Abstract
Objectives
Intratumoral CD8+ lymphocytes (IT-CD8s) have shown promise as a prognostic indicator for Merkel cell carcinoma (MCC). We tested whether IT-CD8s predict survival among a population-based MCC cohort.
Methods
One hundred thirty-seven MCC cases that had not previously been analyzed for IT-CD8s were studied.
Results
Three-year MCC-specific survival rates were 56%, 72%, and 100% for patients with absent (n = 46), low (n = 85), and moderate or strong (n = 6) IT-CD8s, respectively. Increased IT-CD8s were associated with improved MCC-specific survival in a multivariate competing risk-regression analysis including stage, age, and sex (hazards ratio [HR] = 0.5; 95% confidence interval [CI] = 0.3-0.9). Although a similar trend was observed for overall survival, statistical significance was not reached (HR = 0.8; 95% CI = 0.6-1.0), likely because of the high rate of non-MCC deaths among older patients.
Conclusions
This study of prospectively captured MCC cases supports the concept that cellular immunity is important in MCC outcome and that CD8+ lymphocyte infiltration adds prognostic information to conventional staging.
Keywords: T cells, Merkel cell carcinoma, Survival
Merkel cell carcinoma (MCC) is a skin cancer with a reported incidence rate that has almost quadrupled in the past two decades in the United States1 and has an often-aggressive disease course (disease-associated mortality of 46%).2 Although recently adopted consensus staging guidelines2 have brought consistency to MCC staging and improved MCC prognostication, there remains a significant need for biomarkers to further refine MCC risk.
Patients with immune dysfunction such as human immunodeficiency virus (HIV)/AIDS (before effective antiviral therapy), solid organ transplantation and associated immunosuppressive regimens, and chronic lymphocytic leukemia have long been known to be at increased risk of developing MCC.3-5 In 2008, a virus (Merkel cell polyomavirus, MCPyV or MCV) was found to contribute to the etiology of most MCCs.6 It has subsequently been demonstrated that 80% of MCCs express viral oncoproteins7 and that many MCC cell lines require persistent viral oncoprotein expression.8 Furthermore, these nonhuman oncoproteins are recognized by the adaptive immune system in patients with MCC.9,10
Initially in unbiased microarray studies, and then through an independent validation cohort, we demonstrated that intratumoral infiltration by CD8α+ lymphocytes is associated with improved MCC-specific survival.11 Patients with the highest levels of CD8+ infiltration represent a minority of cases but enjoy outstanding disease-specific survival. This association is biologically plausible because CD8α+ lymphocytes (which may include both cytotoxic T cells and natural killer cells) are mediators of antiviral immune responses. CD8+ lymphocyte infiltration is a standard immunohistochemistry assay that may be performed by most pathology laboratories and has appeal both as a prognostic marker and as a potential determinant of which patients may benefit from immunotherapies. However, our initial validation cohort of patients was limited in that it represented patients from a tertiary referral center and, as such, was biased toward patients who are more likely to seek expert consultation (younger and/or with more complicated or advanced disease). We therefore explored CD8+ infiltration and survival in an independent sample that more closely represented the MCC general population.
Kaiser Permanente Northern California (KPNC) is a large, integrated nonprofit health care delivery system caring for more than 3 million persons who are broadly representative of the local and statewide population. Using data from KPNC health plan databases combined with tissue microarray immunohistochemistry findings, we tested the hypothesis that increased CD8+ lymphocyte infiltration is an independent predictor of improved MCC-specific survival.
Materials and Methods
Patient and Data Characteristics
All studies were approved by the relevant institutional review board (IRB; Fred Hutchinson Cancer Research Center IRB#6585; KPNC IRB# CN-09MAsga-03-H) and performed in accordance with Helsinki principles. All patients receiving care at KPNC who were diagnosed with MCC between the years of 1995 to 2009 were considered for inclusion. A waiver of consent was obtained from the KPNC IRB for patients with MCC. A total of 137 patients were included based on the following criteria: MCC diagnosis as assessed by two pathologists, known age at diagnosis, known sex, known diagnosis date, known local-regional-distant stage, presence of follow-up, and availability of adequate formalin-fixed, paraffin-embedded pathology tissues for CD8+ immunohistochemical study. A single patient was identified whose results overlapped with our prior CD8+ infiltration report11; results were unchanged with this patient excluded.
