAcSh.
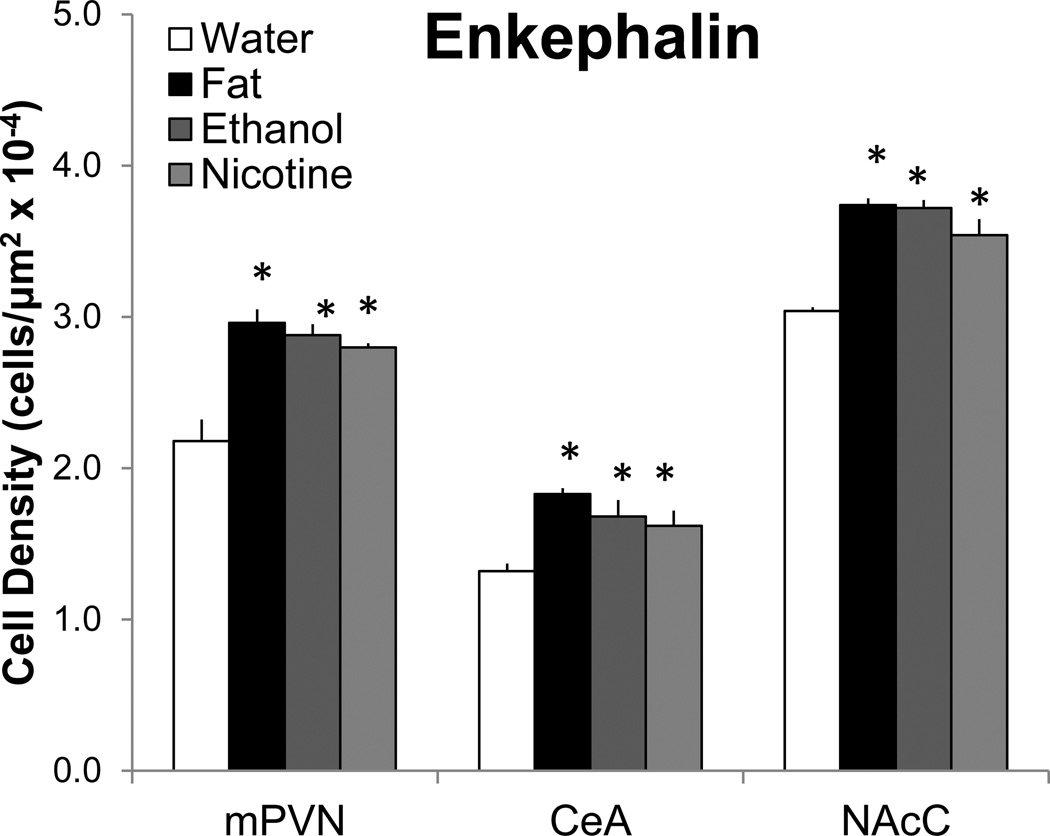
When compared to the water group, the rats administered fat, ethanol, or nicotine exhibited a significant increase in the density of ENK mRNA-expressing neurons. This effect occurred in precisely the same sites within the HYPO, AMYG, and NAc, with the percent increase ranging from 16–40% (Fig. 1) as illustrated for nicotine in the photomicrographs (Fig. 2), and it was rarely evident in certain other nearby regions examined within these brain areas (Table 1). In the HYPO, this effect was seen specifically in the medial parvocellular area of the PVN, at the anterior-posterior level of the anterior PVN (F(3,16)=8.95, p<0.01), middle PVN (F(3,16)=20.09, p<0.01), and posterior PVN (F(3,16)=4.60, p<0.05), but it was not seen in the lateral PVN (F(3,16)=0.00, not significant ns). Post-hoc analyses showed this stimulatory effect to occur most consistently in the middle PVN, where all three substances produced an increase (31–43%, p<0.01) that was similar in magnitude (ns for fat vs. ethanol vs. nicotine) (Fig. 1). This is in contrast to the posterior PVN, where only fat (21%, p<0.05) and ethanol (19%, p<0.05) produced a significant increase in ENK, and also to the anterior PVN, where only ethanol was effective (19%, p<0.01). In the AMYG, this increase in ENK mRNA-expressing neurons was seen for all three substances, specifically in the CeA (F(3,6)=11.18, p<0.01) where it was similar in magnitude (23–39%, p<0.05) (ns for fat vs. ethanol vs. nicotine) (Fig. 1), but it was not evident for any of the substances in the BLA (F(3,6)=0.00, ns). In the NAc, a significant increase was detected specifically in the NAcC (F(3,16)=25.00, p<0.01), where all three substances produced an increase (16–23%, p<0.01) similar in magnitude (ns for fat vs. ethanol vs. nicotine) (Fig. 1), but it was not evident in the NAcSh (F(3,16)=1.06, ns). These results, obtained in the same experiment and using the same treatment paradigm, focus attention on three specific populations of ENK-expressing neurons, located specifically in the middle PVN, CeA, and NAcC, where fat, ethanol, and nicotine have a similar, stimulatory effect on the density of these peptide neurons.
Fig. 1.
Short-term oral gavage of Intralipid (20% v/v), ethanol (12% v/v), or nicotine (0.01% w/v) (3.5 mL each) increases the density of enkephalin mRNA-expressing neurons in the middle paraventricular nucleus (mPVN), central nucleus of the amygdala (CeA), and nucleus accumbens core (NAcC), as measured by digoxigenin-labeled in situ hybridization. Data are mean ± S.E.M., *p<0.05 vs. water, n=5/group.
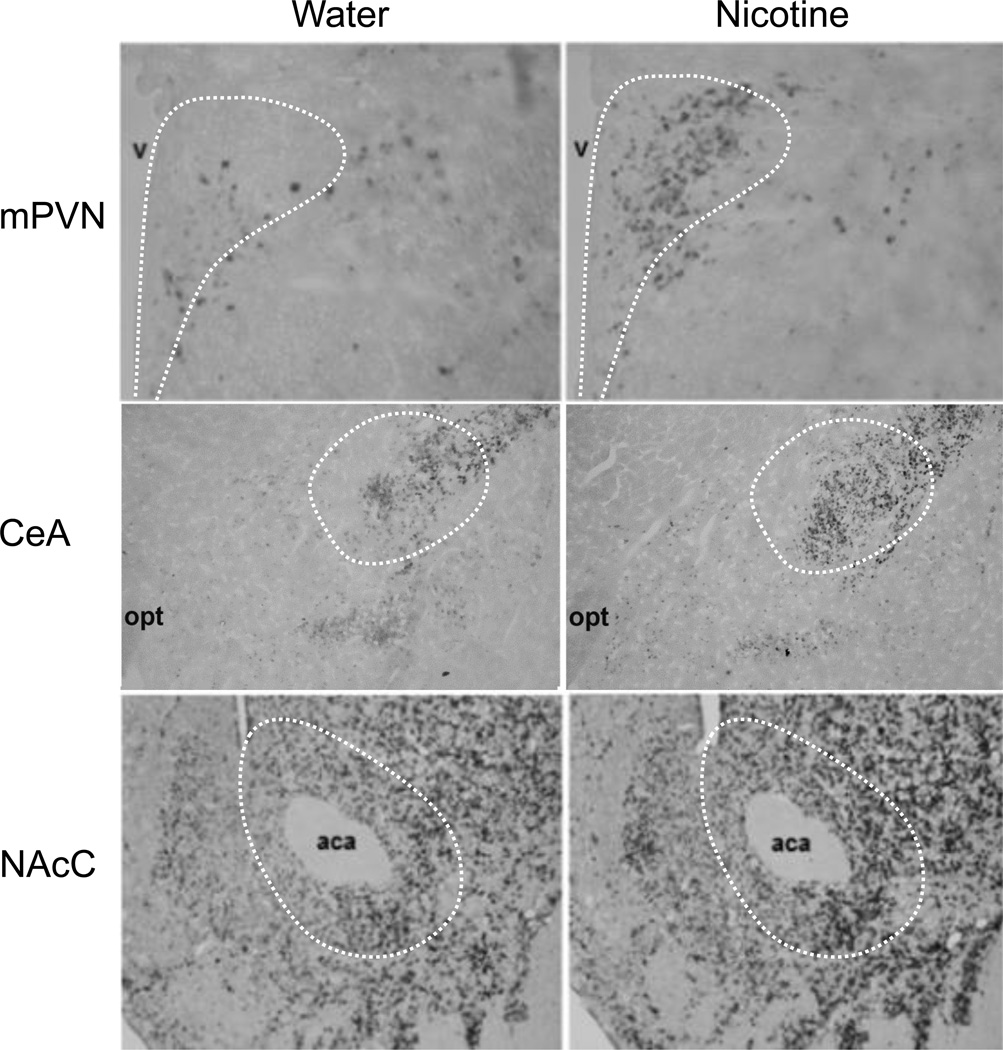
Fig. 2.
