Abstract
Functional variants that contribute to genomewide association study (GWAS) signals are difficult to identify. MicroRNAs could contribute to some of these gene-trait relationships. We compiled a set of GWAS trait gene SNPs that were predicted to affect microRNA regulation of mRNA. Trait associations were tested in a sample of 6725 European-American (EA) and African-American (AA) subjects that were interviewed using the polydiagnostic SSADDA to diagnose major psychiatric disorders. A predicted miR-330-3p target site SNP (rs41305272) in mitogen-activated protein kinase kinase 5 (MAP2K5) mRNA was in LD (d’=1.0, r2=0.02) with a reported GWAS-identified variant for restless legs syndrome (RLS), a disorder frequently comorbid with anxiety and depression, possibly because of a shared pathophysiology. We examined the SNP’s association with mood and anxiety-related disorders. Rs41305272 was associated with agoraphobia (Ag) in EAs (odds ratio[OR]=1.95, p=0.007; 195 cases) and AAs (OR=3.2, p=0.03; 148 cases) and major depressive disorder (MDD) in AAs (OR=2.64, p=0.01; 427 cases), but not EAs (465 cases). Rs41305272*T carrier frequency was correlated with the number of anxiety and depressive disorders diagnosed per subject. RLS was not evaluated in our subjects. Predicted miR-330-3p target genes were enriched in pathways relevant to psychiatric disorders. These findings suggest that microRNA target site information may be useful in the analysis of GWAS signals for complex traits. MiR-330-3p and MAP2K5 are potentially important contributors to mood and anxiety-related traits. With support from additional studies, these findings could add to the large number of risk genes identified through association to medical disorders that have primary psychiatric effects.
Keywords: restless legs syndrome, depression, miR-330, MEK5, GWAS
Introduction
Genomewide association studies (GWAS) have been enormously useful for complex trait gene mapping [Hindorff et al., 2009]; however the functional variants that contribute to GWAS signals are often difficult to identify, and this represents a critical gap in knowledge. Some GWAS loci might harbor multiple variants (rare or common) with independent effects on risk [Eichler et al., 2010]; indeed, multiple independent risk variants were recently described in GWAS-identified risk genes for four complex traits: rheumatoid arthritis (RA), Crohn’s disease (CD), type-1 diabetes (T1D), and type-2 diabetes (T2D) [Ke, 2012]. Variants with independent effects on bipolar disorder risk have also been described at the Ankyrin-3 (ANK3) locus [Schulze et al., 2009]. While identifying the functional variants based on GWAS-identified genes is an important goal of the post-GWAS era, fine mapping associations between complex traits and an increasing number of variants increases the penalty for multiple testing required to control for type-1 error, and many SNPS that have small-to-moderate effects on risk will be difficult to detect without very large samples [Kiezun et al., 2012]. Statistical, as opposed to functional, follow-up studies of GWAS signals are limited in terms of their contribution to understanding the effects of specific alleles on specific biological phenotypes.
Follow-up studies of GWAS gene variants are also complicated because it can be difficult to ascertain their effects, especially those in non-coding regions. Prioritization of genomewide SNP data is generally based on the predicted effects of SNPs on protein structure and function, and variants in non-coding regions are often excluded from follow-up studies a priori. But in addition to these commonly studied functional effects, non-coding (and potentially coding) regions of genes can be evaluated by incorporating information on predicted regulation by microRNAs. MicroRNAs interact with mRNA by base pairing, primarily with the 3’UTR, and function to reduce protein expression [Guo et al., 2010; Bazzini et al., 2012]. The majority of mammalian genes are under selective pressure to maintain microRNA target sites in mRNA [Chen and Rajewsky, 2006; Saunders et al., 2007; Hu and Bruno, 2011]. These regulatory sites can be predicted in mRNA with moderate certainty using computational algorithms [Bartel, 2009]. SNPs in these target sites can affect how microRNAs interact with mRNA, changing gene expression regulation [Martin et al., 2007; Sethupathy et al., 2007; Jensen et al., 2009]. These types of functional SNPs are also associated with various human traits, including disease [Abelson et al., 2005; Martin et al., 2007; Sethupathy et al., 2007], behavior [Jensen et al., 2009; Conner et al., 2010] and molecular traits, such as gene expression [Gamazon et al., 2012; Lu and Clark, 2012].
Recent studies have investigated GWAS data retrospectively for trait-associated SNPs that might affect microRNA regulation of mRNA [Richardson et al., 2011; Thomas et al., 2011; Gamazon et al., 2012]. MicroRNA target site SNPs have been reported to be in high (r2>0.8) linkage disequilibrium (LD) with many previously described GWAS signals. A miR-196 target site variant in IRGM [Brest et al., 2011] and miR-410 target site variant in LPL [Richardson et al., 2013], have been functionally characterized and linked to the development of Crohn’s disease and lipid phenotypes, respectively. Thus, the identification and characterization of the effects of microRNA target site polymorphisms may advance our understanding of the many gene-trait associations identified by GWAS.
In this study, we integrated a microRNA target site algorithm, TargetScan [Garcia et al., 2011], with genomewide SNP array data (~900,000 markers) to identify a set of 3’UTR SNPs that were likely to affect microRNA regulation of mRNA. A subset (n=28) of these SNPs mapped to genes that had previously been linked to human phenotypes by GWAS [Hindorff et al., 2009]. We prioritized SNPs that were associated to behavioral traits. One predicted microRNA:mRNA interaction was disrupted by a SNP in the 3’UTR of the mitogen activated protein kinase gene (MAP2K5), a GWAS gene for restless legs syndrome (RLS). Anxiety and depressive disorders frequently co-occur with RLS, and a common pathophysiology may account for this comorbidity [Sevim et al., 2004; Winkelmann et al., 2005; Winkelman et al., 2006; Lee et al., 2008; Kim et al., 2012].
We used an existing GWAS sample of European Americans (EAs) and African Americans (AAs) that were recruited for genetic studies of substance (SD) and alcohol dependence (AD). The subjects were carefully ascertained for all major psychiatric diagnoses, including anxiety and depressive disorders, using the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA) [Pierucci-Lagha et al., 2005; Pierucci-Lagha et al., 2007]. Anxiety and depression are prevalent in this population, but given the high rate of comorbid SD and AD, this group may have genetic risk factors that are different relative to non-SD and non-AD anxiety and depression cases. The effect of this variant on anxiety and depressive disorder diagnosis and disorder count was examined in EAs and AAs. To understand the function of the predicted effector microRNA, miR-330, we examined the presence of biological pathways and gene groups enriched with miR-330 targets.
