Abstract
Circulating tumor cells (CTCs) represent a surrogate biomarker of hematogenous metastases and thus could be considered as a ‘liquid biopsy’ which reveals metastasis in action. But it is absolutely a challenge to detect CTCs due to their extreme rarity. At present, the most common principle is to take advantage of the epithelial surface markers of CTCs which attach to a specific antibody. Antibody-magnetic nanobeads combine with the epithelial surface markers, and then the compound is processed by washing, separation, and detection. However, a proportion of CTC antigen expressions are down-regulated or lost in the process of epithelial-mesenchymal transition (EMT), and thus, this part of CTCs cannot be detected by classical detection methods such as CellSearch. To resolve this problem, some multiple-marker CTC detections have been developed rapidly. Additionally, nanotechnology is a promising approach to kill CTCs with high efficiency. Implantable nanotubes coated with apoptosis-promoting molecules improve the disease-free survival and overall survival. The review introduces some novel CTC detection techniques and therapeutic methods by virtue of nanotechnology to provide a better knowledge of the progress about CTC study.
Keywords: Nanotechnology, Circulating tumor cell, Metastasis, Detection, Prognosis
Review
Introduction
Circulating tumor cells (CTCs) are shed from primary tumors, enter the circulatory system, and migrate to distant organs to form metastases that ultimately lead to the death of most cancer patients [1]. It is of great importance to carry out further study on CTCs because tumor is the major cause of human beings' death and approximately 90% of death is caused by the secondary tumor [2-4].
Thomas Ashworth firstly proposed the hypothesis of CTCs in 1869 [5], but studies on CTCs have made little progress in the last century mainly due to the limited condition to detect the CTCs. It is a challenging job to detect the CTCs in the peripheral blood since the CTCs are extremely scarce (just a few CTCs mixed with the approximately 10 million leukocytes and 5 billion erythrocytes in 1 ml of blood) [6]. The current studies indicate that there is a close relationship between CTC enumeration and the severity of disease and clinical prognosis, thus initiating further studies. The CTC number has been established as a helpful prognostic biomarker of malignant epithelial tumor including breast cancer [7,8], prostate cancer [9,10], and colon cancer [11]. With the relative technology developed rapidly, the achievements about CTCs are fruitful in the last decade.
The metastatic process is comprised of the following steps: neoangiogenesis, intravasation, circulation in the peripheral blood, extravasation, and ultimately resulting in colonization and growth at distant sites [12,13]. Obviously, CTCs act as the ‘vector’ of metastasis, and the successful capture and analysis of the CTCs throw light on the nature of the primary tumor and characterization of the secondary tumor. However, tumor cells in circulation are heterogeneous. On the one hand, they can exist in the circulatory system in various forms, such as simple cells, clumps of tumor cells, association with blood cells, and non-viable cells, so the CTCs are only a part of the tumor cells in circulation and other forms of tumor cells can also form metastasis [14]. On the other hand, during invasion and metastasis in epithelial tumors, epithelial-mesenchymal transition (EMT), a phenotypic change in epithelial cells may occur. Tumor cells lose the normal cell-to-cell interactions and adopt a more mesenchymal phenotype in the process of EMT, which facilitates their invasion through the vascular endothelium and migration into the circulation. Then CTCs extravasate out of the circulation and invade into tissues to ultimately form a metastasis, and the reverse progress is called mesenchymal-epithelial transition (MET). The epithelial surface markers alter during the process of EMT and MET, so antibody-based methods will lose some malignant tumor cells [14,15]. In addition, there is no single epithelial marker which is expressed in all tumor cells. Although the epithelial cellular adhesion molecule (EpCAM) is the most used tumor surface biomarker in the detection of CTCs, it is not necessarily expressed in all types of epithelial tumor. Furthermore, EpCAM may be lost in the process of EMT, so some CTCs can be missed by CellSearch. Thus, we should take the above properties into account when applying new technologies to detect and manipulate the CTCs. Therefore, simultaneous staining with multiple epithelial markers or negative staining with leukocyte markers is the future trend to detect CTCs.
Nanotechnology, the manipulation of matter on an atomic, molecular, or supramolecular scale, is expected to show great capability for the early detection, accurate diagnosis, and personalized treatment of malignant tumors. Nanoscale particles are typically about several hundreds of nanometers or smaller and are approximately equal to the size of large biological molecules such as enzymes, receptors, and antibodies. These nanoscale devices interact with biomolecules both on the surface of and inside cells with unprecedented efficiency, which may improve disease diagnosis and treatment drastically [16,17]. Many new technologies are derived from the application of nanotechnology, for example, CellSearch System [18], AdnaSystem [19], versatile immunomagnetic nanocarrier platform [20], one-step detection method [21], aptamer application [22], and magnetophoresis technology [23] (Table 1). Nanoscale devices have several advantages over other devices: high sensitivity, microsize, portability, and availability of point-to-care medicine, which will play a crucial role in future translational medicine.
Table 1.
