Abstract
Background
The prognostic impact of primary tumor location on outcomes for patients with upper-tract urothelial carcinoma (UTUC) is still contentious.
Objective
To test the association between tumor location and disease recurrence and cancer-specific survival (CSS) in patients treated with radical nephroureterectomy (RNU) for UTUC.
Design, setting, and participants
Prospectively collected data were retrospectively reviewed from 324 consecutive patients treated with RNU between 1995 and 2008 at a single tertiary referral center. Patients who had previous radical cystectomy, preoperative chemotherapy, previous contralateral UTUC, or metastatic disease at presentation were excluded. This left 253 patients for analysis. Tumor location was categorized as renal pelvis or ureter based on the location of the dominant tumor. Recurrences in the bladder only, in nonbladder sites, and in any site were analyzed.
Intervention
All patients were treated with RNU.
Measurements
Recurrence-free survival and CSS probabilities were estimated using Kaplan-Meier and Cox regression analyses.
Results and limitations
Median follow-up for survivors was 48 mo. The 5-yr recurrence-free probability (including bladder recurrence) and CSS estimates were 32% and 78%, respectively. On multivariable analysis, pathologic stage was the only predictor for disease recurrence (p = 0.01). Tumor location was not an independent predictor for recurrence (hazard ratio: 1.19; p = 0.3), and there was no difference in the probability of disease recurrence between ureteral and renal pelvic tumors (p = 0.18). On survival analysis, we also found no differences between ureteral and renal pelvic tumors on probability of CSS (p = 0.2). On multivariate analysis, pathologic stage (p < 0.0001) and nodal status (p = 0.01) were associated with worse CSS. This study is limited by its retrospective nature.
Conclusions
Our study did not show any differences in recurrence and CSS rates between patients with ureteral and renal pelvic tumors treated with RNU.
Keywords: Nephroureterectomy, Recurrence, Renal pelvis, Survival, Urothelial carcinoma, Ureter
1. Introduction
Upper-tract urothelial carcinoma (UTUC) is a relatively uncommon disease that accounts for approximately 5[en]10% of all renal tumors and 5% of all urothelial carcinomas (UCs) [1-3]. Open radical nephroureterectomy (RNU) with excision of the distal ureter with a bladder cuff is the standard of care for invasive UTUC. Pathologic stage, lymph node metastasis, and tumor grade have been established as prognostic factors for UTUC [4-8]. The primary tumor location, however, represents a controversial risk factor. Some authors report worse prognosis for ureteral compared to renal pelvic tumors, leading to the hypothesis that the thin periureteral layer of adventitia with extensive lymphatic and blood channels make tumor invasion and metastasis easier. In addition, these authors postulated that the renal parenchyma and surrounding adipose tissue act as a barrier to early tumor spread for renal pelvic tumors [9,10].
In contrast, other investigators found that proximally located tumors (renal pelvis and proximal ureter) had worse 5-yr cancer-specific survival (CSS) compared with distal ureteral tumors based on anatomy (thinner muscular layer of the renal pelvis/proximal ureter) [11]. Recently, a large multicenter study and a population-based study both found that renal pelvic tumors present with more advanced pathologic stage than ureteral UTUC [7,12]. Interestingly, both studies failed to show that tumor location had a differential effect on cancer recurrence and survival after adjusting for the effects of pathologic stage, grade, and lymph node metastasis.
The reasons underlying these differences include small sample size for some studies, lack of standardization in surgical approach and pathologic protocol (ie, central slide review), and differences in disease severity and management. Therefore, we decided to assess the effect of tumor location on UTUC outcomes in a large, contemporary cohort of consecutive patients treated with RNU at a single center with dedicated genitourinary surgeons and pathologists. Our hypothesis was that anatomical location of the primary lesion had no prognostic value when other pathologic features were taken into account.