CD8 Immunohistochemistry
Formalin-fixed, paraffin-embedded sections (5- μm) were used. CD8α immunohistochemistry was performed using previously reported methodologies.11 In brief, we used antibody 4B11 (Novocastra, Bannockburn, IL) at 1:200 dilution with heat-induced epitope retrieval and unmasking with pH 8 buffer. Intratumoral CD8+ (IT-CD8+) infiltration was scored semiquantitatively using six bins (scores ranged from 0-5) by a single pathologist who was blinded to patient information or outcome. Although the current study was carried out by only one pathologist, a previous study using the same scoring system tested reproducibility between scorers in a blinded fashion and found them to be reproducible. The observed agreement was 85% and calculated k was 0.65, consistent with substantial agreement between observers.11 A score of 0 to 5 represented average infiltration into the tumor taken as a whole, as opposed to only the densest region of intratumoral infiltration. Only CD8 cells that had infiltrated into the tumor and were not directly in contact with a vessel were counted. Effort was made to avoid counting areas with necrosis to minimize false or nonspecific reactions. Ideally, 8 to 10 representative fields of tumor would be assessed when possible. To provide a more quantitative assessment of these six levels of infiltration, we previously11 determined the approximate numbers of CD8+ cells per mm2 that corresponded to the 0 to 5 scores, representative examples of which are shown in Image 1. Although microscopes vary, a typical high-powered field (×10 ocular and ×40 objective) is 0.15 mm2, meaning there are approximately 7 high-power fields (hpf) per mm2. Our prior attempt11 to quantitate the number of CD8+ cells in each semiquantitative category yielded the following breakdown: score 0: 0 cells/mm2, score 1: 1 to 179 cells/mm2, score 2: 180 to 433 cells/mm2, score 3: 434 to 582 cells/mm2, score 4: 583 to 731 cells/mm2, and score 5: more than or equal to 732 cells/mm2.
Image 1.
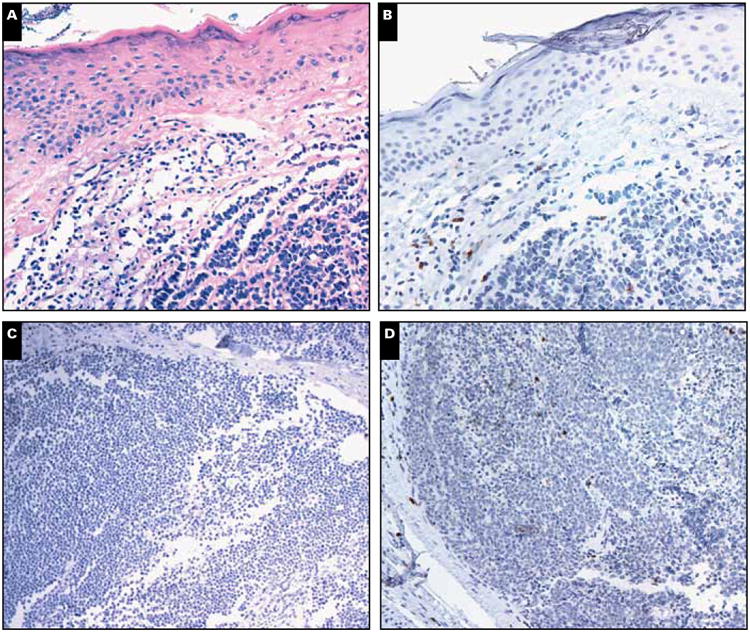
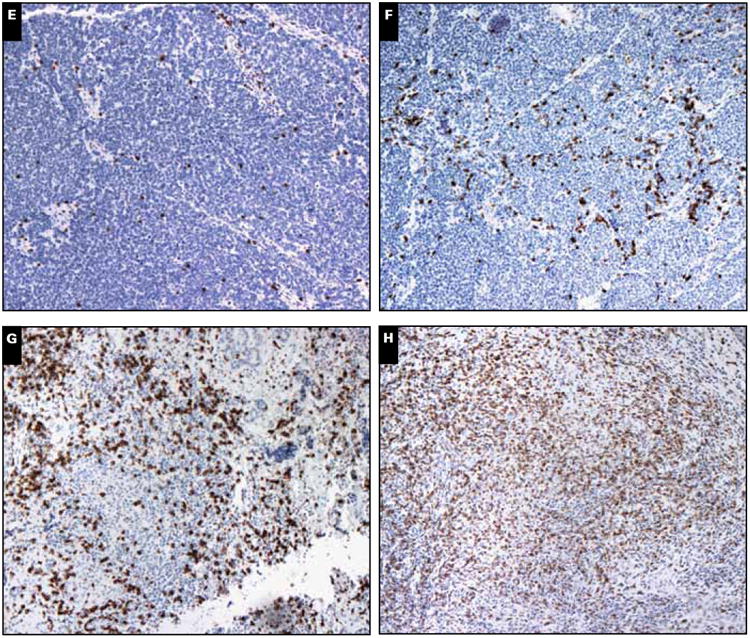
Merkel cell tumor (MCC) tumor scores for intratumoral (IT) CD8+ cells. A, H&E staining of MCC tumor. B, Anti-CD8 staining of the same tumor11 demonstrating peritumoral and IT-CD8+ cells: IT-CD8+ cells must be surrounded by tumor cells and not have direct contact with stroma. MCC tumors showing varying scores of IT-CD8+ cell infiltration per high-power field (hpf) scored semiquantitatively as follows: score 0: 0 cells/mm2 per hpf (C), score 1: approximately 1-179 cells/mm2 per hpf (D), score 2: approximately 180-433 cells/mm2 per hpf.