Characteristics of some CTC detection methods
| Method | Cell viability | Detection level | Advantage | Disadvantage | Reference |
|---|---|---|---|---|---|
| CellSearch System |
No |
Median number of isolated CTCs, 5 CTCs per 7.5 ml of blood |
Adequate clinical evidence, automated enumeration, commercial availability |
Further analysis limited, false-positive and false-negative, applications need to be expanded |
[18,25,26] |
| CellSearch Profile |
No |
Median number of isolated CTCs, about 140 CTCs per 7.5 ml of blood |
Fewer processing steps, better sensitivity, reproducibility |
Manual enumeration, false-positive and false-negative, limited reports |
[32] |
| AdnaSystem |
No |
Sensitivity, 2 CTCs per 5 ml of blood |
Detection of occult or very low number of CTCs, assessment of genomic markers |
False-positive and false-negative, the sensitivity not improved compared to CellSearch System |
[19,37] |
| Immunomagnetic nanocarrier platform |
Yes |
Capture rate, 78% to 93% |
High capture rate, fewer processing steps, altered biomarkers |
Lacks clinical study, limited reports |
[20] |
| Hybrid nanoparticle |
Yes |
Capture rate, 87.5% |
High capture rate, fewer processing steps |
Lacks clinical study, limited reports |
[41] |
| One-step method |
No |
Capture rate, about 3/1,000 |
Convenient process, low cost |
Low capture rate, lacks clinical data, false-negative |
[21] |
| μ-Nuclear magnetic resonance |
No |
Capture rate, 99.2% |
High sensitivity, short measurement time |
Lacks clinical study |
[42,43] |
| Aptamer-conjugated gold nanoparticles |
No |
The limit of detection is 90 cells |
Bare eyes sense the color change, the detection is rapid |
Unable enumeration, CTC detection is still few |
[22,47] |
| CTC microseparator | Yes | Isolates about 90% of CTCs | The step is simple and high throughput, the further can be carried out | Lacks clinical study | [23,49,50] |
The current methods of detection of CTCs mainly constitute three stages, and a corresponding technology is applied in every stage [24]. Due to the rarity of the CTCs, the blood sample should firstly be pretreated to eliminate background cells, such as erythrocytes and leukocytes, or the tumor cells should be roughly separated according to the physical properties or the biological properties. Then different techniques are applied to identify the ‘real CTC’ by different labeling methods in the cellular and genetic levels. Lastly, powerful analytical instruments are required to analyze the superficial or interior biomarkers of the cell and the expression level of relative oncogenes and anti-oncogenes. Classical detection methods usually carry out the three stages in sequence, and nanoparticles prove to be a powerful tool in the process of CTC detection. Herein, nanotechnology will show its special property and function in the following nanoscale devices during the process of CTC detection.
The application of nanotechnology in CTC detection
CellSearch System
CellSearch System, the current gold standard of CTC detection, is the only detective method approved by the US Food and Drug Administration (FDA) [18,25,26]. The CTC enumeration determined by CellSearch System predicts the disease-free survival (DFS) and overall survival (OS) in patients with breast cancer, colorectal cancer, and prostate cancer in multicenter studies, and it proves to be an independent predictive factor [7,11,27,28].
Although CellSearch System is the gold standard to detect CTCs, many limitations still exist yet. In 131 different histological tumor types and subtypes, 33.6% of CTCs have low or positive expression of EpCAM. Only 81% of adenocarcinomas of the colon, 78% of pancreas cancer, and 71% of hormone-refractory adenocarcinomas of the prostate have positive EpCAM expression [29]. Some tumor lines lose the expression of cytokeratins (CK8, CK18, and CK19) during metastasis [30]. Besides, during the treatment of cancer, if normal epithelial cells fall into the peripheral blood, they will mix up with CTCs and it will be difficult to identify them. In addition, the fact that many tumor cells with high-grade malignancy lose surface antigens during epithelial-mesenchymal transition (EMT) indicates that the detection of the antigen of epithelial cells may miss the most malignant tumor cells [6,15,31]. Furthermore, the separated CTCs undergoing centrifugation and permeabilization cannot be cultured and studied further during the process.
The amount of CTCs is also a limiting factor. The CTCs are so rare that 7.5 ml of blood sample of the total 5 l in an adult may not completely reflect the entire tumor cell population. More blood sample means higher positive detection. Of course, it is not a proper way to improve the sensitivity. Notably, the FDA-cleared CellSearch System may underestimate the number of CTCs; an investigative CellSearch Profile approach could nearly detect a 30-fold higher number of CTCs by using the same paired blood samples [32]. As more accurate and sensitive methods are developed, the CTCs can be tested with less blood sample and shorter turnaround time (a 30-min window is preferred) [33]. The current CellSearch System still cannot meet the need for a point-of-care test since the CTC assignment process is not operated as simple and automatic as the pregnancy test and diabetes test. As a new diagnostic method, the clinical significance of screening CTCs needs to be validated and more evidence should be accumulated to form the acknowledged guidelines and diagnosis criterion [24,34].
Therefore, application to routine clinical practice is still controversial. Many constitutions and guidelines are conservative to the application of the CTC enumeration, so more evidences should be collected to validate its utility in tumor diagnosis and treatment [35]. To overcome the limitations of CellSearch System, many new devices have been developed, although most of those are still in the experimental stage.
AdnaSystem
Due to the rarity of the CTCs in the peripheral blood, the detection technique must show high sensitivity, and reverse transcription polymerase chain reaction (RT-PCR) provides that sensitivity and feasibility [36]. AdnaTest BreastCancerSelect/Detect (AdnaGen AG, Langenhagen, Germany) is a new device to detect CTCs. The AdnaTest can detect as few as one tumor cell in 1 ml of mouse blood in a blood spiking experiment [37]. Human breast cancer cells are pre-enriched firstly by immunological nanobeads functionalized with three different antibodies, then the separated cells are lysed, and PCR is performed for HER2, MUC1, and GA733-2. Similarly, PCR for GA733-2, CEA, and epidermal growth factor receptor (EGFR) is performed to diagnose colon cancer and PCR for PSMA, PSA, and EGFR to diagnose prostate cancer [19,38]. Although the AdnaTest has equivalent sensitivity to that of CellSearch System in detecting two or more CTCs, it has several advantages over the latter [38]. Firstly, after recognition of tumor-associated markers, the isolated mRNA from CTCs can further be used for high-throughput gene expression profiling. Secondly, isolation and detection of stem cell and EMT markers can be realized. Thirdly, the CTC selection process and detection process could be used separately and may decrease the cost of detection of CTCs. However, we should put the shortcomings in mind: on the one hand, the AdnaTest is liable to present false-positive due to marker expression on non-tumor cells; on the other hand, enumeration of CTCs is not likely to undertake.