2. Material and methods
2.1. Patient selection and technique
After institutional review board approval, we retrospectively reviewed all the prospectively collected data of the 324 consecutive patients treated with RNU at Memorial Sloan-Kettering Cancer Center (MSKCC) in New York City between 1995 and 2008. We excluded patients treated with previous or concurrent radical cystectomy (n = 45), patients treated with preoperative chemotherapy (n = 43), patients with prior contralateral UTUC (n = 4), and patients with metastatic disease prior to RNU (n = 3). The remaining 253 patients were the subjects of the present analysis. No patient had invasive bladder tumor at the time of RNU.
Surgery was performed by genitourinary surgeons at MSKCC according to the standard criteria for RNU: dissection of the kidney with the entire length of the ureter and adjacent segment of the bladder cuff. The hilar and regional lymph nodes adjacent to the ipsilateral great vessel generally were resected along with enlarged lymph nodes if abnormal on preoperative computed tomography (CT) scans or palpable intraoperatively. Extended lymphadenectomy was not routinely performed.
Tumor location was divided into two groups based on the location (renal pelvis or ureter) of the dominant tumor as identified in the final pathologic specimen. The dominant lesion was defined as that with the highest pathologic tumor stage (pT). For multifocal tumors with the same stage, tumor size was used to define the index lesion for location classification. Thirty-seven patients had primary pelvic tumors with secondary ureteral lesions, and 18 patients had primary ureteral tumors with secondary pelvic lesions; these patients were considered for analysis based on the location of the dominant tumor.
2.2. Pathologic evaluation
All surgical specimens were processed according to standard pathologic procedures at our institution. All specimens were histologically confirmed to be UC. UTUC was defined as UC located in the renal pelvis or calices as well as tumors located within the ureter. Tumors were staged according to the 2002 American Joint Committee on Cancer-Union Internationale Contre le Cancer TNM classification. Tumor grading was assessed according to the 1998 World Health Organization/International Society of Urologic Pathology consensus classification [13].
2.3. Follow-up regimen
Patients were observed every 3 mo for the first year after RNU, every 4 mo for the second year, every 6 mo from the third through fifth years, and annually thereafter. Follow-up consisted of history, physical examination, routine blood work and serum chemistry studies, urinary cytology, chest radiography, cystoscopic evaluation of the urinary bladder, and radiographic evaluation of the contralateral upper urinary tract. Since November 2001, CT urograms have been the standard imaging modality for evaluating the abdomen and pelvis for urothelial recurrence at our institution. Elective bone scans, chest CT, and magnetic resonance imaging scans were performed when clinically indicated.
Disease recurrence was defined as any documented radiograph or pathologically proven failure in the bladder, contralateral kidney, operative site, regional lymph nodes, or distant metastasis. In our analysis, we considered contralateral recurrence as metastatic recurrence. Cause of death was determined by chart review corroborated by death certificates. Most patients who were identified as having died of UTUC had progressive, widely disseminated metastases at the time of death.
2.4. Statistical methods
The Fisher exact test was used to evaluate the association between categorical variables, and the Mann-Whitney test assessed for differences in variables with a continuous distribution across dichotomous categories. Recurrence-free probabilities and CSS were estimated using Kaplan-Meier methods, with differences assessed using the log-rank test. Survival time was calculated from the date of RNU. Univariate and multivariable Cox proportional hazards regression models were used to evaluate the association between tumor location and bladder-only recurrence, nonbladder recurrence (contralateral kidney, operative site, regional lymph nodes, or distant metastasis), and any recurrence as well as cancer-specific mortality after RNU. Patients without disease recurrence were censored at the date of their last follow-up. All reported p values are two-sided, and significance was set at p < 0.05. Statistical analyses were conducted using Stata v.8.0 (StataCorp, College Station, TX, USA).
