Abstract
Pleckstrin homology-like domain family A member 2 (PHLDA2) is a maternally expressed imprinted gene whose elevated expression has been linked to fetal growth restriction in a number of human studies. In mice, Phlda2 negatively regulates placental growth and limits the accumulation of placental glycogen. We previously reported that a three-copy transgene spanning the Phlda2 locus drove a fetal growth restriction phenotype late in gestation, suggesting a causative role for PHLDA2 in human growth restriction. However, in this mouse model, Phlda2 was overexpressed by fourfold, alongside overexpression of a second imprinted gene, Slc22a18. Here, we genetically isolate the role of Phlda2 in driving late fetal growth restriction in mice. We furthermore show that this Phlda2-driven growth restriction is asymmetrical, with a relative sparing of the brain, followed by rapid catch-up growth after birth, classic features of placental insufficiency. Strikingly, fetal growth restriction showed strain-specific differences, being apparent on the 129S2/SvHsd (129) genetic background and absent on the C57BL6 (BL6) background. A key difference between these two strains is the placenta. Specifically, BL6 placentae possess a more extensive endocrine compartment and substantially greater stores of placental glycogen. Taken together, these data support a direct role for elevated Phlda2 in limiting fetal growth but also suggest that growth restriction only manifests when there is limited placental reserve. These findings should be taken into account in interpreting the results from human studies.
KEY WORDS: Phlda2, Fetal growth restriction, Asymmetric
INTRODUCTION
Low birth weight (LBW) is one of the most problematic conditions affecting human populations. It is a very common complication of pregnancy, affecting up to 19% of all births in the developing world and between 5 and 7% of births in developed countries (Valero de Bernabe et al., 2004). Either as a consequence of poor growth in utero, preterm birth or a genetic abnormality, some babies are born too small and are consequently more vulnerable to complications at the time of birth, with increased risk of mortality (Witt et al., 2012). Furthermore, it is increasingly recognised that, although these vulnerable babies can gain weight and seem to recover from their poor start in life, many will experience complications in later life, including alterations in behaviour (ADHD, depression) and metabolism (obesity, type 2 diabetes, heart disease) (Grissom and Reyes, 2013). Identifying the molecular origins of growth restriction is crucial in identifying the most appropriate management of LBW infants to ensure optimal short- and long-term health outcomes.
Genomic imprinting is an epigenetic phenomenon that drives the preferential expression of certain genes from one parental allele (John and Surani, 1996). In humans, a number of childhood growth disorders, including Beckwith-Wiedemann syndrome (BWS; MIM #130650) and Silver-Russell syndrome (SRS; MIM #180860), involve the aberrant expression of imprinted genes (Chiesa et al., 2012). These imprinting disorders are rare at 1 in 13,000 and 1 in 300,000 births, respectively. However, numerous imprinted genes play a key role in regulating fetal growth and placental development in a dosage-sensitive manner, with paternal silencing primarily of growth-restricting genes and maternal silencing of growth-promoting genes (Tunster et al., 2013), which suggests that aberrant imprinting might underlie more common human growth disorders.
Pleckstrin homology-like domain family A member 2 (PHLDA2) was first highlighted as a potential fetal growth restriction gene in a 2006 study that reported elevated expression in the placenta of 9 out of the 38 fetal growth restriction (FGR) placentae (McMinn et al., 2006). Subsequently, several studies have examined PHLDA2 in relation to fetal growth and birth weight (Jensen et al., 2014). Whereas some of these studies have supported a role for elevated PHLDA2 in LBW (Apostolidou et al., 2007; Guo et al., 2008; Lim et al., 2012), others did not find a clear correlation with birth weight (Lambertini et al., 2012; Lewis et al., 2012).
We previously reported on a growth restriction phenotype in mice that was linked to the presence of three copies of a bacterial artificial chromosome (BAC) spanning the murine Phlda2 locus and a second imprinted gene, solute carrier family 22, member 18 (Slc22a18) (Salas et al., 2004; Tunster et al., 2010). Transgenic fetuses were 13% lighter than control littermates late in gestation, with significant placental stunting phenotype and a severe loss of placental glycogen. However, these studies did not establish a direct link between elevated Phlda2 expression and fetal growth restriction, nor did they model the more subtle elevations in expression reported in human studies. Here, we sought to clarify the role of the Phlda2 gene in regulating fetal growth by using transgenic mice carrying a single-copy transgene. We report that as little as twofold increased expression of Phlda2 was sufficient to reduce birth weight by 10%. Importantly, growth restriction was asymmetric, with relative sparing of the brain, followed by rapid catch-up growth after birth, classic features of placental insufficiency.
