Abstract
BACKGROUND
The IMS III Trial did not demonstrate clinical benefit of the endovascular approach compared to IV rt-PA alone for moderate or severe ischemic strokes (NIHSS≥8) enrolled within three hours of stroke onset. Late reperfusion of tissue that is no longer salvageable may be one explanation, as suggested by prior exploratory studies showing an association between time to reperfusion and good clinical outcome. We sought to validate this relationship in the large-scale IMS III trial, and consider its implications for future endovascular trials.
METHODS
The analysis consisted of the endovascular cohort with proximal arterial occlusions in the anterior circulation that achieved angiographic reperfusion (TICI 2–3) during the endovascular procedure (within 7 hours from the onset of symptoms). Logistic regression was used to model good clinical outcome (90-day modified Rankin 0–2) as a function of the time to reperfusion, and prespecified variables were considered for adjustment.
FINDINGS
Among 240 proximal vessel occlusions, angiographic reperfusion (TICI 2–3) was achieved in 182 (76%). Mean time to reperfusion was 325 minutes (range 180–418 minutes). Longer time for reperfusion was associated with a decreased likelihood of good clinical outcome (RR [95% CI] for every 30 minute delay: unadjusted 0·85 [0·77–0·94]; adjusted 0·88 [0·80–0·98]).
INTERPRETATION
We confirm that delay in time to angiographic reperfusion leads to a decreased likelihood of good clinical outcome. Achieving rapid reperfusion may be critical for the successes of future acute endovascular trials.
FUNDING: NIH/NINDS (study sponsor), Genentech Inc. (study drug - intra-arterial t-PA), EKOS Corp. (device), Concentric Inc. (device), Cordis Neurovascular, Inc. (device), and Boehringer Ingelheim (European Investigator Meeting support).
BACKGROUND
Even when acutely treated with IV thrombolysis, over half of all ischemic stroke patients are disabled at three months. 1 This is likely due, in part, to suboptimal rates of recanalization of occluded arteries, especially for more severe strokes caused by larger thrombi. Another important factor may well be late, but technically successful, recanalization of infarcted tissue that is no longer salvageable. 2
The Interventional Management of Stroke III (IMS III) trial tested the hypothesis that endovascular therapy following IV recombinant tissue plasminogen activator (rt-PA) improves outcomes compared to IV rt-PA alone in moderate and severe ischemic strokes (baseline NIHSS ≥8, but with NIHSS of 8 or 9 requiring presence of occlusion on CTA). The trial was stopped after crossing a prespecified futility boundary (primary outcome ≤2: 41% endovascularvs 39% IV rt-PA; p=0.70). 3 One reason for the neutral result mRS = may have been angiographic reperfusion that occurredtoo late to salvage brain tissue.
In the context of IV thrombolysis, clinical outcomes are highly dependent on the rapidity ofrt-PA initiation, and treatment benefit is less likely when rt-PA is initiated beyond 4.5 hours from symptom onset. 4 How this time window translates to the time from symptom onset to actual angiographic reperfusion has been a source of debate. 5 The randomized PROACT II trial of endovascular recombinant pro-urokinase (not commercially available) versus placebo demonstrated clinical benefit with two-hour intra-arterial lytic infusion started within six hours of symptom onset. 6 With the expectation that mechanical devices would recanalize arteries more quickly than pharmacological therapies, pivotal device trials allowed device deployment to begin up to 8 hours from symptom onset. 7 Based on safety and revascularization data, the FDA has 510(k)-cleared recent mechanical embolectomy devices (Penumbra Aspiration, and Solitaire and TREVO2 Stent Retrievers) to remove thrombus within 8 hours of onset. 8–11 However, randomized evidence of a clinical benefit of revascularization therapies initiated beyond six hours is lacking. 12
In a post-hoc analysis of the pooled IMS pilot trials (n=54), longer time to reperfusion was associated with a decreased likelihood of good clinical outcome (OR 0·64, 95% CI 0·42–0·92; RR 0·80, 95% CI 0·64–1·00). 2,13 Specifically, the relative probability of a good outcome declined by 20% for every 30-minute delay in reperfusion. This translated to a 10% absolute decline in likelihood of good outcome (coincidentally the same treatment effect tested in the IMS III trial) for a 30-minute delay from 280 to 310 minutes. The RECANALISE single-center prospective registry showed a similar relationship with 30-minute decrease in time to reperfusion leading to an increased likelihood of good clinical outcome (RR 1·19, 95% CI 1·07–1·32; p=0·0007). 14 Pooling IMS pilot data with five other prospective single-center cohorts, some of which were selected for endovascular therapy based on CT perfusion characteristics, also showed comparable results and, additionally, increased mortality (OR 1·21, 95% CI 1·09–1·34; P<0.001) and intracranial hemorrhage (OR 1·21, 95% CI 1·10–1·33) with delayed reperfusion. 15 Others have also shown a relationship between clinical outcome based on recanalization timing before or after a particular time point. 16,17 One large single-center cohort showed an association between time from onset to endovascular treatment initiation and clinical outcome but only when collateral status was excluded from multivariable modeling.26
We sought to validate the previously demonstrated relationship between the time of angiographic reperfusion and favorable clinical outcome in the independent data set of the large-scale, multicenter IMS III trial. We hypothesized the same association with a higher degree of confidence due to the larger sample size.