Materials and Methods
Subjects
The sample included 6725 unrelated drug dependent and non-drug dependent control subjects (3079 EAs and 3646 AAs) that were recruited at multiple Eastern US sites for genetic studies of alcohol (AD), cocaine (CD) and opioid (OD) dependence. All subjects were interviewed using the SSADDA to obtain diagnoses according to DSM-IV criteria and the additional variables used in the analysis [Pierucci-Lagha et al., 2005; Pierucci-Lagha et al., 2007]. Fifty-five percent of EA subjects and 54% of AA subjects were male. Eighty-one percent of AA subjects were dependent on one or more of three drugs: cocaine (70%), opioids (24%) and alcohol (60%). Seventy-one percent of EA subjects were dependent on one or more of three drugs: cocaine (51%), opioids (44%) and alcohol (55%). Forty-three percent of EA subjects with agoraphobia (Ag) had panic disorder (PD), and 26% of AA subjects with Ag had PD. The number of subjects diagnosed with each disorder of interest is shown in the supplemental (Table 1 and Table S1 and S2).
Table 1.
The effects of rs41305272 genotype on risk for anxiety and depressive disorders.
| rs41305272 genotype counts (frequency) | |||||
|---|---|---|---|---|---|
| Group | CC | CT | TT | Odds Ratio (95%CI), p value |
|
| European Americana | |||||
| Control (n=2010) | 1881 (93.58%) | 127 (6.32%) | 2 (0.1%) | ||
| Agoraphobia (n=195) | 172 (88.21%) | 21 (10.77%) | 2 (1.03%) | 1.95 (1.16–3.15), 0.007 | |
| Panic Disorder (n=255) | 225 (88.24%) | 29 (11.37%) | 1 (0.39%) | 1.94 (1.23–3.00), 0.004 | |
| MDD (n=465) | 427 (91.83%) | 36 (7.74%) | 2 (0.43%) | 1.30 (0.87–1.91), 0.18 | |
| PTSD (n=399) | 367 (91.98%) | 30 (7.52%) | 2 (0.5%) | 1.27 (0.82–1.92), 0.27 | |
| African Americana | |||||
| Control (n=2584) | 2556 (98.92%) | 28 (1.08%) | 0 | ||
| Agoraphobia (n=148) | 143 (96.62%) | 5 (3.38%) | 0 | 3.20 (0.95–8.54), 0.03 | |
| Panic Disorder (n=96) | 94 (97.92%) | 2 (2.08%) | 0 | 1.94 (0.22–7.90), 0.29 | |
| MDD (n=427) | 415 (97.19%) | 12 (2.81%) | 0 | 2.64 (1.21–5.41), 0.01 | |
| PTSD (n=498) | 489 (98.19%) | 9 (1.81%) | 0 | 1.68 (0.69–3.68), 0.18 | |
| Combined sampleb | |||||
| Agoraphobia (n=343) | 2.21 (1.47–3.32), 0.0004 | ||||
| Panic Disorder (n=351) | 1.95 (1.32–2.90), 0.002 | ||||
| MDD (n=892) | 1.53 (1.11–2.11), 0.01 | ||||
| PTSD (n=897) | 1.40 (0.99–1.98), 0.06 | ||||
| Combined sample (onset prior to SUD onset)b | |||||
| Agoraphobia (n=201) | 1.92 (1.11–3.33), 0.03 | ||||
| MDD (n=249) | 1.95 (1.18–3.20), 0.02 | ||||
Fisher's Exact Test of CC and T-carrier,
additive logistic regression model adjusted for sex and ancestry, MDD = major depressive disorder, PTSD= post-traumatic stress disorder, SUD = substance use disorder (alcohol, cocaine, opioid)
Institutional review boards at all participating sites approved the studies and all subjects provided written informed consent to participate. The National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism issued Certificates of Confidentiality to protect study participants.
Genotyping
Genotypes for 1573 EA and 3018 AA subjects were acquired with the Illumina HumanOmni1-Quad v1.0 microarray containing 988,306 autosomal SNPs. A total of 44,644 SNPs on the microarray with call rates < 98% were excluded [Gelernter et al., 2013]. GWAS genotyping was conducted at the Center for Inherited Disease Research (CIDR) and the Yale Center for Genome Analysis (YCGA). Additional subjects (EA, n=1560; AA, n=628) were genotyped specifically for rs41305272 with a 2µl TaqMan allelic discrimination assay (Applied Biosystems, Foster City, CA, USA) described in the supplement methods.
Data analysis
The TargetScan algorithm was used to identify microRNA targets. TargetScan is a well-validated method to identify microRNA targets [Friedman et al., 2009]. The algorithm’s predictions primarily require continuous annealing between nucleotides 2–7 of the microRNA (the “seed”) and the mRNA molecule [Grimson et al., 2007]. For EA and AA subjects with GWAS data, ancestries were assigned based on the first two principal components of the GWAS determined with Eigensoft [Patterson et al., 2006; Price et al., 2006] using HapMap 3 CEU, YRI, and CHB reference populations as described elsewhere [Gelernter et al., 2013]. For subjects without GWAS data, population assignments were determined by an ancestry informative STR and SNP method [Yang et al., 2005], with additional informative SNPs, rs1540771 (6p23.5), rs1805007 (MC1R), rs12896399 (SLC24A4) and rs1426654 (SLC24A5)) using Structure [Pritchard et al., 2000] or an ancestry informative panel of 96 SNPs that differentiate African, Asian, European, and Mexican populations using a Bayesian variable partition method. MAP2K5 genotypes did not deviate from Hardy-Weinberg equilibrium expectations. Data analysis was performed using JMP 9.0.0 software (Cary, NC, USA), GraphPad Prism (GraphPad Software, Inc., La Jolla, CA), PLINK [Purcell et al., 2007] and the SNPassoc package [Gonzalez et al., 2007] in R version 2.15.1 as described in the text. MetaCore (GeneGo Inc., Saint Joseph, Michigan), which uses a manually curated database of biological networks and interactions, was used to test for pathway enrichment. The p-value is the probability that the miR-330-3p target gene set intersects the curated gene set given a hypergeometric distribution. For the analysis of the Ag, PD, generalized anxiety disorder (GAD), social phobia (SocP), obsessive-compulsive disorder (OCD), post-traumatic stress disorder (PTSD) and major depressive disorder (MDD) diagnosis count, subjects with unknown diagnoses were not included as controls. Subjects with an unknown diagnosis were included in the diagnosis count if they met criteria for at least one of the seven disorders. For example a subject without PD, SocP or GAD, but unknown for Ag, would be excluded from the analysis, but a subject that was negative for Ag, unknown for MDD, and diagnosed with PD and SocP would have a diagnosis count of two.