RESULTS
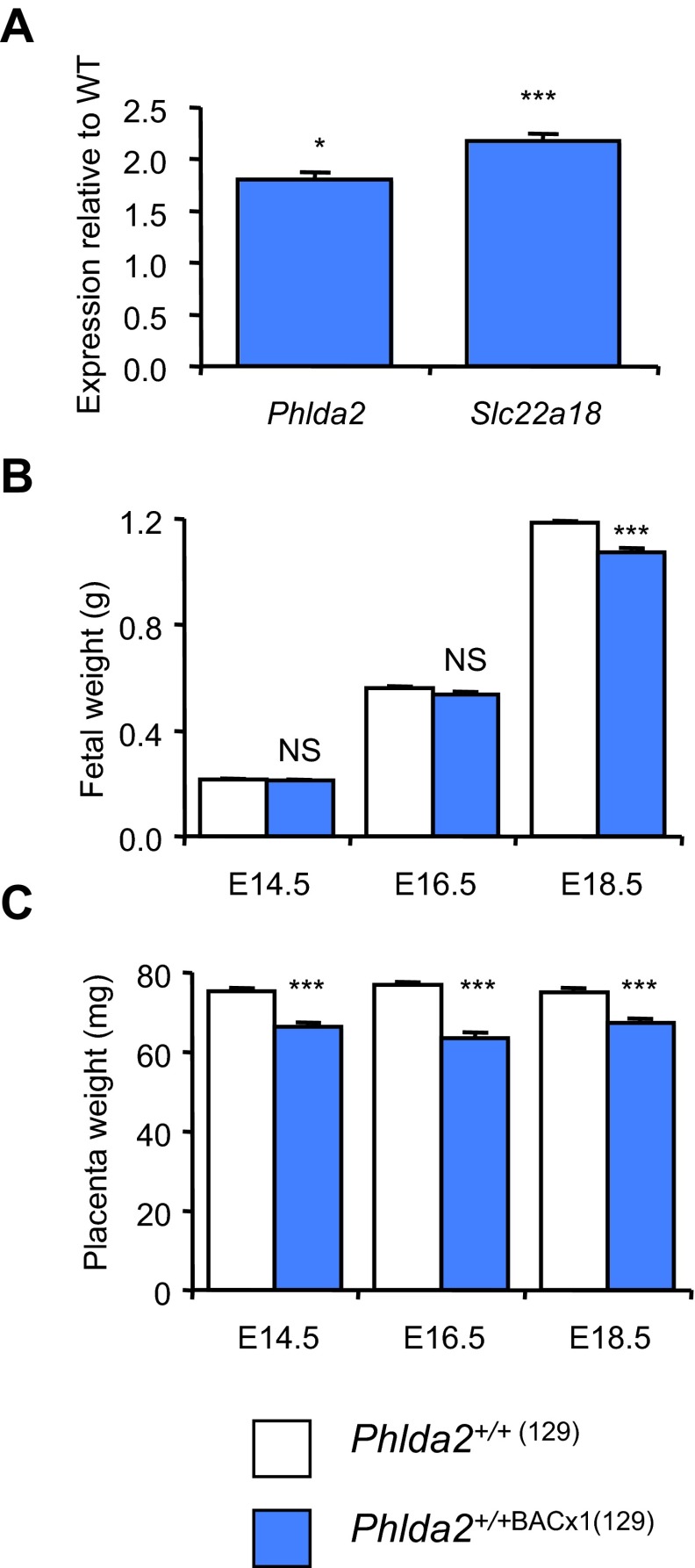
In our previous paper, gene expression analysis of a newly generated BAC transgenic line on mixed genetic background suggested the presence of at least two copies of the BAC transgene (Tunster et al., 2010). However, when we bred this line fully into the 129S2/SvHsd (129) genetic background (>eight generations) and re-examined gene expression, the data was more consistent with the presence of a single copy (Fig. 1A). When we examined fetal weight in the context of twofold gene expression, growth restriction of Phlda2+/+BACx1 fetuses was apparent at embryonic day 18.5 (E18.5; 1184.4±8.7 mg versus 1073.8±18.5 mg, P=5.33×10−6) but not at E14.5 or E16.5 (Fig. 1B), similar to the growth profile we reported in response to fourfold expression (Tunster et al., 2010). Transgenic placentae were 10–20% lighter than non-transgenic placentae at each time point (Fig. 1C), similar to the weight reduction we reported for a single-copy transgenic line spanning Phlda2, Slc22a18 and Cdkn1c (Tunster et al., 2010).
Fig. 1.
Characterisation of the single-copy line BACx1 on a 129 strain background. (A) QPCR analysis of Phlda2 and Slc22a18 expression in E14.5 Phlda2+/+BACx1(129) placentae. (B) Fetal wet weights on the 129 genetic background at E14.5, E16.5 and E18.5. Phlda2+/+BACx1(129) (2×) fetuses (n=36) were significantly lighter at E18.5, weighing 90.7% of the weight of their non-transgenic counterparts (P=5.33×10−6). (C) Placental wet weights on the 129 genetic background. Phlda2+/+BACx1(129) (2×) placentae were significantly lighter that non-transgenic placentae at each time point. Numerical data is given in supplementary material Table S1. NS, not significant (P>0.05), *P<0.05, ***P<0.005.
TRANSLATIONAL IMPACT.
Clinical issue
Low birth weight is one of the most intractable and yet clinically relevant complications affecting human pregnancies. Being born small increases the risk of complications at the time of birth and can lead to early mortality. In addition, although low-birth-weight babies often gain weight and seem to recover from their poor start in life, babies with accelerated postnatal weight gain after in utero growth restriction have a greatly increased risk of developing type 2 diabetes, obesity and cardiovascular disease in later life compared with normal-birth-weight babies. Although the causes of low birth weight are numerous and include both genetic and environmental factors, elevated placental expression of the imprinted gene PHLDA2 has been reported in a number of studies on low birth weight. However, because correlation does not always equal causation, determination of the relevance of elevated PHLDA2 expression using an animal model is of crucial importance.
Results
In this study, the authors generated a mouse model in which Phlda2 expression was elevated twofold, a similar increase in expression to that reported in human studies. The authors observed late fetal growth restriction in the transgenic mice, with the pups being born with a low birth weight. Crucially, growth restriction was asymmetric, with relative sparing of the brain, followed by rapid catch-up growth after birth, which are classic features of placental insufficiency. Moreover, growth restriction showed strain-specific differences and the effects of Phlda2 overexpression were only apparent in mouse strains in which the placenta was already working at maximum capacity.
Implications and future directions
These findings support a causal role for elevated PHLDA2 in the aetiology of human low birth weight. Moreover, the observation of both asymmetrical growth restriction and rapid catch-up growth in this new mouse model of Phlda2 overexpression suggests that human infants born with a low birth weight owing to this specific alteration might be at higher risk of later-life health complications than infants born with a low birth weight for other reasons. This possibility can be investigated by reexamining data from human cohorts and through further work on the animal model. Finally, these findings suggest that elevated PHLDA2 expression might be most harmful in scenarios in which the placenta is working at maximum capacity, such as in pregnancies subject to prenatal adversity.