METHODS
The NIH-funded, international, randomized IMS III trial tested IV rt-PA followed by protocol-approved endovascular treatment, as compared with standard IV rt-PA alone. The protocol stipulated IV rt-PA initiation within three hours, endovascular therapy initiation within five hours, and procedure termination within seven hours of onset. Ethics committee approvals were obtained at all participating sites, and informed consents were obtained for all enrolled participants. Detailed methods and primary results are published. 3,18
For this analysis, to limit variability and thereby isolate the role of time to angiographic reperfusion, we selected the as-treated endovascular cohort with relatively homogenous occlusion locations on baseline angiography – the proximal middle cerebral artery (M1 and M2) or internal carotid artery terminus (ICAT) occlusions. Cases with continued intervention beyond 7 hours (n=25; a protocol violation in the trial) or missing time data (n=1) were excluded. This methodology was chosen to maintain consistency with our prior post-hoc IMS pilot trial analysis.2
Angiographic reperfusion was assessed by central readers blinded to clinical outcome and defined as “at least partial restoration of blood flow to the distal arterial bed of the occluded artery achieved during the interventional procedure,” or modified Thrombolysis in Cerebral Infarction (TICI) grade 2 or more (i.e., 2a, 2b, or 3); this was the prespecified revascularization endpoint of the IMS III trial. 3 Time to angiographic reperfusion was defined as time from stroke onset to procedure termination. Procedure end was deemed to be a reasonable surrogate for reperfusion timing since angiographic assessments were required after successive 15-minute infusions of IA rt-PA or after each device deployment, and investigators were encouraged to abort the procedure upon achieving TICI 2b reperfusion (i.e., half or more of the vascular distribution of the occluded artery). Good clinical outcome was defined as the modified Rankin Scale (mRS) 0–2 at 3 months, the primary and blinded endpoint of the IMS III trial.
The effect of time on good clinical outcome is described via relative risks ratios for every 30-minute delay. The relative risks (RRs) are estimated via generalized linear model using the log link, as these are more clinically meaningful than odds ratios derived from logistic regression analysis. P-values reported are from the logistic regression analyses.
We prespecified variables believed to be potentially associated with clinical outcome to be considered for adjustment. These included the following from our original analysis: age (continuous), baseline NIH Stroke Scale (NIHSS) score (8–19 vs ≥20), sex, and baseline glucose level (continuous). Given the larger dataset, we also considered baseline systolic blood pressure (continuous), premorbid disability (mRS ≥2), the presence of early ischemic changes on baseline CT scan as assessed by the Alberta Stroke Program Early CT Score (ASPECTS 0–4 vs 5–10) by the Imaging Core Lab, and presence of collaterals using the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) five-point collateral score (0–2 vs 3–4). We also considered other procedural time variables including time from symptom onset to IV rt-PA bolus, time from IV rt-PA start to groin puncture, time from groin puncture to start of endovascular therapy, and duration of endovascular therapy. Variables with potential association (p<0.20) were considered in multivariable modeling. Results were considered statistically significant if p<0.05.
Stepwise methodology was used for variable selection, with alpha 0.10 required for entry and 0.05 to remain. Model goodness of fit was assessed via the Hosmer and Lemeshow test. Interaction between time and the following variables were also explored: baseline ASPECTS score (0–4, 5–7, vs 8–10; or 0–4 vs 5–10), NIHSS strata (8–19 vs ≥20), and ASITN/SIR collateral score (0–2 vs 3–4).
Additional prespecified secondary analyses were performed using the more stringent definition of reperfusion of TICI 2b/3 (defined as restoration of specifically ≥50% blood flow) and in the cohort of subjects without M2 occlusions (i.e., ICAT and M1 only). To consider mechanisms by which time to reperfusion may influence clinical outcome, the relationships of time to reperfusion with symptomatic intracranial hemorrhage, mortality, and serious adverse events (SAEs) were also evaluated.
Role of Funding Source: IMS III was an independent, investigator-initiated study. External support from the National Institutes of Health/National Institute of Neurological Disorders and Stroke was received to cover costs of study enrollment. The study sponsor had no role in data collection or analysis, or writing of the manuscript. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication.