Versatile immunomagnetic nanocarrier platform
The biomarkers of CTCs have changed during EMT, for example, EpCAM might be down-regulated and expression of vimentin is present, so EpCAM-based methods miss some CTCs [39]. Supposing more useful markers are applied to detect the CTCs, the capture efficiency would be improved significantly. Deng et al. report that the assay method combining CK and EpCAM detects 15% to 111% more CTCs than the CellSearch™ method in patients with higher CTC counts (>20 CTCs per 7.5 ml of blood) [40].
The immunomagnetic nanocarrier platform is a nanoparticle with a gold shell and iron oxide core (Figure 1) [20]. The gold shell is coated with PEG-SH and a heterofunctional linker conjugated with a specific antibody directly. After adding magnetic nanobeads labeled with anti-EpCAM, anti-HER2, anti-EGFR, and anti-CK antibodies into different cell lines, the cell surface markers are identified by dark-field microscopy. The CTC capture efficiency is improved by combining microliquid technology to make full use of the intracellular and extracellular biomarkers. In a tumor cell spiking experiment, peripheral blood from healthy volunteers and a certain amount of CTCs are mixed up, and then the sample is passed through a microfluidic chamber at a continuous rate of 2.5 ml/h in the magnetic field and then identified using fluorescent staining. Notably, the CTC capture efficiency is 70% to 80% by using a nanoparticle coated with anti-CK antibody similar to the FDA-approved CellSearch System. But the CTC capture efficiency can be enhanced by utilizing two different nanoparticles. In addition, the combined tests improve the capture efficiency of EpCAM-low expressed cells. In this way, the EpCAM-low expressed cells are detected efficiently. The immunomagnetic nanocarrier platform captures CTCs efficiently, enumerates CTCs accurately, and analyzes the surface molecules in detail. The process eliminates multiple preprocessing steps such as plasma replacement, centrifugation, and sample transfer between tubes which are commonly used in other assays, for example, the CellSearch method. Thus, the simple and efficient platform is a promising CTC detection method.
Figure 1.
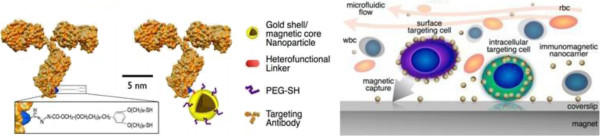
Schematic of an antibody molecule combined with immunomagnetic particle and versatile immunomagnetic nanocarrier platform. Left: schematic of an antibody molecule combined with an immunomagnetic particle. The nanoparticle is composed of a gold shell and an iron oxide core. The surface is functionalized with a heterofunctional linker and polyethylene glycol terminated with dithiol group (PEG-SH) which are used to link the antibody and the nanoparticle. Right: a versatile immunomagnetic nanocarrier platform in microfluidics for capturing CTCs. (Adapted from [20]).
Recently, Lee et al. demonstrate capture, in situ protein expression analysis, and cellular phenotype identification of CTCs simultaneously by using hybrid nanoparticles (HNPs) [41]. Each HNP constitutes three parts: antibodies that bind specifically to a known biomarker for CTCs, a quantum dot that emits fluorescence signals, and biotinylated DNA which is bound with streptavidin-coated quantum dots. They test three different breast cancer subtypes, and the average capture efficiency of CTCs is 87.5% with an identification accuracy of 92.4%. Subsequently, captured cells are released at efficiencies of 86.1% by cleaving the DNA portion with a restriction enzyme. Further study indicates that the released cells are viable and proliferative in vitro. The method has several advantages: it could efficiently capture heterogeneous CTCs, including those with low EpCAM expression; the progress without organic dye could not kill CTCs; and the ‘interested’ cells are selectively released and then cultured in vitro, enabling additional studies possible, such as drug screening and gene analysis. Although the above two methods seem to be perfect for capturing and analyzing CTCs, there is still a long way to apply the new technology to routine clinical practice.
Rapid detection method
During a series of processes, such as erythrocyte lysis, cell centrifugation, and washing, many CTCs get lost and much detection time and money are spent, so it is a trend to study rapid detection. Kim et al. [21] report a rapid one-step method to detect ovarian cancer CTCs. They develop a fluorescent nanoprobe with enhanced fluorescent intensity (magnetic NP [MNP]-SiO2 [rhodamine B isothiocyanate (RITC)]) and then combine it with the MUC1 monoclonal antibody. The compound is added into the sample directly, and after washing and fixation, approximately 107 cells are analyzed in the FCM. The number of cells with a positive signal indicative of OVCAR-3 cells is counted as 23, 32, 58, and 387 for the unspiked blood samples and the indirect blood models with 100, 1,000, and 10,000 OVCAR-3 cells, respectively. This method needs no enrichment and fewer washing, and it also detects CTCs rapidly and feasibly. But the method should be validated via clinical trials. Obviously, the capture rate is so low that the application is limited too much; if more specific monoclonal antibodies are attached to the magnetic nanoparticles, the result will be more satisfying.
Micro-nuclear magnetic resonance (μNMR) is a novel method to detect circulating tumor cells; it consists of solenoidal microcoils, a portable permanent magnet, and custom-built NMR hardware. The measurement time is typically less than 30 min; the detection starts with cell separation, fixation, and pretreatment; then cells are incubated with monoclonal antibodies against the protein of interest coated with TCO-NHS (trans-cyclooctene), and then are reacted with synthesized Tz-MNP (magnetic nanoparticles); and finally, the sample is located in the coil to get detected [42]. The sensitivity and specificity of ovarian cancer and lung cancer diagnosis are more than 90% by means of a quadruple marker subset (MUC1 + EGFR + HER2 + EpCAM) (Figure 2) [43,44]. The quad marker detection of cancer cells in blood is much more sensitive than conventional EpCAM-based detection. The use of μNMR technology for direct measurements of rare CTCs in whole blood is quite creative, and the preclinical validation of a quadruple CTC marker signature harbors promising clinical utility. The key question is whether the CTC levels and characters would be beneficial to the clinical decision.
