Abstract
Background
The age-association of cardiovascular disease (CVD) may be partially because its metabolic risk factors tend to rise with age. Few studies have analyzed age-associations of multiple metabolic risks in the same population, especially in nationally representative samples. We examined worldwide variations in the age associations of systolic blood pressure (SBP), total cholesterol (TC), and fasting plasma glucose (FPG).
Methods and Results
We used individual records from 83 nationally or sub-nationally representative health examination surveys in 52 countries to fit a linear model to risk factor data between ages 30-64 years for SBP and FPG, and between 30-54 years for TC. We report the cross-country variation of the slope and intercept of this relationship. We also assessed non-linear associations in older ages.
Between 30 and 64 years of age, SBP increased by 1.7-11.6 mmHg per ten years of age and FPG increased by 0.8-20.4 mg/dL per ten years of age in different countries and in the two sexes. Between 30 and 54 years of age, TC increased by 0.2-22.4 mg/dL per ten years of age in different surveys and in the two sexes. For all risk factors and in most countries, risk factor levels rose more steeply among women than among men, especially for TC. On average, there was a flattening of age-SBP relationship in older ages; TC and FPG age associations reversed in older ages, leading to lower levels in older ages than in middle ages.
Conclusions
The rise with age of major metabolic CVD risk factors varies substantially across populations, especially for FPG and TC. TC rises more steeply in high-income countries and FPG in the Oceania countries, the Middle East, and the US. The SBP age association had no specific income or geographical pattern.
Keywords: Aging, systolic blood pressure, serum total cholesterol, fasting plasma glucose, Global health
Introduction
Cardiovascular disease (CVD) rates tend to rise with age in most populations, although the age-association of CVD varies across countries and has changed overtime 1-3. It is believed that this association is partly because major CVD risk factors including blood pressure, cholesterol, and diabetes rise with age, and possibly due to the accumulation of risk factor effects over life course 1. Despite this commonly-held view, some studies have found that populations with low CVD risk, e.g. some rural populations in developing countries, have attenuated age gradients of blood pressure and cholesterol compared to urban and industrialized populations, attributed partly to lower consumption of salt and animal products and lower body mass index (BMI) 4-11. Most prior studies focused on a single risk factor and, with the notable exception of the Intersalt study, were in one or a small number of populations. To our knowledge, there is no quantitative analysis of the age association of multiple CVD risk factors across nationally representative samples from countries in different regions and at different stages of economic development.
We used health examination surveys in populations worldwide to conduct consistent cross-population analyses of the age associations of blood pressure, cholesterol, and glucose. We examined the age associations in young-middle ages as well as how it changes in older ages. Following the analyses of Intersalt data 10, we also examined whether the age association slopes are correlated with average population risk factor levels. Finally, we examined whether all risks rise more or less steeply in the same populations, and whether the slopes are associated with national income.
Methods
Our analysis was on systolic blood pressure (SBP), serum total cholesterol (TC) and fasting plasma glucose (FPG) because these factors were measured in a larger number of surveys than other markers of risk such as LDL cholesterol or postprandial glucose.
Data sources
The data for this analysis were de-identified individual records in 83 health examination surveys in 52 countries that all covered ages 30-64 with measured SBP, TC, and FPG. The details of the surveys included in the analysis are provided in Table S1. All surveys had data on men and women. Fifty-four surveys were national and the other 29 covered large sub-national regions (e.g. nine provinces in China). Of the latter group 16 were from England and Scotland and were designed with all the features of a national survey; these surveys are labeled as subnational in Table S1 because we considered United Kingdom as the parent country. We excluded 6 other surveys for reasons detailed in Table S1. All surveys had data on SBP, 32 on TC, and 22 on FPG. Fifty-two surveys were from low-and middle-income countries and 31 from high-income countries.
Statistical analysis
We dropped 0.7% of participant for SBP, 0.3% for TC, and 0.6% for FPG because they did not have information on age. When SBP was measured more than once (all but one survey), we dropped the first SBP measurement because it was significantly higher than subsequent ones, and used the average of the other measurements as each subject's SBP. All analyses were done separately for men and women.