Results
A predicted microRNA target site SNP in MAP2K5, a GWAS gene for RLS
We first collected a set of probable microRNA target sites by exporting from the May 2004, March 2006, and February 2009 builds of the UCSC genome browser [Dreszer et al., 2012] the genomic coordinates for 128,047 microRNA targets site predicted by the TargetScan algorithm [Lewis et al., 2005; Grimson et al., 2007]. We integrated the genomic coordinates for these microRNA target sites with genomewide SNP data that were obtained with the HumanOmni1-Quad v1.0 microarray (Illumina, San Diego, CA). We identified a set of 226 SNPs that were predicted to affect how microRNA interacts with mRNA. These SNPs mapped to the 3’UTRs of 224 different genes. Consistent with prior studies suggesting that there is selection against microRNA target site polymorphisms [Chen and Rajewsky, 2006; Saunders et al., 2007; Hu and Bruno, 2011], the population frequency of the SNPs within these elements was significantly lower than the average frequency for SNPs interrogated by the HumanOmni1-Quad v1.0 microarray. The average minor allele frequency (MAF) of the predicted microRNA target site SNPs was 0.18 in EAs and 0.17 in AAs, compared to the average MAF of all SNPs assayed, which was 0.21 in EAs and 0.22 in AAs (EA and AA p<0.001, Student’s T-Test)
A subset of the microRNA target site SNPs occurred in genes previously reported to be associated with a trait via GWAS [Hindorff et al., 2009]. There were 28 genes with at least one SNP (not necessarily the microRNA target site SNP) associated to a trait with a P value less than 1×10−8, and with the association replicated in at least one additional data set. We investigated the reported traits to determine whether any of these genes could be predicted to function in pathways affecting psychiatric traits that were ascertained in our studies of drug and alcohol dependence. Most traits were seemingly unrelated to psychiatric disorder risk (Table S1 in Supplement 1). However, one exception was a GWAS result for restless legs syndrome (RLS) and SNPs that mapped to MAP2K5. Two studies reported associations for variants in the MAP2K5 gene and RLS in populations of European ancestry [Winkelmann et al., 2007; Winkelmann et al., 2011] The most recent (and largest) GWAS study by Winkelmann et al., reported the lowest p value (1.37×10−22) for rs12593813, which maps to an intron towards the 3’ end of MAP2K5 [Winkelmann et al., 2011]. Anxiety and depression are hallmarks of RLS [Sevim et al., 2004]. Subjects with RLS are >3 times more likely to have comorbid anxiety or depression and at even greater risk for having comorbid anxiety with depression [Winkelmann et al., 2005; Winkelman et al., 2006; Lee et al., 2008; Kim et al., 2012].
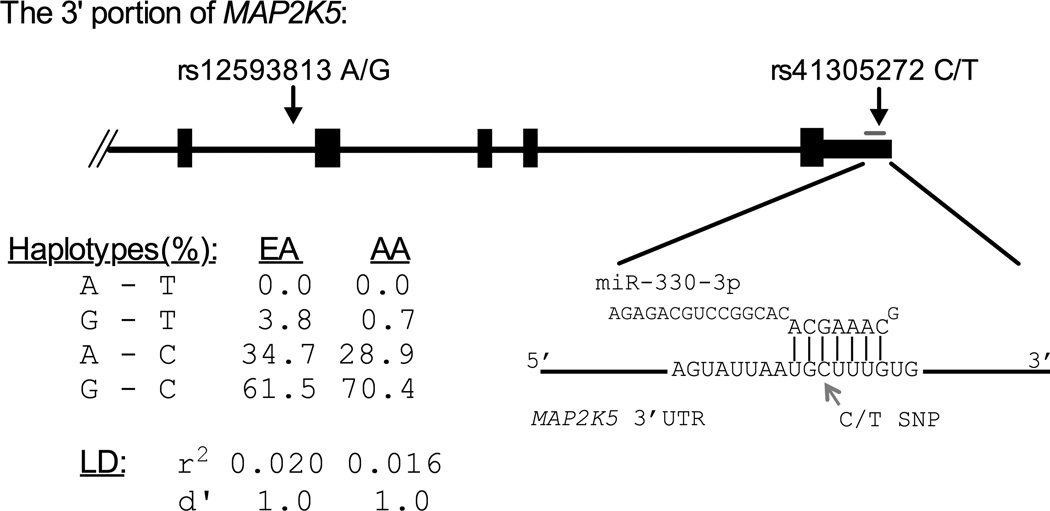
We used our own GWAS array data to investigate the linkage disequilibrium and phasing between the MAP2K5 SNP with the best GWAS signal for RLS, rs12593813, and the predicted microRNA target site SNP, rs41305272. The more common GWAS allele (the risk-allele), rs12593813*G, and the predicted microRNA target site allele, rs41305272*T, were in phase in both EA and AAs. The relative positions of each SNP in MAP2K5, haplotype predictions, and linkage disequilibrium (LD) reported by PLINK [Purcell et al., 2007] are shown in Figure 1. The C/T polymorphism alters the base pairing predicted between MAP2K5 mRNA and the “seed” sequence of miR-330-3p (Figure 1). MiR-330-3p and MAP2K5 mRNA are brain expressed and relatively abundant in hippocampus–a key region for anxiety and depression, based on rodent studies [Di Benedetto et al., 2007; He et al., 2007]. Together, these findings, the RLS GWAS, the functional predictions, and the hippocampal expression, suggested that the 3’UTR SNP might affect RLS-related phenotypes such as anxiety and depression.
Figure 1.