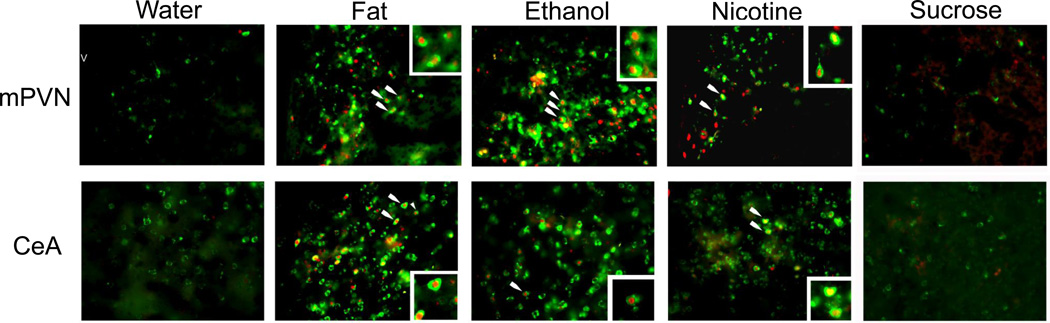
Photomicrographs illustrate changes in the density of enkephalin mRNA-expressing neurons in the middle paraventricular nucleus (mPVN, 10× magnification), central nucleus of the amygdala (CeA, 4× magnification), and nucleus accumbens core (NAcC, 10× magnification), in response to oral gavage of nicotine (0.01% w/v) or water (3.5 mL each), as measured by digoxigenin-labeled in situ hybridization. aca: anterior commissure, opt: optic tract, v: third ventricle.
Table 1.
Density of enkephalin-expressing neurons, measured by digoxigenin-labeled in situ hybridization, in brain areas showing few changes after short-term oral gavage of Intralipid (20% v/v), ethanol (12% v/v), or nicotine (0.01% w/v) (3.5 mL each). These generally unresponsive brain areas include the anterior paraventricular nucleus (aPVN), posterior paraventricular nucleus (pPVN), lateral paraventricular nucleus (lPVN), basolateral amygdala (BLA), and nucleus accumbens shell (NAcSh). Data are mean ± S.E.M.
| Enkephalin Cell Density (cells/µm2 × 10−4) | |||||
|---|---|---|---|---|---|
| Brain areas | Water | Fat | Ethanol | Nicotine | |
| aPVN | 0.87 ± 0.04 | 0.93 ± 0.02 | 1.02 ± 0.03* | 0.91 ± 0.05 | |
| pPVN | 2.68 ± 0.11 | 3.16 ± 0.23* | 3.12 ± 0.29* | 3.01 ± 0.25 | |
| lPVN | 0.35 ± 0.01 | 0.37 ± 0.02 | 0.36 ± 0.02 | 0.35 ± 0.02 | |
| BLA | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | |
| NAcSh | 2.91 ± 0.16 | 3.27 ± 0.11 | 3.19 ± 0.07 | 3.16 ± 0.09 | |
p<0.05 vs. water, n=5/group.
1.3.2 Experiment 2: Effect of fat, ethanol, and nicotine on c-Fos immunofluorescent cells
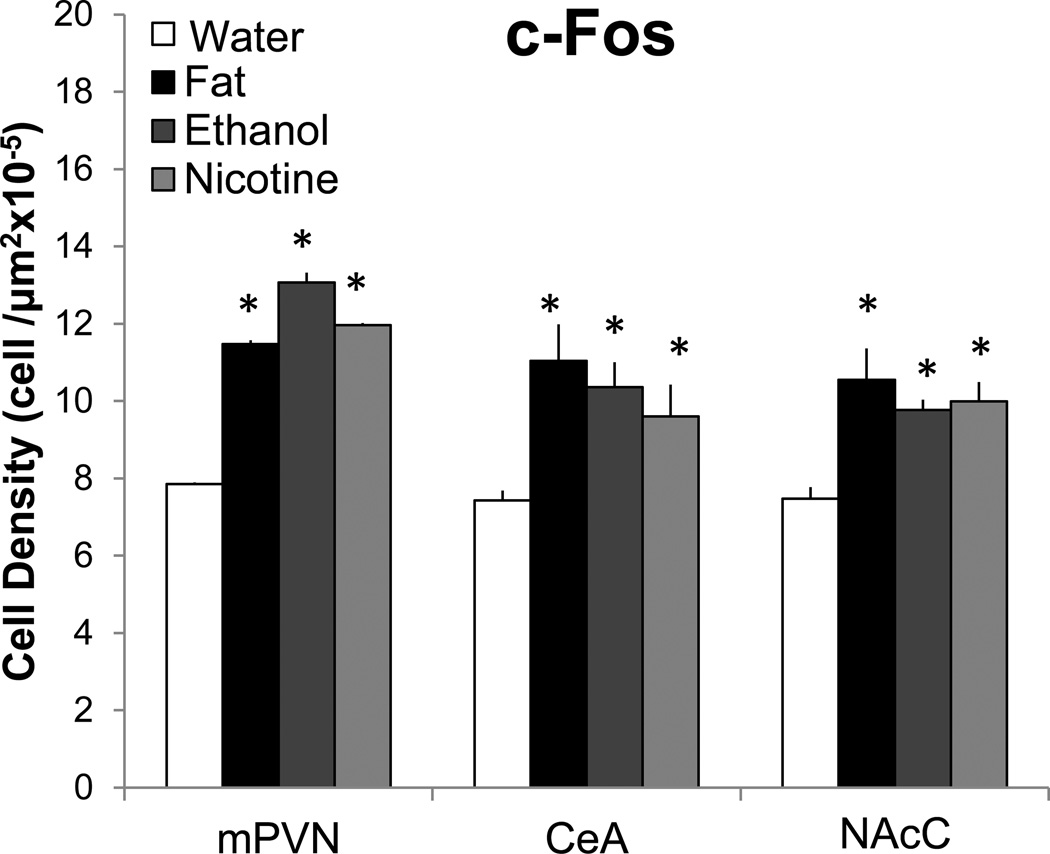
To more precisely localize the neuronal populations activated by these different reinforcing substances, this next experiment used immunofluorescent histochemistry to examine in a second set of rats (n=4–5/group) c-Fos-ir cells in the different brain areas. As compared to the water control animals which had generally low c-Fos-ir, short-term administration of fat, ethanol, or nicotine each produced a significant increase in c-Fos-ir cells (Fig. 3) in the same areas where their administration altered ENK mRNA, while having little effect (Table 2) in the areas where ENK was unaffected. In the PVN, this stimulatory effect was once again restricted to the medial parvocellular part of this nucleus, at the anterior-posterior levels of the middle PVN (F(3,14)=5.42, p<0.05) and posterior PVN (F(3,13)=4.04, p<0.05) but not the anterior PVN (F(3,12)=0.09, ns), and it was not evident in the lateral PVN (F(3,12)=2.39, ns). Post-hoc analyses showed this stimulatory effect in the middle PVN to be significant (p<0.01) for all three substances, where it was similar in magnitude (46–92%, ns for fat vs. ethanol vs. nicotine) (Fig. 3). However, in the posterior PVN, it was evident only for fat (16%, p<0.01). In the AMYG, the increase in c-Fos-ir cells induced by the three substances occurred exclusively in the CeA (F(3, 12)=6.59, p<0.01) (Fig. 3), with no change observed in the BLA (F(3, 12)=0.00, ns), and in the NAc, it was evident in the NAcC (F(3,12)=5.83, p<0.05) (Fig. 3) but not in the NAcSh (F(3,12)=0.00, ns). In the CeA and NAcC, post-hoc analyses showed the three substances to produce a significant increase (p<0.01) in c-Fos-ir cells that was similar in magnitude in the CeA (30–50%, ns for fat vs. ethanol vs. nicotine) and NAcC (30–40%, ns for fat vs. ethanol vs. nicotine). Thus, these results reveal neuronal activation in the same brain sites, middle PVN, CeA, and NAcC, where fat, ethanol, and nicotine were found in Experiment 1 to increase the density of ENK-expressing neurons.
Fig. 3.
Short-term oral gavage of Intralipid (20% v/v), ethanol (12% v/v), or nicotine (0.01% w/v) (3.5 mL each) increases the density of c-Fos-immunoreactive cells in the middle paraventricular nucleus (mPVN), central nucleus of the amygdala (CeA), and nucleus accumbens core (NAcC). Data are mean ± S.E.M., *p<0.05 vs. water, n=4–5/group.
Table 2.