RESULTS
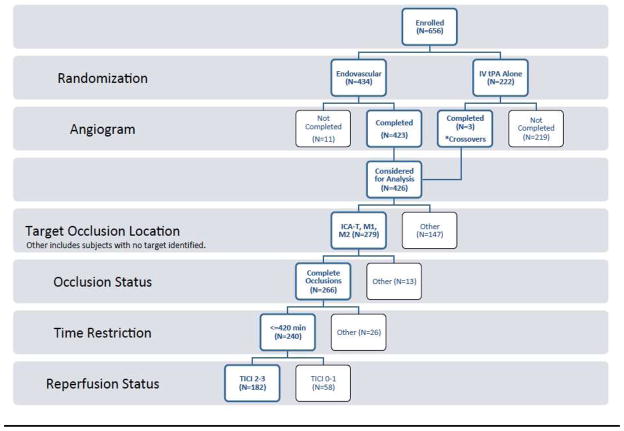
In the IMS III trial, 434 subjects were randomized to endovascular therapy, and three subjects randomized to IV rt-PA alone crossed over to the endovascular arm. Of these, 240 (55%) with complete ICAT, M1, and M2 occlusions identified on initial angiograms received endovascular therapy that was completed within the protocol-mandated seven hours from symptom onset (mean age 66 years [SD 12·3], median NIHSS 18 [IQR 8], and 35 (14·6%) with ASPECTS ≤4). Among this cohort, 182 (76%) achieved angiographic reperfusion (reperfusion cohort), including 33 (18%) ICAT, 98 (54%) M1, and 51 (28%) M2 occlusions. See flow chart (Figure 1).
FIGURE 1.
Flow Chart
Among the reperfusion cohort, 12 (6·6%) suffered symptomatic intracerebral hemorrhages (sICH), 35 (19%) died, and 73 (40%) had good 90-day outcomes. Endovascular modalities consisted of 63 (35%) MERCI Retriever, 61 (34%) intra-arterial rt-PA only, 32 (18%) Penumbra Aspiration, 12 (7%) EKOS Ultrasound, and 4 (2%) Solitaire Retriever cases. Ten (5%) cases had device protocol violations (i.e., use of unapproved devices or multiple devices in the same patient). The mean time from symptom onset to reperfusion was 325 ± 51·5 minutes (SD). See Figure 2 for additional descriptive time parameters.
FIGURE 2.
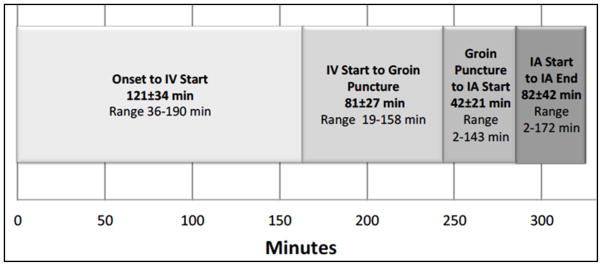
Time Points from Stroke Symptom Onset to Angiographic Reperfusion in IMS III Reperfusion Cohort (n=182)
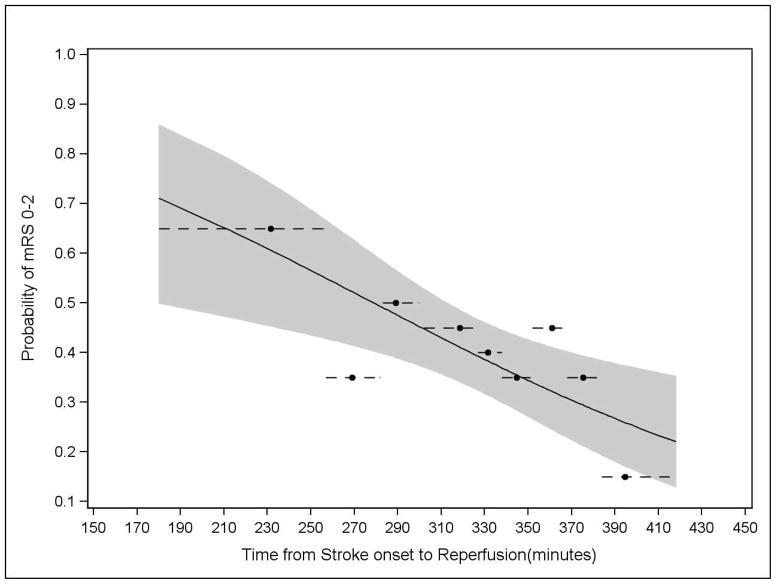
Longer time to reperfusion was associated with a reduced likelihood of good outcome in the unadjusted analysis (RR 0·85; 95% CI 0·77–0·94; p=0·003). Observed and expected values are shown in Figure 3, and mRS distributions by tertiles of time to reperfusion are shown in Figure 4. Univariate analyses of additional variables are shown in Table 1.
FIGURE 3. Probability of Good Clinical Outcome Over Time as Predicted by Unadjusted Analysis (p=0·003).
The reperfusion cohort was divided into groups of approximately 20 subjects each. The dot reflects the observed good outcome proportion (y-axis) and the mean time to reperfusion (x-axis), whereas the dashed line depicts the range of time included in the corresponding group. The solid line shows the model results from the logistic regression analysis, and the shaded area defines the corresponding 95% confidence bands.
FIGURE 4.
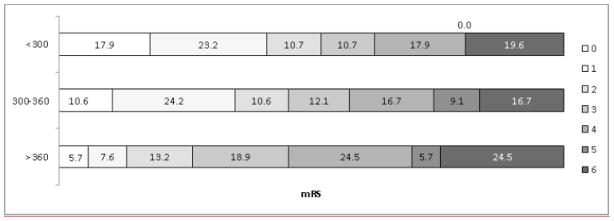
Distributions of 90-Day modified Rankin Scores Based on Time to Reperfusion (<300 vs 300–360 vs>360 Minutes)
Table 1.