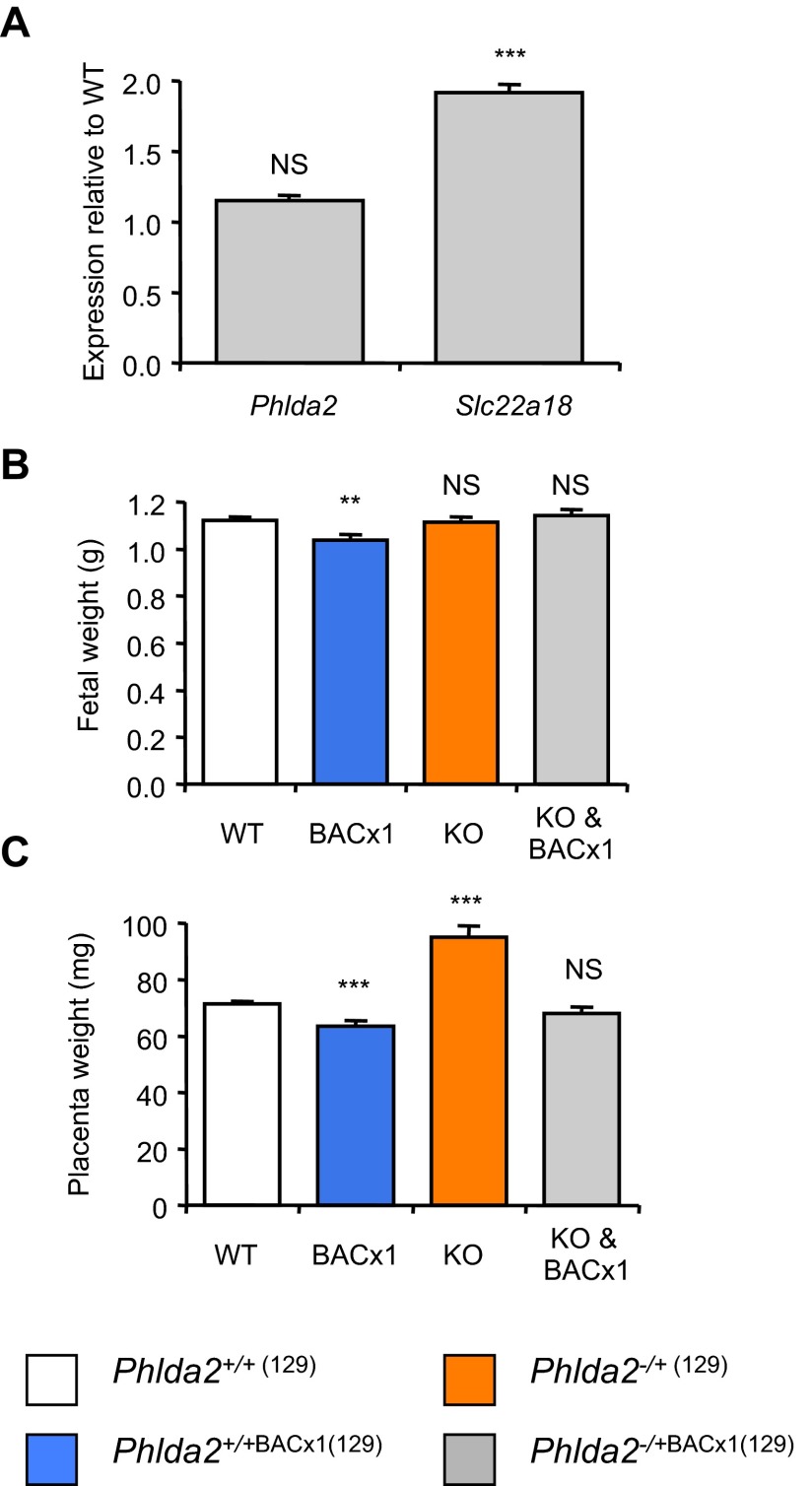
To formally test whether the line carried a single copy of the transgene, we crossed males carrying the BAC with females carrying a paternally inherited targeted allele of Phlda2, also bred for >eight generations into the 129 genetic background. This cross generated four genotypes: Phlda2+/+(129) (one active copy of Phlda2 and Slc22a18), Phlda2+/+BACx1(129) (two active copies Phlda2 and Slc22a18), Phlda2−/+(129) (maternal-knockout Phlda2, equivalent to loss of function) and Phlda2−/+BACx1(129) (double transgenic; one active copy of Phlda2 and two active copies of Slc22a18). At E14.5, Phlda2 was expressed at wild-type levels in double-transgenic fetuses, whereas Slc22a18 remained elevated, confirming that the line carried a single extra copy of Phlda2 (Fig. 2A). Importantly, these double-transgenic fetuses exposed to a single dose of Phlda2 and a double dose of Slc22a18 were not significantly different in weight to controls at E18.5 (1123.3±13.8 mg versus 1144.3±23.4 mg, P=0.415; Fig. 2B), whereas fetuses carrying the single-copy BAC transgene, exposed to a double dose of both genes (Phlda2+/+BACx1(129)), weighed less than controls (1123.3±13.8 mg versus 1038.7±23.4 mg, P=0.00180; Fig. 2B). This finding genetically assigned growth restriction to the twofold-elevated Phlda2 expression. As observed with a different single-copy BAC transgene (Tunster et al., 2010), normalising the dose of Phlda2 also returned placental weights to normal (Fig. 2C).
Fig. 2.
Isolating a causative role for elevated Phlda2 in inducing late fetal growth restriction (129 strain background). (A) QPCR analysis of Phlda2 and Slc22a18 expression in E14.5 Phlda2+/+(129) (wild type; non-transgenic) versus Phlda2−/+BACx1(129) (1×) (double transgenic) from litters containing all four genotypes. Phlda2 expression was normal in double-transgenic placenta. (B) E18.5 fetal wet weights for Phlda2+/+(129) (WT), Phlda2+/+BACx1(129) (BACx1; 2×), Phlda2−/+(129) (KO; 0×) and Phlda2−/+BACx1(129) (KO & BACx1; 1×) from litters containing all four genotypes on the 129 genetic background. Fetal growth restriction was attenuated in double-transgenic fetuses. Phlda2−/+(129) (0×) fetuses were similar in weight to controls. (C) E18.5 placental wet weights for the litters used to generate data in Fig. 2B. Placental growth restriction was apparent only when Phlda2 expression was elevated (Phlda2+/+BACx1(129)). Phlda2−/+(129) (0×) placentae were 33% heavier than controls. Numerical data is given in supplementary material Table S2. NS, not significant (P>0.05), **P<0.01, ***P<0.005.