Shown are the relative positions of rs12593813, a Restless Leg Syndrome (RLS) GWAS SNP, and rs41305272, a predicted microRNA target site SNP in MAP2K5. Rs41305272 alters the interaction predicted between the MAP2K5 mRNA and the critical “seed” sequence of miR-330-3p. The haplotypes and LD predicted for rs12593813-rs41305272 in EA and AA populations show that rs12593813*G is in phase with rs41305272*T
MAP2K5 rs41305272 is associated with anxiety and depressive disorders in EAs and AAs
We hypothesized that functional MAP2K5 variants might affect risk for depressive and anxiety disorders, based on the high prevalence of these psychiatric traits in RLS patients. We investigated the association of rs41305272 to anxiety and depressive disorders in subjects ascertained with the SSADDA, including PD, Ag, PTSD, SocP, OCD, GAD and MDD. We focused initially on the EA GWAS population because the minor allele frequency in AAs was very low (EA MAF = 3.6%, AA MAF =0.7%), and we focused our initial association testing on MDD and anxiety disorders that were well represented in our initial sample: namely, PTSD (n=270), PD (n=192) and Ag (n=146). The significance level was p=0.0125 after testing 4 phenotypes. The prevalence of each disorder for EAs and AAs is shown in Table S2 (Supplement 1). The rates of anxiety disorders were generally lower in AAs than EAs. Similar differences between EAs and AAs have been noted by Asnaani et al., who found social anxiety disorder, GAD and PD to be more common in EAs than AAs [Asnaani et al., 2010].
In this initial analysis, there were significant associations between rs41305272 and each trait except MDD in EAs. The association signal was most robust based on the Cochran-Armitage Trend Test for Ag (Z=3.26, p=0.001), followed by PD (Z=3.02, p=0.0025) and PTSD (Z=2.67, p=0.0075). There was no risk effect for MDD (Z=0.032, p=0.97). The high comorbidity among the anxiety disorders made it difficult to distinguish whether risk was associated with a specific diagnosis or broadly distributed. Encouraged by these findings, we genotyped rs41305272 in additional EA and AA subjects (EA n=1560, AA n=628), so that we could complete a more comprehensive analysis of the SNP and anxiety and depression. These subjects were part of the same set of genetic studies of drug and alcohol dependence, but the second wave of genotype data for EAs was enriched with non-drug dependent controls, and the prevalence of anxiety and depression was lower than in the initial EA GWAS subjects (e.g., Ag prevalence was 3.2% versus 9.4% in the two subsamples). For the analysis presented in Table 1, the control subjects were negative for all anxiety disorders ascertained by the SSADDA (PD, Ag, PTSD, SocP, OCD, and GAD) and MDD. EAs that carried the T-allele were at higher risk for Ag (Fisher Exact test (FET), OR=1.95 [95%CI=1.16–3.15), p= 0.007) and PD (OR=1.94 [95%CI=1.23–3.00), p=0.004) (Table 1). In an additive logistic regression models adjusted for sex, the effects were similar (Ag, OR=2.06 [95%CI=1.32–3.2], p=0.003; PD, OR=1.96 [95%CI=1.30–2.94], p=0.003). The effect of this SNP on the risk of Ag was also evident in the AA population (FET, OR=3.2 [95%CI=0.95–8.54], p=0.03). The SNP was associated with MDD in AAs (FET, OR=2.64 [95%CI=1.21–5.41], p=0.01), but not in EAs. We used logistic regression to estimate the effect of genotype on risk in the combined sample. In a model that controlled for sex and ancestry (coded as dichotomous variables), rs41305272*T was associated with elevated risk for Ag (OR=2.22 [95%CI 1.48–3.34], p=0.0004), PD (OR=1.95 [95%CI 1.32–2.90], p=0.002) and MDD (OR=1.48 [95%CI 1.05–2.07], p=0.01) (Table 1). We restricted our analysis to the subset of subjects that reported the onset of Ag (n=201) or MDD (n=249) prior to the onset of AD, CD or OD. The effect of genotype was significant in this sub-group in an additive regression model that controlled for ancestry and sex (Ag, OR=1.92 [95%CI 1.11–3.33], p=0.03; MDD, OR=1.95 [95%CI 1.18–3.20, p=0.016). Rs41305272 genotype was not significantly associated with AD, CD, OD or nicotine dependence (ND) in EAs or AAs. The minor allele frequency for EA non-drug dependent subjects was 3.8% (n=860) and 3.8%, 3.5%, 3.7% and 3.7% for EA AD (n=1392), EA ND (n=1609), EA CD (n=1532) and EA OD (n=1323) subjects, respectively. The minor allele frequency for AA non-drug dependent subjects was 0.9% (n=666) and 0.6%, 0.6%, 0.6% and 0.6% for AA AD (n=1893), AA ND (n=1981), AA CD (n=2498) and AA OD (n=868) subjects, respectively.