Density of c-Fos-labeled cells in brain areas showing no change after short-term oral gavage of Intralipid (20% v/v), ethanol (12% v/v), or nicotine (0.01% w/v) (3.5 mL each). These unresponsive brain areas include the anterior paraventricular nucleus (aPVN), posterior paraventricular nucleus (pPVN), lateral paraventricular nucleus (lPVN), basolateral amygdala (BLA), and nucleus accumbens shell (NAcSh). Data are mean ± S.E.M.
| c-Fos Cell Density (cells/um2 × 10−5) | ||||
|---|---|---|---|---|
| Brain areas | Water | Fat | Ethanol | Nicotine |
| aPVN | 7.85 ± 1.06 | 8.05 ± 0.46 | 8.21 ± 0.42 | 8.25 ± 0.70 |
| pPVN | 7.53 ± 0.25 | 8.75 ± 0.46* | 7.66 ± 0.29 | 7.53 ± 0.33 |
| lPVN | 3.43 ± 0.69 | 3.93 ± 0.57 | 3.84 ± 0.92 | 3.63 ± 0.64 |
| BLA | 0.74 ± 0.03 | 0.78 ± 0.01 | 0.79 ± 0.01 | 0.76 ± 0.02 |
| NAcSh | 7.42 ± 0.44 | 7.47 ± 0.33 | 7.51 ± 0.32 | 7.35 ± 0.44 |
p<0.05 vs. water, n=4–5/group.
1.3.3 Experiment 3: Effect of fat, ethanol, and nicotine on ENK mRNA and c-Fos colocalization
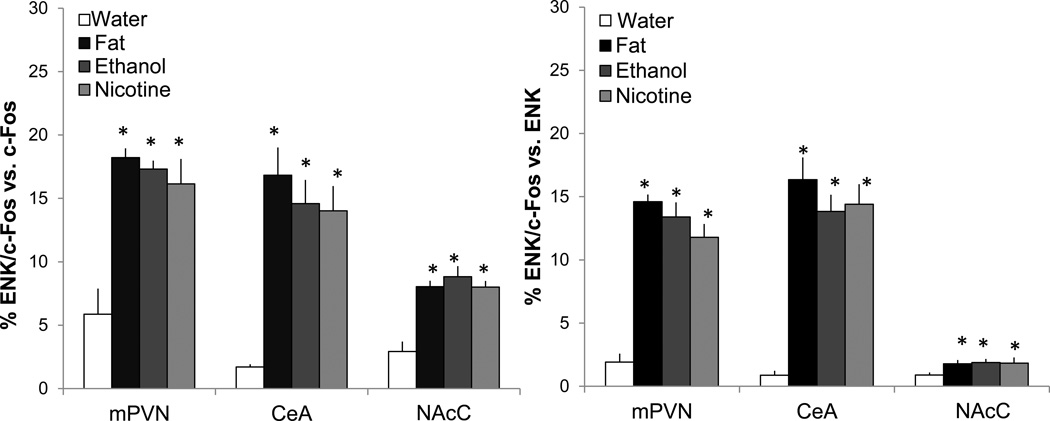
To determine whether the neurons that are activated by fat, ethanol, and nicotine are in fact those that express ENK, we examined in alternate slices from the same set of rats used in Experiment 2 (n=4/group) the double-labeling of ENK mRNA using FISH and c-Fos-ir using immunofluorescent histochemistry in the middle PVN, CeA and NAcC. In the water control group, only a few neurons (3–5%) in these areas exhibited ENK and c-Fos colocalization. As compared to this group, the rats administered fat, ethanol, or nicotine exhibited significant differences with measurements of cells co-expressing ENK mRNA and c-Fos relative to single-labeled cells (Fig. 4), as illustrated in the photomicrographs (Fig. 5). Specifically, an increase in double-labeled neurons was observed in the middle PVN relative to single-labeled ENK+ (F(3,12)=41.89, p<0.01) and c-Fos+ (F(3,12)=14.74, p<0.01) neurons, in the CeA relative to single-labeled ENK+ (F(3,12)=11.19, p<0.01) and c-Fos+ (F(3,12)=17.68, p<0.01) neurons, and in the NAcC relative to single-labeled ENK+ (F(3,12)=4.49, p<0.01) and c-Fos+ (F(3,12=13.8, p<0.01) neurons. For all three substances, post-hoc analyses revealed in each brain region significant, stimulatory effects on double-labeled neurons (p<0.01) relative to single-labeled neurons, with the effects of these substances similar in magnitude within each brain area (ns for fat vs. ethanol vs. nicotine). Thus, fat, ethanol, and nicotine are each found to activate the same population of ENK-expressing neurons in specific areas of the HYPO, AMYG, and NAc, underscoring the similarity of their actions in the brain.
Fig. 4.
Short-term oral gavage of Intralipid (20% v/v), ethanol (12% v/v), or nicotine (0.01% w/v) (3.5 mL each) increases the percentage of neurons that double-label for enkephalin (ENK) mRNA and c-Fos-immunoreactivity in the middle paraventricular nucleus (mPVN), central nucleus of the amygdala (CeA), and nucleus accumbens core (NAcC), as measured by immunofluorescence and fluorescence in situ hybridization. Left panel: Double-labeled neurons vs. single-labeling of c-Fos. Right panel: Double-labeled neurons vs. single-labeling of ENK mRNA. Data are mean ± S.E.M., *p<0.05 vs. water, n=4/group.
Fig. 5.
Photomicrographs illustrate double-labeling of c-Fos (red) and enkephalin mRNA (green) in the middle paraventricular nucleus (mPVN) and central nucleus of the amygdala (CeA) in response to oral gavage of water (3.5 mL), Intralipid (20% v/v), ethanol (12% v/v), nicotine (0.01% w/v), and sucrose (22% w/v) as labeled with immunofluorescence and fluorescence in situ hybridization. White arrows indicate double-labeled neurons depicted in inset images. Main images: 20× magnification; insets: 40× magnification.
1.3.4 Experiment 4: Effect of sucrose on ENK gene expression and c-Fos immunoreactivity
The consistency and similarity of the stimulatory effect of fat, ethanol, and nicotine on ENK- and c-Fos-labeled neurons in the middle PVN, CeA, and NAcC encouraged us to examine, by testing sucrose for comparison, whether the reinforcing properties of these substances are an essential component of this effect. Using the same experimental procedures as with fat, ethanol, and nicotine, we found in an additional set of rats (n=5/group) that sucrose had little effect on single-labeling of ENK mRNA in the middle PVN (t(8)=0.07, ns), CeA (t(8)=0.14, ns) or NAcC (t(8)=1.03, ns) and also on single-labeling of c-Fos-ir in the middle PVN (t(8)=0.91, ns), CeA (t(8)=1.10, ns), or NAcC (t(8)=0.87, ns) as compared to the water control group (Table 3). These results, clearly distinguishing sucrose from fat, ethanol, and nicotine in their ability to stimulate ENK and c-Fos-ir in these specific brain sites, suggest that other factors besides the reinforcing properties are involved in the activation of the ENK neurons.
Table 3.
Density of enkephalin (ENK)-expressing neurons and c-Fos immunoreactive cells in the middle paraventricular nucleus (mPVN), central nucleus of the amygdala (CeA), and nucleus accumbens core (NAcC) after short-term oral gavage of sucrose (22% w/v) compared to water (3.5 mL). Data are mean ± S.E.M., n=5/group.
| ENK cell density (× 10−5) | c-Fos cell density (× 10−5) | |||
|---|---|---|---|---|
| Brain areas | Water | Sucrose | Water | Sucrose |
| mPVN | 2.34 ± 0.07 | 2.41 ± 0.05 | 7.87 ± 0.34 | 7.73 ± 0.32 |
| CeA | 1.41 ± 0.06 | 1.46 ± 0.02 | 7.55 ± 0.24 | 7.75 ± 0.26 |
| NAcC | 3.19 ± 0.03 | 3.25 ± 0.03 | 6.90 ± 0.11 | 6.93 ± 0.14 |
1.3.5 Experiment 5: Effect of fat, ethanol, and nicotine on stress-related behaviors and circulating factors
The question addressed in this experiment is whether short-term, intraoral administration of fat, ethanol, and nicotine, at the same low doses used in Experiments 1–3 (n=6/group), produces behavioral changes indicative of a stress response that may be related to the stimulation of ENK neurons. The behaviors measured were locomotor activity (line crossing), exploratory behavior (rearing), and arousal (grooming) in a novel open field, and also anxiety and locomotor activity in an EPM (Table 4). As compared to the control group, the rats administered fat, ethanol, or nicotine exhibited a significant increase in locomotor activity (F(3,24)=3.76, p<0.05), rearing (F(3,24)=3.91, p<0.05), and grooming (F(3,24)=10.14, p<0.01) in the open field. Post-hoc analyses showed that the stimulatory effect on locomotor activity occurred only in the ethanol-treated rats (45%, p<0.05), with no effect in the fat- and nicotine-treated groups (ns vs. control). The stimulatory effect on rearing and grooming occurred only in the nicotine-treated rats (42–173%, p<0.05), with no effect in the fat- and ethanol-treated groups (ns vs. control). The EPM test revealed no anxiety in response to fat, ethanol, or nicotine, as indicated by no group difference in the percent time spent in the open arms (F(3,24)=1.89, ns) or in the number of open arm entries (F(3,24)=3.15, ns), and also no difference in locomotor activity in the EPM, as measured by the number of closed and total arm entries (F(3,24)=1.15, ns). Thus, the three substances, fat, ethanol, and nicotine, have very different effects on these stress-related behavioral measures, suggesting that psychological stress may not be an essential factor in their neurochemical effects.