3. Results
3.1. Patient characteristics
Overall, 171 patients (69%) had a renal pelvic tumor, and 78 patients (31%) had a ureteral tumor (Table 1). The median age was 72 yr (interquartile range [IQR]: 64-77). Patients with ureteral tumors were more likely to have a previous history of non-muscle-invasive bladder tumor (40% vs 30%) and positive urinary cytology; however, the differences were not statistically significant. Patients with microscopic or gross hematuria were more likely to have pelvic tumor lesions (p = 0.04). In contrast, patients with ureteric tumor were more likely to have hydronephrosis than patients with renal pelvic tumor (p < 0.0001). Although patients with ureteral tumor were more likely to have pT2 disease (32% vs 18%), those with renal pelvic tumor were more likely to have non-muscle-invasive UTUC (54% vs 46%; Table 2). There were no differences between the two groups in the rates of non-organ-confined disease (pT3/pT4 vs pTa/pTis/pT1/pT2; p = 0.35) and lymph node metastasis (p = 0.91). Presence of concomitant carcinoma in situ (CIS; p = 0.04) was more common in the patients with ureteral lesions. No pathologic evidence of malignancy was found in four patients (1.5%), all of whom had previously undergone endoscopic tumor ablation.
Table 1.
Descriptive preoperative characteristics of 253 patients treated with radical nephroureterectomy and ipsilateral bladder cuff for upper-tract urothelial carcinoma
| Variable | All patients (n = 253) |
Tumor location* |
||
|---|---|---|---|---|
| Renal pelvis (n = 171) |
Ureter (n = 78) |
p value | ||
| Median age, yr (IQR) | 72 (64-77) |
71 (63-78) | 73 (66-77) | 0.7 |
| Gender, No. (%) | ||||
| Male | 159 (63) | 104 (61) | 52 (67) | 0.4 |
| Female | 94 (37) | 67 (39) | 26 (33) | - |
| Race, No. (%) | ||||
| White | 233 (92) | 157 (92) | 72 (92) | 0.8 |
| Other | 20 (8) | 14 (8) | 6 (8) | - |
| ASA score, No. (%) | ||||
| 1 | 7 (3) | 4 (2) | 3 (4) | 0.8 |
| 2 | 126 (50) | 86 (50) | 39 (50) | - |
| 3 | 119 (47) | 80 (47) | 36 (46) | - |
| Smoking history, No. (%) | 186 (74) | 128 (75) | 54 (69) | 0.4 |
| Previous non-muscle-invasive bladder tumor, No. (%) |
86 (34) | 52 (30) | 31 (40) | 0.2 |
| Previous endoscopic tumor ablation, No. (%) |
20 (8) | 10 (6) | 6 (8) | 0.6 |
| Hematuria, No. (%) | ||||
| No | 63 (25) | 36 (21) | 27 (35) | 0.04 |
| Microhematuria | 28 (11) | 22 (13) | 5 (6) | - |
| Gross hematuria | 162 (64) | 113 (66) | 46 (59) | - |
| Positive void cytology, No. (%) | 191 (75) | 126 (74) | 64 (82) | 0.3 |
| Hydronephrosis on preoperative imaging, No. (%) |
125 (49) | 62 (36) | 62 (79) | <0.000 1 |
| Parenchymal/sinus fat extension or extraureter extension on imaging, No. (%) |
26 (10) | 30 (18) | 6 (8) | 0.05 |
| Suspicious nodes on imaging, No. (%) | 21 (8) | 16 (9) | 5 (6) | 0.6 |
| High-grade disease, No. (%)† | 181 (73) | 121 (71) | 58 (74) | 0.6 |
IQR = interquartile range; ASA = American Society of Anesthesiologists.
n = 4, pT0 patients (submitted to previous endoscopic tumor ablation).
Based on biopsy and wash/brush cytology.
Table 2.