Figure 2.
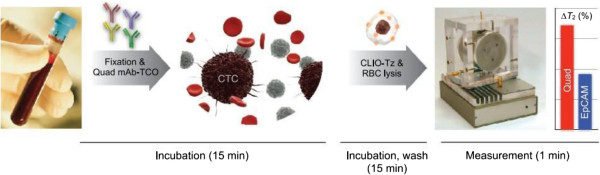
Schematic of the quad-μNMR system. After being incubated with four different mono-antibodies, the sample is spun down in the μNMR device and the sensitivity is improved significantly. (Adapted from [43]).
Aptamer-associated detection strategy
Aptamers are single-stranded DNA or RNA oligonucleotides that bind to target molecules with robust binding affinities. They are generated though repeated rounds of processes termed systematic evolution of ligands by exponential enrichment (SELEX) and bind to a wide range of target molecules, including extracellular ligands and cell surface proteins, small interfering RNAs (siRNAs), chemotherapeutic agents, cell toxins, and nanoparticles [45]. Moreover, man-made aptamers possess several advantages over natural-made antibodies. The man-made aptamers can be generated rapidly and conveniently according to various diagnostic and therapeutic needs and can be stored with long-term stability as dry powder or in solution and act with fast tissue penetration. These chemical properties make aptamers ideal candidates as probes for detection and targeted therapy and may replace the antibody in research, diagnostic methods, and therapeutics [46].
Huang et al. design multiple aptamers conjugated on Au-Ag nanorods, up to 80 fluorophore-labeled aptamers attached on a 12 nm × 56 nm nanorod [47]. This leads to an affinity at least 26-fold higher than the intrinsic affinity of the original aptamer probes, and the fluorescence signal increases by 300-fold compared with those labeled by the individual aptamer probe. Researchers have already designed a creative method to detect CTCs without a fluorochrome by taking advantage of aptamer technology [22]. Due to their plasmon resonance, when the gold nanoparticles come into proximity with one another, their absorption spectra and their scattering profiles change. Therefore, the color of aptamer-conjugated gold nanoparticles (ACGNPs) bound with targeted cells shifts while the unbound ACGNPs show no change, so the bare eyes as well as a colorimetric assay identify the color change and it is likely to be a very useful assay technique for point-of-care diagnostics.
Besides, aptamers have a wide application in tumor therapy. As aptamers are an excellent intermedium, they combine various organic molecules to form chimeras, for example, aptamer-antibody chimera, aptamer-protein chimera, aptamer-siRNA chimera, and aptamer-miRNA chimera and then combine with nanoparticle as a carrier to be absorbed by the CTCs to perform pharmacological action [46,48].
Magnetophoresis technology to separate CTCs
That the magnetic particle is drove to a certain direction in the magnetic field is defined as magnetophoresis, and the course can be compared with electrophoresis course, which is an efficient and high-throughput separation technology in recent years [49,50]. It is an easy, rapid, and accurate method to separate RNA and tumor cells [51-53]. Herein, we introduce a novel CTC separation device applying the lateral magnetophoresis principle designed by Kim et al. [23]. The device is mainly fabricated by two pieces of glass slides, and the core which consists of a ferromagnetic permalloy wire array is hidden between them. The device detects CTCs effectively and efficiently since experimental results indicate that the CTC microseparator isolates approximately 90% of CTCs spiked into blood samples with a flow rate of up to 5 ml/h and the purity of separated CTCs is 97%; the overall isolation procedure can be completed within 15 min for 200 μL of peripheral blood. Besides, clinical practice has demonstrated that it can monitor the therapeutic effect and recurrence of tumor.
The process is quite simple and only needs three steps: buffer injection only, sample and buffer at the same flow rate, and a second injection of buffer only. So the CTC microseparator platform is easy to establish and automate. The technology has many unique properties. Firstly, the separation method maintains intactness and contributed to further study about CTCs. Secondly, the simple procedure reduces CTC loss during the enrichment process, improving the sensitivity. Thirdly, the CTC microseparator can be simply applied for the separation of various CTCs by using other tumor-specific antibodies and changing the size of the magnetic nanobeads to optimize the magnetic force to detect specific tumor CTCs. As it can be combined with other advanced genetic detection methods (e.g., single-cell RT-PCR), it pushes the development of an automated platform for CTC-based cellular and molecular assays.
The application of nanotechnology in CTC therapy
Nanoparticles have many unique and excellent physical properties, and we can take advantage of the properties to overcome the limitations of traditional diagnostic and therapeutic methods [54]. The nanoparticles applied in tumor therapy currently include liposomes, polymeric nanoparticles, protein nanoparticles, ceramic nanoparticles, metallic nanoparticles, and carbon nanotubes [55], but only a few are approved by the FDA, such as pegylated liposomal doxorubicin (Doxil in the USA and Caelyx outside the USA), liposomal daunorubicin (DaunoXome), non-pegylated liposomal doxorubicin (Myocet), and albumin-bound paclitaxel nanoparticles [56]. Faltas has already concluded some CTC therapeutic methods, but many are still in the experimental stage and there are many problems to be solved before being applied in routine clinical practice [57]. Herein, we introduce some novel methods for CTC therapy.
The nanoparticles and the halloysite nanotube fixed on the substrate significantly increase the number of combined selectin and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), thus improving the capture efficiency for targeting CTCs [58,59]. Besides, TRAIL induces a death signal via the caspase pathway [60,61]. There is no significant effect of TRAIL on hematopoietic stem cells and other normal blood cells, but it induces apoptosis in a wide variety of cancer cells. Rana et al. design a capillary flow chamber by a bionic method [62]. The surface of the flow capillary chamber is functionalized with TRAIL and E-selectin and takes 1 h to kill 30% of the captured cells, but in the static condition, it will take 4 h to kill 30% of the cells. Different protein molecules could be applied in the device to capture various CTCs to induce apoptosis; the same technology can be applied in clinical practice to capture rare cells in the peripheral blood. The technology applied in clinical practice will significantly decrease the load of the disseminated tumor cells in cancer patients.