Loss of function of Phlda2 has previously been characterised on the C57BL/6 genetic background (Frank et al., 2002; Salas et al., 2004). We similarly observed substantial placental overgrowth on the 129 background. Phlda2−/+(129) placentae weighed 33% more than control placentae at E18.5 (94.9±4.1 mg versus 71.5±0.9 mg; P=8.41×10−8; Fig. 2C). As in the study of Phlda2 loss of function on the BL6 background, Phlda2−/+(129) fetuses, which lacked the maternal Phlda2 allele, were not advantaged by the possession of a larger placenta and weighed the same as their non-transgenic counterparts at E18.5 (1116.1±19.1 mg versus 1123.3±13.8; P=0.753; Fig. 2B). These data formally assigned the late fetal growth restriction phenotype to twofold-elevated expression of Phlda2 and confirmed that loss of function of Phlda2 does not induce fetal overgrowth.
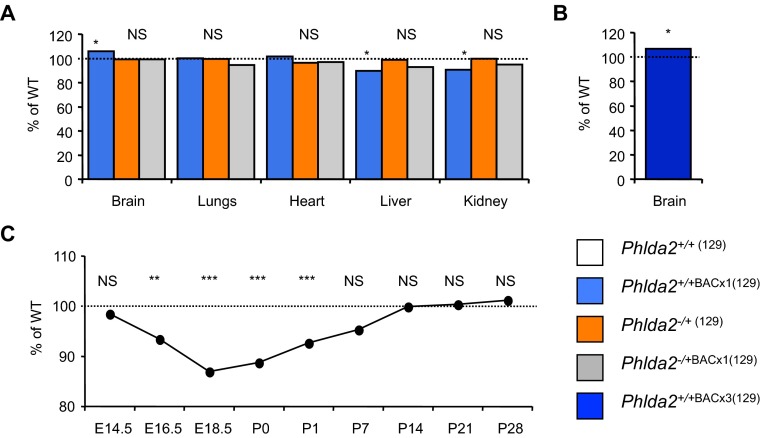
Fetal growth restriction due to placental insufficiency is often asymmetric, with a relative reduction in fetal kidney, liver, pancreas and lung size but a sparing of the brain. When organ weights of the four genotypes were compared at E18.5, the brain weights of the Phlda2+/+BACx1 fetuses were 10% heavier relative to body weight as compared to the non-transgenic fetuses, suggestive of brain sparing (Fig. 3A). The lungs and heart were proportionately growth restricted, whereas the liver and kidney, sites of embryonic Phlda2 expression (Qian et al., 1997), were growth restricted as a proportion of body weight (Fig. 3A). Brain sparing was similarly apparent in newborn mice carrying three copies of the transgene (Phlda2+/+BACx3) examined on the 129 genetic background (Fig. 3B). Normalising the dose of Phlda2 restored symmetry to the fetus (Fig. 3A). These data genetically demonstrated that twofold elevated Phlda2 restricts fetal growth asymmetrically. Moreover, loss of function of Phlda2 had no significant consequence for these organ weights, excluding a reciprocal function for Phlda2 in regulating organ weights.
Fig. 3.
Phlda2-induced fetal growth restriction is asymmetric (129 strain background). (A) Ratio of brain, lungs, heart, liver and kidney wet weights at E18.5 expressed as a proportion of body weight taken from samples described in Fig. 2. Brain weight was heavier as a proportion of body weight, compared with controls, whereas liver and kidney, sites of embryonic Phlda2 expression, were lighter. Normalising Phlda2 expression restored organ weight to non-transgenic proportions. (B) Ratio of brain as a proportion of body weight in E18.5 fetuses carrying three copies of the BAC transgene, Phlda2+/+BACx3(129) (4×). (C) Fetal and postnatal weight data for the line Phlda2+/+BACx3(129) (4×). Transgenic fetuses were lighter at birth but rapidly gained weight such that there was no significant difference in weight to their non-transgenic counterparts by P7. Numerical data is given in supplementary material Table S3. NS, not significant (P>0.05), *P<0.05, **P<0.01, ***P<0.005.
Catch-up growth is a key feature of extrinsically driven fetal growth restriction (Saenger et al., 2007). When postnatal weights for line Phlda2+/+BACx3 were examined from birth (P0) until 4 weeks of age (P28), pups were born weighing 11% less than their non-transgenic counterparts (1.25±0.014 g versus 1.41±0.009 g; P=3.29×10−17). Within 7 days, there was no significant difference in the weights between transgenic and non-transgenic pups (P=0.147; Fig. 3C).
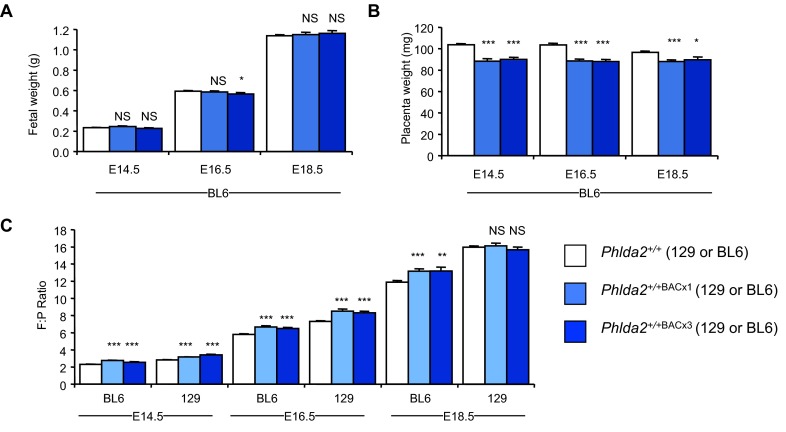
Placental weight and fetal:placental (F:P) ratios are widely used as a parameter of placental functional capacity (Fowden et al., 2009). The F:P ratio for mice of the 129 genetic background at E18.5 is relatively high, at 16.0±0.23, whereas that of C57BL/6 (BL6) mice is lower, at 12.3±0.18 (Tunster et al., 2012). This suggests that BL6 placenta might have a greater reserve capacity than 129 placenta. To investigate whether Phlda2 similarly restrained fetal growth in the context of a more favourable F:P ratio, we bred Phlda2+/+BACx1 and Phlda2+/+BACx3 (three copy line) mice onto the BL6 genetic background for >eight generations. We observed no difference in fetal weight at E14.5, E16.5 or E18.5 (Fig. 4A) despite transgenic placentae weighing 10–15% less than non-transgenic placentae at each time point (Fig. 4B). When we examined F:P ratios, there was a significant difference from normal at every time point due to the reduction in weight of the placenta but not the fetus on the BL6 background (Fig. 4C). F:P ratios were also significantly different from normal at E14.5 and E16.5 on the 129 background. At E18.5, there was no significant difference in F:P ratio because, at this later time point, both placental and fetal weights were reduced. This suggests that 129 placentae function at maximum capacity late in gestation and consequently cannot compensate for the reduction in capacity induced by elevated Phlda2, resulting in fetal growth restriction
Fig. 4.