Univariate and Multivariable Results
| Variable | Univariate p-value (from Logit-Link) | RR (95% CI) (from Log-Link) | Multi-variable p-value | RR (95% CI) | |
|---|---|---|---|---|---|
| TICI 2–3 (N=182) | |||||
| Time to reperfusion (continuous) | 0·003 | 30 min: 0·85 (0·77–0·94) | 0·02 | 30 min: 0·89 (0·80–0·98) | |
| Age (continuous) | 0·0168 | 1 year: 0·98 (0·98–1·00) | |||
| NIHSS Strata: 8–19 vs ≥20 | 0·001 | 1·96 (1·25–3·05) | 0·01 | 1·65 (1·09–2·51) | |
| Baseline SBP (continuous) | 0·19 | 0·99 (0·99–1·003) | |||
| Baseline Glucose (continuous) | 0·025 | 0·99 (0·99–1·00) | |||
| Premorbid disability: No versus yes | 0·0176 | 2·62 (1·05–6·56) | 0·01 | 2·62 (1·05–6·50) | |
| ASPECTS: >4 versus <=4 | 0·004 | 3·85 (1·30–11·42) | 0·006 | 3·64 (1·23–10·81) | |
| Sex: Male vs Female | 0·88 | 0·97 (0·68–1·39) | |||
| Time from Onset to IV (continuous) | 0·44 | 30 min: 0·95 (0·81–1·10) | |||
| Time from IV to puncture (continuous) | 0·61 | 0.95 (0·79–1·15) | |||
| Time from groin puncture to IA (continuous) | 0·08 | 30 min: 0·75 (0·55–1·03) | |||
| Time from IA to Reperfusion (continuous) | 0·08 | 30 min: 0·89 (0·78–1·005) | |||
| Collateral status: 3–4 vs 0–2 | 0·004 | 1.70 (1·17–2·45) |
In adjusted analyses, longer time to reperfusion remained significantly associated with a reduced likelihood of good clinical outcome (RR 0·88; 95% CI 0·80–0·98; p=0·02; r2=0·18). Notably, the other time variables did not remain associated with clinical outcome after adjustment. The final multivariable model is shown in Table 1. Goodness of fit was demonstrated.
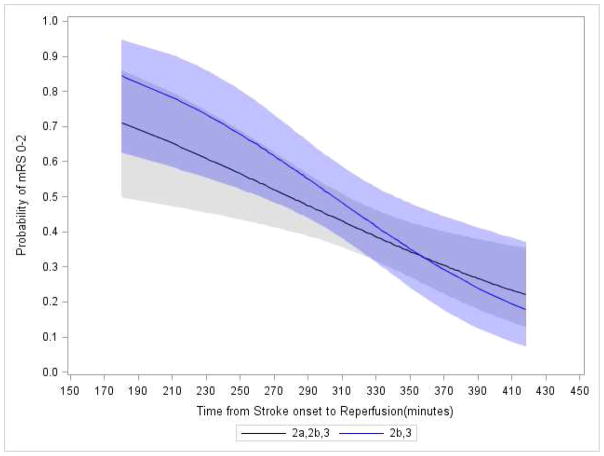
Additionally, no effect modification was demonstrated by the degree of early ischemic changes on the baseline CT scan (ASPECTS 0–4 vs 5–7 vs 8–10; RR 0·88 vs 0·88 vs 0·87; p=0·95) or by stroke severity (NIHSS strata 8–19 vs ≥20; RR 0·86 vs 0·83; p=0·76). The relationship with time was maintained after adjustment for age (RR 0.88; 95% CI 0.80–0.98; p=0.02) and replacing NIHSS for clot location (RR 0.88 95% CI 0.80–0.97), and in the subcohort of ICAT and M1 only cases (adjusted RR 0.82; 95% CI 0.72–0.93; p=0.002). The relationship with time was also maintained after adjustment for reperfusion status (2a vs 2b/3; RR 0.91; 95% CI 0.81–1.01; p=0.07), and when restricted to subjects achieving 2b/3 status (adjusted RR 0.87; 95% CI 0.75–1.01; p=0.008).as shown in Figure 5.
FIGURE 5. Probability of Good Clinical Outcome Over Time as Predicted by Unadjusted Analysis for Reperfusion Cohorts Defined by TICI 2a/2b/3 Vs. 2b/3.
The solid blue line shows the model results from the logistic regression analysis, and the associated shaded area indicate the corresponding 95% confidence bands, for the cohort with the more stringently defined threshold for angiographic reperfusion of TICI 2b/3. For comparison, the solid back line and associated shaded area shows the result for the broader definition of angiographic reperfusion of TICI 2/3 used in the original IMS pilot studies to generate the time hypothesis.