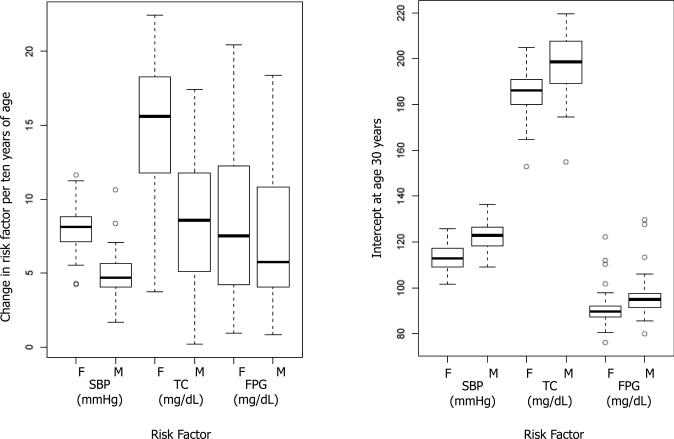
For each survey and risk factor, we estimated the slope and intercept of the linear relationship between risk factor and age by fitting a linear regression model to individual records of participants who were between 30 and 64 years old for SBP and FPG, and between 30 and 54 years old for TC. All analyses used survey sample weights when available. Hereafter we refer to the coefficient of age in this linear regression as “slope” and to the regression constant as “intercept”. We used 30 years as the start age because some surveys did not have measurement below this age and there was indication that there may also be non-linearity below 30 years, making earlier ages incomparable across surveys. We restricted the analysis to 64 and 54 years because the slope may significantly change in older ages, making the linear model inappropriate; exploratory analyses showed that slopes changed at a younger age for TC than for SBP and FPG. We examined the distributions of the slopes and intercepts across populations and report their descriptive statistics (Figure 1).
Figure 1.
Distributions of age-association slopes and intercepts of SBP, TC, and FPG. Each box plot shows distributions for all analyzed surveys.
In each survey, we also calculated mean risk factor levels in 5-year age groups, i.e. 30-34, 35-39, ..., 85+ years and used these mean levels to calculate an age-standardized mean for men and women in each survey for ages 30 to 64 for SBP and FPG and 30 to 54 for TC; we used the world population in 2000 as the standard population. Thus, we created a dataset in which each observation was one survey- sex and the variables were the slope and the intercept of the age association and the age-standardized mean for the same age range. We fitted a simple linear regression, separately for each sex, with the slope as the dependent and the age-standardized mean as the independent variable. The dependent variable in this regression is a measure of how steep/shallow the age association is; the independent variable measures how low/high the risk factor level is in the population across ages. A previous analysis of Intersalt had used sample median risk factor level instead of the age-standardized mean 10. We did not use sample median because the survey populations had different age distributions, which could affect the comparability of the median. We also considered regression models that included whether a country was high- vs. low-and-middle-income, but did not include that variable in the final model because its coefficients were not statistically significant at the 0.05 level. We conducted a similar analysis with the intercept (at age 30 years) as the dependant variable. Both regressions included a country-level random intercept to account for repeated surveys in the same countries.
Because there is a simple linear relationship between the intercept and slope of age-association and the age-standardized mean, at a constant age-standardized mean any change in the coefficient of slope is accompanied by a change in the opposite direction in the coefficient of intercept and vice versa. At the extreme, if change in age-standardized mean is only due to a change in intercept (i.e. shifting the whole line up/down), then the coefficient of regression of slope on mean would on average be 0 and the coefficient of regression of intercept on mean would be 1.0. On the other hand, if the change in age-standardized mean is only due to a shift in slope (rotating the regression line around the same intercept), the coefficient of regression of the intercept on mean would be 0 and that of slope would be or approximately 0.06.
To analyze risk factors patterns in older ages, we used surveys that had data above 65 years of age: 38 surveys for SBP; 17 for TC; and 8 for FPG (Table S1). We divided the surveys into tertiles of slope for SBP and to those below and above median-slope for TC and FPG. In each tertile or median group, we estimated mean risk factor level for 5-year age groups across all surveys, adjusting for differences across surveys using a fixed-effects regression model.
All analyses were done using STATA 10 (StataCorp, Texas, USA)
Results
The age associations of SBP, TC, and FPG
The distributions of slopes and intercepts for SBP, TC and FPG are shown in Figure 1. Slopes varied across surveys, with TC and FPG having larger inter-quartile ratio (IQR) than SBP (Figure 1a). The intercepts at age 30 varied less than the slopes, with intercept IQR ranging from 30% of that of slope for male TC to 86% for female SBP (Figure 1b).
The median slope for SBP was 4.7 and 8.1 mmHg per ten years of age for men and women, respectively (Figure 1). At the low end, SBP increased 1.7-2.1 mmHg per ten years of age for men in surveys such as Cape Verde 2007, Tonga 2001, Tokelau 2005, and India 2007. At the high-end, the slope was above 11 mmHg per ten years of age for women in Nauru 2004 and Sao Tome et Principe 2009 (Table S2). The lowest slope in our analysis (1.7 mmHg per ten years) was about 2-3 times as large as the lowest Intersalt slopes and the highest slope in our analysis was about 10% lower than the highest Intersalt slope 10. Median intercept at age 30 for men was 123.1mmHg, ranging from below 115 mmHg to above 131 mmHg in Sao Tome et Principe 2009, South Africa SAGE 2009 and Cape Verde 2007 (Table S2). Women had a lower median intercept at 30 years of age (113.1 mmHg), ranging from below 105 mmHg in a number of countries in Asia and the Pacific to above 125 mmHg in Niger 2007 (Table S2). SBP slopes and intercepts in high-income and low-and-middle-income countries were generally similar (Table 1 and Figure 2).