Absence of fetal growth restriction on the BL6 genetic background. (A) Fetal wet weights on the BL6 genetic background at E14.5, E16.5 and E18.5 for Phlda2+/+BACx1(BL6) (2×), and at E18.5 for Phlda2+/+BACx3(BL6) (4×). Fetuses were the same weight as their non-transgenic counterparts at each time point. (B) Placental wet weights on the BL6 genetic background at E14.5, E16.5 and E18.5 for Phlda2+/+BACx1(BL6) (2×) and at E18.5 for Phlda2+/+BACx3(BL6) (4×). Placentae were significantly lighter that non-transgenic placentae at each time point. (C) Fetal:placental (F:P) weight ratios. Ratios were significantly different compared with non-transgenic mice at each time point on the BL6 genetic background, but only at E14.5 and E16.5 on the 129 genetic background. Numerical data is given in supplementary material Table S4. NS, not significant (P>0.05), *P<0.05, **P<0.01, ***P<0.005.
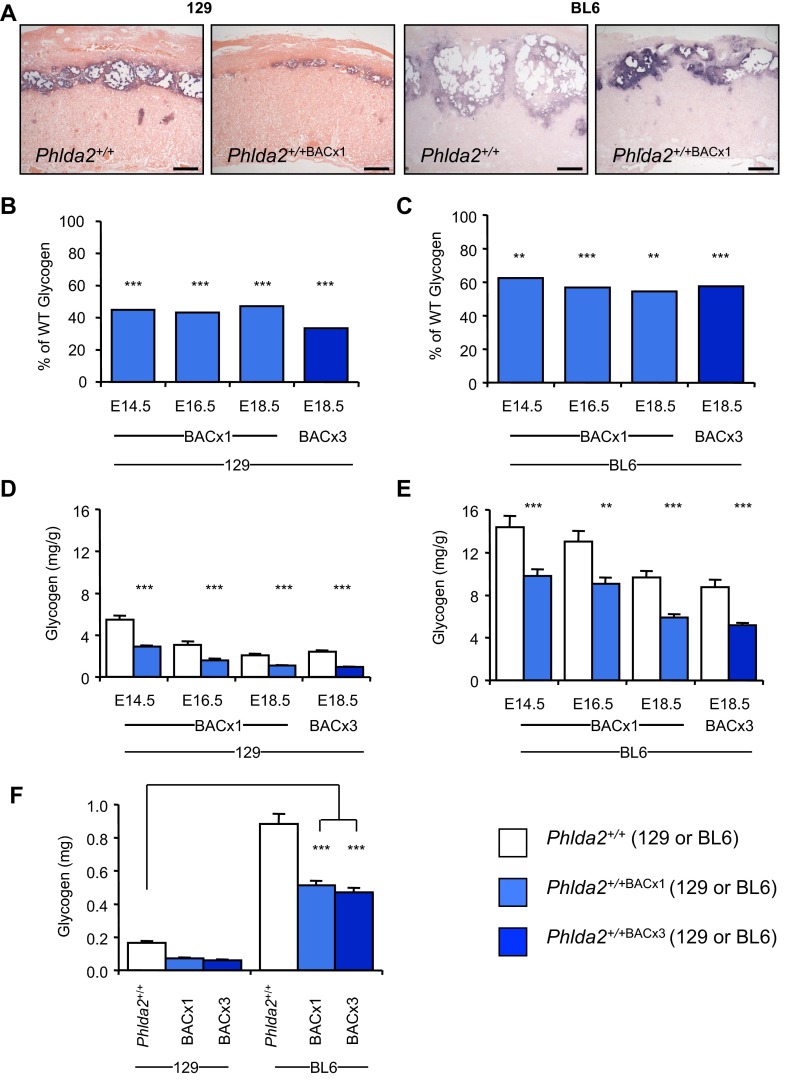
A reduced junctional zone and loss of placental glycogen have been linked to fetal growth restriction in several studies (Hitz et al., 2005; Oh-McGinnis et al., 2011; Withington et al., 2006; Zheng-Fischhöfer et al., 2007). Furthermore, placental glycogen has been suggested as an important source of easily utilisable energy to support late embryonic growth (Coan et al., 2006). In addition to the difference in F:P ratios, BL6 placentae have a disproportionately larger junctional zone and significantly greater stores of placental glycogen than 129 placentae (Tunster et al., 2012). In situ hybridisation with trophoblast specific protein alpha (Tpbpa), a marker for the junctional zone (Lescisin et al., 1988), revealed a reduction of the junctional zone at E14.5 on both the 129 and the BL6 genetic backgrounds for line Phlda2+/+BACx1 (Fig. 5A). To establish to what extent elevated Phlda2 compromised glycogen accumulation on the two genetic backgrounds, a biochemical determination of glycogen was performed at E14.5, E16.5 and E18.5. Twofold expression of Phlda2 induced a 50–60% decrease in total glycogen on the 129 background and 40–45% decrease on the BL6 background, relative to the respective non-transgenic counterparts, at each time point (Fig. 5B,C). A similar reduction was apparent for the three-copy line at E18.5 on both backgrounds (Fig. 5B,C). Thus, elevated Phlda2 similarly limited the accumulation of glycogen on both genetic backgrounds. However, although the amplitude of the effect was similar, a comparison of the amount of glycogen stored, expressed either relative to the weight of the placenta (mg/g) or as a total amount (mg), highlighted a striking finding. Whereas glycogen stores were compromised on both genetic backgrounds by elevated Phlda2, the compromised BL6 placentae (Phlda2+/+BACx1(BL6)) still contained three times more glycogen than the genetically uncompromised 129 placentae (Phlda2+/+(129)) (Fig. 5D–F).
