Abstract
Purpose of review
Because early findings indicated that native low density lipoprotein (LDL) did not substantially increase macrophage cholesterol content during in vitro incubations, investigators presumed that LDL must be modified in some way to trigger its uptake by the macrophage. The purpose of this review is to discuss recent findings showing that native unmodified LDL can induce massive macrophage cholesterol accumulation mimicking macrophage foam cell formation that occurs within atherosclerotic plaques.
Recent findings
Macrophages that show high rates of fluid-phase pinocytosis also show similar high rates of uptake of native unmodified LDL through non-receptor mediated uptake within both macropinosomes and micropinosomes. Non-saturable fluid-phase uptake of LDL by macrophages converts the macrophages into foam cells. Different macrophage phenotypes demonstrate either constitutive fluid-phase pinocytosis or inducible fluid-phase pinocytosis. Fluid-phase pinocytosis has been demonstrated by macrophages within mouse atherosclerotic plaques indicating that this pathway contributes to plaque macrophage cholesterol accumulation.
Summary
Contrary to what has been believed previously, macrophages can take up large amounts of native unmodified LDL by receptor-independent, fluid-phase pinocytosis converting these macrophages into foam cells. Thus, targeting macrophage fluid-phase pinocytosis should be considered when investigating strategies to limit macrophage cholesterol accumulation in atherosclerotic plaques.
Keywords: LDL, macrophages, fluid-phase pinocytosis, cholesterol, macropinocytosis
Introduction
Macrophage accumulation of cholesterol that transforms the macrophages into so called foam cells within atherosclerotic plaques is considered to be a key pathologic event in the development of plaques. Macrophages may take up cholesterol that has accumulated in various physical forms within the extracellular spaces of plaques [1]. This cholesterol burden is comprised in part by lipoproteins such as LDL that enter the vessel wall from the blood and remain unbound. Unbound LDL levels in the intima, the innermost layer of the vessel wall where atherosclerotic plaques form, are twice that of plasma LDL levels [2, 3], a finding that is not widely appreciated, but which has important implications for studies of how macrophages accumulate cholesterol. Macrophage uptake of extracellular bound cholesterol may be necessary to initiate reverse cholesterol transport of this cholesterol out of the vessel wall. On the other hand, macrophage uptake of extracellular unbound LDL may directly contribute to vessel wall cholesterol accumulation by preventing the LDL from exiting the vessel wall through transport either back into the blood or into the vessel lymphatics. Although it is not clear to what extent macrophage uptake of cholesterol contributes to vessel wall cholesterol accumulation, macrophage cholesterol accumulation has pathologic consequences. Macrophage accumulation of cholesterol promotes macrophage release of metalloproteinases and expression of tissue factor, processes that can promote plaque rupture and subsequent plaque thrombosis, respectively [4–6].
Because macrophage foam cell formation could not be achieved in earlier studies through incubation of cultured macrophages with even high levels of native LDL, investigators concluded that LDL must undergo some type of modification that would promote macrophage binding and uptake of the modified LDL [7]. In this regard, the LDL receptor cannot explain macrophage foam cell formation because patients and animals that lack the LDL receptor nevertheless show foam cells in their atherosclerotic plaques, and macrophage LDL receptors appear to be down regulated in atherosclerotic plaques reflecting LDL receptor down-regulation in the presence of excess cholesterol [8]. Various enzymatic and non-enzymatic modifications to LDL or complexing of LDL with other potential macrophage ligands were shown to promote macrophage uptake of LDL producing various levels of macrophage cholesterol accumulation [9]. The most extensively studied foam cell formation hypothesis is that oxidative modification of LDL facilitates its recognition by macrophage scavenger receptors that mediate macrophage uptake of the oxidized LDL. However, certain findings challenge this hypothesis. LDL isolated from human aorta is not oxidized sufficiently to trigger macrophage uptake through scavenger receptors [10]; macrophage foam cells form in mice with genetic deletion of those scavenger receptors that mediate mouse macrophage uptake of oxidized LDL [11••]; and macrophage uptake of oxidized LDL produces mostly lysosomal rather than mostly lipid droplet accumulation of cholesterol [12–14], the latter a characteristic of most plaque macrophage foam cells.