Table 1.
Mean (SD) slopes and intercepts by national income.
| Low-and-middle-income | High-income | |||
|---|---|---|---|---|
| Slope of risk factor age association (per decade of age) |
||||
| Male | Female | Male | Female | |
| SBP (mmHg)* | 4.8 (1.6) | 8.0(1.6) | 4.8 (1.0) | 8.1 (1.0) |
| TC (mg/dL)† | 5.7 (3.8) | 12.0 (3.7) | 9.6 (4.9) | 16.3 (4.9) |
| FPG (mg/dL)‡ | 8.6 (5.3) | 11.6 (6.0) | 5.4 (1.8) | 5.5 (2.4) |
| Intercept at age 30 of risk factor age association |
||||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| SBP (mmHg)* | 121.7 (6.0) | 113.6 (5.4) | 123.9 (4.5) | 112.7 (4.7) |
| TC (mg/dL)† | 186.4 (14.4) | 180.6 (10.7) | 202.9 (9.1) | 186.2 (9.4) |
| FPG (mg/dL)‡ | 100.5 (15.3) | 94.7 (13.7) | 93.9 (2.8) | 89.7 (1.6) |
Systolic blood pressure
Total cholesterol
Fasting plasma glucose
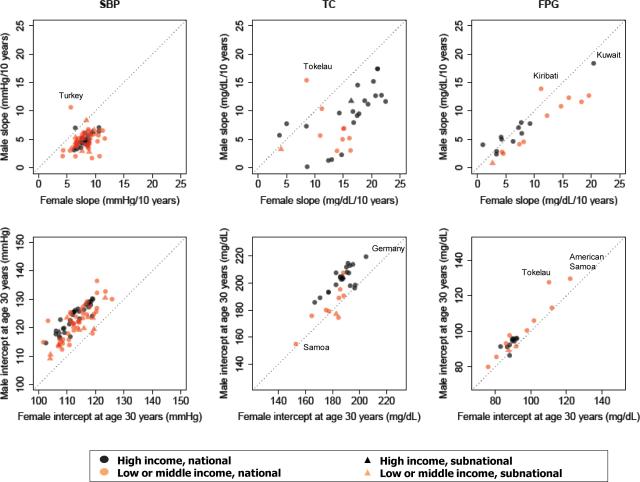
Figure 2.
Age-association slopes and intercepts of SBP, TC, and FPG for males vs. females. Each data point represents an analyzed survey. The outliers and extremes are identified. See Table S2 for numerical information for all surveys.
Median FPG slope was slightly higher among women than men (7.7 vs. 6.1 mg/dL per ten years of age); however, men had higher median intercept at age 30 than women (95.2 vs. 89.8mg/dL) (Table 1 and Figure 2). Men and/or women in a number of surveys had relatively shallow rise in FPG, below 3 mg/dL per ten years of age in Algeria 2003, Colombia NHS 2007, England HSE 2003, Korea NHANES 2001, Thailand NHES I 1991 and United States (US) NHANES II 1976-1980 (Table S2). The steepest rise in FPG per ten years of age, those above 15 mg/dL (and as high as 20.4 mg/dL), were in men and women in Kuwait 2006, American Samoa 2004, Tokelau 2005, and Nauru 2004. Aside from populations in Oceania, the next largest FPG slopes were in later US surveys of NHANES 2005-2006 for women (9.2 mg/dL/10 years) and NHANES 2003-2004 for men (8.0 mg/dL/10 years). Intercepts ranged from below 85 mg/dL for both men and women in Colombia 2007 and Nauru 2004 to above 110 mmHg for men and women in Kiribati 2004, Tokelau 2005, and American Samoa 2004. FPG slopes as well as intercepts were higher in low-and-middle-income countries than in high-income ones (Table 1 and Table S2); the differences were not statistically significant, possibly due to the relatively small number of surveys with FPG data.