Besides, the microtube flow device proves to kill CTCs more efficiently if the specimen is pretreated with a particular drug. Clinical trials indicate that aspirin prevents the occurrence of colorectal cancer [63-66]. Aspirin treatment alone kills only about 3% of Colo205 cells when compared to untreated control. When cells pretreated with aspirin are perfused through the microtube flow device for 1 h and analyzed 18 h later, the kill rate is 44.32% while perfusing untreated cells for 1 h shows a kill rate of 17%. The kill rate of cells perfused through the microtube following aspirin pretreatment for 1 h is found to be similar to that of untreated cells perfused over the combined surface for 2 h. This means that the CTCs pretreated with aspirin can be killed more rapidly and the microtube functionalized with TRAIL and E-selectin can be combined with drugs to kill cancer cells to reduce the cells more quickly and efficiently [67].
Another novel nanodevice has been developed to kill CTCs. The strong absorbance of single-walled carbon nanotubes (SWCNTs) in 700 to 1,100-nm near-infrared (NIR) light can be used for optical stimulation of nanotubes inside living cells to afford various useful functions. When the nanotubes are coated with folacin, they can combine with tumor cells with folacin specially, and then consistent NIR light radiation causes cell death without harming receptor-free normal cells. So SWCNT combined with specific chemical matter under NIR light radiation is a novel way to convey drugs and treat tumors [68,69]. Neves et al. [70] design a SWCNT-annexin V (AV) conjugate by taking advantage of the phenomenon that AV combines specially with the anionic phospholipids expressed externally on the surface of tumor cells and endothelial cells that line the tumor vasculature. It kills most 4 T1 mouse mammary tumors for the majority of the animals by 11 days since the irradiation is at a wavelength of 980 nm. The combination of photothermal therapy with the immunoadjuvant cyclophosphamide results in an increased survival rate. Also, in vivo results suggest that the SWCNT-AV/NIR treatment is a promising approach to treat cancer. Moreover, Hossain et al. utilize iron oxide nanoparticles and bismuth nanoparticles to combine the folate receptor and then kill CTCs under X-ray radiation [71]. The systemic toxicity associated with conventional therapy may thus be significantly reduced in targeted photodynamic therapy, but a series of problems should be solved before entering clinical practice, such as the toxicity of nanoparticles and how to eliminate them in the body after use [72].
One new trend of manipulating CTCs is to kill CTCs in vivo. Researchers have already developed a medical device, functionalized structured medical wire (FSMW), that offers opportunity of capturing CTCs from the circulating blood of cancer patients (Figure 3) [73]. The device is based on a stainless steel medical wire of 0.5 mm in diameter and 160 mm in length. The first 20 mm is plated with a thick gold layer, and a synthetic polycarboxylate and anti-EpCAM are attached to the gold layer. The EpCAM-functionalized FSMW surface dwells in the lumen of the vein for 30 min, and the total volume of blood that comes in contact with the FSMW is estimated to be 1.5 to 3 l. The capture rate of CTCs is 10/12 for patients with breast cancer with a median of 5.5 CTCs and 12/12 for patients with non-small lung cell cancer (NSCLC) with a median of 16 CTCs. As an in vivo device to capture CTCs, it is very unique and paves the way for future study. It is not only a way to capture CTCs but also offers a potential therapeutic trend which will initiate ‘dialysis therapy’ for cancer therapy just as dialysis therapy for renal failure.
Figure 3.
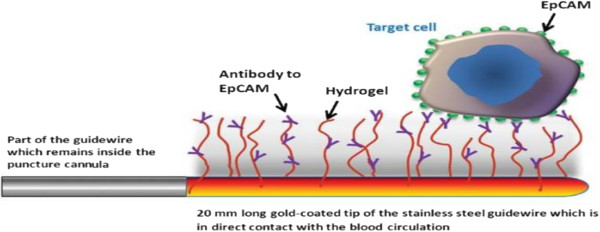
Schematic drawing of the functionalized tip of the FSMW. The gold-coated tip of the stainless steel captures CTCs in the circulating blood for 30 min. (Adapted from [73]).
As the CTCs shed from the primary tumor inconstantly, the blood samples extracted from the patients only reflected a temporal condition [6]. In order to realize consistent detection and treatment, the implantable vascular shunt device is an ideal design. Wojciechowski et al. have already used the device to capture CD34+ hematopoietic stem and progenitor cells (HSPCs) [74]. Similarly, we can design a vascular shunt to capture CTCs. The CTCs should be captured firstly and then induce apoptosis to decrease the CTC load and inhibit the tumor metastatic progress. Then we capture specific CTCs by means of different molecules, such as CD44, CK, and E-selectin. Apoptotic molecules including TRAIL [62,75], Fas-L [76-78], TNF [79,80], and liposomes with encapsulated siRNA [81] will make a difference in the kill of CTCs.
Scarberry et al. have demonstrated that targeted removal of migratory tumor cells by functionalized magnetic nanoparticles impedes metastasis and tumor progression [82]. They mix the magnetic nanoparticles (MNPs) functionalized with ephrin-A1 mimetic peptides selective for the EphA2 receptor with the peritoneal fluid withdrawn from female C57BL/6 mice injected with a murine ovarian cancer cell line. Then the processed peritoneal fluid is re-introduced into the peritoneal cavity. They found that tumor progression of the experimental group is 10.77 times slower than that of the control group which receives no intervention, and the median time to endpoint for the experimental group (49 days) is 32.4% longer than that for the control group (37 days), which indicates that the intervention is quite efficient. We envision that the technology could be applied to cancer patients. Firstly, the body liquid (including blood and peritoneal fluid) is pumped out and then mixes with the MNPs to discharge the malignant cells; the purged liquid is then returned to the body. Once the protocol is accomplished at a satisfying level, it shall be an amazing breakthrough in tumor therapy.