Ironically, while Brown and Goldstein first suggested the modified LDL hypothesis of macrophage foam cell formation that led to subsequent decades of related research, these investigators also described non-receptor mediated, fluid-phase uptake of LDL by mutant fibroblasts that lacked the LDL receptor [15, 16]. Fluid-phase uptake by cells occurs when cells engulf extracellular fluid and any material such as a lipoprotein contained in that fluid. Thus, uptake of lipoprotein by fluid-phase pinocytosis (also called bulk-phase endocytosis) does not involve cell binding of the lipoprotein. Uptake of the lipoprotein is linearly related to its concentration and does not show saturation of uptake that is characteristic of receptor-mediated uptake processes (Figure 1). Also, in contrast to receptor-mediated uptake processes, fluid-phase mediated uptake of labeled lipoprotein is not competed by excess unlabeled lipoprotein. These characteristics are summarized in Table 1.
Figure 1. Comparison of receptor-mediated uptake of acetylated LDL and fluid-phase-mediated uptake of LDL.
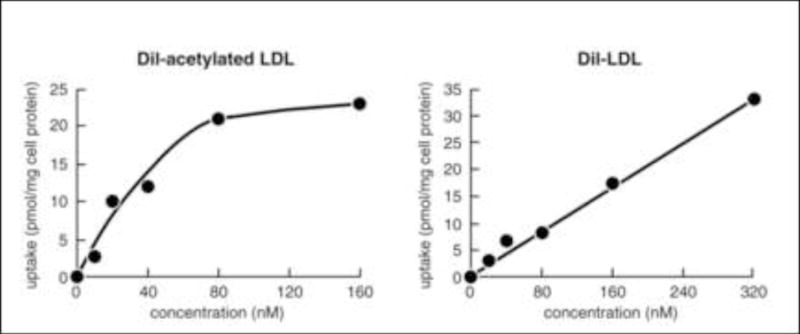
M-CSF-differentiated human monocyte-derived macrophages were incubated with increasing concentrations of either fluorescent DiI-labeled acetylated LDL, which binds the scavenger receptor, or DiI-LDL for 5 hours. Then, macrophage uptake of the fluorescent lipoproteins was determined. DiI-acetylated LDL uptake shows saturation at a lipoprotein concentration of 80 nM (i.e., 44 ug/ml) consistent with receptor-mediated endocytosis, while DiI-LDL uptake does not show saturation, consistent with fluid-phase pinocytosis. Data adapted from [17••].
Table 1.
Comparison of fluid-phase micropinocytosis and macropinocytosis
| Characteristic | Micropinocytosis | Macropinocytosis |
|---|---|---|
| Size of pinosome | < 0.1 μm | 0.2 – 5.0 μm |
| Multiple pathways | yes | yes |
| Actin-dependent | 1some pathways | all pathways |
| Constitutive | usually | in some cell types |
| Signaling mediators | vary with cell type | vary with cell type |
usually at a very low level in most cells and at a high level in the M-CSF phenotype macrophages
Fluid-phase pinocytosis can occur by either micropinocytosis in small micropinosome vesicles less than 0.1 μm, or macropinocytosis in large macropinosome vacuoles typically greater than 0.2 μm (Figure 2). Because of the large size of the forming macropinosomes, this uptake mechanism can be observed by phase-contrast light microscopy (see supplemental online material, Figure S1). Macropinocytosis is an actin-dependent endocytic pathway carried out by some cell types including macrophages in which ruffling plasma membranes fuse to enclose fluid and contained solute within the macropinosome vacuoles. Macropinocytosis is thus different from actin-dependent phagocytosis triggered by plasma membrane binding of large particles that are then engulfed by cells within phagocytic vacuoles. Phagocytic vacuoles are relatively free of fluid because of the tight apposition of the engulfed particle with the cell’s plasma membrane. The characteristics of micropinocytosis and macropinocytosis are summarized in Table 2.
Figure 2. Micropinocytosis and macropinocytosis mediate fluid-phase pinocytosis of LDL.
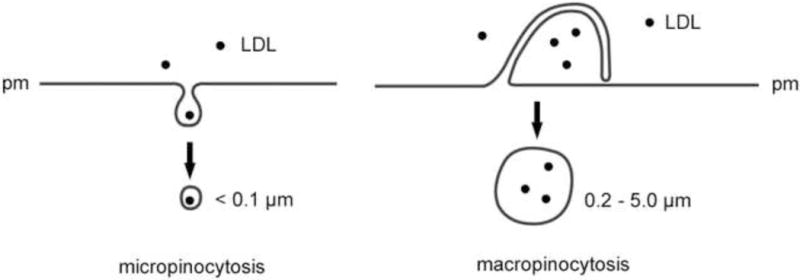
Micropinocytosis includes uptake of fluid into small vesicles by clathrin-mediated, caveolae-mediated, and clathrin and caveolae-independent pinocytosis. Micropinocytosis may be actin-dependent or independent. Macropinocytosis is an actin-dependent pinocytic pathway by which macrophages can engulf droplets of extracellular fluid within large vacuoles formed by fusion of a plasma membrane extension with non-extended plasma membrane (pm) as shown.