The age associations for TC had two salient features: First, the TC slopes were markedly higher in women than in men (Figure 1a), with medians being 15.1 mg/dL and 7.8 mg/dL per ten years of age, respectively. In contrast, median intercept at age 30 of 185.9 mg/dL in women was lower than the 198.6mg/dL median among men. Second, TC generally was higher at age 30 and rose more steeply with age in high-income populations than in low-and-middle-income countries (Figure 2 and Table 1). Despite this general pattern, some recent surveys in high-income countries also had relatively low slope. For example, the steepest slopes were 16.8-22.5 mg/dL per ten years of age for men in England HSE 1993/4 and Ireland SLAN 1998 and 21.7-22.4 mg/dL for women in England HSE 1993/4 and US NHANES II. Men in Kuwait 2006, Nauru 2004 and Korea NHANES 1998, 2001, 2005 had relatively little rise in TC with age. The smallest intercepts at age 30 for men and women were in Samoa (below 155 mg/dL) while the largest, above 200 mg/dL for women and above 215 mg/dL for men were in Germany BGS 1998. TC slope and intercept were correlated with per-capita availability of animal fats in the food supply (using data from the food balance sheets of the Food and Agriculture Organization of the United Nations), with correlation coefficients ranging 0.47-0.74 for intercept and slope in the two sexes.
Although risk factor slopes and intercepts differed between men and women, male and female values were moderately correlated across countries, with correlation coefficients ranging from 0.29 for SBP to 0.91 for FPG for slopes and from 0.79 for SBP to 0.95 for FPG for intercepts (Figure 2). There was little correlation among the slopes and intercepts for different risk factors (pairwise correlations ranged -0.26 to 0.36 ), i.e. at least some of the determinants of each risk factor vary across populations independently of those of other risk factors.
An important finding in some of the countries with multiple survey rounds over long periods was that age associations of risk factors have changed over time. For example, the age associations of SBP and TC became shallower over multiple rounds of US NHANES and England HSE 12, while that of FPG became substantially steeper over multiple NHANES rounds (Table S2).
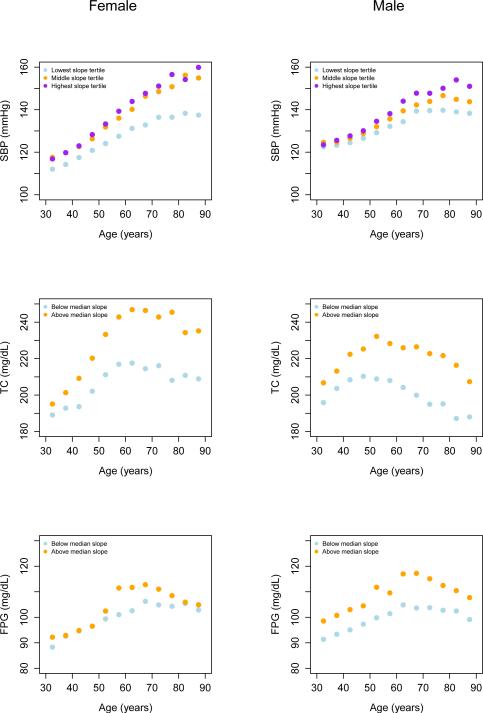
Figure 3 shows that on average there was a flattening of age-SBP relationship around age 70 years, especially among men. FPG and TC age associations reversed in older ages (~55-60 for TC and 60-65 for FPG), leading to lower levels in older ages than in middle ages. The FPG reversal was more modest for those surveys with below-median slope. In secondary analyses, we tested this flattening or reversal of age-association at older ages in individual surveys by fitting a series of 2-peice linear regressions to those surveys that had data above 65 years. This analysis showed that SBP continued to rise with age beyond 65 years in some surveys while the rise became more attenuated or stopped in others. TC and FPG either increased at a slower rate or began to decline in older ages in most surveys.
Figure 3.
Average risk factor level by age in groups of surveys. Survey groups were formed based on whether slope between 30 and 64 years for SBP and FPG (30-54 years for TC) was in the lowest, middle, or highest tertile (SBP) or below/above median (TC and FPG).
The relationship between slope and average risk factor level in the population
The strongest relationship between slope and age-standardized mean was seen for FPG with the regression coefficient being 0.21 for each decade of life (95% CI 0.12, 0.29) for men and 0.28 (95% CI 0.17, 0.39) for women (Table 2). In other words, populations that had 10 mg/dL higher age-standardized mean FPG were those in which FPG rose by an additional 2.1 and 2.8 mg/dL for each decade of age for men and women, respectively. Mirroring this, the coefficients of the regression of intercept at 30 on age-standardized mean FPG were 0.72 (95% CI 0.60, 0.83) for men and 0.64 (95% CI 0.49, 0.80) for women, which was smaller than those of SBP and TC, (Table 2). Together, these results indicate that differences in slope account for about one third to one half of the variations in age-standardized mean FPG across populations. For example, a population with an age-standardized mean FPG of about 150 mg/dL (about the same level as American Samoa in 2004) vs. one that had average FPG of 95 mg/dL (about the same level as England in 2003) would have an additional 1.2-1.5 mg/dL rise in FPG for each year of age, or 42-53 mg/dL over 35 years. Since intercept is also higher, by age 65, the latter group would have a mean FPG that is higher by 80-88 mg/dL (Figure 2). SBP slope had the weakest association with age-standardized mean SBP, with a regression coefficient of 0.09 (95% CI 0.04, 0.15) for each decade of life for men and 0.09 ( 0.04, 0.14) for women. Consequently, SBP intercept had the largest association with its age-standardized mean among the three risk factors, with regression coefficients being 0.86 for women and men (Table 2) 10.