Later time to reperfusion was associated with a higher rate of SAEs (RR 1·13; 95% 1·02–1·26; p=0·02). We were unable to demonstrate a relationship between time and either sICH or mortality (sICH RR 1·15, 95% CI 0·82–1·61; mortality RR 1·10, 95% CI 0·91–1·33).
To put our findings in the context of the broader IMS III trial results, we identified the good outcome rates of proximal occlusions with no angiographic reperfusion (6/58; 10%; 95% CI 4%–21%), the overall IV rt-PA-only arm (86/222; 39%; 95% CI 32%–45%), the overall endovascular arm (177/434; 41%; 95% CI 36%–46%), and the subset in the IV arm with baseline CTAs demonstrating ICAT, M1, or M2 occlusions (32/83; 39%; 95% CI 28%–50%). Table 2 shows baseline characteristics for these subgroups. Endovascular subjects with proximal occlusions and reperfusion had better clinical outcomes than those without reperfusion (40% vs 10%; p<0·0001).
Table 2.
Baseline and Clinical Features of Key IMS III Cohorts
| Endovascular Treated Arm | IV rt-PA Arm | ||
|---|---|---|---|
| ICAT, M1, or M2 Occlusions on Baseline Angiography | ICAT, M1, or M2 Occlusions on Baseline CTA | ||
| REPERFUSION COHORT | NO REPERFUSION COHORT | ||
| n | 1821 | 582 | 833 |
| Age:Mean (SD) | 66·2 (12·2) | 65·6 (12·7) | 67·4 (11·5) |
| NIHSS: Median (IQR) | 18 (14–21) | 17 (16–22) | 17·5 (14–21) |
| ASPECTS >4 (%)[Exact 95% CI] | 153 (85·5) [79·5–90·3] | 48 (84·2) [72·1–92·5] | 68 (81·9) [72·0–89·5] |
| mRS 0–2 (% ) [Exact 95% CI] | 73 (40·1) [32·9–47·6] | 6 (10·3) [3·9–21·2] | 32 (38·6) [28·1–49·9] |
1 subject missing NIHSS; 1 subject missing ASPECTS
3 subjects missing ASPECTS
1 subject missing NIHSS
DISCUSSION
In this preplanned analysis of the IMS III trial, we demonstrate that the time from stroke symptom onset to angiographic reperfusion is highly associated with the likelihood of good clinical outcome. Thus, we validate prior exploratory analyses of the IMS pilot trials and the RECANALISE registry, and replicate the phenomenon with a higher degree of confidence. In this analysis, every 30-minute delay in angiographic reperfusion reduced the relative likelihood of a good clinical outcome by 15% in unadjusted analysis and 12% in adjusted analysis. In absolute terms, this translated to a 10% decline in the likelihood of a good outcome for a 45-minute delay, from 280 to 325 minutes, in the unadjusted analysis.
A limitation of this analysis is that time to angiographic reperfusion was defined as time from stroke onset to procedure termination. This may underestimate the impact of time if clinically good reperfusion (ex: TICI 2b) was achieved, and then the operator continued the procedure to achieve a higher degree of reperfusion (ex: TICI 3). This may also overestimate the role of time if the longer procedure resulted in a poorer outcome, although this seems less likely since procedure duration was not associated with outcome in this analysis. Additionally, we focused on a relatively small, specialized subcohort with major occlusions and successful angiographic reperfusion, in order to isolate the role of time. Outcome modeling in this subgroup may not apply more broadly. For example, we did not identify a significant association of time to IV start, but this association is well established in broader and larger stroke cohorts.4 Also, we do not intend to discount other known influences on outcome, such as age, stroke severity, and revascularization. Finally, the width of the confidence intervals reminds us that additional measured and unmeasured factors contribute to clinical outcome, and that the current sample size is relatively small.
Nevertheless, the findings of this analysis are robust. The time relationship is observed even with a more restrictive definition of reperfusion, a smaller subset of occlusion types, regardless of stroke severity or level of ischemic changes on baseline CT imaging, and when cases with time protocol violations are included. It is also noteworthy that this time finding was replicated despite the use of different endovascular modalities in the IMS III trial versus the IMS pilot trials. The IMS pilot trials used intra-arterial thrombolysis (and low-energy ultrasound in a subset), while the IMS III trial used one of four mechanical embolectomy devices and/or intra-arterial thrombolysis.
This time analysis suggests that healthcare systems should heavily prioritize expeditious acute stroke treatment. Developing faster stroke systems of care may have the same magnitude of impact in reducing the burden of stroke as treatment advances that our large-scale acute stroke trials have been trying to achieve. The US American Heart/Stroke Association registry data have demonstrated modest improvements in median door-to-needle times (i.e., time from Emergency Department arrival to initiation of IV rt-PA), from 85 minutes in 2003 to 75 minutes in 2009. 19 In contrast, in a single Finnish center, dramatic improvement in median door-to-needle time of 105 minutes (1998), to 60 minutes (2003), and then to 20 minutes (2011) have been achieved, suggesting that faster treatment is indeed achievable with better healthcare organization.20 Improving times in the endovascular setting presents unique challenges, including the need to transfer patients from the initial emergency department to a hospital with endovascular capabilities, mobilization of neuroangiography technicians and operators to the suite, and possibly intubation for general anesthesia.21,27 Of note, the IMS III protocol recommendation of IV rt-PA bolus to IA groin puncture under 90 minutes was indeed achieved in the trial (mean 85 minutes); this may not have been fast enough.