Conclusions
At present, the most important applications of the CTC technology are the monitoring of the CTC account of cancer patients and evaluation of the metastasis of malignant tumor [6,7,83]. But the present devices are not ideal enough to meet the application needs because the problems of insufficient capture, low purity, and narrow detection spectrum still need to be addressed. Manipulating CTCs in vivo directly is an attractive direction; it denotes that the process can reduce tumor metastasis and even cut metastasis while dealing with the primary tumor, and it also means that we should overcome some great challenges: temporal heterogeneity of dissemination and sample size limitations for in vivo techniques [25], so much more effort should be paid to enhance the practical application of CTCs. Nanotechnology has already been studied a lot in CTCs, and it probably has a wider application in tumor diagnosis and treatment. We can design a long therapeutical guidewire by virtue of nanotechnology and CTC theory which is placed in the vessel by means of interventional methods. The surface of the guidewire is covered with various kinds of nanoparticles decorated with adhesion molecules and apoptosis-inducing ligands for the capture and kill of CTCs. In order to realize personal therapy, the nanoparticles can be modified according to the characteristics of the CTCs. The therapy outcomes can be quantified by comparing the number of CTCs before and after the process. If we keep the number of CTCs at quite a low level, patients can survive longer even if they live with tumor [7].
In a word, with the advent of translational medicine and multiple-discipline treatment (MDT), the basic research products about nanotechnology and CTCs are supposed to offer a better guide for clinical decision. Meanwhile, we should also pay attention on the side effects of nanotechnology while exploiting it. In the future, the use of nanotechnology for CTC detection and kill is promising, and it will offer a multifunctional, potable platform in the field of anticancer therapy.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YG collected and reviewed the data and drafted the manuscript. ZY modified the draft in the first version and after revision. Both authors helped in drafting the manuscript. Both authors read and approved the final manuscript.
Contributor Information
Yang Gao, Email: gaoyang8992@163.com.
Zhou Yuan, Email: zhouyuan851@163.com.
Acknowledgements
The authors thank The Sixth People's Hospital Affiliated to Shanghai Jiao Tong University for the technical assistance during this study.
References
- Dong Y, Skelley AM, Merdek KD, Sprott KM, Jiang C, Pierceall WE, Lin J, Stocum M, Carney WP, Smirnov DA. Microfluidics and circulating tumor cells. J Mol Diagn. 2013;15:149–157. doi: 10.1016/j.jmoldx.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, Bray F. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Wittekind C, Neid M. Cancer invasion and metastasis. Oncology. 2005;69(Suppl 1):14–16. doi: 10.1159/000086626. [DOI] [PubMed] [Google Scholar]
- Ashworth TR. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aust Med J. 1869;14:146–147. [Google Scholar]
- Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253:180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC, Fritsche HA, Hortobagyi GN, Terstappen LW. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, Scher HI. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- O'Flaherty JD, Gray S, Richard D, Fennell D, O'Leary JJ, Blackhall FH, O'Byrne KJ. Circulating tumour cells, their role in metastasis and their clinical utility in lung cancer. Lung Cancer. 2012;76:19–25. doi: 10.1016/j.lungcan.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Bonnomet A, Brysse A, Tachsidis A, Waltham M, Thompson EW, Polette M, Gilles C. Epithelial-to-mesenchymal transitions and circulating tumor cells. J Mammary Gland Biol Neoplasia. 2010;15:261–273. doi: 10.1007/s10911-010-9174-0. [DOI] [PubMed] [Google Scholar]
- Cai W, Gao T, Hong H, Sun J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnology, Science and Applications. 2008;1:17–32. doi: 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- He S, Li P, Long T, Zhang N, Fang J, Yu Z. Detection of circulating tumour cells with the Cell Search system in patients with advanced-stage head and neck cancer: preliminary results. J Laryngol Otol. 2013;127:788–793. doi: 10.1017/S0022215113001412. [DOI] [PubMed] [Google Scholar]
- Andreopoulou E, Yang LY, Rangel KM, Reuben JM, Hsu L, Krishnamurthy S, Valero V, Fritsche HA, Cristofanilli M. Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect™ versus Veridex Cell Search™ system. Int J Cancer. 2012;130:1590–1597. doi: 10.1002/ijc.26111. [DOI] [PubMed] [Google Scholar]
- Wu CH, Huang YY, Chen P, Hoshino K, Liu H, Frenkel EP, Zhang JX, Sokolov KV. Versatile immunomagnetic nanocarrier platform for capturing cancer cells. ACS Nano. 2013;7:8816–8823. doi: 10.1021/nn403281e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Chung HH, Jeong MS, Song MR, Kang KW, Kim JS. One-step detection of circulating tumor cells in ovarian cancer using enhanced fluorescent silica nanoparticles. Int J Nanomedicine. 2013;8:2247–2257. doi: 10.2147/IJN.S45059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medley CD, Smith JE, Tang Z, Wu Y, Bamrungsap S, Tan W. Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Anal Chem. 2008;80:1067–1072. doi: 10.1021/ac702037y. [DOI] [PubMed] [Google Scholar]
- Kim S, Han SI, Park MJ, Jeon CW, Joo YD, Choi IH, Han KH. Circulating tumor cell microseparator based on lateral magnetophoresis and immunomagnetic nanobeads. Anal Chem. 2013;85:2779–2786. doi: 10.1021/ac303284u. [DOI] [PubMed] [Google Scholar]