Table 4.
Few effects occurred after short-term oral gavage of Intralipid (20% v/v), ethanol (12% v/v), or nicotine (0.01% w/v) compared to water (3.5 mL) on behavior in an open field and elevated plus maze. Data are mean ± S.E.M.
| Behaviors | Water | Fat | Ethanol | Nicotine |
|---|---|---|---|---|
| Open Field | ||||
| Line crossings (#) | 66.0 ± 4.6 | 67.0 ± 4.5 | 95.0 ± 7.0* | 55.0 ± 8.9 |
| Rearing episodes (#) | 16.0 ± 1.3 | 17.0 ± 2.3 | 18.5 ± 1.2 | 22.7 ± 0.7* |
| Grooming episodes (#) | 1.8 ± 0.5 | 2.0 ± 0.6 | 2.3 ± 0.4 | 5 .0 ± 0.4* |
| Elevated Plus Maze | ||||
| Open arm time (%) | 11.0 ± 2.6 | 10.8 ± 3.3 | 18.6 ± 0.9 | 19.7 ± 5.4 |
| Open arm entries (#) | 2.7 ± 0.7 | 2.0 ± 0.4 | 5.2 ± 0.9 | 4.0 ± 0.9 |
| Closed arm entries (#) | 7.0 ± 1.5 | 8.0 ± 1.6 | 5.3 ± 1.0 | 10.0 ± 0.4 |
p<0.05 vs. water, n=6/group.
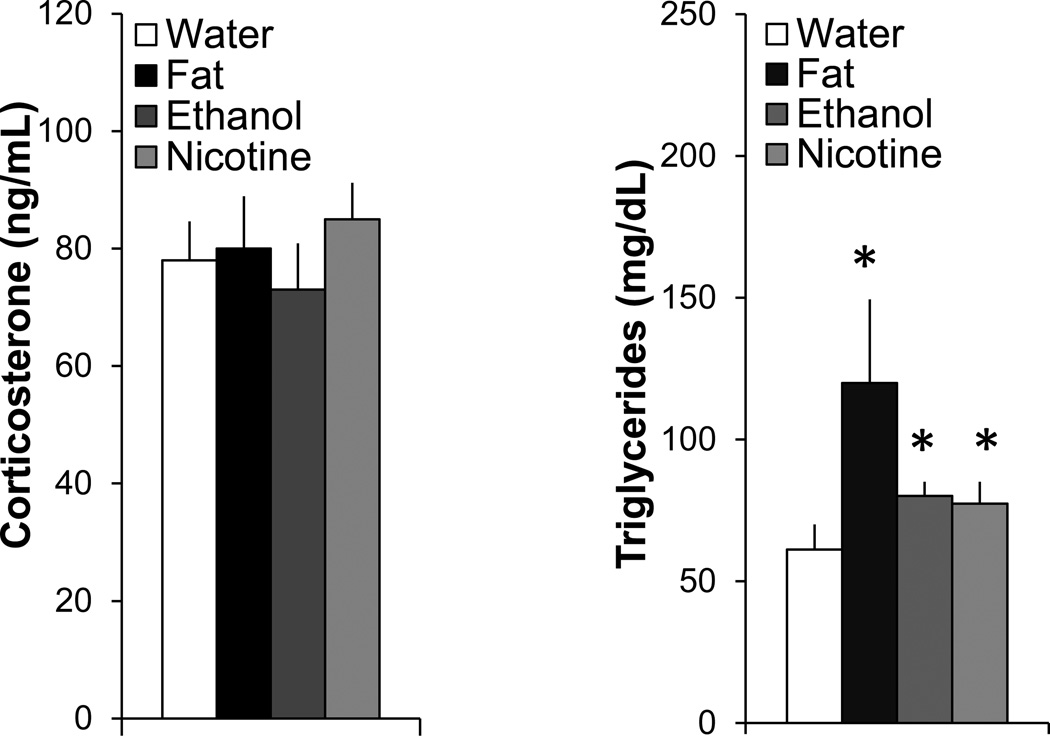
To assess their possible relation to certain physiological parameters, measurements were taken of circulating levels of CORT and different metabolites. Compared to the water control group, measurements of serum CORT levels (130±33 ng/ml in this group) showed little change (F(3,24)=0.87, ns) after short-term intraoral administration of fat, ethanol, or nicotine (Fig. 6), again suggesting that these substances in this paradigm had little impact on stress. In addition to causing elevated blood ethanol levels after ethanol gavage (61±5 mg/dl) and blood nicotine levels after nicotine gavage (39±5 ng/mL), the three substances each produced a significant rise in levels of TG (F(3,24)=12.80, p<0.001) (Fig. 6). Compared to the water control group, levels of TG were increased most strongly by fat (+96%, p<0.05) but also by ethanol (+26.5%, p<0.05) and nicotine (+31%, p<0.05), with no significant difference evident between the experimental groups (ns for fat vs. ethanol vs. nicotine). Thus, while having little impact on the stress hormone CORT, short-term administration of fat, ethanol, and nicotine each causes an increase in circulating TG.
Fig. 6.
Short-term oral gavage of Intralipid (20% v/v), ethanol (12% v/v), or nicotine (0.01% w/v) (3.5 mL each) increases circulating levels of triglycerides while have no effect on levels of corticosterone. Data are mean ± S.E.M., *p<0.05 vs. water, n=6/group.
1.4 DISCUSSION
Studies have shown that administration or consumption of fat, ethanol, or nicotine can stimulate endogenous ENK gene expression in certain brain areas (Mathieu et al., 1996; Chang et al., 2004; Chang et al., 2010a; Can et al., 2012) and that peripheral and local injections of ENK agonists can, in turn, stimulate consummatory behavior (Sanger and McCarthy, 1981; Simmons and Self, 2009; Barson et al., 2010), suggesting a positive relationship between ENK and consumption of food and drugs of abuse. The goal of the present study was to identify more precisely the anatomical location of the ENK-responsive neurons involved in this feedback circuit and determine whether these three substances do in fact act on the same population of neurons. By administering fat, ethanol, or nicotine at relatively low doses, using the same mode and short-term duration, and by closely examining the specific brain sites affected, the results clearly demonstrate that these three reinforcing substances similarly increase, by 20–40%, the gene expression of ENK in the same population of neurons in particular areas. Within the HYPO, this effect was observed in the PVN and found for each substance to be strongest and most consistent in the medial region of this nucleus, at the anterior-posterior level of the middle PVN. It was also detected in the AMYG, where the anatomical analysis identified a population of ENK neurons in the CeA, particularly its lateral subregion and lateral capsular area, as being most responsive, in contrast to those in the BLA which were unaffected. Further, in the NAc, the present results clearly distinguished the two major subregions of this structure, revealing a significant increase in the density of ENK neurons specifically in the NAcC but not in the NAcSh, consistent with effects described with repeated administration of nicotine (Mathieu et al., 1996). Thus, within the HYPO, AMYG, and NAc, these findings identify three populations of ENK neurons in specific sites, the middle PVN, CeA, and NAcC, which are similarly responsive to the stimulatory effect of fat, ethanol, and nicotine, and they exclude populations of neurons in other areas, specifically the lateral PVN, BLA and NAcSh, which are unresponsive to these substances. Interestingly, the density of c-Fos-ir cells was affected by fat, ethanol, and nicotine, which caused a 40–80% increase, in precisely the same brain areas where the density of ENK-expressing neurons was altered. This evidence underscores the anatomical specificity of this stimulatory effect of these substances on different subpopulations of neurons in general and on ENK neurons in particular.