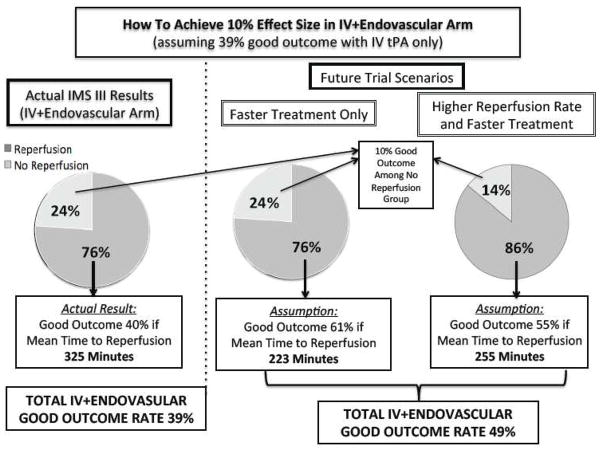
The current data allow us to consider how a trial restricted to patients with large vessel occlusions and with faster reperfusion times might demonstrate superiority of the endovascular approach solely by increasing speed, as illustrated in Figure 6. In IMS III, the IV rt-PA-only arm with ICAT, M1 and M2 occlusions on baseline CTA achieved a 39% good outcome rate; the hypothesized 10% effect size would then require 49% good outcome in the endovascular arm. Based on the observed 10% good outcome rate among the 24% of endovascular cases without angiographic reperfusion in IMS III, a 61% good outcome rate among the 76% of endovascular cases with reperfusion would be needed to achieve an overall 49% good outcome rate in the endovascular arm. According to our analysis, a 61% good outcome rate corresponds to a mean time to reperfusion of 223 minutes (compared to 325 minutes observed in the IMS III reperfusion cohort). Mathematically, a higher reperfusion rate (85%) in a future trial might allow for achieving reperfusion as late as 255 minutes from symptom onset, or 70 minutes faster than IMS III. Since decreasing time from onset to IV treatment may increase the good outcome rate in the IV-only group as well, this 70-minute improvement may need to occur during the time between IV start and IA procedure termination. This modeling is limited by assumptions of no increased risk incurred with increasing speed to reperfusion and achieving higher reperfusion rates. This modeling also makes the simplifying assumption that baseline CTA proximal occlusions are going to persist as baseline angiographic proximal occlusions. It also assumes that the CTA and non-CTA cohorts are comparable, but there may be selection bias among those who did versus those who did not receive CTAs. A detailed decision analysis model is currently being developed. In the cardiac literature, among patients presenting within three hours of symptom onset, IV thrombolysis is recommended over an endovascular approach if the door to balloon time is expected to exceed 90 minutes; our data suggest similar constraints may become applicable in the acute ischemic stroke setting. 22
FIGURE 6. Theoretical Trial Scenarios for Demonstrating Superiority of the Endovascular Approach Based on Varying Only Time to Angiographic Reperfusion and Rates of Angiographic Reperfusion.
Based on the association of time to reperfusion and clinical outcome observed in the IMS III trial endovascular cohort with proximal occlusions on baseline angiogram, we consider scenarios in which varying time to angiographic reperfusion and rates of angiographic reperfusion might influence final outcome in the endovascular arm of a trial. We assume that the medical arm will have a good outcome rate of 39% based on the observed rate in the IV rt-PA cohort of the IMS III with proximal occlusions on baseline CTA.
Newly initiated, randomized trials comparing combined IV/endovascular versus IV rt-PA alone have planned mRS 0–2 effect sizes of 10% or more, including the ongoing THERAPY (10.6%), REVASCAT (15%), PISTE (15%), and ESCAPE (20%)trials.23–25 Given the large treatment effect sizes planned, these trials will likely need to shorten times to angiographic reperfusion significantly to have the potential to show superiority of endovascular therapy. Future avenues of research, such as the penumbral imaging, may identify subgroups of IV rtPA-treated patients that will benefit from later angiographic reperfusion. However, it remains possible that these approaches will select patients who will also have relatively better outcomes after IV rt-PA alone, and therefore will be prognostic biomarkers and not modifiers of endovascular treatment effect.12
In a post hoc manner, we demonstrate that endovascular subjects with angiographic reperfusion of proximal occlusions had better outcomes than those without reperfusion. This association was also identified in the overall IMS III cohort (TICI 0 12.7%, 1 27.6%, 2A 34.3%, 2B 47.9%, and 3 71.4%; p<0.001).3 This finding must be interpreted with caution because confounding factors can lead patients with reperfusion to have better outcomes as well. Only randomized trials with intention-to-treat analyses can determine the true benefit of endovascular therapy.