(E), score 3: approximately 434-582 cells/mm2 per hpf (F), score 4: approximately 583-731 cells/mm2 per hpf (G) and score 5: ≥732 cells/mm2 per hpf (H).
Statistical Analysis
All statistical analyses were performed on Stata software version 11.0 for Macintosh (StataCorp, College Station, TX). A P value of .05 was considered to be statistically significant. Univariate and multivariate analyses were performed. All multivariate analyses included age at diagnosis, sex, and local-regional-distant stage in addition to degree of CD8 infiltration and used robust standard errors. For cause-specific survival analyses, competing risks regression was performed with deaths from MCC (n = 36) or probable MCC (n = 10) considered to be events and deaths from known non-MCC causes (n = 37) considered to be competing events. Patients who were alive at last follow-up were censored on their last day of follow-up (n = 38; median follow-up among this group, 7 years) and patients who died of unknown causes (n = 16) were censored on their day of death. For overall survival analyses, Cox regression was performed; deaths from any cause (including unknown) were considered to be events. In both the competing risk regression (disease-specific survival [DSS]) and standard (overall survival [OS]) Cox models, the semiquantitative CD8 score (which ranges from 0-5) was treated as a continuous variable. In this case, the hazard ratio (HR) measures each single point increase (eg, from 0 to 1, or from 1 to 2). An HR in which the 95% confidence interval (CI) did not cross 1.0 and the P value was less than .05 was considered to be statistically significant. For the purposes of survival data visualization, Kaplan-Meier graphics were created; groupings of absent (CD8 infiltration score of 0), low infiltration (score of 1 or 2), or moderate-strong infiltration (score of 3-5) were selected a priori.
Results
Patient and Tumor Characteristics
A total of 137 patients were included, with a median follow-up of 2.3 years (7.0 years among patients alive at last contact date) and a total follow-up of 493 years. Of these, 87 (63.5%) were male and 50 (36.5%) female. Age at diagnosis ranged from 31 to 96 years, with a median and mean age of 78 and 75 years, respectively. These demographics are similar to those of recently reported US national registry data (61.5% male, mean age 76 years for women and 74 years for men).1 Of these patients, 85 (62%) had local disease, 41 (30%) had regional/nodal disease, and 11 (8%) had distant metastases. As is typical for MCC, 18 (13%) patients had nodal or distant MCC spread but no identifiable skin primary site.