Table 2.
Coefficients of the regression of the risk factor age-association slope/intercept on age-standardized means.
| Slope (per decade of life) of risk factor age association |
||||
|---|---|---|---|---|
| Male | Female | |||
| Coefficient (95% CI) | P | Coefficient (95% CI) | p | |
| SBP (mmHg)* | 0.09 (0.04, 0.15) | 0.002 | 0.09 (0.04, 0.14) | 0.001 |
| TC (mg/dL)† | 0.10 (0.01, 0.20) | 0.048 | 0.19 (0.06, 0.32) | 0.005 |
| FPG (mg/dL)‡ | 0.21 (0.12, 0.29) | <0.001 | 0.28 (0.17, 0.39) | <0.001 |
| Intercept at age 30 of risk factor age association |
||||
|---|---|---|---|---|
| Male | Female | |||
| Coefficient (95% CI) | P | Coefficient (95% CI) | p | |
| SBP (mmHg)* | 0.86 (0.78, 0.94) | <0.001 | 0.86 (0.79, 0.93) | <0.001 |
| TC (mg/dL)† | 0.85 (0.74, 0.95) | <0.001 | 0.75 (0.59, 0.90) | <0.001 |
| FPG (mg/dL)‡ | 0.72 (0.60, 0.83) | <0.001 | 0.64 (0.49, 0.80) | <0.001 |
Systolic blood pressure
Total cholesterol
Fasting plasma glucose
Discussion
Our analysis of health examination surveys found that the age association of three major CVD risk factors, characterized by their intercept at age 30 years and slope in the subsequent decades of life, varied substantially among different populations. SBP had the smallest variation in slope relative to that of its intercept at 30 and FPG had the opposite pattern. In other words, currently more of the cross-population differences in SBP occur by age 30 compared to that of FPG which occur mostly in adult life. We also found that the rise in SBP, TC, and FPG with age generally became attenuated or even reversed in older ages, as hypothesized in previous studies using more limited data 13-15. The age at which change in slope occurred was smallest for TC (~55-60 years) and largest for SBP (~65-70 years). The reasons for flattening or reversal in older ages requires further investigation and may include selective early mortality among those with high risk factor levels, increased use of medication in older ages 16, 17, or possibly cohort effects.
Previous studies, using samples from specific communities and cohorts, found that some communities had flat or shallow age associations for these three risk factors 4-11. However, some of these communities were selected specifically because they had low CVD risk or diets that were low in salt or animal products, thus, by design, were not representative of national populations. None of the populations in our data had flat SBP age curves, possibly because national populations (vs. smaller individual communities) are all affected by major determinants of SBP, especially salt intake. Some populations in our analysis had shallow age associations for FPG in both sexes and for TC in men.
Western high-income countries had higher slopes and intercepts for TC, especially in surveys prior to 2000, possibly because TC slope is affected by intake of animal fats, often associated with higher income 18, 19. However TC slope declined in later surveys in high-income countries, possibly due to changes in diet or an increase in the use of statins in middle-aged and older people. There was no association between national income and the slopes of SBP and FPG. This may be because salt intake, a major determinant of SBP in populations 11, 20, 21, is determined more by cultural and geographical factors than by national income. Further, high-income countries may have higher coverage of blood pressure screening and anti-hypertensive drug use which would help attenuate the age association of SBP. There may also be an independent effect of aging on SBP, e.g. due to vascular stiffness, which would lead to some similarities across populations.22 This does not explain the nearly flat age association in some previous studies such as Intersalt.4, 6, 10 We found the largest FPG slopes in countries in the Middle East and Oceania, which also have some of the highest BMI levels in the world 23. The age gradients of blood glucose and diabetes are influenced by body weight and body composition although an independent age effect has also been observed 24-30. Similarly, between late 1970s and mid 2000s, when mean BMI in the US increased, FPG age association shifted from one of the shallowest to one of the steepest worldwide.