Pathology characteristics of 253 patients treated with radical nephroureterectomy and ipsilateral bladder cuff for upper tract urothelial carcinoma
| Variable | All patients (n = 253) |
Tumor location* |
||
|---|---|---|---|---|
| Renal pelvis (n = 171) |
Ureter (n = 78) |
p value | ||
| pT stage, No. (%) | ||||
| pT0 | 4 (1.6) | - | - | - |
| pTis | 11 (4) | 7 (4) | 4 (5) | 0.06 |
| pTa | 70 (28) | 54 (31.6) | 16 (20.5) | - |
| pT1 | 47 (19) | 31 (18) | 16 (20.5) | - |
| pT2 | 56 (22) | 31 (18) | 25 (32) | - |
| pT3 | 59 (23) | 42 (25) | 17 (22) | - |
| pT4 | 6 (2.4) | 6 (3.5) | 0 (0) | - |
| pN stage, No. (%) | ||||
| Nx | 92 (36) | 62 (36) | 27 (35) | 0.91 |
| N0 | 138 (55) | 94 (55) | 43 (55) | - |
| N+ | 23 (9) | 15 (9) | 8 (10) | - |
| Tumor grade, No. (%) | ||||
| Low | 63 (25) | 41 (24) | 18 (23) | 1.0 |
| High | 190 (75) | 130 (76) | 60 (77) | - |
| Tumor focality, No. (%) | ||||
| Unifocal | 189 (75) | 133 (78) | 52 (67) | 0.08 |
| Multifocal | 64 (25) | 38 (22) | 26 (33) | - |
| Concomitant CIS, No. (%) | ||||
| No | 183 (72) | 130 (76) | 49 (63) | 0.04 |
| Yes | 64 (25) | 41 (24) | 29 (37) | - |
CIS = carcinoma in situ.
*n = 4, pT0 patients (submitted to previous endoscopic tumor ablation).
3.2. Disease recurrence
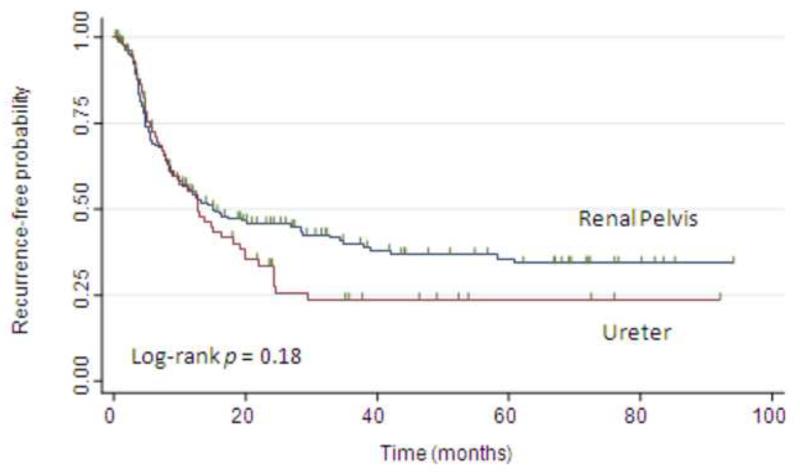
Overall, 151 patients (60%) experienced recurrence of their disease, with a median follow-up for survivors at last follow-up of 48 mo (IQR: 23, 92). The 2- and 5-yr recurrence-free probabilities were 42% and 32%, respectively. Figure 1 shows the probability of freedom from disease recurrence following RNU stratified by tumor location (p = 0.18 by log-rank test). On univariate analyses, female gender, pathologic stage, and positive lymph nodes but not tumor location were associated with disease recurrence (Table 3). On multivariable analysis, only advanced pathologic stage was associated with disease recurrence (p = 0.01). Tumor location was not associated with disease recurrence (adjusted hazard ratio [HR]: 1.19 for ureter vs renal pelvis; 95% confidence interval [CI], 0.83-1.70; p = 0.32).
Fig. 1.
Kaplan-Meier estimates of recurrence-free probabilities (any recurrence) following nephroureterectomy, stratified by tumor location.
Table 3.