Anxiety and depressive disorder count correlates with T-carrier frequency
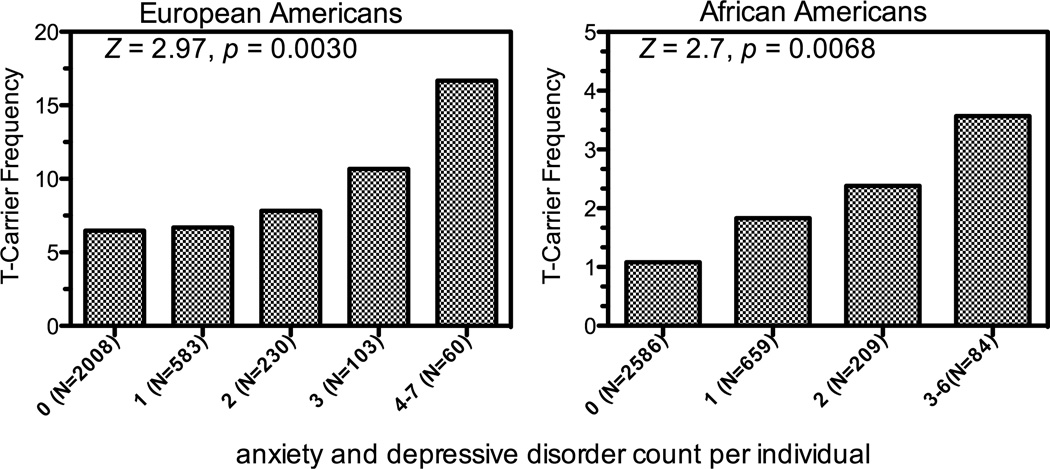
To test the effect of rs41305272 on anxiety and depression severity, we created a diagnosis count that included the 6 anxiety disorders assessed by the SSADDA (Ag, PD, SocP, GAD, OCD, PTSD) and MDD. The presence of multiple diagnoses may, to some extent, reflect the severity of anxiety and depression [Noyes, 2001; Tukel et al., 2002; Kessler et al., 2005]. Thirty-three percent of EAs and 27% of AAs were affected with at least one anxiety or depressive disorder. The distribution of disorder counts is shown in Table S3 (Supplement 1). As show in Figure 2, the frequency of the T-carrier genotype increased as a function of the number of disorders. The effect was evident in both EA and AA subjects (Cochran-Armitage Trend Test, EAs: Z=2.97, p=0.003; AAs: Z=2.71, p= 0.0068). For EA subjects, the T-carrier genotype frequency increased monotonically: 6.47% (n=2008), 6.69% (n=583), 7.83% (n=230), 10.68% (n=103), 14% (n=50), and 42.86% (n=7) as the diagnosis count increased from 0–5 (the numbers of individuals with 6 or 7 disorders were 2 and 1, respectively). For AAs, the T-carrier frequency increased monotonically: 1.04% (n=2586), 1.97% (n=659), 2.39% (n=30), and 4.62% (n=65) as the diagnosis count increased from 0–3 (the numbers of individuals with 4, 5, or 6 disorders were 16, 2, and 1, respectively). The disorder counts were grouped at the higher diagnosis count for Figure 2, but the statistical test was done with subjects ungrouped. We investigated this effect separately for the subset of subjects diagnosed with AD, ND, CD or OD. There were no remarkable differences between each EA subset. The effect was slightly more robust in the OD group (Z=4.36, p<0.0001, n=1272) followed by the ND group (Z=4.02, p<0.0001, n=1544), AD group (Z=3.11, p=0.0019, n=1342) and CD group (Z=2.78, p<0.0055, n=1475). Despite the low allele frequency and reduced sample size, the effect, though not consistently significant, remained evident when the AA subgroups were analyzed separately (CD, Z=2.40, p=0.016, n=2409; AD, Z=2.00, p=0.046, n=1829; ND, Z=1.35, p=0.18, n=1898; OD, Z=1.48, p=0.14, n=828). Only 5 subjects had the “TT” genotype (all EA), and 3 of these (60%) were affected with at least one anxiety or depressive disorder, which is approximately twice the rate for the EA subjects in our sample overall (33%).
Figure 2.
The relationship between rs41305272 and anxiety and depressive disorder count. The T-carrier frequency was positively correlated with disorder count in EA and AA subjects. A change in T-carrier frequency across all counts (EA=0-7 and AA=0-6) was tested with the Cochran Armitage Trend Test (EA, z=2.97, p=0.003; AA, z=2.7, p=0.0068).
We considered that this variant might be in LD with other variants at the MAP2K5 locus that were associated with risk for anxiety and depression. Using the subset of EA (n=1573) and AA subjects (n=3018) that had GWAS SNP array information for 159 SNPs in or flanking MAP2K5 (a 797 kb window), we found that rs41305272 was associated with diagnosis count in an additive regression model adjusted for sex and ancestry (coded as a dichotomous variable) (p=1.33×10−6), but there was not a comparable effect for any other SNP (lowest p=1.2×10−4).
Gene sets enriched with predicted miR-330-3p targets
Single microRNAs can directly regulate groups of genes that function in related biologically processes [Giraldez et al., 2005; Krutzfeldt et al., 2005]. To better understand miR-330-3p function, we used MetaCore™ (GeneGo Inc.) [Ekins et al., 2006; Ekins et al., 2007] pathway analysis software to investigate biological processes that might be enriched with miR-330-3p target genes. TargetScan predicted 875 conserved miR-330-3p target genes and all were included in the analysis. The top 3 enriched gene sets reported for four domains, Pathway Maps, Gene Ontology Process, Process network and Disease are shown in Table 2. Each domain included a miR-330-3p target-enriched gene set that was relevant to the neurobiology of anxiety and depression. The gene set most significantly enriched with miR-330-3p targets was “nervous system development” in the Gene Ontology process category (p=2.73×10−36). In the Gene Ontology process category, “neurogenesis” was another noteworthy gene set enriched with miR-330 targets (p=6.26×10−31).
Table 2.
GeneGo analysis of predicted miR-330 target genes.
| Enrichment Analysis | rank | genes (N) |
miR-330 targets (N) |
p value |
|---|---|---|---|---|
| Pathway Maps: | ||||
| Development-Role of HDAC and calcium/calmodulin-dependent kinase (CaMK) in control of skeletal myogenesis |
1 | 54 | 13 | 1.77E-08 |
| Cytoskeleton remodeling-TGF, WNT and cytoskeletal remodeling | 2 | 111 | 18 | 2.61E-08 |
| Development-Neurotrophin family signaling | 3 | 40 | 11 | 5.10E-08 |
| Gene Ontology Processes: | ||||
| Nervous system development | 1 | 2361 | 242 | 2.73E-36 |
| System development | 2 | 4304 | 352 | 2.07E-33 |
| Neurogenesis | 3 | 1613 | 180 | 6.26E-31 |
| Process Networks: | ||||
| Development-Neurogenesis-Axonal guidance | 1 | 230 | 38 | 9.60E-10 |
| Reproduction-FSH-beta signaling pathway | 2 | 160 | 29 | 1.21E-08 |
| Cardiac development-FGF-ErbB signaling | 3 | 124 | 25 | 1.38E-08 |
| Diseases (by Biomarkers): | ||||
| Mental Disorders | 1 | 3101 | 234 | 5.89E-12 |
| Psychiatry and Psychology | 2 | 3120 | 234 | 1.13E-11 |
| Schizophrenia | 3 | 984 | 99 | 2.29E-11 |
Discussion
We report the association of a predicted miR-330-3p target site SNP in MAP2K5 with anxiety and depression in EA and AA populations. Our findings extend prior research on two fronts. First, our results support the findings of other groups regarding the utility of using predicted microRNA target site variants to understand the functionality of GWAS-identified alleles [Brest et al., 2011; Richardson et al., 2011; Thomas et al., 2011; Gamazon et al., 2012]. This analysis strategy holds promise for further use in this area of investigation. Second, these findings advance our understanding of the genetic risk for anxiety and depression, and potentially the genetic risk that is shared among these disorders. Additional studies will be needed to confirm these association findings.