Fig. 5.
Placental glycogen attenuates fetal growth restriction. (A) In situ hybridisation of Tpbpa riboprobe to E14.5 sections from Phlda2+/+(129) and Phlda2+/+BACx1(129) (2×) placenta in comparison to the same genotypes on the BL6 genetic background. Scale bars: 200 μm. (B) Biochemical determination of glycogen at E14.5, E16.5 and E18.5 for Phlda2+/+BACx1 and at E18.5 for Phlda2+/+BACx3 (4×) on the 129 background expressed as a percentage of values in non-transgenic placenta. (C) Biochemical determination of glycogen at E14.5, E16.5 and E18.5 for Phlda2+/+BACx1 and at E18.5 for Phlda2+/+BACx3 on the BL6 background expressed as a percentage of values in control placenta. (D) 129 glycogen data from panel B expressed as mg/g of placenta. (E) BL6 glycogen data from panel C expressed as mg/g of placenta. (F) Comparison of total glycogen stored in E18.5 placenta on the 129 versus BL6 background. Compromised (Phlda2 overexpression) BL6 placentae contain three times more glycogen than that found in the uncompromised (Phlda2 wild-type expression) 129 placentae. Numerical data is given in supplementary material Table S5. **P<0.01, ***P<0.005.
Taken together, these data genetically define a role for elevated Phlda2 in asymmetrically restricting late fetal growth but suggests that the growth-restricting properties of Phlda2 manifest only in unfavourable circumstances in which the placenta is already functioning at its upper limit to support the growth demands of the rapidly growing fetus.
DISCUSSION
Elevated expression of the imprinted gene PHLDA2 has been reported in a number of studies on LBW and fetal growth restriction, but correlation does not always equal causation. Here, we have demonstrated, using a mouse model, that twofold elevated expression of Phlda2 can restrain fetal growth late in gestation. Moreover, we have also shown that Phlda2-induced growth restriction is asymmetric, with a relative sparing of the brain alongside rapid postnatal catch-up growth. However, we did not observe a similar growth restriction on a different genetic background.
Mouse and human placentae differ substantially not only with respect to histologically classified lineages but also with respect to gene expression (Carter, 2012). Nonetheless, our data support a causal role for elevated PHLDA2 in restricting fetal growth in human pregnancies. In the study by McMinn et al., ~25% of the placentae from growth-restricted babies showed increased expression of PHLDA2 (McMinn et al., 2006). Kumar et al. similarly reported elevated placental PHLDA2 in relation to fetal growth restriction, and Diplas et al. reported a threefold increase in placental PHLDA2 in very LBW babies (Kumar et al., 2012; Diplas et al., 2009). These data suggest that elevated PHLDA2 might be a relatively common cause of LBW in human populations. Our findings in the mouse model that Phlda2-induced growth restriction was asymmetric, with relative sparing of the brain, followed by rapid catch-up growth, is consequently of significant importance. Human babies with these characteristics, particularly those with accelerated postnatal weight gain after in utero growth restriction, have a greatly enhanced risk of developing type 2 diabetes, obesity and cardiovascular disease [Barker, 1990; Barker, 1994; Barker, 2004; Barker et al., 2006; Barker et al., 1991; Barker, 2001; Barker and Medical Research Council, Environmental Epidemiology Unit (UK), 1992; Lackland et al., 2000; Lackland et al., 2001]. These diseases are thought to reflect the programming of metabolic abnormalities in utero in response to limited nutrient supply acerbated by optimal or even excessive nutrition after birth. This suggests that babies with elevated placental PHLDA2 might be at risk of these later-life health complications. Further work is required to follow up the later-life outcomes for babies with elevated PHLDA2 and also our transgenic mice to identify the predictive value of PHLDA2 as a biomarker.
Several human studies suggest that PHLDA2 expression inversely correlates with birth weight even within normal pregnancies (Apostolidou et al., 2007; Guo et al., 2008; Lim et al., 2012). Moreover, in a study of >7000 samples over three cohorts, a PHLDA2 promoter polymorphism was positively correlated with birth weight (Ishida et al., 2012). An inverse correlation with LBW was not reproduced in two other studies (Lambertini et al., 2012; Lewis et al., 2012), although these studies only looked at ~100 samples. In our previous investigation of PHLDA2 expression in placentae from the Southampton Women’s Study, we did not find a significant inverse association between placental PHLDA2 and birth weight, but we did observe a slowdown in fetal femur growth late in gestation and a significant loss of bone density at age 4 years (Lewis et al., 2012). In this current study, we found that the growth-restricting properties of Phlda2 were apparent on the 129 and not the BL6 genetic background, despite similar proportional reductions in placental weight and glycogen stores. Both the human data and our mouse data can be reconciled if elevated PHLDA2 only restricts fetal growth under certain circumstances, i.e. when the placenta is working at maximum capacity or when other factors, such as environmental exposures, limit placental capacity extrinsically. LBW is particularly prevalent in populations under severe socioeconomic stress; this could be due to a multitude of factors including poor diet, maternal stress and smoking. Human placental PHLDA2 has been reported to be upregulated in response to maternal smoking; additionally, in an experimental rat model, both maternal calorie restriction and alcohol were found to result in significantly upregulated placental Phlda2 (Bruchova et al., 2010; Shukla et al., 2011). We did not identify a correlation between placental PHLDA2 expression and maternal lifestyle in our relatively small study (Lewis et al., 2012), but it might be pertinent to further explore the data on PHLDA2 both with respect to asymmetry and catch-up growth while taking into account the degree of prenatal adversity experienced by the mother.
In summary, our work has identified a causative role for elevated Phlda2 in inducing asymmetrical late fetal growth restriction. Importantly, our work also suggests that elevated Phlda2 might be more harmful in scenarios where the placenta is working at maximum capacity, such as in pregnancies subject to prenatal adversity.