Men and women in the same populations were generally on the low or high side of the distribution of slopes, but slopes tended to be higher among women than men, especially for TC, with the opposite pattern for intercepts. Since the data for men and women are from the same surveys, differences in measurement methods are unlikely to account for this finding. We hypothesize two potential explanations for the differences in male-female intercept and slope for SBP, TC, and FPG. First, these differences may be a consequence of differences in dietary and environmental factors between men and women in the same country 31-33.
Second, the higher slope among women may be due to real physiologic differences, e.g. those associated with menopause 34. It is well-known that prior to menopause women have lower CVD rates and this sex difference narrows after menopause 35, 36 but the evidence for the role of hormonal factors on post-menopausal increase in CVD risk factors remains limited and equivocal.37-41 Understanding the role of dietary, environmental, and reproductive factors in male-female blood pressure, cholesterol and glucose differences at various ages requires individual level follow-up data or at least surveys with consistent and comparable data on such determinants including time since menopause.
The strengths and innovations of this analysis include the inclusion of three major CVD risk factors; a relatively large number of nationally representative surveys from high- as well as low-and-middle-income countries; and estimating slope and intercept using individual records. The limitations of our analysis include the fact that fewer surveys had measured TC and FPG than SBP; some regions of the world were under-represented in the data, e.g. Latin America; it was not possible to investigate age associations before 30 years of age; and there were few countries with repeated surveys over a few decades. The lack of repeated surveys in most countries restricted our ability to examine cohort effects which may be an important determinant of cross-sectional age patterns. For example, data from multiple rounds of NHANES , the only survey that covered > 25 years, show that SBP, TC, and FPG had both age and cohort effects in the US. Specifically, at any given age, SBP and TC were generally lower in later birth cohorts than in earlier ones; FPG was generally higher in later birth cohorts. A previous analysis used an age and cohort model on NHANES data and found a continuous decline in SBP levels and age slope in subsequent birth cohorts in the US 12.
Median SBP decreased by 2.4 mmHg per decade of birth year but remained smaller than age effects. The SBP decrease by birth cohort was larger in women than men. Results for TC were similar with median TC declining by 0.12 mmol/L per decade of birth year.42 It is well-established that for individuals as well as populations, CVD risk factors are affected by interactions of genetic factors; diet and adiposity; behavioral and psychosocial factors; and treatment access and utilization. Based on our findings, an important research need is to use individual records from longitudinal studies to examine how these risk factors change over life course 43, especially in relation to dietary, behavioral, psychosocial and healthcare determinants. For example, a recent analysis of data from multiple cohorts in the UK found that blood pressure rose through late adulthood, when the rise slowed; some of the rise and the intercept in younger ages were associated with BMI 44. Furthermore, repeated population health surveys with measurements of CVD risk factors would help examine how changes in factors like salt and animal fat intake, adiposity, and the use of antihypertensive drugs and statins influence the levels and age patterns of risk factors at the population level.
Supplementary Material
Acknowledgements and funding
Majid Ezzati is supported by a strategic award from the UK Medical Research Council (MRC) and by the National Institute for Health Research Comprehensive Biomedical Research Centre at Imperial College London and Imperial College Healthcare NHS Trust. The analysis of metabolic risk factors was undertaken as a part of the Global Burden of Diseases, Injuries, and Risk Factors Study. A grant from the Bill and Melinda Gates Foundation supported the Study's core activities and partially supported the analyses in this paper. The results in this paper are prepared independently of the final estimates of the Global Burden of Diseases, Injuries, and Risk Factors Study. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit manuscript.
Footnotes
Disclosures
None of the authors have a competing interest.