When these three responsive areas were examined using double-labeling immunofluorescence, some of the neurons that were activated by fat, ethanol, or nicotine were found to have an ENK phenotype. With short-term oral administration, these three substances each significantly increased the density of double-labeled neurons, which accounted for up to 18% of the single-labeled ENK or c-Fos-ir neurons. The largest increase in c-Fos+ENK double-labeled neurons was observed in the medial parvocellular region of the middle PVN and the lateral subdivision of the CeA, consistent with published reports examining the PVN and CeA after acute nicotine injection (Loughlin et al., 2006) and the CeA after acute ethanol injection (Criado and Morales, 2000). While not previously studied, the NAcC exhibited a similar effect as the PVN and CeA, although it was smaller in magnitude, with the number of double-labeled neurons accounting for only 8% of the single-labeled ENK or c-Fos-ir neurons. The c-Fos-ir neurons that failed to double-label ENK may express a variety of other neurochemicals, such as the amino acid neurotransmitter, γ-aminobutyric acid (GABA). Brain levels of GABA are increased by all three substances (Fisler et al., 1989; De Witte, 1996; Benowitz, 2009), and GABA neurons are found to be dense in the PVN (Vincent et al., 1982), CeA (Sun and Cassell, 1993), and NAcC (Rogard et al., 1993). The present results reveal, at least with short-term administration, a clear similarity between fat, ethanol, and nicotine in their ability to activate specific populations of ENK-expressing neurons in these subregions of the hypothalamus and limbic system. With these neurochemical changes induced by post-ingestive stimuli rather than by taste, they may differ somewhat from results produced by voluntary oral intake. This is exemplified by the finding that the density of c-fos-ir in cells of the hypothalamic arcuate nucleus that express the opioid β-endorphin precursor pro-opiomelanocortin is increased by intragastric administration of Intralipid at a later time than by oral ingestion of Intralipid (Matsumura et al., 2012).
While sucrose is similar to fat, nicotine, and ethanol in being overconsumed and having reinforcing properties(Corrigall et al., 1994; Czachowski and Samson, 1999; Czachowski et al., 2003; Ackroff and Sclafani, 2014), the results obtained here clearly differentiate this sweet substance from the others tested. Whereas there is little information on the effects of acute sucrose exposure, studies with chronic sucrose administration or drinking have described a suppression of ENK mRNA in the NAc (Kelley et al., 2003), mixed effects on c-Fos in the BLA (Bachtell et al., 1999; Ulrich-Lai et al., 2007), and little change in c-Fos-ir in the PVN, CeA, or NAcC (Bachtell et al., 1999; Pomonis et al., 2000; Ulrich-Lai et al., 2007; Mitra et al., 2010). Our findings with short-term oral administration fail to reveal any effect of sucrose on ENK-expressing neurons or c-Fos-ir in the middle PVN, CeA, or NAcC, in marked contrast to the strong stimulatory effect observed with fat, ethanol, and nicotine. In addition to clearly differentiating these reinforcing substances, this finding suggests that the opioid ENK may have relatively little role in mediating the effects and behavioral consequences of sucrose on the brain. This is consistent with other evidence showing that PVN injection of an ENK analog, which increases the consumption of fat or ethanol (Barson et al., 2010; Chang et al., 2010b), has little impact on the consumption of sucrose (Naleid et al., 2007). In contrast to ENK, sucrose may have a stronger effect on levels of another opioid peptide, β-endorphin. Both consumption and sham-feeding of sucrose are found to be reduced by an antagonist of the mu-opioid receptor, which is targeted by β-endorphin, but are generally unaffected by antagonists of the delta-opioid receptor, which is targeted by ENK (Beczkowska et al., 1992; Leventhal et al., 1995).
In addition to reinforcing properties, results obtained here with fat, ethanol, and nicotine suggest that psychological stress, sometimes induced by these substances (Scheufele et al., 2000; Walker et al., 2010; Can et al., 2012), may not be critical to their stimulatory effect on ENK-expressing neurons. Under some circumstances, stress is found to stimulate ENK mRNA (Ceccatelli and Orazzo, 1993; Christiansen et al., 2011) and c-Fos or c-fos (Honkaniemi et al., 1992; Miyata et al., 1995) in the HYPO, AMYG, and NAc, and the administration of fat, ethanol, and nicotine, particularly at chronic and high doses, can produce behavioral signs of stress (Scheufele et al., 2000; Walker et al., 2010; Can et al., 2012). The results of the present study, which examined the effects of these three substances after short-term oral administration at relatively low doses, provide little evidence of stress-related behaviors and reveal few similarities between the behavioral effects these substances do have. Oral administration of fat produced little change in the different behaviors measured, locomotor activity, rearing, grooming, or anxiety, indicating little effect on stress. Also, ethanol gavage produced effects that are inconsistent with stress, namely, no change in anxiety in the EPM, no difference in rearing or grooming, and a small increase in locomotor activity in a novel open field that indicates an increase in exploratory behavior, similar to previous results with low to moderate ethanol doses (Paivarinta and Korpi, 1993; Sharko et al., 2013). Nicotine differed from ethanol in having no effect on locomotor activity but stimulating both rearing and grooming as previously reported (Collins et al., 1988; Zarrindast et al., 1998; Jandova et al., 2013), while it was similar to ethanol in having no impact on anxiety in the EPM as previously reported (Irvine et al., 2001). Importantly, the administration of fat, ethanol, or nicotine produced little change in circulating levels of the stress hormone, CORT, when measured one hour later, at a time when acute stress would lead these levels to be in the descending phase of their elevation (Rittenhouse et al., 2002) but when levels of fatty acids, ethanol, and nicotine should still be in the rising or plateau phase after gavage (Ferraro et al., 1991; Crooks et al., 1997; Adalsteinsson et al., 2006). Thus, in addition to revealing clear differences between the three substances in their effects on stress-related behaviors, these findings provide evidence that, when orally administered short-term, the substances have little lasting impact on stress hormones.
There are a variety of studies indicating that fat, ethanol, and nicotine have a common feature of increasing circulating levels of lipids (Scheufele et al., 2000; Chattopadhyay and Chattopadhyay, 2008; Barson et al., 2009). Our findings are consistent with this evidence, showing short-term oral administration of these substances to significantly increase levels of TG, with fat producing the strongest effect. While circulating TG from Intralipid invariably come from the lipid emulsion itself (Chen, 1984), those from ethanol and nicotine are increased due to de novo synthesis in the liver (Tsukamoto et al., 1984; Ma et al., 2014) or a reduction of basal lipolysis (Ashakumary and Vijayammal, 1997; Szkudelski et al., 2004). There is evidence that lipids can contribute to the stimulation of ENK gene expression, as indicated in studies showing ENK mRNA in the PVN to be increased by acute injection of Intralipid or by the fatty acid linoleic acid and also by the consumption of a fat-rich meal (Oomura et al., 1975; Chang et al., 2004; Chang et al., 2007). This elevated PVN ENK may, in turn, further stimulate consummatory behavior, as shown in rats that have spontaneously high post-meal TG (Karatayev et al., 2009) and subsequently eat more fat and drink more ethanol when offered these substances (Karatayev et al., 2009; Karatayev et al., 2010). Although further studies are needed to characterize the mechanisms mediating the stimulatory effect of lipids on ENK, there is evidence showing that lipids can alter, either directly or indirectly, a variety of central neural processes. This was demonstrated in vitro with short-chain fatty acids, which increase ENK gene expression in PC12 rat cells via the PKA signaling pathway (Mally et al., 2004), suggesting that lipid-activated transcription factors may be involved. Lipids may also influence the ENK system by modulating the binding of opioid peptides to their receptor (Murphy et al., 1987; Remmers et al., 1990) and by producing oxidative stress in cells (Rosenberger et al., 2001; Amin et al., 2011). Together, these studies provide initial evidence supporting the idea that the stimulatory effect that fat, ethanol, and nicotine each have on ENK-expressing neurons, specifically in the middle PVN, CeA, and NAcC, may be mediated, in part, by their common effect of elevating circulating lipids.
1.5 Conclusions
The results of this study demonstrate for the first time that fat, ethanol, and nicotine can act in an anatomically specific manner to stimulate a common neurochemical system that promotes consumption. This system is found to involve ENK, which is enhanced specifically in the middle PVN, CeA, and NAcC, areas known to have appetitive-related functions (Kalra and Kalra, 2004; Cybulska-Klosowicz et al., 2009; Tandon et al., 2012), but is unaffected in nearby areas, the BLA or NAcSh, which are more closely related to reward (Soderman and Unterwald, 2008; Lintas et al., 2012). This stimulatory effect of these reinforcing substances on ENK expression in these appetite-controlling brain sites may have a variety of behavioral consequences, as evidenced by studies showing peripheral or central injection of ENK to stimulate the consumption of fat, ethanol, and nicotine (Naleid et al., 2007; Barson et al., 2010; Liu and Jernigan, 2011). Thus, the population of ENK neurons stimulated in these areas, perhaps acting in conjunction with circulating lipids, may direct appetitive actions that increase the chances of these three substances being consumed again, and they may also increase chow intake, although this remains to be tested using the current dosing paradigm. This evidence, showing increased activation of ENK-expressing neurons in specific nuclei with a high degree of interconnectivity, suggests that the positive relationship which exists between ENK and these reinforcing substances is a defining commonality that allows the intake of one of these three substances to stimulate intake of the others (Carrillo et al., 2004; Barson et al., 2009; Bito-Onon et al., 2011), ultimately leading to overconsumption and potential abuse of any or all of the substances.
Highlights.