Our data show that the risk associated with MAP2K5 variation may be generalizable to different forms of anxiety and depression, which is not surprising considering that many studies show considerable genetic overlap among these traits [Andrews et al., 1990; Kendler et al., 1992; Roy et al., 1995; Kendler, 1996; Hettema et al., 2005; Mosing et al., 2009], and little of this genetic variation has been identified and characterized. But, as reviewed by Hettema (2001), twin and family studies of MDD and anxiety do not always converge on the same genetic diathesis model for all trait combinations [Hettema et al., 2001]. Larger studies using approaches similar to the recent mega-analysis of Smoller et al. may be required to detect these shared genetic effects [Cross-Disorder Group of the Psychiatric Genomics and Genetic Risk Outcome of Psychosis, 2013]. Moreover, phenotypes based on DSM-IV may not have sufficient resolution to reveal the effects of MAP2K5 on complex behaviors related to anxiety and depression.
We observed these effects in two different populations: EA and AA. However, the subjects analyzed were recruited for studies of AD, CD and OD, and anxiety and depression were analyzed as secondary phenotypes. Study subjects were heavily affected with anxiety and depression. In addition to AD, CD and/or OD, 29% percent had a comorbid anxiety or depressive disorder. Thirty-six percent of subjects with an anxiety or depression disorder had more than one such diagnosis and 13% had more than two. It is possible that the effects of this variant differ in clinical populations that are not as severely affected as ours. For example, the genotype correlation that we observed with disorder count may reflect a relationship with a specific cluster of disorders and not severity. Our sample may favor the detection of some of these effects because the frequencies of these comorbidities might differ from those of primary anxiety disorder or in epidemiological samples. We cannot exclude the possibility that our findings are the result of type-1 error, especially given the low minor allele frequency of rs41305272 (AA=0.7%, EA=3.6%). This being the case, our results rest on relatively few observations of this variant. As with all association studies, replication and extension of these findings will be important.
Apart from the association to RLS, MAP2K5 is an intriguing gene to consider for anxiety and depression risk. The MAP2K5 protein product, MEK5, is activated by phosphorylation via MEKK2 (encoded by the MAP3K2 gene). Activated MEK5 phosphorylates ERK5, which then translocates into the nuclease to activate gene transcription. The MEKK2-MEK5-ERK5 signaling cascade can be triggered by neurotrophic factors, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) [Kamakura et al., 1999]. It is possible that the behavioral effects of MAP2K5 variants are BDNF dependent, given the important role of BDNF in controlling anxiety and depressive behaviors [Martinowich et al., 2007].
This study also poses many new questions regarding the function of miR-330-3p, about which little is known. Although miR-330-3p target genes are enriched in pathways relevant to anxiety and depression, in vitro and in vivo studies that directly test miR-330 regulation of these genes, especially MAP2K5, in brain regions linked to anxiety and depression are needed to increase our biological understanding of the statistical effect that we report here. Also, studies of microRNA expression in the context of anxiety and depression may yield clues as to how miR-330-3p expression is controlled.
Our approach was not entirely comprehensive with regard to SNPs at the MAP2K5 locus. Other MAP2K5 variants that may affect risk for anxiety, depression will likely be discovered with denser genotyping arrays or re-sequencing. SNPs with MAF <5% were excluded from the RLS analysis of [Winkelmann et al., 2011] and no effects were reported for rs41305272. RLS was not ascertained for our subjects, so our results cannot address the possible existence of common genetic determinants for these disorders, via different functional variants. It is unclear whether the high rate of anxiety and depression observed in RLS is attributable to shared pathophysiology or a consequence of RLS impairment. Winkelmann et al. found that 40% of RLS subjects with comorbid PD reported the onset of PD prior to RLS, and 23% of subjects with comorbid MDD reported MDD prior to RLS [Winkelmann et al., 2005]. Based on the finding that some psychiatric symptoms predate RLS symptoms, only a fraction of depression and anxiety symptoms could be attributable to the RLS–related stressful effects. More research will be needed to investigate the relationship between MAP2K5 gene variants and the different clinical features of RLS.
Supplementary Material
Acknowledgements
This work was supported in part by NIH grants DA12849, DA12690, DA18432, AA017535, DA028909, AA11330, MH64122, a VA MERIT award, and T32 MH014276. Genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University (contract number N01-HG-65403). Dr. Kranzler has been a consultant or advisory board member for Alkermes, Lilly, Lundbeck, Pfizer, and Roche. He is also a member of the American Society of Clinical Psychopharmacology's Alcohol Clinical Trials Initiative, which is supported by Lilly, Lundbeck, Abbott, and Pfizer. Dr. Stein is Co-Editor-in-Chief for UpToDate in Psychiatry and Deputy Editor for the journal Depression and Anxiety.
Footnotes
Drs. Gelernter and Jensen have nothing to disclose.