Univariate and multivariable Cox regression models predicting disease recurrence (any recurrence) in 253 patients treated with radical nephroureterectomy and ipsilateral bladder cuff for upper tract urothelial carcinoma
| Univariate |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 0.99 | 0.98-1.00 | 0.39 | 0.99 | 0.98-1.00 | 0.52 |
|
| ||||||
| Gender | ||||||
| Male | Reference | - | - | Reference | - | - |
| Female | 0.71 | 0.50-0.99 | 0.04 | 0.71 | 0.49-1.02 | 0.06 |
|
| ||||||
| Previous bladder tumor | 1.29 | 0.93-1.80 | 0.11 | 1.12 | 0.79-1.61 | 0.50 |
|
| ||||||
| Tumor focality | ||||||
| Unifocal | Reference | - | - | Reference | - | - |
| Multifocal | 1.34 | 0.94-1.91 | 0.10 | 1.16 | 0.79-1.71 | 0.43 |
|
| ||||||
| Concomitant CIS | 1.12 | 0.78-1.59 | 0.51 | 1.02 | 0.69-1.49 | 0.91 |
|
| ||||||
| Postoperative chemotherapy |
1.35 | 0.68-2.65 | 0.38 | 0.69 | 0.31-1.53 | 0.36 |
|
| ||||||
| Tumor location | ||||||
| Renal pelvis | Reference | - | - | Reference | - | - |
| Ureter | 1.25 | 0.89-1.75 | 0.19 | 1.19 | 0.83-1.70 | 0.32 |
|
| ||||||
| Tumor grade | ||||||
| Low | Reference | - | - | Reference | - | - |
| High | 1.18 | 0.81-1.72 | 0.36 | 0.96 | 0.62-1.48 | 0.85 |
|
| ||||||
| pT classification | ||||||
| pT0/pTis/pTa/pT1 | Reference | - | - | Reference | - | - |
| pT2 | 1.37 | 0.92-2.06 | 0.11 | 1.29 | 0.84-1.98 | 0.23 |
| pT3/pT4 | 1.73 | 1.19-2.52 | 0.004 | 1.69 | 1.11-2.60 | 0.01 |
|
| ||||||
| pN classification | ||||||
| Nx | Reference | - | - | Reference | - | - |
| N0 | 1.21 | 0.85-1.72 | 0.27 | 1.24 | 0.85-1.80 | 0.24 |
| N+ | 2.20 | 1.28-3.77 | 0.004 | 1.79 | 0.92-3.51 | 0.08 |
HR = hazard ratio; CI = confidence interval; CIS = carcinoma in situ.
The most common location of disease recurrence was within the bladder, occurring in 84 patients (49%) with pelvic tumors and 41 patients (53%) with ureteral lesions. When we reran the analyses with bladder-only recurrences, there were no significant differences in outcomes. Kaplan-Meier analysis revealed no difference in disease recurrence between renal pelvic and ureteral tumors (p = 0.3 by log-rank test). In univariate analyses, female gender (HR: 0.64; 95% CI, 0.44-0.94; p = 0.02) and multifocality (HR: 1.49; 95% CI, 1.02-2.19; p = 0.03) were associated with bladder-only recurrence. Tumor location was not associated with bladder-only recurrence in any of the analyses (univariate or multivariable).
When we reran the analyses with nonbladder recurrences, 40 patients (23%) with renal pelvic tumor and 23 patients (29%) with ureteral tumor developed nonbladder recurrences. The 5-yr nonbladder recurrence-free probability was 71%. There was no significant difference in nonbladder recurrence between ureteral and renal pelvic tumors (p = 0.3 by log-rank test). In univariate analyses, postoperative chemotherapy (HR: 4.28; 95% CI, 2.02-9.09; p < 0.0001), tumor grade (HR: 2.79 for high vs low grade; 95% CI, 1.32-5.86; p = 0.007), pathologic stage (HR: 2.28 for pT2 vs non-muscle invasive; 95% CI, 1.08-4.79; p = 0.03 and HR: 7.05 for pT3/pT4 vs non-muscle invasive; 95% CI, 3.84-12.9; p < 0.0001), and nodal status (HR: 8.77 for pN+ vs pNx; 95% CI, 4.38-17.5; p < 0.0001) were associated with nonbladder recurrence. In multivariable analysis, pathologic stage (adjusted HR: 4.75 for pT3/pT4 vs non-muscle invasive; 95% CI, 2.43-9.27; p < 0.0001) and nodal status (adjusted HR: 3.23 for pN+ vs pNx; 95% CI, 1.42-7.33; p = 0.005) remained independently associated with nonbladder recurrence.