- Hong B, Zu Y. Detecting circulating tumor cells: current challenges and new trends. Theranostics. 2013;3:377–394. doi: 10.7150/thno.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AD, King MR. Nanobiotechnology for the capture and manipulation of circulating tumor cells. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:291–309. doi: 10.1002/wnan.168. [DOI] [PubMed] [Google Scholar]
- Lowes LE, Hedley BD, Keeney M, Allan AL. User-defined protein marker assay development for characterization of circulating tumor cells using the Cell Search® system. Cytometry A. 2012;81:983–995. doi: 10.1002/cyto.a.22158. [DOI] [PubMed] [Google Scholar]
- Krell J, Stebbing J. Circulating tumour cells as biomarkers in early breast cancer. Lancet Oncol. 2012;13:653–654. doi: 10.1016/S1470-2045(12)70245-0. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:1223–1229. doi: 10.1093/annonc/mdn786. [DOI] [PubMed] [Google Scholar]
- Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35:122–128. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Willipinski-Stapelfeldt B, Riethdorf S, Assmann V, Woelfle U, Rau T, Sauter G, Heukeshoven J, Pantel K. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;11:8006–8014. doi: 10.1158/1078-0432.CCR-05-0632. [DOI] [PubMed] [Google Scholar]
- Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, Martens JW, Gratama JW, Sleijfer S, Foekens JA. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst. 2009;101:61–66. doi: 10.1093/jnci/djn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores LM, Kindelberger DW, Ligon AH, Capelletti M, Fiorentino M, Loda M, Cibas ES, Janne PA, Krop IE. Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer. Br J Cancer. 2010;102:1495–1502. doi: 10.1038/sj.bjc.6605676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore ML, Steindel SJ, Smith JA. Evaluating stat testing options in an academic health center: therapeutic turnaround time and staff satisfaction. Clin Chem. 1998;44:1597–1603. [PubMed] [Google Scholar]
- Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- Nagaiah G, Abraham J. Circulating tumor cells in the management of breast cancer. Clin Breast Cancer. 2010;10:209–216. doi: 10.3816/CBC.2010.n.028. [DOI] [PubMed] [Google Scholar]
- Hampton R, Walker M, Marshall J, Juhl H. Differential expression of carcinoembryonic antigen (CEA) splice variants in whole blood of colon cancer patients and healthy volunteers: implication for the detection of circulating colon cancer cells. Oncogene. 2002;21:7817–7823. doi: 10.1038/sj.onc.1205906. [DOI] [PubMed] [Google Scholar]
- Gorges TM, Tinhofer I, Drosch M, Rose L, Zollner TM, Krahn T, von Ahsen O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. doi: 10.1186/1471-2407-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulova V, Kolostova K, Zima T. Methods for detection of circulating tumour cells and their clinical value in cancer patients. Folia Biol. 2011;57:151–161. doi: 10.14712/fb2011057040151. [DOI] [PubMed] [Google Scholar]
- Danila DC, Pantel K, Fleisher M, Scher HI. Circulating tumors cells as biomarkers: progress toward biomarker qualification. Cancer J. 2011;17:438–450. doi: 10.1097/PPO.0b013e31823e69ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Herrler M, Burgess D, Manna E, Krag D, Burke JF. Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients. Breast Cancer Res. 2008;10:R69. doi: 10.1186/bcr2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Cho HY, Oh JH, Namkoong K, Lee JG, Park JM, Lee SS, Huh N, Choi JW. Simultaneous capture and in situ analysis of circulating tumor cells using multiple hybrid nanoparticles. Biosens Bioelectron. 2013;47:508–514. doi: 10.1016/j.bios.2013.03.040. [DOI] [PubMed] [Google Scholar]
- Haun JB, Castro CM, Wang R, Peterson VM, Marinelli BS, Lee H, Weissleder R. Micro-NMR for rapid molecular analysis of human tumor samples. Sci Transl Med. 2011;3:71ra16. doi: 10.1126/scitranslmed.3002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazani AA, Castro CM, Gorbatov R, Lee H, Weissleder R. Sensitive and direct detection of circulating tumor cells by multimarker micro-nuclear magnetic resonance. Neoplasia. 2012;14:388–395. doi: 10.1596/neo.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazani AA, Pectasides M, Sharma A, Castro CM, Mino-Kenudson M, Lee H, Shepard JA, Weissleder R. Molecular characterization of scant lung tumor cells using iron-oxide nanoparticles and micro-nuclear magnetic resonance. Nanomedicine: Nanotechnology, Biology, and Medicine. 2014;10:661–668. doi: 10.1016/j.nano.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas AS, Mi J, Clary BM, White RR. Aptamer applications for targeted cancer therapy. Future Oncol. 2010;6:1117–1126. doi: 10.2217/fon.10.67. [DOI] [PubMed] [Google Scholar]
- Fang X, Tan W. Aptamers generated from cell-SELEX for molecular medicine: a chemical biology approach. Acc Chem Res. 2010;43:48–57. doi: 10.1021/ar900101s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YF, Chang HT, Tan W. Cancer cell targeting using multiple aptamers conjugated on nanorods. Anal Chem. 2008;80:567–572. doi: 10.1021/ac702322j. [DOI] [PubMed] [Google Scholar]
- Kanwar JR, Roy K, Kanwar RK. Chimeric aptamers in cancer cell-targeted drug delivery. Crit Rev Biochem Mol Biol. 2011;46:459–477. doi: 10.3109/10409238.2011.614592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinjing C, Qianhong W. Bioseparation and bioanalysis based on magnetophoresis. Prog Chem. 2006;2:344–348. [Google Scholar]
- Suwa M, Watarai H. Magnetophoretic velocimetry of manganese(II) in a single emulsion droplet at the femtomole level. Anal Chem. 2001;73:5214–5219. doi: 10.1021/ac010418v. [DOI] [PubMed] [Google Scholar]