References
- 1.Sniderman AD, Furberg CD. Age as a modifiable risk factor for cardiovascular disease. Lancet. 2008;371:1547–1549. doi: 10.1016/S0140-6736(08)60313-X. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970-2002. Jama. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 3.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho JJ, Baruzzi RG, Howard PF, Poulter N, Alpers MP, Franco LJ, Marcopito LF, Spooner VJ, Dyer AR, Elliott P, Stamler J, Stamler R. Blood pressure in four remote populations in the INTERSALT Study. Hypertension. 1989;14:238–246. doi: 10.1161/01.hyp.14.3.238. [DOI] [PubMed] [Google Scholar]
- 5.Connor WE, Cerqueira MT, Connor RW, Wallace RB, Malinow MR, Casdorph HR. The plasma lipids, lipoproteins, and diet of the Tarahumara indians of Mexico. Am J Clin Nutr. 1978;31:1131–1142. doi: 10.1093/ajcn/31.7.1131. [DOI] [PubMed] [Google Scholar]
- 6.He J, Klag MJ, Whelton PK, Chen JY, Mo JP, Qian MC, Mo PS, He GQ. Migration, blood pressure pattern, and hypertension: the Yi Migrant Study. Am J Epidemiol. 1991;134:1085–1101. doi: 10.1093/oxfordjournals.aje.a116012. [DOI] [PubMed] [Google Scholar]
- 7.Beaglehole R, Foulkes MA, Prior IA, Eyles EF. Cholesterol and mortality in New Zealand Maoris. Br Med J. 1980;280:285–287. doi: 10.1136/bmj.280.6210.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauletto P, Caroli M, Pessina AC, Dal Palu C. Hypertension prevalence and age- related changes of blood-pressure in semi-nomadic and urban Oromos of Ethiopia. Eur J Epidemiol. 1994;10:159–164. doi: 10.1007/BF01730365. [DOI] [PubMed] [Google Scholar]
- 9.Poulter N, Khaw KT, Hopwood BE, Mugambi M, Peart WS, Rose G, Sever PS. Blood pressure and associated factors in a rural Kenyan community. Hypertension. 1984;6:810–813. doi: 10.1161/01.hyp.6.6.810. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez BL, Labarthe DR, Huang B, Lopez-Gomez J. Rise of blood pressure with age. New evidence of population differences. Hypertension. 1994;24:779–785. doi: 10.1161/01.hyp.24.6.779. [DOI] [PubMed] [Google Scholar]
- 11.Elliott P, Marmot M, Dyer A, Joossens J, Kesteloot H, Stamler R, Stamler J, Rose G. The INTERSALT study: main results, conclusions and some implications. Clin Exp Hypertens A. 1989;11:1025–1034. doi: 10.3109/10641968909035389. [DOI] [PubMed] [Google Scholar]
- 12.Goff DC, Howard G, Russell GB, Labarthe DR. Birth cohort evidence of population influences on blood pressure in the United States, 1887-1994. Ann Epidemiol. 2001;11:271–279. doi: 10.1016/s1047-2797(00)00224-6. [DOI] [PubMed] [Google Scholar]
- 13.Lawes CM, Vander Hoorn S, Law M, Elliot P, MacMahon S, Rodgers A. High Blood Pressure. In: Ezzati M, Lopez A, Rodgers A, Murray CJM, editors. Comparative Quantification of Health Risks. Vol. 1. World Health Organization; Genva: 2004. pp. 281–358. Volume 1. [Google Scholar]
- 14.Lawes CM, Vander Hoorn S, Law M, Elliot P, MacMahon S, Rodgers A. High Cholesterol. In: Ezzati M, Lopez A, Rodgers A, Murray CJM, editors. Comparative Quantification of Health Risks. Vol. 1. World Health Organization; Geneva: 2004. pp. 391–496. [Google Scholar]
- 15.Danaei G, Lawes CM, Vander Hoorn S, Murray CJ, Ezzati M. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet. 2006;368:1651–1659. doi: 10.1016/S0140-6736(06)69700-6. [DOI] [PubMed] [Google Scholar]
- 16.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. Jama. 2011;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 17.Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Ann Pharmacother. 2008;42:1208–1215. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 18.Law M. Dietary fat and adult diseases and the implications for childhood nutrition: an epidemiologic approach. Am J Clin Nutr. 2000;72:1291S–1296S. doi: 10.1093/ajcn/72.5.1291s. [DOI] [PubMed] [Google Scholar]
- 19.Ezzati M, Vander Hoorn S, Lawes CM, Leach R, James WP, Lopez AD, Rodgers A, Murray CJ. Rethinking the “diseases of affluence” paradigm: global patterns of nutritional risks in relation to economic development. PLoS Med. 2005;2:e133. doi: 10.1371/journal.pmed.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law MR, Frost CD, Wald NJ. By how much does dietary salt reduction lower blood pressure? I--Analysis of observational data among populations. Bmj. 1991;302:811–815. doi: 10.1136/bmj.302.6780.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, Marmot M. Bmj. Vol. 312. Intersalt Cooperative Research Group; 1996. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. pp. 1249–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steppan J, Barodka V, Berkowitz DE, Nyhan D. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res Pract. 2011;2011:263585. doi: 10.4061/2011/263585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9. 1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imbeault P, Prins JB, Stolic M, Russell AW, O'Moore-Sullivan T, Despres JP, Bouchard C, Tremblay A. Aging per se does not influence glucose homeostasis: in vivo and in vitro evidence. Diabetes Care. 2003;26:480–484. doi: 10.2337/diacare.26.2.480. [DOI] [PubMed] [Google Scholar]