Table 2.
Comparison of fluid-phase and receptor-mediated endocytosis of LDL
| Characteristic | Fluid-phase | Receptor-mediated |
|---|---|---|
| LDL binds to cell | no | yes |
| LDL uptake shows saturation | no | yes |
| Uptake of 125I-LDL can be competed by unlabeled LDL | no | yes |
| Uptake of 125I-LDL and 125I-albumin are similar1 | yes | no |
when added at the same protein concentration
Brown and Goldstein showed that human skin fibroblasts take up LDL both through the LDL receptor and through fluid-phase pinocytosis [15, 16]. In fibroblasts, LDL taken up through the LDL receptor led to cellular cholesterol accumulation, while LDL taken up by fluid-phase pinocytosis was likewise degraded by lysosomes, but its cholesterol was excreted rather than being retained by the fibroblast. Other early studies showed non-saturable uptake of LDL that did not stimulate cholesteryl ester accumulation in unactivated mouse peritoneal macrophages and unactivated human LDL receptor-negative, monocyte-derived macrophages differentiated with human serum [19, 20]. While most of the non-saturable LDL uptake could be attributed to fluid-phase pinocytosis [15, 21], it is likely that LDL uptake in these studies was mediated by fluid-phase micropinocytosis rather than macropinocytosis because macropinocytosis does not occur in these cells without some form of activation. J774 mouse macrophages show cholesterol esterification induced by native LDL taken up through both LDL receptor and non-LDL receptor-mediated mechanisms. Some of the LDL uptake possibly occurred by fluid-phase pinocytosis in this macrophage cell line [22].
Because fluid-phase pinocytosis of LDL in these early studies did not result in substantial cellular cholesterol accumulation, for decades there was little subsequent attention paid to fluid-phase pinocytosis as a mechanism for macrophage foam cell formation. This review will discuss recent studies that show fluid-phase pinocytosis is an important mechanism for cellular cholesterol accumulation, especially for macrophages, a cell type that can show extremely high levels of fluid-phase pinocytosis of LDL converting these cells into foam cells.
Macrophages take up large quantities of native LDL by fluid-phase pinocytosis
The long delay in the recognition of fluid-phase pinocytosis as an important mechanism for macrophage cholesterol accumulation also can be explained by the fact that most studies of macrophage LDL uptake have been carried out with rather low concentrations of LDL, typically 50 ug protein/ml or less, reflecting the concentrations that saturate receptors for uptake of native or modified LDL. As mentioned above, much higher levels of LDL that can exceed 2 mg/ml exist in the vessel intima [2, 3]. Fluid-phase pinocytosis shows substantial levels of LDL uptake compared to receptor-mediated uptake only at these higher LDL levels. Secondly, cultured macrophages show phenotype heterogeneity depending on the macrophage source including tissue of origin (e.g., blood monocytes, bone marrow, peritoneum, and spleen), species of origin, and culture conditions especially with respect to differentiation factors such as granulocyte-macrophage colony stimulating factor (GM-CSF) and macrophage colony stimulating factor (M-CSF)[23, 24]. Not all macrophages necessarily show constitutive high levels of fluid-phase pinocytosis, but activation of this process can occur in these macrophages similar to the activation of macropinocytosis in some non-macrophage cells following stimulation with various growth factors [25, 26].
For example, human monocytes differentiated in fetal bovine serum with added GM-CSF or with human serum without added GM-CSF generate macrophages with a rounded “fried-egg” appearing morphology (i.e., GM-CSF phenotype), and show low levels of fluid-pinocytosis unless activated with phorbol 12-myristate 13-acetate (PMA) or other activators of protein kinase C [27••, 28•]. On the other hand, mouse bone marrow-derived macrophages differentiated with GM-CSF show high levels of fluid-phase pinocytosis of LDL that is constitutive not requiring macrophage activation [29]. PMA-activation of human of macrophages having the GM-CSF phenotype increases fluid-phase pinocytosis to levels about 5 times that shown by unactivated macrophages [27••, 28•]. Incubation of these PMA-activated macrophages with native LDL results in a non-saturable, concentration-dependent uptake and lysosomal degradation of LDL that transforms the macrophages into foam cells because of the massive amount of cholesterol that accumulates due to the stimulated LDL uptake [24, 27••, 28•]. Protein kinase C isoforms delta and beta each mediate about half the cholesterol accumulation suggesting that macropinocytosis may function through multiple signaling pathways [30].