References
- Abelson JF, Kwan KY, O'Roak BJ, Baek DY, Stillman AA, Morgan TM, Mathews CA, Pauls DL, Rasin MR, Gunel M, Davis NR, Ercan-Sencicek AG, Guez DH, Spertus JA, Leckman JF, Dure LSt, Kurlan R, Singer HS, Gilbert DL, Farhi A, Louvi A, Lifton RP, Sestan N, State MW. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005;310(5746):317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- Andrews G, Stewart G, Allen R, Henderson AS. The genetics of six neurotic disorders: a twin study. J Affect Disord. 1990;19(1):23–29. doi: 10.1016/0165-0327(90)90005-s. [DOI] [PubMed] [Google Scholar]
- Asnaani A, Richey JA, Dimaite R, Hinton DE, Hofmann SG. A cross-ethnic comparison of lifetime prevalence rates of anxiety disorders. J Nerv Ment Dis. 2010;198(8):551–555. doi: 10.1097/NMD.0b013e3181ea169f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzini AA, Lee MT, Giraldez AJ. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science. 2012;336(6078):233–237. doi: 10.1126/science.1215704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brest P, Lapaquette P, Souidi M, Lebrigand K, Cesaro A, Vouret-Craviari V, Mari B, Barbry P, Mosnier JF, Hebuterne X, Harel-Bellan A, Mograbi B, Darfeuille-Michaud A, Hofman P. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn's disease. Nat Genet. 2011;43(3):242–245. doi: 10.1038/ng.762. [DOI] [PubMed] [Google Scholar]
- Chen K, Rajewsky N. Natural selection on human microRNA binding sites inferred from SNP data. Nat Genet. 2006;38(12):1452–1456. doi: 10.1038/ng1910. [DOI] [PubMed] [Google Scholar]
- Conner TS, Jensen KP, Tennen H, Furneaux HM, Kranzler HR, Covault J. Functional polymorphisms in the serotonin 1B receptor gene (HTR1B) predict self-reported anger and hostility among young men. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):67–78. doi: 10.1002/ajmg.b.30955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics C and Genetic Risk Outcome of Psychosis C. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto B, Hitz C, Holter SM, Kuhn R, Vogt Weisenhorn DM, Wurst W. Differential mRNA distribution of components of the ERK/MAPK signalling cascade in the adult mouse brain. J Comp Neurol. 2007;500(3):542–556. doi: 10.1002/cne.21186. [DOI] [PubMed] [Google Scholar]
- Dreszer TR, Karolchik D, Zweig AS, Hinrichs AS, Raney BJ, Kuhn RM, Meyer LR, Wong M, Sloan CA, Rosenbloom KR, Roe G, Rhead B, Pohl A, Malladi VS, Li CH, Learned K, Kirkup V, Hsu F, Harte RA, Guruvadoo L, Goldman M, Giardine BM, Fujita PA, Diekhans M, Cline MS, Clawson H, Barber GP, Haussler D, James Kent W. The UCSC Genome Browser database: extensions and updates 2011. Nucleic Acids Res. 2012;40(Database issue):D918–D923. doi: 10.1093/nar/gkr1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, Nadeau JH. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet. 2010;11(6):446–450. doi: 10.1038/nrg2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S, Bugrim A, Brovold L, Kirillov E, Nikolsky Y, Rakhmatulin E, Sorokina S, Ryabov A, Serebryiskaya T, Melnikov A, Metz J, Nikolskaya T. Algorithms for network analysis in systems-ADME/Tox using the MetaCore and MetaDrug platforms. Xenobiotica. 2006;36(10–11):877–901. doi: 10.1080/00498250600861660. [DOI] [PubMed] [Google Scholar]
- Ekins S, Nikolsky Y, Bugrim A, Kirillov E, Nikolskaya T. Pathway mapping tools for analysis of high content data. Methods Mol Biol. 2007;356:319–350. doi: 10.1385/1-59745-217-3:319. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamazon ER, Ziliak D, Im HK, LaCroix B, Park DS, Cox NJ, Huang RS. Genetic architecture of microRNA expression: implications for the transcriptome and complex traits. Am J Hum Genet. 2012;90(6):1046–1063. doi: 10.1016/j.ajhg.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol. 2011;18(10):1139–1146. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Sherva R, Koesterer R, Almasy L, Zhao H, Kranzler HR, Farrer L. Genome-wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, Cinalli RM, Glasner ME, Enright AJ, Thomson JM, Baskerville S, Hammond SM, Bartel DP, Schier AF. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308(5723):833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- Gonzalez JR, Armengol L, Sole X, Guino E, Mercader JM, Estivill X, Moreno V. SNPassoc: an R package to perform whole genome association studies. Bioinformatics. 2007;23(5):644–645. doi: 10.1093/bioinformatics/btm025. [DOI] [PubMed] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27(1):91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Zhang Q, Liu Y, Pan X. Cloning and identification of novel microRNAs from rat hippocampus. Acta Biochim Biophys Sin (Shanghai) 2007;39(9):708–714. doi: 10.1111/j.1745-7270.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158(10):1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62(2):182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, Manolio TA. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106(23):9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Bruno AE. The Influence of 3'UTRs on MicroRNA Function Inferred from Human SNP Data. Comp Funct Genomics. 2011;2011 doi: 10.1155/2011/910769. (910769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, Covault J, Conner TS, Tennen H, Kranzler HR, Furneaux HM. A common polymorphism in serotonin receptor 1B mRNA moderates regulation by miR-96 and associates with aggressive human behaviors. Mol Psychiatry. 2009;14(4):381–389. doi: 10.1038/mp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem. 1999;274(37):26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- Ke X. Presence of multiple independent effects in risk loci of common complex human diseases. Am J Hum Genet. 2012;91(1):185–192. doi: 10.1016/j.ajhg.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. Major depression and generalised anxiety disorder. Same genes, (partly)different environments--revisited. Br J Psychiatry Suppl. 1996;30:68–75. [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder. Same genes, (partly) different environments? Arch Gen Psychiatry. 1992;49(9):716–722. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiezun A, Garimella K, Do R, Stitziel NO, Neale BM, McLaren PJ, Gupta N, Sklar P, Sullivan PF, Moran JL, Hultman CM, Lichtenstein P, Magnusson P, Lehner T, Shugart YY, Price AL, de Bakker PI, Purcell SM, Sunyaev SR. Exome sequencing and the genetic basis of complex traits. Nat Genet. 2012;44(6):623–630. doi: 10.1038/ng.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WH, Kim BS, Kim SK, Chang SM, Lee DW, Cho MJ, Bae JN. Restless legs syndrome in older people: a community-based study on its prevalence and association with major depressive disorder in older Korean adults. Int J Geriatr Psychiatry. 2012;27(6):565–572. doi: 10.1002/gps.2754. [DOI] [PubMed] [Google Scholar]
- Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438(7068):685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- Lee HB, Hening WA, Allen RP, Kalaydjian AE, Earley CJ, Eaton WW, Lyketsos CG. Restless legs syndrome is associated with DSM-IV major depressive disorder and panic disorder in the community. J Neuropsychiatry Clin Neurosci. 2008;20(1):101–105. doi: 10.1176/jnp.2008.20.1.101. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lu J, Clark AG. Impact of microRNA regulation on variation in human gene expression. Genome Res. 2012;22(7):1243–1254. doi: 10.1101/gr.132514.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MM, Buckenberger JA, Jiang J, Malana GE, Nuovo GJ, Chotani M, Feldman DS, Schmittgen TD, Elton TS. The human angiotensin II type 1 receptor +1166 A/C polymorphism attenuates microRNA-155 binding. J Biol Chem. 2007;282(33):24262–24269. doi: 10.1074/jbc.M701050200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9):1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Gordon SD, Medland SE, Statham DJ, Nelson EC, Heath AC, Martin NG, Wray NR. Genetic and environmental influences on the co-morbidity between depression, panic disorder, agoraphobia, and social phobia: a twin study. Depress Anxiety. 2009;26(11):1004–1011. doi: 10.1002/da.20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes R., Jr Comorbidity in generalized anxiety disorder. Psychiatr Clin North Am. 2001;24(1):41–55. doi: 10.1016/s0193-953x(05)70205-7. [DOI] [PubMed] [Google Scholar]
- Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2(12):e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Chan G, Arias A, Cubells JF, Farrer L, Kranzler HR. Reliability of DSM-IV diagnostic criteria using the semi-structured assessment for drug dependence and alcoholism (SSADDA) Drug Alcohol Depend. 2007;91(1):85–90. doi: 10.1016/j.drugalcdep.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, Farrer L, Kranzler HR. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA) Drug Alcohol Depend. 2005;80(3):303–312. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K, Lai CQ, Parnell LD, Lee YC, Ordovas JM. A genome-wide survey for SNPs altering microRNA seed sites identifies functional candidates in GWAS. BMC Genomics. 2011;12(504) doi: 10.1186/1471-2164-12-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K, Nettleton JA, Rotllan N, Tanaka T, Smith CE, Lai CQ, Parnell LD, Lee YC, Lahti J, Lemaitre RN, Manichaikul A, Keller M, Mikkila V, Ngwa J, van Rooij FJ, Ballentyne CM, Borecki IB, Cupples LA, Garcia M, Hofman A, Ferrucci L, Mozaffarian D, Perala MM, Raitakari O, Tracy RP, Arnett DK, Bandinelli S, Boerwinkle E, Eriksson JG, Franco OH, Kahonen M, Nalls M, Siscovick DS, Houston DK, Psaty BM, Viikari J, Witteman JC, Goodarzi MO, Lehtimaki T, Liu Y, Zillikens MC, Chen YD, Uitterlinden AG, Rotter JI, Fernandez-Hernando C, Ordovas JM. Gain-of-function lipoprotein lipase variant rs13702 modulates lipid traits through disruption of a microRNA-410 seed site. Am J Hum Genet. 2013;92(1):5–14. doi: 10.1016/j.ajhg.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MA, Neale MC, Pedersen NL, Mathe AA, Kendler KS. A twin study of generalized anxiety disorder and major depression. Psychol Med. 1995;25(5):1037–1049. doi: 10.1017/s0033291700037533. [DOI] [PubMed] [Google Scholar]
- Saunders MA, Liang H, Li WH. Human polymorphism at microRNAs and microRNA target sites. Proc Natl Acad Sci U S A. 2007;104(9):3300–3305. doi: 10.1073/pnas.0611347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TG, Detera-Wadleigh SD, Akula N, Gupta A, Kassem L, Steele J, Pearl J, Strohmaier J, Breuer R, Schwarz M, Propping P, Nothen MM, Cichon S, Schumacher J, Consortium NGIBD, Rietschel M, McMahon FJ. Two variants in Ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Mol Psychiatry. 2009;14(5):487–491. doi: 10.1038/mp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethupathy P, Borel C, Gagnebin M, Grant GR, Deutsch S, Elton TS, Hatzigeorgiou AG, Antonarakis SE. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3' untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am J Hum Genet. 2007;81(2):405–413. doi: 10.1086/519979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevim S, Dogu O, Kaleagasi H, Aral M, Metin O, Camdeviren H. Correlation of anxiety and depression symptoms in patients with restless legs syndrome: a population based survey. J Neurol Neurosurg Psychiatry. 2004;75(2):226–230. [PMC free article] [PubMed] [Google Scholar]
- Thomas LF, Saito T, Saetrom P. Inferring causative variants in microRNA target sites. Nucleic Acids Res. 2011;39(16):e109. doi: 10.1093/nar/gkr414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukel R, Polat A, Ozdemir O, Aksut D, Turksoy N. Comorbid conditions in obsessive-compulsive disorder. Compr Psychiatry. 2002;43(3):204–209. doi: 10.1053/comp.2002.32355. [DOI] [PubMed] [Google Scholar]
- Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7(7):545–552. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Winkelmann J, Czamara D, Schormair B, Knauf F, Schulte EC, Trenkwalder C, Dauvilliers Y, Polo O, Hogl B, Berger K, Fuhs A, Gross N, Stiasny-Kolster K, Oertel W, Bachmann CG, Paulus W, Xiong L, Montplaisir J, Rouleau GA, Fietze I, Vavrova J, Kemlink D, Sonka K, Nevsimalova S, Lin SC, Wszolek Z, Vilarino-Guell C, Farrer MJ, Gschliesser V, Frauscher B, Falkenstetter T, Poewe W, Allen RP, Earley CJ, Ondo WG, Le WD, Spieler D, Kaffe M, Zimprich A, Kettunen J, Perola M, Silander K, Cournu-Rebeix I, Francavilla M, Fontenille C, Fontaine B, Vodicka P, Prokisch H, Lichtner P, Peppard P, Faraco J, Mignot E, Gieger C, Illig T, Wichmann HE, Muller-Myhsok B, Meitinger T. Genome-wide association study identifies novel restless legs syndrome susceptibility loci on 2p14 and 16q12.1. PLoS Genet. 2011;7(7):e1002171. doi: 10.1371/journal.pgen.1002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J, Prager M, Lieb R, Pfister H, Spiegel B, Wittchen HU, Holsboer F, Trenkwalder C, Strohle A. "Anxietas tibiarum". Depression and anxiety disorders in patients with restless legs syndrome. J Neurol. 2005;252(1):67–71. doi: 10.1007/s00415-005-0604-7. [DOI] [PubMed] [Google Scholar]
- Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, Fulda S, Putz B, Eckstein G, Hauk S, Trenkwalder C, Zimprich A, Stiasny-Kolster K, Oertel W, Bachmann CG, Paulus W, Peglau I, Eisensehr I, Montplaisir J, Turecki G, Rouleau G, Gieger C, Illig T, Wichmann HE, Holsboer F, Muller-Myhsok B, Meitinger T. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39(8):1000–1006. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- Yang BZ, Zhao H, Kranzler HR, Gelernter J. Practical population group assignment with selected informative markers: characteristics and properties of Bayesian clustering via STRUCTURE. Genet Epidemiol. 2005;28(4):302–312. doi: 10.1002/gepi.20070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.