- 25.Ferrannini E, Vichi S, Beck-Nielsen H, Laakso M, Paolisso G, Smith U. Insulin action and age. European Group for the Study of Insulin Resistance (EGIR). Diabetes. 1996;45:947–953. doi: 10.2337/diab.45.7.947. [DOI] [PubMed] [Google Scholar]
- 26.Yang YC, Lu FH, Wu JS, Chang CJ. Age and sex effects on HbA1c. A study in a healthy Chinese population. Diabetes Care. 1997;20:988–991. doi: 10.2337/diacare.20.6.988. [DOI] [PubMed] [Google Scholar]
- 27.Maneatis T, Condie R, Reaven G. Effect of age on plasma glucose and insulin responses to a test mixed meal. J Am Geriatr Soc. 1982;30:178–182. doi: 10.1111/j.1532-5415.1982.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 28.Meneilly GS, Elahi D, Minaker KL, Sclater AL, Rowe JW. Impairment of noninsulin-mediated glucose disposal in the elderly. J Clin Endocrinol Metab. 1989;68:566–571. doi: 10.1210/jcem-68-3-566. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto Y, Futamura A, Ikushima M. Effect of aging on HbA1c in a working male Japanese population. Diabetes Care. 1995;18:1337–1340. doi: 10.2337/diacare.18.10.1337. [DOI] [PubMed] [Google Scholar]
- 30.Iozzo P, Beck-Nielsen H, Laakso M, Smith U, Yki-Jarvinen H, Ferrannini E. Independent influence of age on basal insulin secretion in nondiabetic humans. European Group for the Study of Insulin Resistance. J Clin Endocrinol Metab. 1999;84:863–868. doi: 10.1210/jcem.84.3.5542. [DOI] [PubMed] [Google Scholar]
- 31.Lawlor DA, Ebrahim S, Davey Smith G. Sex matters: secular and geographical trends in sex differences in coronary heart disease mortality. Bmj. 2001;323:541–545. doi: 10.1136/bmj.323.7312.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawlor DA, Ebrahim S, Davey Smith G. Role of endogenous oestrogen in aetiology of coronary heart disease: analysis of age related trends in coronary heart disease and breast cancer in England and Wales and Japan. Bmj. 2002;325:311–312. doi: 10.1136/bmj.325.7359.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikiforov SV, Mamaev VB. The development of sex differences in cardiovascular disease mortality: a historical perspective. Am J Public Health. 1998;88:1348–1353. doi: 10.2105/ajph.88.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, Sutton-Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 36.Kannel WB, Levy D. Menopause, hormones, and cardiovascular vulnerability in women. Arch Intern Med. 2004;164:479–481. doi: 10.1001/archinte.164.5.479. [DOI] [PubMed] [Google Scholar]
- 37.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women's Health Across the Nation. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–18. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- 39.Willeit J, Kiechl S, Egger G, Oberhollenzer M, Oberhollenzer F, Muggeo M, Poewe W, Bonora E. The role of insulin in age-related sex differences of cardiovascular risk profile and morbidity. Atherosclerosis. 1997;130:183–189. doi: 10.1016/s0021-9150(96)06038-8. [DOI] [PubMed] [Google Scholar]
- 40.Cifkova R, Pitha J, Lejskova M, Lanska V, Zecova S. Blood pressure around the menopause: a population study. J Hypertens. 2008;26:1976–1982. doi: 10.1097/HJH.0b013e32830b895c. [DOI] [PubMed] [Google Scholar]
- 41.Casiglia E, Tikhonoff V, Caffi S, Bascelli A, Schiavon L, Guidotti F, Saugo M, Giacomazzo M, Martini B, Mazza A, D'Este D, Pessina AC. Menopause does not affect blood pressure and risk profile, and menopausal women do not become similar to men. J Hypertens. 2008;26:1983–1992. doi: 10.1097/HJH.0b013e32830bfdd9. [DOI] [PubMed] [Google Scholar]
- 42.Goff DC, Jr., Labarthe DR, Howard G, Russell GB. Primary prevention of high blood cholesterol concentrations in the United States. Arch Intern Med. 2002;162:913–919. doi: 10.1001/archinte.162.8.913. [DOI] [PubMed] [Google Scholar]
- 43.Tate RB, Manfreda J, Cuddy TE. The effect of age on risk factors for ischemic heart disease: the Manitoba Follow-Up Study, 1948-1993. Ann Epidemiol. 1998;8:415–421. doi: 10.1016/s1047-2797(98)00011-8. [DOI] [PubMed] [Google Scholar]
- 44.Wills AK, Lawlor DA, Matthews FE, Aihie Sayer A, Bakra E, Ben-Shlomo Y, Benzeval M, Brunner E, Cooper R, Kivimaki M, Kuh D, Muniz-Terrera G, Hardy R. Life Course Trajectories of Systolic Blood Pressure Using Longitudinal Data from Eight UK Cohorts. PLoS Med. 2011;8:e1000440. doi: 10.1371/journal.pmed.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.