- Lee H, Jung J, Han SI, Han KH. High-speed RNA microextraction technology using magnetic oligo-dT beads and lateral magnetophoresis. Lab Chip. 2010;10:2764–2770. doi: 10.1039/c005145d. [DOI] [PubMed] [Google Scholar]
- Forbes TP, Forry SP. Microfluidic magnetophoretic separations of immunomagnetically labeled rare mammalian cells. Lab Chip. 2012;12:1471–1479. doi: 10.1039/c2lc40113d. [DOI] [PubMed] [Google Scholar]
- Schneider T, Karl S, Moore LR, Chalmers JJ, Williams PS, Zborowski M. Sequential CD34 cell fractionation by magnetophoresis in a magnetic dipole flow sorter. Analyst. 2010;135:62–70. doi: 10.1039/b908210g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 2008;26:57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Faltas B. Cornering metastases: therapeutic targeting of circulating tumor cells and stem cells. Front Oncol. 2012;2:68. doi: 10.3389/fonc.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Allio BA, Foster DG, King MR. Nanoparticle coatings for enhanced capture of flowing cells in microtubes. ACS Nano. 2010;4:174–180. doi: 10.1021/nn900442c. [DOI] [PubMed] [Google Scholar]
- Hughes AD, King MR. Use of naturally occurring halloysite nanotubes for enhanced capture of flowing cells. Langmuir. 2010;26:12155–12164. doi: 10.1021/la101179y. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- Plasilova M, Zivny J, Jelinek J, Neuwirtova R, Cermak J, Necas E, Andera L, Stopka T. TRAIL (Apo2L) suppresses growth of primary human leukemia and myelodysplasia progenitors. Leukemia. 2002;16:67–73. doi: 10.1038/sj.leu.2402338. [DOI] [PubMed] [Google Scholar]
- Rana K, Liesveld JL, King MR. Delivery of apoptotic signal to rolling cancer cells: a novel biomimetic technique using immobilized TRAIL and E-selectin. Biotechnol Bioeng. 2009;102:1692–1702. doi: 10.1002/bit.22204. [DOI] [PubMed] [Google Scholar]
- Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- Berman KS. Aspirin and the prevention of colorectal cancer. N Engl J Med. 2003;348:2466–2467. author reply 2466–2467. [PubMed] [Google Scholar]
- Rana K, Reinhart-King CA, King MR. Inducing apoptosis in rolling cancer cells: a combined therapy with aspirin and immobilized TRAIL and E-selectin. Mol Pharm. 2012;9:2219–2227. doi: 10.1021/mp300073j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam NW, O'Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Xing D, Ou Z, Wu B, Resasco DE, Chen WR. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J Biomed Opt. 2009;14:021009. doi: 10.1117/1.3078803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves LF, Krais JJ, Van Rite BD, Ramesh R, Resasco DE, Harrison RG. Targeting single-walled carbon nanotubes for the treatment of breast cancer using photothermal therapy. Nanotechnology. 2013;24:375104. doi: 10.1088/0957-4484/24/37/375104. [DOI] [PubMed] [Google Scholar]
- Hossain M, Luo Y, Sun Z, Wang C, Zhang M, Fu H, Qiao Y, Su M. X-ray enabled detection and eradication of circulating tumor cells with nanoparticles. Biosens Bioelectron. 2012;38:348–354. doi: 10.1016/j.bios.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Mir Y, Elrington SA, Hasan T. A new nanoconstruct for epidermal growth factor receptor-targeted photo-immunotherapy of ovarian cancer. Nanomedicine. 2013;9:1114–1122. doi: 10.1016/j.nano.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo-Zeni N, Mewes S, Niestroj R, Gasiorowski L, Murawa D, Nowaczyk P, Tomasi T, Weber E, Dworacki G, Morgenthaler NG, Jansen H, Propping C, Sterzynska K, Dyszkiewicz W, Zabel M, Kiechle M, Reuning U, Schmitt M, Lücke K. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int J Oncol. 2012;41:1241–1250. doi: 10.3892/ijo.2012.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski JC, Narasipura SD, Charles N, Mickelsen D, Rana K, Blair ML, King MR. Capture and enrichment of CD34-positive haematopoietic stem and progenitor cells from blood circulation using P-selectin in an implantable device. Br J Haematol. 2008;140:673–681. doi: 10.1111/j.1365-2141.2007.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder RN, El-Deiry WS. Caspase-8 regulation of TRAIL-mediated cell death. Exp Oncol. 2012;34:160–164. [PubMed] [Google Scholar]
- Villa-Morales M, Fernandez-Piqueras J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:85–101. doi: 10.1517/14728222.2011.628937. [DOI] [PubMed] [Google Scholar]
- O'Brien DI, Nally K, Kelly RG, O'Connor TM, Shanahan F, O'Connell J. Targeting the Fas/Fas ligand pathway in cancer. Expert Opin Ther Targets. 2005;9:1031–1044. doi: 10.1517/14728222.9.5.1031. [DOI] [PubMed] [Google Scholar]
- Yang C, Liu HZ, Fu ZX. PEG-liposomal oxaliplatin induces apoptosis in human colorectal cancer cells via Fas/FasL and caspase-8. Cell Biol Int. 2012;36:289–296. doi: 10.1042/CBI20100825. [DOI] [PubMed] [Google Scholar]
- Waters JP, Pober JS, Bradley JR. Tumour necrosis factor and cancer. J Pathol. 2013;230:241–248. doi: 10.1002/path.4188. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Huang Z, King MR. An immobilized nanoparticle-based platform for efficient gene knockdown of targeted cells in the circulation. Gene Ther. 2009;16:1271–1282. doi: 10.1038/gt.2009.76. [DOI] [PubMed] [Google Scholar]
- Scarberry KE, Mezencev R, McDonald JF. Targeted removal of migratory tumor cells by functionalized magnetic nanoparticles impedes metastasis and tumor progression. Nanomedicine (Lond) 2011;6:69–78. doi: 10.2217/nnm.10.103. [DOI] [PubMed] [Google Scholar]
- Riethdorf S, Wikman H, Pantel K. Review: biological relevance of disseminated tumor cells in cancer patients. Int J Cancer. 2008;123:1991–2006. doi: 10.1002/ijc.23825. [DOI] [PubMed] [Google Scholar]