3.3. Cancer-specific survival
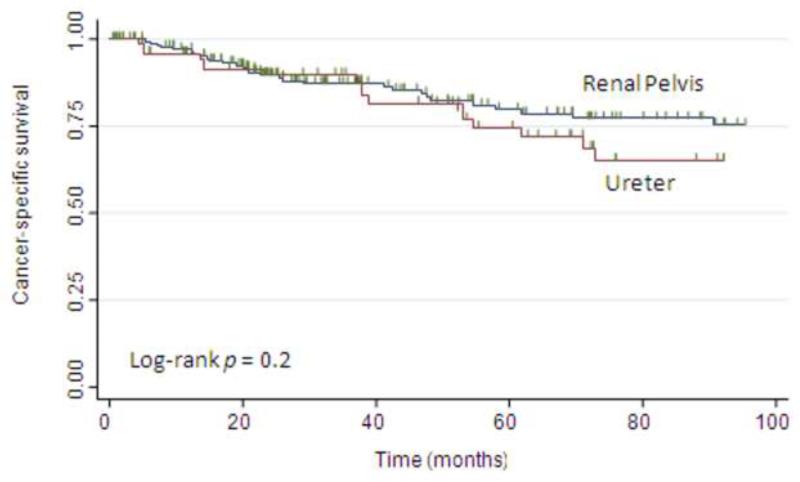
Death from UTUC occurred in 48 patients (19%). Thirty patients (18%) with renal pelvic tumors and 18 patients (23%) with ureteral tumors died of UTUC. The 5-yr CSS estimate was 78%. Figure 2 shows the CSS estimates following RNU stratified by tumor location. There was no difference in the probability of CSS between the groups (p = 0.2, log-rank test). On univariate analyses, advanced age, postoperative chemotherapy, high tumor grade, pathologic stage, and metastasis to lymph nodes were associated with CSS (Table 4). In multivariable analysis, pathologic stage and metastasis to lymph nodes remained associated with worse CSS. Tumor location was not associated with CSS in either univariate or multivariable analysis.
Fig. 2.
Kaplan-Meier estimates of cancer-specific survival following nephroureterectomy, stratified by tumor location.
Table 4.
Univariate and multivariable Cox regression models predicting cancer-specific survival in 253 patients treated with radical nephroureterectomy and ipsilateral bladder cuff for upper tract urothelial carcinoma
| Univariate |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 1.03 | 1.00-1.06 | 0.02 | - | - | - |
|
| ||||||
| Gender | ||||||
| Male | Reference | - | - | - | - | - |
| Female | 0.84 | 0.46-1.53 | 0.57 | - | - | - |
|
| ||||||
| Previous bladder tumor |
1.11 | 0.62-2.00 | 0.71 | - | - | - |
|
| ||||||
| Tumor focality | ||||||
| Unifocal | Reference | - | - | - | - | - |
| Multifocal | 0.92 | 0.47-1.80 | 0.81 | - | - | - |
|
| ||||||
| Concomitant CIS | 1.65 | 0.91-2.99 | 0.09 | - | - | - |
|
| ||||||
| Postoperative chemotherapy |
4.56 | 2.03-10.2 | <0.0001 | 0.77 | 0.29-1.99 | 0.59 |
|
| ||||||
| Tumor location | ||||||
| Renal pelvis | Reference | - | - | Reference | - | - |
| Ureter | 1.38 | 0.77-2.48 | 0.27 | 1.30 | 0.72-2.37 | 0.33 |
|
| ||||||
| Tumor grade | ||||||
| Low | Reference | - | - | Reference | - | - |
| High | 3.09 | 1.31-7.29 | 0.01 | 1.92 | 0.76-4.80 | 0.16 |
|
| ||||||
| pT classification | ||||||
| pT0/pTis/pTa/pT1 | Reference | - | - | Reference | - | - |
| pT2 | 4.17 | 1.61-10.7 | 0.003 | 3.43 | 1.31-8.98 | 0.01 |
| pT3/pT4 | 11.6 | 5.10-26.5 | <0.0001 | 7.38 | 3.04-17.9 | <0.0001 |
|
| ||||||
| pN classification | ||||||
| Nx | Reference | - | - | Reference | - | - |
| NO | 1.29 | 0.64-2.62 | 0.46 | 0.90 | 0.43-1.88 | 0.78 |
| N+ | 10.4 | 4.80-22.5 | <0.0001 | 2.95 | 1.24-7.03 | 0.01 |
HR = hazard ratio; CI = confidence interval; CIS = carcinoma in situ.