MATERIALS AND METHODS
Mouse strains and genotyping
Animal studies and breeding were approved by the Universities of Cardiff ethical committee and performed under a UK Home Office project licence (RMJ). All mice were housed together in one room throughout the study on a 12-hour light-dark cycle with lights coming on at 06.00 hour and a temperature range of 21±2°C with free access to water (tap water) and standard chow. The Phlda2 transgenic lines Phlda2+/+BACx1(129) (previously Tg10–10) and Phlda2+/+BACx3(129) (previously Tg10–15) were bred for >eight generations onto either the C57BL6 (Harlan, BL6) or the 129S2/SvHsd (Harlan, 129) strain backgrounds for phenotypic assessment. The Phlda2 targeted allele was either maintained by paternal transmission on the BL6 background or crossed into the 129 background for >eight generations.
Weighing studies and biochemical determination of placental glycogen concentration
Fetal and placental wet weights were taken at the stated time points after a discernible plug. Genotyping data was obtained from yolk sac DNA as previously described (Frank et al., 2002; John et al., 2001). Glycogen was extracted from whole placenta, and resuspended in 1 ml of H2O and assayed according to the method of Lo et al. (Lo et al., 1970) at a dilution of 1 in 2 (129) or 1 in 10 (BL6).
Quantitative RNA analysis
Quantitative PCR of reverse-transcribed RNA (QRT-PCR) was performed and analysed as described (Schmittgen and Livak, 2008; Tunster et al., 2010).
Statistical analyses
Statistical significance (probability values) was determined using the Student’s t-test (two-tailed distribution and two-sample unequal variance) or the Mann-Whitney U-test.
Supplementary Material
Acknowledgments
We thank Mary Cleaton and Charlotte Taylor for help with glycogen extraction, and Hugo Creeth for organ weighing.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
S.J.T. performed the bulk of the experiments, analysed data and contributed to the writing of the manuscript. M.V.D.P. performed some of the animal work. R.M.J. conceived and designed the experiment, interpreted the data and wrote the paper.
Funding
S.J.T. was supported by BBSRC grant BB/J015156; M.V.D.P. was supported by a BBSRC doctoral training grant BB/F016557/1.
Supplementary material
Supplementary material available online at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.017079/-/DC1
References
- Apostolidou S., Abu-Amero S., O’Donoghue K., Frost J., Olafsdottir O., Chavele K. M., Whittaker J. C., Loughna P., Stanier P., Moore G. E. (2007). Elevated placental expression of the imprinted PHLDA2 gene is associated with low birth weight. J. Mol. Med. 85, 379–387 [DOI] [PubMed] [Google Scholar]
- Barker D. J. (1990). The fetal and infant origins of adult disease. BMJ 301, 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. (1994). Maternal and fetal origins of coronary heart disease. J. R. Coll. Physicians Lond. 28, 544–551 [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. P. (2001). Fetal Origins of Cardiovascular and Lung Disease. New York, NY: Marcel Dekker [Google Scholar]
- Barker D. J. (2004). The developmental origins of well-being. Philos. Trans. R. Soc. B 359, 1359–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. P., Medical Research Council, Environmental Epidemiology Unit (UK) (1992). Fetal and Infant Origins of Adult Disease: Papers. London: British Medical Journal [Google Scholar]
- Barker D. J., Godfrey K. M., Fall C., Osmond C., Winter P. D., Shaheen S. O. (1991). Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 303, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J., Bagby S. P., Hanson M. A. (2006). Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2, 700–707 [DOI] [PubMed] [Google Scholar]
- Bruchova H., Vasikova A., Merkerova M., Milcova A., Topinka J., Balascak I., Pastorkova A., Sram R. J., Brdicka R. (2010). Effect of maternal tobacco smoke exposure on the placental transcriptome. Placenta 31, 186–191 [DOI] [PubMed] [Google Scholar]
- Carter A. M. (2012). Evolution of placental function in mammals: the molecular basis of gas and nutrient transfer, hormone secretion, and immune responses. Physiol. Rev. 92, 1543–1576 [DOI] [PubMed] [Google Scholar]
- Chiesa N., De Crescenzo A., Mishra K., Perone L., Carella M., Palumbo O., Mussa A., Sparago A., Cerrato F., Russo S., et al. (2012). The KCNQ1OT1 imprinting control region and non-coding RNA: new properties derived from the study of Beckwith-Wiedemann syndrome and Silver-Russell syndrome cases. Hum. Mol. Genet. 21, 10–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan P. M., Conroy N., Burton G. J., Ferguson-Smith A. C. (2006). Origin and characteristics of glycogen cells in the developing murine placenta. Dev. Dyn. 235, 3280–3294 [DOI] [PubMed] [Google Scholar]
- Diplas A. I., Lambertini L., Lee M. J., Sperling R., Lee Y. L., Wetmur J., Chen J. (2009). Differential expression of imprinted genes in normal and IUGR human placentas. Epigenetics 4, 235–240 [DOI] [PubMed] [Google Scholar]
- Fowden A. L., Sferruzzi-Perri A. N., Coan P. M., Constancia M., Burton G. J. (2009). Placental efficiency and adaptation: endocrine regulation. J. Physiol. 587, 3459–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D., Fortino W., Clark L., Musalo R., Wang W., Saxena A., Li C. M., Reik W., Ludwig T., Tycko B. (2002). Placental overgrowth in mice lacking the imprinted gene Ipl. Proc. Natl. Acad. Sci. USA 99, 7490–7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N. M., Reyes T. M. (2013). Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics. Int. J. Dev. Neurosci. 31, 406–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Choufani S., Ferreira J., Smith A., Chitayat D., Shuman C., Uxa R., Keating S., Kingdom J., Weksberg R. (2008). Altered gene expression and methylation of the human chromosome 11 imprinted region in small for gestational age (SGA) placentae. Dev. Biol. 320, 79–91 [DOI] [PubMed] [Google Scholar]