In conclusion, our demonstration of a time to reperfusion effect in the endovascular therapy arm of the IMS III trial suggests that endovascular therapy does influence clinical outcome. The critical question remains, however, whether endovascular therapy can lead to superior outcomes compared to reperfusion by IV thrombolysis alone. Current evidence suggests that, on average, faster times to reperfusion will lead to better clinical outcomes, whether accomplished by IV rt-PA, endovascular therapy, or both. This serves as a reminder to clinicians that it is critical to improve infrastructure to start IV therapy more quickly, given the potential public heath impact of earlier reperfusion to reduce the burden of stroke. More directly, it challenges clinical trialists to design future trials of novel reperfusion therapies with speed in mind.
RESEARCH IN CONTEXT.
Systematic review
We searched PubMed for human, clinical trials published in English from January 1, 2000 to October 31, 2013, with the search terms “reperfusion,” “stroke/therapy,” “time factors,” and “clinical outcome.” This search yielded 23 publications of which two studies analyzed the association of time to angiographic reperfusion on clinical outcome in patients without penumbral imaging selection. The first was a post hoc analysis of the pooled multicenter IMS pilot trials, which demonstrated an association between time to reperfusion and good clinical outcome within 7 hours of stroke onset; this analysis, along with an analysis of the single-center prospective RECANALISE registry, provide the pilot data for the current validation study. The second study identified in the systematic review was a post hoc analysis of the pooled multicenter MERCI trial and Multi MERCI trials; this study suggested a trend towards an association between time to reperfusion in adjusted analysis and good clinical outcome in a later cohort (40% ≥6·9 hours).
Interpretation
Using the same methodology as our prior IMS pilot analysis, we validate the hypothesis that longer time to angiographic reperfusion leads to a decreased likelihood of good clinical outcome in a preplanned analysis of the largest trial of endovascular therapy to date. Our findings suggest that future endovascular trials must treat patients much more rapidly to achieve benefit compared to IV rt-PA alone.
Acknowledgments
SOURCES OF FUNDING:
NIH/NINDS Grant Numbers: UC U01NS052220; MUSC U01NS054630 and U01NS077304. Genentech Inc. supplied study drug used for intra-arterial t-PA in the endovascular group. EKOS Corp., Concentric Inc., Cordis Neurovascular, Inc. supplied study catheters during Amendments 1–3. In Europe, IMS III investigator meeting support was provided in part by Boehringer Ingelheim.
Author contributions
PK: study design, grant writing, study planning, data collection, data analysis, data interpretation, writing of the first draft of the report, and report revision
SDY: study design, grant writing, study planning, data collection, data analysis, data interpretation, writing of the first draft of the report, and report revision
MM: data collection, data interpretation, and report revision
JPB: study design, grant writing, study planning, data collection, data analysis, data interpretation, and report revision
DSL: data collection, data interpretation, and report revision
AMD: study design, grant writing, study planning, data collection, data analysis, data interpretation, writing of the first draft of the report, and report revision
PA: data collection, data interpretation, and report revision
JC: study design, grant writing, study planning, data collection, data analysis, data interpretation, and report revision
JS: study design, grant writing, study planning, data collection, data analysis, and data interpretation
LDF: data analysis
MG: data collection, data interpretation, and report revision
MDH: study design, grant writing, study planning, data collection, data analysis, data interpretation, and report revision
YYP: Head statistician and lead at the statistical and data coordination unit who contributed to the conception of the study, study design, grant writing, study planning, data analysis, data interpretation, and report revision
ECJ: study planning, data collection, data analysis, data interpretation, and report revision
ECH: data collection, data interpretation, and report revision
AV: data interpretation, and report revision
TAT: study design, grant writing, study planning, data collection, data analysis, data interpretation, and report revision
Conflicts of Interest
PK: Penumbra Inc, research support to UC, Department of Neurology forrole as Neurology PI of THERAPY Trial; Genentech Inc, research support to UC, Department of Neurology for role as PI of PRISMS Trial.
SDY: Genentech, Inc for role as PRISMS Steering Committee Member.
MM: Consultant for BoerhingerIngelheim
JPB: Genentech Inc. (Supplier of Alteplase for NINDS-funded CLEARER, JMS Ill trials); $65,000 Educational grant to the American Academy of Neurology for 2012 annual meeting program 2AC.007 “What’s in a Stroke Center: Members, Services, Organization and Roles” as course director; Research Grant- PRISMS study; EKOS Corporation supplied catheter devices for IMS HI clinical trial; Schering Plough supplies drug for NINDS-funded CLEARER Trial
DSL: Consultant to Stryker and Covidien
AMD: Consultant to Covidien for speaking engagements.
PA: Receipt of research grant support and lecture fees from Pfizer, Sanofi, Bristol-Myers-Squibb, Merck, AstraZeneca, Boehringer-Ingelheim, and consultancy fees from Pfizer, BMS, Merck, Boehringer-Ingelheim, AstraZeneca, Bayer, Daiichi-Sankyo, Lundbeck, Edwards, Boston Scientific, Kowa, lecture fees from Bayer, Boston Scientific, St-Jude Medical, and research grants from the French government
JC: None
JAS: None
LF: None
MG: Consultant for Covidien/EV3 for teaching engagement, trial design, and execution.