CD8+ Lymphocyte Infiltration
Tumors from 46 patients (34%) lacked appreciable CD8+ lymphocyte infiltration. Among the 66% of patients with CD8+ infiltration, most had low (n = 77) or low-moderate (n = 8) infiltration and only a few (n = 6) had robust infiltration with scores of moderate or stronger. A breakdown of patients by individual score is presented in Table 1.
Table 1. One- and Three-Year Disease-Specific Survival (DSS) for Each CD8 Score Group.
| CD8 Intratumoral Semiquantitative Score | No. of Patients in the Bin | 1-y DSS | 3-y DSS |
|---|---|---|---|
| 0 | 46 | 77 | 56 |
| 1 | 77 | 90 | 73 |
| 2 | 8 | 100 | 64 |
| 3 | 3 | 100 | 100 |
| 4 | 2 | 100 | 100 |
| 5 | 1 | Not calculablea | Not calculablea |
Patient was alive at last follow-up at 259 days.
DSS
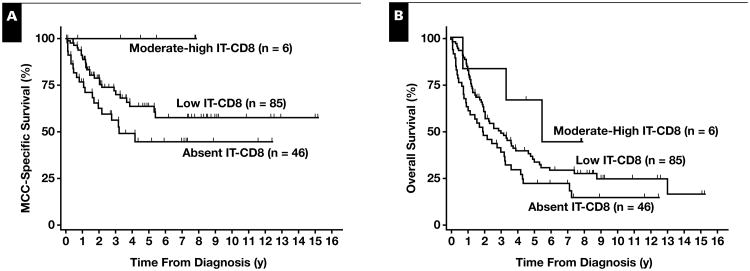
Each 1-point increase of CD8+ infiltration on the 0-5 point IT-CD8 scale was associated with significantly improved MCC-specific survival in a univariate model (HR = 0.6 per increase; P = .02) Table 2. Also significant in the univariate model were regional stage (vs local stage) and distant stage (vs local stage). Age and sex were not predictive of MCC-specific survival. MCC-specific 3-year survival rates among patients with absent (score = 0), low (score = 1-2), and moderate-strong CD8+ lymphocyte infiltration (score = 3-5) were 56%, 72%, and 100%, respectively Figure 1A (Table 1). In the multivariate model including stage, age, and sex in addition to IT-CD8, degree of IT-CD8+ lymphocyte infiltration remained a significant predictor of MCC-specific survival (HR = 0.5 per increase; P = .01), as did stage (Table 2).
Table 2. MCC-Specific Survivala.
| Univariate | Multivariateb | |||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age at diagnosis (per year increase) | 1.0 (1.0-1.0) | .57 | 1.0 (1.0-1.0) | .53 |
| Female sex (vs male) | 1.1 (0.6-2.0) | .76 | 1.2 (0.6-2.2) | .62 |
| Stage at diagnosis | ||||
| Regional (vs local) | 2.4 (1.3-4.5) | <.01 | 2.8 (1.4-5.5) | <.01 |
| Distant (vs local) | 3.5 (1.2-10.4) | .02 | 4.7 (1.5-14.4) | .01 |
| CD8 infiltration (per increase on 0-5 scale) | 0.6 (0.4-0.9) | .02 | 0.5 (0.3-0.9) | .01 |
CI, confidence interval; HR, hazard ratio; MCC, Merkel cell carcinoma.
Results of competing risks Cox regression (deaths from MCC or probable MCC were considered events, deaths from non-MCC causes competing events; n = 137). P < .05 was considered statistically significant.
Multivariate analysis considered all listed variables.
Figure 1.
CD8+ lymphocyte infiltration and Merkel cell carcinoma (MCC) survival. A, MCC-specific survival rates using Kaplan-Meier curves for patients with absent, low, and moderate-strong intratumoral CD8+ infiltration, respectively, were 77%, 91%, 100% at 1 year; 56%, 72%, 100% at 3 years; and 45%, 64%, 100% at 5 years. B, Overall survival rates for patients with absent, low, and moderate-strong intratumoral CD8+ infiltration, respectively, were 63%, 84%, 83% at 1 year; 39%, 49%, 83% at 3 years; and 22%, 34%, 67% at 5 years. Data are shown for purposes of visualization. (See Tables 2 and 3 for univariate and multivariate Cox regression statistical analysis.)