Fat, ethanol, and nicotine but not sucrose activate neurons in the same hypothalamic and limbic areas
Fat, ethanol, and nicotine but not sucrose activate enkephalin in the same neuronal populations
Fat, ethanol, and nicotine dosing regimens have little effect on stress-related behaviors or levels of cortisol
Fat, ethanol, and nicotine increase circulating levels of the lipids, triglycerides
ACKNOWLEDGEMENTS
This research was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Numbers R01AA12882 (SFL) and K99AA021782 (JRB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We would like to thank the Proteomics Resource Center at The Rockefeller University for determining plasma nicotine levels with HPLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ackroff K, Sclafani A. Post-oral fat stimulation of intake and conditioned flavor preference in C57BL/6J mice: A concentration-response study. Physiology & behavior. 2014;129C:64–72. doi: 10.1016/j.physbeh.2014.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adalsteinsson E, Sullivan EV, Mayer D, Pfefferbaum A. In vivo quantification of ethanol kinetics in rat brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:2683–2691. doi: 10.1038/sj.npp.1301023. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Garcia MM, Harlan RE. Glucocorticoid regulation of preproenkephalin gene expression in the rat forebrain. Brain Res Mol Brain Res. 1992;16:119–127. doi: 10.1016/0169-328x(92)90201-l. [DOI] [PubMed] [Google Scholar]
- Amin KA, Kamel HH, Abd Eltawab MA. The relation of high fat diet, metabolic disturbances and brain oxidative dysfunction: modulation by hydroxy citric acid. Lipids Health Dis. 2011;10:74. doi: 10.1186/1476-511X-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon CM, Pesold CN, Amit Z. Ethanol-induced motor activity in normal and acatalasemic mice. Alcohol. 1992;9:207–211. doi: 10.1016/0741-8329(92)90055-f. [DOI] [PubMed] [Google Scholar]
- Ashakumary L, Vijayammal PL. Effect of nicotine on lipoprotein metabolism in rats. Lipids. 1997;32:311–315. doi: 10.1007/s11745-997-0038-8. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain research. 1999;847:157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Balerio GN, Aso E, Maldonado R. Involvement of the opioid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology. 2005;181:260–269. doi: 10.1007/s00213-005-2238-y. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. Neural mechanisms underlying nicotine dependence. Addiction. 1994;89:1419–1423. doi: 10.1111/j.1360-0443.1994.tb03738.x. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Khullar AK, Longden A. Effects of nicotine on plasma corticosterone and brain amines in stressed and unstressed rats. Pharmacology, biochemistry, and behavior. 1975;3:179–184. doi: 10.1016/0091-3057(75)90145-8. [DOI] [PubMed] [Google Scholar]
- Barrett ST, Bevins RA. A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand. Behav Pharmacol. 2012;23:781–789. doi: 10.1097/FBP.0b013e32835a38d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Rada P, Leibowitz SF, Hoebel BG. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcoholism, clinical and experimental research. 2010;34:214–222. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Chang GQ, Poon K, Morganstern I, Leibowitz SF. Galanin and the orexin 2 receptor as possible regulators of enkephalin in the paraventricular nucleus of the hypothalamus: relation to dietary fat. Neuroscience. 2011a;193:10–20. doi: 10.1016/j.neuroscience.2011.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Karatayev O, Chang GQ, Johnson DF, Bocarsly ME, Hoebel BG, Leibowitz SF. Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: counteraction by lipid-lowering drugs. Alcohol. 2009;43:433–441. doi: 10.1016/j.alcohol.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF. Similarities in hypothalamic and mesocorticolimbic circuits regulating the overconsumption of food and alcohol. Physiology & behavior. 2011b;104:128–137. doi: 10.1016/j.physbeh.2011.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beczkowska IW, Bowen WD, Bodnar RJ. Central opioid receptor subtype antagonists differentially alter sucrose and deprivation-induced water intake in rats. Brain research. 1992;589:291–301. doi: 10.1016/0006-8993(92)91289-q. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol. 2011;16:440–449. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can OD, Ulupinar E, Ozkay UD, Yegin B, Ozturk Y. The effect of simvastatin treatment on behavioral parameters, cognitive performance, and hippocampal morphology in rats fed a standard or a high-fat diet. Behav Pharmacol. 2012;23:582–592. doi: 10.1097/FBP.0b013e328356c3f2. [DOI] [PubMed] [Google Scholar]
- Carrillo CA, Leibowitz SF, Karatayev O, Hoebel BG. A high-fat meal or injection of lipids stimulates ethanol intake. Alcohol. 2004;34:197–202. doi: 10.1016/j.alcohol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Orazzo C. Effect of different types of stressors on peptide messenger ribonucleic acids in the hypothalamic paraventricular nucleus. Acta Endocrinol (Copenh) 1993;128:485–492. doi: 10.1530/acta.0.1280485. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Barson JR, Karatayev O, Chang SY, Chen YW, Leibowitz SF. Effect of chronic ethanol on enkephalin in the hypothalamus and extra-hypothalamic areas. Alcoholism, clinical and experimental research. 2010a;34:761–770. doi: 10.1111/j.1530-0277.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz SF. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol Endocrinol Metab. 2007;292:E561–E570. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Barson JR, Chang SY, Leibowitz SF. Increased enkephalin in brain of rats prone to overconsuming a fat-rich diet. Physiology & behavior. 2010b;101:360–369. doi: 10.1016/j.physbeh.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145:3904–3912. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay K, Chattopadhyay BD. Effect of nicotine on lipid profile, peroxidation & antioxidant enzymes in female rats with restricted dietary protein. Indian J Med Res. 2008;127:571–576. [PubMed] [Google Scholar]
- Chen WJ. Utilization of exogenous fat emulsion (Intralipid) in septic rats. JPEN J Parenter Enteral Nutr. 1984;8:14–17. doi: 10.1177/014860718400800114. [DOI] [PubMed] [Google Scholar]
- Chepulis LM, Starkey NJ, Waas JR, Molan PC. The effects of long-term honey, sucrose or sugar-free diets on memory and anxiety in rats. Physiology & behavior. 2009;97:359–368. doi: 10.1016/j.physbeh.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Christiansen AM, Herman JP, Ulrich-Lai YM. Regulatory interactions of stress and reward on rat forebrain opioidergic and GABAergic circuitry. Stress. 2011;14:205–215. doi: 10.3109/10253890.2010.531331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hamelink C, Brunnquell M, Thorsell A, Heilig M, Eskay RL. Binge-like ethanol consumption increases corticosterone levels and neurodegneration whereas occupancy of type II glucocorticoid receptors with mifepristone is neuroprotective. Addict Biol. 2014;19:27–36. doi: 10.1111/j.1369-1600.2012.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Romm E, Wehner JM. Nicotine tolerance: an analysis of the time course of its development and loss in the rat. Psychopharmacology. 1988;96:7–14. doi: 10.1007/BF02431526. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain research. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Criado JR, Morales M. Acute ethanol induction of c-Fos immunoreactivity in pre-pro-enkephalin expressing neurons of the central nucleus of the amygdala. Brain research. 2000;861:173–177. doi: 10.1016/s0006-8993(99)02468-3. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Li M, Dwoskin LP. Metabolites of nicotine in rat brain after peripheral nicotine administration. Cotinine, nornicotine, and norcotinine. Drug Metab Dispos. 1997;25:47–54. [PubMed] [Google Scholar]
- Cybulska-Klosowicz A, Zakrzewska R, Kossut M. Brain activation patterns during classical conditioning with appetitive or aversive UCS. Behav Brain Res. 2009;204:102–111. doi: 10.1016/j.bbr.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Assessment of sucrose and ethanol reinforcement: the across-session breakpoint procedure. Physiology & behavior. 2003;78:51–59. doi: 10.1016/s0031-9384(02)00963-0. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Breakpoint determination and ethanol self-administration using an across-session progressive ratio procedure in the rat. Alcoholism, clinical and experimental research. 1999;23:1580–1586. [PubMed] [Google Scholar]
- De Witte P. The role of neurotransmitters in alcohol dependence: animal research. Alcohol and alcoholism. 1996;31:13–16. [PubMed] [Google Scholar]
- Ferraro TN, Carrozza DP, Vogel WH. In vivo microdialysis study of brain ethanol concentrations in rats following oral self-administration. Alcoholism, clinical and experimental research. 1991;15:504–507. doi: 10.1111/j.1530-0277.1991.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Fisler JS, Shimizu H, Bray GA. Brain 3-hydroxybutyrate, glutamate, and GABA in a rat model of dietary obesity. Physiology & behavior. 1989;45:571–577. doi: 10.1016/0031-9384(89)90075-9. [DOI] [PubMed] [Google Scholar]