MDH: Grant from Covidien, Heart & Stroke Foundation Alberta, Alberta Innovates Health Solutions, Hotchkiss Brain Institute, Department of Clinical Neurosciences for a clinical trial of endovascular stroke therapy.
YP: None
ECJ: None.
ECH: Consultancy with Glaxo Smith Kline.
AV: None
TAT: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NINDS rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA IMS I and II Investigators. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73(13):1066–72. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broderick JP, Tomsick TA, Palesch YY. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368(25):2432–3. doi: 10.1056/NEJMc1304759. [DOI] [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 5.Nogueira RG, Smith WS, Sung G, et al. Effect of Time to Reperfusion on Clinical Outcome of Anterior Circulation Strokes Treated With Thrombectomy: Pooled Analysis of the MERCI and Multi MERCI Trials. Stroke. 2011;42(11):3144–9. doi: 10.1161/STROKEAHA.111.624163. [DOI] [PubMed] [Google Scholar]
- 6.Furlan A, Higashida R, Wechsler L, Schulz G Investigators ftPI. PROACT II: Recombinant prourokinase (r-ProUK) in acute cerebral thromboembolism. Initial Trial Results. 24th AHA International Conference on Stroke and Cerebral Circulation; Nashville, TN. 1999. [Google Scholar]
- 7.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36(7):1432–8. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 8.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 9.Bose A, Henkes H, Alfke K, et al. The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol. 2008;29(7):1409–13. doi: 10.3174/ajnr.A1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. The Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 11.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. The Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA IMS I and II Investigators. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73(13):1066–72. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazighi M, Serfaty JM, Labreuche J, et al. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8(9):802–9. doi: 10.1016/S1474-4422(09)70182-6. [DOI] [PubMed] [Google Scholar]
- 15.Mazighi M, Chaudhry SA, Ribo M, et al. Impact of onset-to-reperfusion time on stroke mortality: a collaborative pooled analysis. Circulation. 2013;127(19):1980–5. doi: 10.1161/CIRCULATIONAHA.112.000311. [DOI] [PubMed] [Google Scholar]
- 16.Christou I, Alexandrov AV, Burgin WS, Wojner AW, Felberg RA, Malkoff M, Grotta JC. Timing of recanalization after tissue plasminogen activator therapy determined by transcranial doppler correlates with clinical recovery from ischemic stroke. Stroke. 2000;31(8):1812–6. doi: 10.1161/01.str.31.8.1812. [DOI] [PubMed] [Google Scholar]
- 17.Goyal M, Menon BK, Coutts SB, Hill MD, Demchuk AM. Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke. 2011;42(1):93–7. doi: 10.1161/STROKEAHA.110.594481. [DOI] [PubMed] [Google Scholar]
- 18.Khatri P, Hill MD, Palesch YY, et al. Methodology of the Interventional Management of Stroke (IMS) III Trial. Int J of Stroke. 2007 In Press. [Google Scholar]
- 19.Fonarow GC, Smith EE, Saver JL, et al. Timeliness of Tissue-Type Plasminogen Activator Therapy in Acute Ischemic Stroke Clinical Perspective Patient Characteristics, Hospital Factors, and Outcomes Associated With Door-to-Needle Times Within 60 Minutes. Circulation. 2011;123(7):750–8. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 20.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79(4):306–13. doi: 10.1212/WNL.0b013e31825d6011. [DOI] [PubMed] [Google Scholar]
- 21.Sun CJ, Nogueira RG, Glenn BA, et al. “Picture to puncture”: a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.112.000506. [DOI] [PubMed] [Google Scholar]
- 22.Boersma E, Maas ACP, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. The Lancet. 348(9030):771–5. doi: 10.1016/S0140-6736(96)02514-7. [DOI] [PubMed] [Google Scholar]
- 23.Fiehler J, Söderman M, Turjman F, et al. Future trials of endovascular mechanical recanalisation therapy in acute ischemic stroke patients-A position paper endorsed by ESMINT and ESNR. Neuroradiology. 2012;54(12):1303–12. doi: 10.1007/s00234-012-1076-y. [DOI] [PubMed] [Google Scholar]
- 24.Personal Communication ESCAPE Trial, Michael D Hill. Oct 31, 2013.
- 25.Personal Communication, THERAPY Trial, Siu Po Sit. Oct 31, 2013.
- 26.Galimanis A, Jung S, Mono ML, et al. Endovascular therapy of 623 patients with anterior circulation stroke. Stroke. 2012;43(4):1052–1057. doi: 10.1161/STROKEAHA.111.639112. [DOI] [PubMed] [Google Scholar]
- 27.Almekhlafi MA, Hockley A, Desai JA, et al. Overcoming the evening/weekend effects on time delays and outcomes of endovascular stroke therapy: the Calgary Stroke Program experience. Journal of Neurointerventional Surgery. 2013 Dec 5; doi: 10.1136/neurintsurg-2013-011000. Epub ahead of print. [DOI] [PubMed] [Google Scholar]