OS
In the univariate model, each 1-point increase in CD8+ infiltration was associated with improved survival (HR = 0.8; P = .07) Table 3; however, this did not reach statistical significance. Three-year OS rates were 39%, 49%, and 83% among patients with absent, low, and moderate-strong CD8+ lymphocyte infiltration, respectively Figure 1B. Age and distant disease were found to be significant predictors of OS, whereas sex and regional stage (vs local stage) were not. In the multivariate analysis, although lower stage and stronger IT-CD8 were associated with better survival, only age was found to be a significant predictor of OS (Table 3). This may reflect the increased rate of non-MCC death among older patients (3-year non-MCC death rate of 14% among patients younger than the median age at diagnosis of 78 years vs 38% non-MCC death rate among patients aged ≥78 years).
Table 3. Overall Survival (n = 137).
| Univariate | Multivariateb | |||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age at diagnosis (per year increase) | 1.03 (1.0-1.1) | <.01 | 1.03 (1.0-1.1) | .01 |
| Female sex (vs male) | 0.7 (0.5-1.1) | .14 | 0.8 (0.5-1.2) | .19 |
| Stage at diagnosis | ||||
| Regional (vs local) | 1.2 (0.8-1.9) | .42 | 1.5 (0.9-2.4) | .14 |
| Distant (vs local) | 2.1 (1.1-4.3) | .03 | 1.7 (0.8-3.8) | .16 |
| CD8 infiltration (per increase on 0-5 scale) | 0.8 (0.6-1.0) | .07 | 0.7 (0.5-1.0) | .08 |
CI, confidence interval; HR, hazard ratio.
P < .05 was considered statistically significant.
Multivariate analysis included all listed variables.
Discussion
MCC is a virus-associated malignancy of increasing clinical impact, and there remains a need for biomarkers to improve prognostication. Here we report that in a population representative cohort of 137 patients with MCC, IT-CD8+ infiltration is an independent predictor of MCC-specific survival and that a greater degree of IT-CD8+ infiltration portends better prognosis.
Two independent US cohorts of patients with MCC (including the present study) used the same CD8 scoring methods and demonstrated an association between CD8+ lymphocyte infiltration and improved MCC-specific survival.11 An additional population-based cohort study from Finland using a different approach further supports an independent prognostic role for IT-CD8+ as well as CD3+ lymphocytes in MCC.12 Classic H&E evaluation of tumor-infiltrating lymphocytes (TILs) has previously shown univariate but not independent significance11,13; these studies are limited by technical difficulties in differentiating small, round, blue tumor cells from small, round, blue lymphocytes, particularly in the tumor field. We therefore suggest that immunohistochemical evaluation of cytotoxic intratumoral T-cell infiltration is a more sensitive means to assess the antitumor response.
There is no consensus in the literature for how TIL scoring should best be performed, in part because there are many variables that must be considered, such as the tumor type, the stain used, the region that is assessed (tumor vs stroma vs both), etc. However, a few patterns have emerged in the recent TIL literature such as less prognostic significance of TIL infiltration into stromal or peritumoral regions than for TIL infiltration into the tumor itself.14,15 Nevertheless, the extent and quality of intratumoral T-cell infiltration has been associated with prognosis in multiple other cancers including colorectal, breast, ovarian, and endometrial carcinomas.16-23 Studies have demonstrated that colorectal cancer prognosis is influenced by the presence of memory effector T cells in the stroma, within the invasive front and in the parenchyma.16,24 Immune infiltration of hepatic metastasis from colorectal cancer was also associated with better prognosis.25 In addition, several studies have shown that cancer prognosis is improved by a Th1 (type 1 adaptive) response of the tumor-infiltrating cells14,26 whereas a Th2 response tends to be detrimental.27,28 Seo et al29 noted that CD8+ TILs independently predict treatment response to systemic therapy in breast cancer (n = 153). A high ratio of IT-CD8 T cells to T-regulatory cells (Tregs) was an independent prognostic factor for both improved disease-specific survival (P = .001) and overall survival (P < .0001) in hepatocellular carcinoma30 and had a favorable prognosis in ovarian cancer.17 Indeed, these findings have led to the proposal for an immune-based parameter (quantifying the extent of cytotoxic and memory T cells at the core and invasive margin of tumors) to be added to tumor staging.31 We suggest that MCC may also benefit from the addition of an immune score to the standard staging algorithm, and look forward to further developments in these areas.