- Hitz C., Vogt-Weisenhorn D., Ruiz P., Wurst W., Floss T. (2005). Progressive loss of the spongiotrophoblast layer of Birc6/Bruce mutants results in embryonic lethality. Genesis 42, 91–103 [DOI] [PubMed] [Google Scholar]
- Ishida M., Monk D., Duncan A. J., Abu-Amero S., Chong J., Ring S. M., Pembrey M. E., Hindmarsh P. C., Whittaker J. C., Stanier P., et al. (2012). Maternal inheritance of a promoter variant in the imprinted PHLDA2 gene significantly increases birth weight. Am. J. Hum. Genet. 90, 715–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A. B., Tunster S. J., John R. M. (2014). The significance of elevated placental PHLDA2 in human growth restricted pregnancies. Placenta 35, 528–532 [DOI] [PubMed] [Google Scholar]
- John R. M., Surani M. A. (1996). Imprinted genes and regulation of gene expression by epigenetic inheritance. Curr. Opin. Cell Biol. 8, 348–353 [DOI] [PubMed] [Google Scholar]
- John R. M., Ainscough J. F., Barton S. C., Surani M. A. (2001). Distant cis-elements regulate imprinted expression of the mouse p57(Kip2) (Cdkn1c) gene: implications for the human disorder, Beckwith–Wiedemann syndrome. Hum. Mol. Genet. 10, 1601–1609 [DOI] [PubMed] [Google Scholar]
- Kumar N., Leverence J., Bick D., Sampath V. (2012). Ontogeny of growth-regulating genes in the placenta. Placenta 33, 94–99 [DOI] [PubMed] [Google Scholar]
- Lackland D. T., Bendall H. E., Osmond C., Egan B. M., Barker D. J. (2000). Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch. Intern. Med. 160, 1472–1476 [DOI] [PubMed] [Google Scholar]
- Lackland D. T., Egan B. M., Fan Z. J., Syddall H. E. (2001). Low birth weight contributes to the excess prevalence of end-stage renal disease in African Americans. J. Clin. Hypertens. (Greenwich) 3, 29–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini L., Marsit C. J., Sharma P., Maccani M., Ma Y., Hu J., Chen J. (2012). Imprinted gene expression in fetal growth and development. Placenta 33, 480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescisin K. R., Varmuza S., Rossant J. (1988). Isolation and characterization of a novel trophoblast-specific cDNA in the mouse. Genes Dev. 2 12A, 1639–1646 [DOI] [PubMed] [Google Scholar]
- Lewis R. M., Cleal J. K., Ntani G., Crozier S. R., Mahon P. A., Robinson S. M., Harvey N. C., Cooper C., Inskip H. M., Godfrey K. M., et al. Southampton Women’s Survey Study Group (2012). Relationship between placental expression of the imprinted PHLDA2 gene, intrauterine skeletal growth and childhood bone mass. Bone 50, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A. L., Ng S., Leow S. C., Choo R., Ito M., Chan Y. H., Goh S. K., Tng E., Kwek K., Chong Y. S., et al. (2012). Epigenetic state and expression of imprinted genes in umbilical cord correlates with growth parameters in human pregnancy. J. Med. Genet. 49, 689–697 [DOI] [PubMed] [Google Scholar]
- Lo S., Russell J. C., Taylor A. W. (1970). Determination of glycogen in small tissue samples. J. Appl. Physiol. 28, 234–236 [DOI] [PubMed] [Google Scholar]
- McMinn J., Wei M., Schupf N., Cusmai J., Johnson E. B., Smith A. C., Weksberg R., Thaker H. M., Tycko B. (2006). Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta 27, 540–549 [DOI] [PubMed] [Google Scholar]
- Oh-McGinnis R., Bogutz A. B., Lefebvre L. (2011). Partial loss of Ascl2 function affects all three layers of the mature placenta and causes intrauterine growth restriction. Dev. Biol. 351, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian N., Frank D., O’Keefe D., Dao D., Zhao L., Yuan L., Wang Q., Keating M., Walsh C., Tycko B. (1997). The IPL gene on chromosome 11p15.5 is imprinted in humans and mice and is similar to TDAG51, implicated in Fas expression and apoptosis. Hum. Mol. Genet. 6, 2021–2029 [DOI] [PubMed] [Google Scholar]
- Saenger P., Czernichow P., Hughes I., Reiter E. O. (2007). Small for gestational age: short stature and beyond. Endocr. Rev. 28, 219–251 [DOI] [PubMed] [Google Scholar]
- Salas M., John R., Saxena A., Barton S., Frank D., Fitzpatrick G., Higgins M. J., Tycko B. (2004). Placental growth retardation due to loss of imprinting of Phlda2. Mech. Dev. 121, 1199–1210 [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D., Livak K. J. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- Shukla P. K., Sittig L. J., Ullmann T. M., Redei E. E. (2011). Candidate placental biomarkers for intrauterine alcohol exposure. Alcohol. Clin. Exp. Res. 35, 559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunster S. J., Tycko B., John R. M. (2010). The imprinted Phlda2 gene regulates extraembryonic energy stores. Mol. Cell. Biol. 30, 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunster S. J., Van de Pette M., John R. M. (2012). Impact of genetic background on placental glycogen storage in mice. Placenta 33, 124–127 [DOI] [PubMed] [Google Scholar]
- Tunster S. J., Jensen A. B., John R. M. (2013). Imprinted genes in mouse placental development and the regulation of fetal energy stores. Reproduction 145, R117–R137 [DOI] [PubMed] [Google Scholar]
- Valero De, Bernabé J., Soriano T., Albaladejo R., Juarranz M., Calle M. E., Martínez D., Domínguez-Rojas V. (2004). Risk factors for low birth weight: a review. Eur. J. Obstet. Gynecol. Reprod. Biol. 116, 3–15 [DOI] [PubMed] [Google Scholar]
- Withington S. L., Scott A. N., Saunders D. N., Lopes Floro K., Preis J. I., Michalicek J., Maclean K., Sparrow D. B., Barbera J. P., Dunwoodie S. L. (2006). Loss of Cited2 affects trophoblast formation and vascularization of the mouse placenta. Dev. Biol. 294, 67–82 [DOI] [PubMed] [Google Scholar]
- Witt W. P., Wisk L. E., Cheng E. R., Hampton J. M., Hagen E. W. (2012). Preconception mental health predicts pregnancy complications and adverse birth outcomes: a national population-based study. Matern. Child Health J. 16, 1525–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng-Fischhöfer Q., Kibschull M., Schnichels M., Kretz M., Petrasch-Parwez E., Strotmann J., Reucher H., Lynn B. D., Nagy J. I., Lye S. J., et al. (2007). Characterization of connexin31.1-deficient mice reveals impaired placental development. Dev. Biol. 312, 258–271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.