4. Discussion
We found that ureteral tumors were more likely to present with hydronephrosis and that renal pelvic tumors were more likely to present with hematuria. However, we did not find any difference in established prognostic features such as pathologic stage, tumor grade, and lymph node status between ureteral and renal pelvic tumors. Moreover, we found no association between tumor location and disease recurrence or CSS in patients treated with RNU for UTUC.
The 5-yr nonbladder recurrence-free survival (RFS) and CSS (71% and 78%, respectively) are within the range reported in the literature [7,10,12,14]. In accordance with previous studies, we found that pathologic stage and lymph node metastasis are the strongest predictors of recurrence and survival in UTUC.
We confirmed that tumor location is not able to predict outcomes in a large, single-center series of consecutive patients treated with RNU for UTUC. This is in agreement with several previous studies. In a large multicenter study of >1200 patients, Raman et al found that although renal pelvic tumors presented with more advanced stage, tumor location had no effect on disease recurrence or survival after controlling for the effects of tumor stage and lymph node metastasis [7]. Subgroup analyses in patients with organ-confined disease or without adjuvant chemotherapy did not change the findings. In an analysis of 2824 patients treated with RNU for UTUC within nine National Cancer Institute Surveillance, Epidemiology and End Results Program registries between 1988 and 2004, Isbarn et al found that renal pelvic tumors were of higher stage and had a higher rate of lymph node metastases. However, after multivariable adjustment, tumor location failed to reach independent predictor status for cancer-specific mortality [12]. In a study of 149 UTUC patients, Van der Poel et al also found that renal pelvic and proximal ureteral tumors were more likely to grow beyond muscular layers compared with distally located ureteral tumors [11]. Conversely, in a study of 224 patients, Park et al found that ureteral tumors were more frequently associated with higher stages than pelvic tumors at diagnosis and had a worse prognosis than renal pelvic tumors, which was mainly attributable to pT3 tumor outcomes in which renal parenchyma invasion had lower local failure and higher survival rates than those invading peripelvic or periureteral fat [10]. Some of these studies were limited by a lack of multivariable analysis and small sample size. Although the multicenter and population-based studies addressed these issues, their lack of centralized pathologic evaluation and heterogeneity in disease severity and treatment may have limited the statistical power of the study. Finally, in contrast to previous studies, we assessed bladder-only recurrence as an end point in addition to nonbladder recurrence. Although bladder-only recurrence may not affect survival, it will result in differences in disease management and quality of life for the patient.
Our study has several limitations. It represents a retrospective analysis of a database from a single institution and, thus, our results are subject to the inherent biases associated with high-volume tertiary care centers. We also identified a high proportion of patients with high-grade disease. As our cohort comprises only patients submitted to RNU, the reason behind this finding is probably related to a preoperative selection of patients suspicious for more aggressive and high-grade disease, who are more suitable for radical surgery. However, both groups had a similar proportion of patients with high-grade disease (76% for renal pelvis vs 77% for ureter) and, therefore, it could not influence the outcomes results. Despite these limitations, our study has some strength, such as centralized pathologic review and standardized follow-up.