Our findings, combined with the presence of oncogenic viral proteins in most MCCs, and prior epidemiologic links to immune dysfunction,3-5 suggest a critical role for cytotoxic T-cell immunity in controlling progression of this cancer. T-cell immune-stimulating therapies will soon be explored in MCC, and evaluation of lymphocyte infiltration may help to determine which patients could benefit most from such treatments. Identifying the underlying CD8+ lymphocyte evasion strategies used by this virus-driven cancer holds exciting potential for both scientific and clinical impact.
Acknowledgments
We thank Eric Chang and Greg Bischak for database support and Farinaz Shokri and Elizabeth Donato for technical support.
This study was funded by grants NIH-RC2CA147820 (P.N.), NIH-K24-CA139052 (P.N.), and NIH-F30ES017385 (K.P.) from the National Institutes of Health, Bethesda, MD; RSG-08-115-01-CCE from the American Cancer Society (P.N.); Cora May Poncin Foundation (K.P.); MCC Patient Gift Fund; David & Rosalind Bloom Fund; and Michael Piepkorn Endowment.
References
- 1.Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2009;37:20–27. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 2.Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63:751–761. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engels EA, Frisch M, Goedert JJ, et al. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- 4.Penn I, First MR. Merkel's cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717–1721. doi: 10.1097/00007890-199912150-00015. [DOI] [PubMed] [Google Scholar]
- 5.Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–381. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuda M, Arora R, Kwun HJ, et al. Human Merkel cell polyomavirus infection I: MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. 2009;125:1243–1249. doi: 10.1002/ijc.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houben R, Shuda M, Weinkam R, et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to Merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70:8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer JG, Afanasiev OK, McClurkan C, et al. Merkel cell polyomavirus-specific CD8+ and CD4+ T-cell responses identified in Merkel cell carcinomas and blood. Clin Cancer Res. 2011;17:6671–6680. doi: 10.1158/1078-0432.CCR-11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulson KG, Iyer JG, Tegeder AR, et al. Transcriptome-wide studies of Merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011;29:1539–1546. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sihto H, Bohling T, Kavola H, et al. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res. 2012;18:2872–2881. doi: 10.1158/1078-0432.CCR-11-3020. [DOI] [PubMed] [Google Scholar]
- 13.Andea AA, Coit DG, Amin B, et al. Merkel cell carcinoma: histologic features and prognosis. Cancer. 2008;113:2549–2558. doi: 10.1002/cncr.23874. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher K, Haensch W, Roefzaad C, et al. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 15.Fridman WH, Galon J, Pages F, et al. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71:5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 16.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 17.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 19.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 21.Matkowski R, Gisterek I, Halon A, et al. The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer Res. 2009;29:2445–2451. [PubMed] [Google Scholar]
- 22.de Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–110. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Townsend KN, Spowart JE, Huwait H, et al. Markers of T cell infiltration and function associate with favorable outcome in vascularized high-grade serous ovarian carcinoma. PloS One. 2013;8:e82406. doi: 10.1371/journal.pone.0082406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 25.Halama N, Michel S, Kloor M, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 26.Simonson WTN, Allison KH. Tumour-infiltrating lymphocytes in cancer: implications for the diagnostic pathologist. Diagnos Histopathol. 2011;17:80–90. [Google Scholar]
- 27.De Monte L, Reni M, Tassi E, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Divella R, Daniele A, Savino E, et al. Circulating levels of transforming growth factor-betaeta (TGF-beta) and chemokine (C-X-C motif) ligand-1 (CXCL1) as predictors of distant seeding of circulating tumor cells in patients with metastatic breast cancer. Anticancer Res. 2013;33:1491–1497. [PubMed] [Google Scholar]
- 29.Seo AN, Lee HJ, Kim EJ, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. 2013;109:2705–2713. doi: 10.1038/bjc.2013.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 31.Galon J, Pages F, Marincola FM, et al. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]