- Forger NG, Morin LP. Reproductive state modulates ethanol intake in rats: effects of ovariectomy, ethanol concentration, estrous cycle and pregnancy. Pharmacology, biochemistry, and behavior. 1982;17:323–331. doi: 10.1016/0091-3057(82)90087-9. [DOI] [PubMed] [Google Scholar]
- Fornari A, Pedrazzi P, Lippi G, Picciotto MR, Zoli M, Zini I. Nicotine withdrawal increases body weight, neuropeptide Y and Agouti-related protein expression in the hypothalamus and decreases uncoupling protein-3 expression in the brown adipose tissue in high-fat fed mice. Neuroscience letters. 2007;411:72–76. doi: 10.1016/j.neulet.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain research. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur J Neurosci. 2008;27:1912–1922. doi: 10.1111/j.1460-9568.2008.06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, Mayfield RD, Camp MC, Alcantara AA. Ethanol-induced Fos immunoreactivity in the extended amygdala and hypothalamus of the rat brain: focus on cholinergic interneurons of the nucleus accumbens. Alcoholism, clinical and experimental research. 2004;28:588–597. doi: 10.1097/01.alc.0000122765.58324.6d. [DOI] [PubMed] [Google Scholar]
- Hilario MR, Turner JR, Blendy JA. Reward sensitization: effects of repeated nicotine exposure and withdrawal in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2661–2670. doi: 10.1038/npp.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkaniemi J, Kainu T, Ceccatelli S, Rechardt L, Hokfelt T, Pelto-Huikko M. Fos and jun in rat central amygdaloid nucleus and paraventricular nucleus after stress. Neuroreport. 1992;3:849–852. doi: 10.1097/00001756-199210000-00007. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Tolerance to nicotine’s effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacology, biochemistry, and behavior. 2001;68:319–325. doi: 10.1016/s0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa J, Ogawa Y, Owada Y, Ishikawa A. Hyperlocomotor activity and stress vulnerability during adulthood induced by social isolation after early weaning are prevented by voluntary running exercise before normal weaning period. Behav Brain Res. 2014;264:197–206. doi: 10.1016/j.bbr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Jandova K, Maresova D, Pokorny J. Fast and delayed locomotor response to acute high-dose nicotine administration in adult male rats. Physiol Res. 2013;62(Suppl 1):S81–S88. doi: 10.33549/physiolres.932610. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. Overlapping and interactive pathways regulating appetite and craving. Journal of addictive diseases. 2004;23:5–21. doi: 10.1300/J069v23n03_02. [DOI] [PubMed] [Google Scholar]
- Karatayev O, Barson JR, Carr AJ, Baylan J, Chen YW, Leibowitz SF. Predictors of ethanol consumption in adult Sprague-Dawley rats: relation to hypothalamic peptides that stimulate ethanol intake. Alcohol. 2010;44:323–334. doi: 10.1016/j.alcohol.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatayev O, Gaysinskaya V, Chang GQ, Leibowitz SF. Circulating triglycerides after a high-fat meal: predictor of increased caloric intake, orexigenic peptide expression, and dietary obesity. Brain research. 2009;1298:111–122. doi: 10.1016/j.brainres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18:2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- Kesse E, Clavel-Chapelon F, Slimani N, van Liere M, Group EN. Do eating habits differ according to alcohol consumption? Results of a study of the French cohort of the European Prospective Investigation into Cancer and Nutrition (E3N-EPIC) The American journal of clinical nutrition. 2001;74:322–327. doi: 10.1093/ajcn/74.3.322. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucuk A, Golgeli A, Saraymen R, Koc N. Effects of age and anxiety on learning and memory. Behav Brain Res. 2008;195:147–152. doi: 10.1016/j.bbr.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Langen B, Dietze S, Fink H. Acute effect of ethanol on anxiety and 5-HT in the prefrontal cortex of rats. Alcohol. 2002;27:135–141. doi: 10.1016/s0741-8329(02)00219-7. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Akabayashi A, Alexander JT, Wang J. Gonadal steroids and hypothalamic galanin and neuropeptide Y: role in eating behavior and body weight control in female rats. Endocrinology. 1998;139:1771–1780. doi: 10.1210/endo.139.4.5867. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Akabayashi A, Wang J, Alexander JT, Dourmashkin JT, Chang GQ. Increased caloric intake on a fat-rich diet: role of ovarian steroids and galanin in the medial preoptic and paraventricular nuclei and anterior pituitary of female rats. J Neuroendocrinol. 2007;19:753–766. doi: 10.1111/j.1365-2826.2007.01584.x. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Lucas DJ, Leibowitz KL, Jhanwar YS. Developmental patterns of macronutrient intake in female and male rats from weaning to maturity. Physiology & behavior. 1991;50:1167–1174. doi: 10.1016/0031-9384(91)90578-c. [DOI] [PubMed] [Google Scholar]
- Leriche M, Mendez M, Zimmer L, Berod A. Acute ethanol induces Fos in GABAergic and non-GABAergic forebrain neurons: a double-labeling study in the medial prefrontal cortex and extended amygdala. Neuroscience. 2008;153:259–267. doi: 10.1016/j.neuroscience.2008.01.069. [DOI] [PubMed] [Google Scholar]
- Leventhal L, Kirkham TC, Cole JL, Bodnar RJ. Selective actions of central mu and kappa opioid antagonists upon sucrose intake in sham-fed rats. Brain research. 1995;685:205–210. doi: 10.1016/0006-8993(95)00385-4. [DOI] [PubMed] [Google Scholar]
- Levin BE. Factors promoting and ameliorating the development of obesity. Physiology & behavior. 2005;86:633–639. doi: 10.1016/j.physbeh.2005.08.054. [DOI] [PubMed] [Google Scholar]
- Lewis MJ. Alcohol reinforcement and neuropharmacological therapeutics. Alcohol and alcoholism. 1996;31(Suppl 1):17–25. [PubMed] [Google Scholar]
- Lintas A, Chi N, Lauzon NM, Bishop SF, Sun N, Tan H, Laviolette SR. Inputs from the basolateral amygdala to the nucleus accumbens shell control opiate reward magnitude via differential dopamine D1 or D2 receptor transmission. Eur J Neurosci. 2012;35:279–290. doi: 10.1111/j.1460-9568.2011.07943.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Jernigan C. Activation of the opioid mu1, but not delta or kappa, receptors is required for nicotine reinforcement in a rat model of drug self-administration. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:146–153. doi: 10.1016/j.pnpbp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin SE, Islas MI, Cheng MY, Lee AG, Villegier AS, Leslie FM. Nicotine modulation of stress-related peptide neurons. The Journal of comparative neurology. 2006;497:575–588. doi: 10.1002/cne.20999. [DOI] [PubMed] [Google Scholar]
- Ma N, Nicholson CJ, Wong M, Holloway AC, Hardy DB. Fetal and neonatal exposure to nicotine leads to augmented hepatic and circulating triglycerides in adult male offspring due to increased expression of fatty acid synthase. Toxicol Appl Pharmacol. 2014;275:1–11. doi: 10.1016/j.taap.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Mally P, Mishra R, Gandhi S, Decastro MH, Nankova BB, Lagamma EF. Stereospecific regulation of tyrosine hydroxylase and proenkephalin genes by short-chain fatty acids in rat PC12 cells. Pediatr Res. 2004;55:847–854. doi: 10.1203/01.PDR.0000119365.21770.45. [DOI] [PubMed] [Google Scholar]
- Mathieu AM, Caboche J, Besson MJ. Distribution of preproenkephalin, preprotachykinin A, and preprodynorphin mRNAs in the rat nucleus accumbens: effect of repeated administration of nicotine. Synapse. 1996;23:94–106. doi: 10.1002/(SICI)1098-2396(199606)23:2<94::AID-SYN5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Eguchi A, Okafuji Y, Tatsu S, Mizushige T, Tsuzuki S, Inoue K, Fushiki T. Dietary fat ingestion activates beta-endorphin neurons in the hypothalamus. FEBS letters. 2012;586:1231–1235. doi: 10.1016/j.febslet.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Matta SG, Foster CA, Sharp BM. Nicotine stimulates the expression of cFos protein in the parvocellular paraventricular nucleus and brainstem catecholaminergic regions. Endocrinology. 1993;132:2149–2156. doi: 10.1210/endo.132.5.8386611. [DOI] [PubMed] [Google Scholar]
- McLaughlin CL, Baile CA, Della-Fera MA. Changes in brain met-enkephalin concentrations with peripheral CCK injections in Zucker rats. Physiology & behavior. 1986;36:681–686. doi: 10.1016/0031-9384(86)90354-9. [DOI] [PubMed] [Google Scholar]