5. Conclusions
We did not find any difference in outcomes between ureteral and renal pelvic tumors in a large, single-institution cohort of patients treated with RNU for UTUC. Therefore, clinical decisions regarding adjuvant therapy or follow-up protocol should not differ between patients with renal pelvic or ureteral UTUC.
Acknowledgment statement
The authors would like to thank The Sidney Kimmel Center for Prostate and Urologic Cancers for its support.
Funding/Support and role of the sponsor: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Guido Dalbagni had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Favaretto, Shariat, Dalbagni, Kaag.
Acquisition of data: Favaretto, Adamy, Chade, Godoy.
Analysis and interpretation of data: Dalbagni, Favaretto, Shariat.
Drafting of the manuscript: Favaretto, Shariat.
Critical revision of the manuscript for important intellectual content: Dalbagni, Shariat, Coleman, Bochner.
Statistical analysis: Favaretto, Shariat, Kaag, Adamy.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Dalbagni, Bochner, Coleman.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- [1].Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- [2].Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164:1523–5. [PubMed] [Google Scholar]
- [3].Tawfiek ER, Bagley DH. Upper-tract transitional cell carcinoma. Urology. 1997;50:321–9. doi: 10.1016/S0090-4295(97)00230-6. [DOI] [PubMed] [Google Scholar]
- [4].Huben RP, Mounzer AM, Murphy GP. Tumor grade and stage as prognostic variables in upper tract urothelial tumors. Cancer. 1988;62:2016–20. doi: 10.1002/1097-0142(19881101)62:9<2016::aid-cncr2820620924>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [5].Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- [6].Roscigno M, Cozzarini C, Bertini R, et al. Prognostic value of lymph node dissection in patients with muscle-invasive transitional cell carcinoma of the upper urinary tract. Eur Urol. 2008;53:794–802. doi: 10.1016/j.eururo.2008.01.008. [DOI] [PubMed] [Google Scholar]
- [7].Raman JD, Ng CK, Scherr DS, et al. Impact of tumor location on prognosis for patients with upper tract urothelial carcinoma managed by radical nephroureterectomy. Eur Urol. 2009;57:1072–9. doi: 10.1016/j.eururo.2009.07.002. [DOI] [PubMed] [Google Scholar]
- [8].Kikuchi E, Margulis V, Karakiewicz PI, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol. 2009;27:612–8. doi: 10.1200/JCO.2008.17.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Park S, Hong B, Kim CS, Ahn H. The impact of tumor location on prognosis of transitional cell carcinoma of the upper urinary tract. J Urol. 2004;171:621–5. doi: 10.1097/01.ju.0000107767.56680.f7. [DOI] [PubMed] [Google Scholar]
- [10].Park J, Ha SH, Min GE, et al. The protective role of renal parenchyma as a barrier to local tumor spread of upper tract transitional cell carcinoma and its impact on patient survival. J Urol. 2009;182:894–9. doi: 10.1016/j.juro.2009.05.040. [DOI] [PubMed] [Google Scholar]
- [11].van der Poel HG, Antonini N, van Tinteren H, Horenblas S. Upper urinary tract cancer: location is correlated with prognosis. Eur Urol. 2005;48:438–44. doi: 10.1016/j.eururo.2005.03.009. [DOI] [PubMed] [Google Scholar]
- [12].Isbarn H, Jeldres C, Shariat SF, et al. Location of the primary tumor is not an independent predictor of cancer specific mortality in patients with upper urinary tract urothelial carcinoma. J Urol. 2009;182:2177–81. doi: 10.1016/j.juro.2009.07.035. [DOI] [PubMed] [Google Scholar]
- [13].Epstein JI, Amin MB, Reuter VR, Mostofi FK. Bladder Consensus Conference Committee. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Am J Surg Pathol. 1998;22:1435–48. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- [14].Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224–33. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]