- Mitra A, Gosnell BA, Schioth HB, Grace MK, Klockars A, Olszewski PK, Levine AS. Chronic sugar intake dampens feeding-related activity of neurons synthesizing a satiety mediator, oxytocin. Peptides. 2010;31:1346–1352. doi: 10.1016/j.peptides.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Itoh T, Lin SH, Ishiyama M, Nakashima T, Kiyohara T. Temporal changes of c-fos expression in oxytocinergic magnocellular neuroendocrine cells of the rat hypothalamus with restraint stress. Brain Res Bull. 1995;37:391–395. doi: 10.1016/0361-9230(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Barson JR, Leibowitz SF. Regulation of drug and palatable food overconsumption by similar peptide systems. Curr Drug Abuse Rev. 2011;4:163–173. doi: 10.2174/1874473711104030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Ye Z, Liang S, Fagan S, Leibowitz SF. Involvement of cholinergic mechanisms in the behavioral effects of dietary fat consumption. Brain research. 2012;1470:24–34. doi: 10.1016/j.brainres.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MG, Moak CM, Rao BG. Effects of membrane polyunsaturated fatty acids on opiate peptide inhibition of basal and prostaglandin E1-stimulated cyclic AMP formation in intact N1E-115 neuroblastoma cells. Biochem Pharmacol. 1987;36:4079–4084. doi: 10.1016/0006-2952(87)90564-8. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. American journal of physiology Regulatory, integrative and comparative physiology. 2007;293:R99–R105. doi: 10.1152/ajpregu.00675.2006. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Noh SJ, Kang DW, Yoo SB, Lee JY, Kim JY, Kim BT, Lee JH, Jahng JW. Stress-responsive hypothalamic-nucleus accumbens regulation may vary depending on stressors. Indian J Exp Biol. 2012;50:447–454. [PubMed] [Google Scholar]
- Olausson P, Ericson M, Lof E, Engel JA, Soderpalm B. Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur J Pharmacol. 2001;417:117–123. doi: 10.1016/s0014-2999(01)00903-7. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Nakamura T, Sugimori M, Yamada Y. Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiology & behavior. 1975;14:483–486. doi: 10.1016/0031-9384(75)90015-3. [DOI] [PubMed] [Google Scholar]
- Paivarinta P, Korpi ER. Voluntary ethanol drinking increases locomotor activity in alcohol-preferring AA rats. Pharmacology, biochemistry, and behavior. 1993;44:127–132. doi: 10.1016/0091-3057(93)90289-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, C.A: Elsevier Academic Press; 2005. [Google Scholar]
- Petruzziello F, Falasca S, Andren PE, Rainer G, Zhang X. Chronic nicotine treatment impacts the regulation of opioid and non-opioid peptides in the rat dorsal striatum. Mol Cell Proteomics. 2013;12:1553–1562. doi: 10.1074/mcp.M112.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Interaction of ethanol and stress: research with experimental animals--an update. Alcohol and alcoholism. 1990;25:263–276. doi: 10.1093/oxfordjournals.alcalc.a045000. [DOI] [PubMed] [Google Scholar]
- Pomonis JD, Jewett DC, Kotz CM, Briggs JE, Billington CJ, Levine AS. Sucrose consumption increases naloxone-induced c-Fos immunoreactivity in limbic forebrain. American journal of physiology Regulatory, integrative and comparative physiology. 2000;278:R712–R719. doi: 10.1152/ajpregu.2000.278.3.R712. [DOI] [PubMed] [Google Scholar]
- Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiology & behavior. 1996;60:1039–1042. doi: 10.1016/0031-9384(96)00135-7. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Barson JR, Hoebel BG, Leibowitz SF. A high-fat meal, or intraperitoneal administration of a fat emulsion, increases extracellular dopamine in the nucleus accumbens. Brain Sci. 2012;2:242–253. doi: 10.3390/brainsci2020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Remmers AE, Nordby GL, Medzihradsky F. Modulation of opioid receptor binding by cis and trans fatty acids. J Neurochem. 1990;55:1993–2000. doi: 10.1111/j.1471-4159.1990.tb05787.x. [DOI] [PubMed] [Google Scholar]
- Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–318. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- Rogard M, Caboche J, Julien JF, Besson MJ. The rat nucleus accumbens: two levels of complexity in the distribution of glutamic acid decarboxylase (67 kDa) and preproenkephalin messenger RNA. Neuroscience letters. 1993;155:81–86. doi: 10.1016/0304-3940(93)90678-e. [DOI] [PubMed] [Google Scholar]
- Rosenberger J, Petrovics G, Buzas B. Oxidative stress induces proorphanin FQ and proenkephalin gene expression in astrocytes through p38- and ERK-MAP kinases and NF-kappaB. J Neurochem. 2001;79:35–44. doi: 10.1046/j.1471-4159.2001.00520.x. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2:32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- Salminen O, Seppa T, Gaddnas H, Ahtee L. Effect of acute nicotine on Fos protein expression in rat brain during chronic nicotine and its withdrawal. Pharmacology, biochemistry, and behavior. 2000;66:87–93. doi: 10.1016/s0091-3057(00)00203-3. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, McCarthy PS. Increased food and water intake produced in rats by opiate receptor agonists. Psychopharmacology. 1981;74:217–220. doi: 10.1007/BF00427097. [DOI] [PubMed] [Google Scholar]
- Scheufele PM, Faraday MM, Grunberg NE. Nicotine administration interacts with housing conditions to alter social and non-social behaviors in male and female Long-Evans rats. Nicotine Tob Res. 2000;2:169–178. doi: 10.1080/713688133. [DOI] [PubMed] [Google Scholar]
- Sharko AC, Kaigler KF, Fadel JR, Wilson MA. Individual differences in voluntary ethanol consumption lead to differential activation of the central amygdala in rats: relationship to the anxiolytic and stimulant effects of low dose ethanol. Alcoholism, clinical and experimental research. 2013;37(Suppl 1):E172–E180. doi: 10.1111/j.1530-0277.2012.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji H, Mizoguchi K. Acute and repeated stress differentially regulates behavioral, endocrine, neural parameters relevant to emotional and stress response in young and aged rats. Behav Brain Res. 2010;211:169–177. doi: 10.1016/j.bbr.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Acute nicotine enhances c-fos mRNA expression differentially in reward-related substrates of adolescent and adult rat brain. Neuroscience letters. 2007;418:286–291. doi: 10.1016/j.neulet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderman AR, Unterwald EM. Cocaine reward and hyperactivity in the rat: sites of mu opioid receptor modulation. Neuroscience. 2008;154:1506–1516. doi: 10.1016/j.neuroscience.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulis G, Papalexi E, Kittas C, Kitraki E. Early impact of a fat-enriched diet on behavioral responses of male and female rats. Behavioral neuroscience. 2007;121:483–490. doi: 10.1037/0735-7044.121.3.483. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. The Journal of comparative neurology. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Szkudelski T, Bialik I, Szkudelska K. Adipocyte lipolysis, hormonal and metabolic changes in ethanol-drinking rats. J Anim Physiol Anim Nutr (Berl) 2004;88:251–258. doi: 10.1111/j.1439-0396.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- Takeda A, Tamano H, Kan F, Itoh H, Oku N. Anxiety-like behavior of young rats after 2-week zinc deprivation. Behav Brain Res. 2007;177:1–6. doi: 10.1016/j.bbr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Tandon S, Simon SA, Nicolelis MA. Appetitive changes during salt deprivation are paralleled by widespread neuronal adaptations in nucleus accumbens, lateral hypothalamus, and central amygdala. J Neurophysiol. 2012;108:1089–1105. doi: 10.1152/jn.00236.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O'Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology. 2009;206:303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O'Dell LE. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcoholism, clinical and experimental research. 2014;38:108–115. doi: 10.1111/acer.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013;7:28. doi: 10.3389/fnbeh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Lew G, Larkin EC, Largman C, Rao GA. Hepatic origin of triglycerides in fatty livers produced by the continuous intragastric infusion of an ethanol diet. Lipids. 1984;19:419–422. doi: 10.1007/BF02537403. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegier AS, Gallager B, Heston J, Belluzzi JD, Leslie FM. Age influences the effects of nicotine and monoamine oxidase inhibition on mood-related behaviors in rats. Psychopharmacology. 2010;208:593–601. doi: 10.1007/s00213-009-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR, Hokfelt T, Wu JY. GABA neuron systems in hypothalamus and the pituitary gland. Immunohistochemical demonstration using antibodies against glutamate decarboxylase. Neuroendocrinology. 1982;34:117–125. doi: 10.1159/000123288. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmaier EP, Rosen K, Abbott B. Free fatty acids activate the hypothalamic-pituitary-adrenocortical axis in rats. Endocrinology. 1992;131:2313–2318. doi: 10.1210/endo.131.5.1330498. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Chang GQ, Davydova Z, Leibowitz SF. Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia. American journal of physiology Regulatory, integrative and comparative physiology. 2003;284:R1454–R1465. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Sedaghati F, Borzouyeh F. Nicotine-induced grooming: a possible dopaminergic and/or cholinergic mechanism. J Psychopharmacol. 1998;12:375–379. doi: 10.1177/026988119801200408. [DOI] [PubMed] [Google Scholar]