Human monocytes and mouse bone marrow-derived macrophages differentiated with fetal bovine serum and added M-CSF generate elongated macrophages (i.e., M-CSF phenotype) that show high levels of constitutive fluid-phase pinocytosis. As a result, these macrophages show constitutive fluid-phase uptake of LDL and cholesterol accumulation transforming these macrophages into foam cells [31••](Barthwal, M, Anzinger, JJ, Kruth, HS, unpublished data). Consistent with the LDL uptake occurring through fluid-phase rather than a binding mechanism in M-CSF and GM-CSF macrophage phenotypes, 125I- LDL uptake shows a non-saturable concentration-dependent uptake, 125I- LDL uptake cannot be competed by excess unlabeled LDL, and 125I- LDL uptake can be completely accounted for by the level of fluid-phase pinocytosis determined with a fluid-phase tracer [27••, 31••]. In the case of the M-CSF macrophage phenotype, micropinocytosis and macropinocytosis each contribute to about one-half the LDL uptake and cholesterol accumulation [18•]. This could be shown with the use of bafilomycin and SU6656 that inhibit respectively, micropinocytosis and macropinocytosis in these cells. In contrast, in the case of the GM-CSF macrophage phenotype, macropinocytosis mediates most of the fluid-phase pinocytosis [28•, 29].
Interestingly, how cholesterol is delivered to macrophages, either through receptor-mediated compared with fluid-phase pinocytosis, has been shown to influence the mechanism of efflux of cholesterol from macrophages [32••]. When cholesterol is delivered to mouse bone-marrow derived macrophages by scavenger receptor-mediated uptake of acetylated LDL, subsequent cholesterol efflux occurs predominantly through an apo (apolipoprotein)A-I/ ATP-binding cassette transporter A1(ABCA1) mediated efflux mechanism. In contrast, macrophage uptake of LDL cholesterol by fluid-phase pinocytosis results in cholesterol efflux that is predominantly independent of apoA-I/ABCA1 and likely due to diffusional cholesterol efflux mechanisms.
Regulation of fluid-phase pinocytosis
Recent findings show that liver X receptors (LXRs), which bind oxysterols, regulate fluid-phase uptake of LDL by human M-CSF differentiated monocyte-derived macrophages [33•]. When monocytes are differentiated into macrophages in the presence of the LXR agonists T0901317 or 22(R)-hydroxycholesterol, both fluid-phase uptake of LDL and cholesterol accumulation by the M-CSF differentiated macrophages are decreased by greater than 50%. The LXR-agonist decrease in the uptake of LDL is completely accounted for by a similar decrease in the uptake of the fluid-phase tracer albumin. The LXR-agonist treatment effect is specific for fluid-phase pinocytosis because the treatment has no effect on macrophage receptor-mediated uptake of acetylated LDL. Although it was not quantitatively determined to what degree LXR-agonist treatment downregulated fluid-phase micropinocytosis compared with fluid-phase macropinocytosis, LXR-agonist treatment substantially decreases macropinocytosis assessed by time-lapse phase microscopy (Anzinger JJ, Kruth HS, unpublished data).
While it remains to be determined by what mechanism LXRs regulate fluid-phase pinocytosis in macrophages, the LXR up-regulated gene, ABCA1, has been shown to affect fluid-phase pinocytosis in other cell types. Fluid-phase pinocytosis is increased in cultured Tangier disease fibroblasts [34], which are genetically deficient in ABCA1, a protein best known for its function in cellular cholesterol export. In contrast, increased expression of ABCA1 in cultured MDCK2 epithelial cells causes reduced fluid-phase pinocytosis [35]. Thus, the anti-atherogenic properties of LXR agonists may be not only due to their well known stimulation of macrophage cholesterol efflux, but also be contributed to by an LXR agonist decrease in macrophage fluid-phase uptake of LDL. In contrast to LXRs that decrease fluid-phase pinocytosis, overexpression of another oxysterol-binding protein, oxysterol-binding protein-related protein 2 (ORP2), in HeLa epithelial cells causes an increase in fluid-phase pinocytosis [36]. The increased fluid-phase pinocytosis may mediate some of the increased LDL uptake observed in ORP2 overexpressing HeLa cells.
While fluid-phase pinocytosis can be constitutive in M-CSF phenotype macrophages and dendritic cells [31••, 37], fluid-phase pinocytosis can be induced in other macrophage phenotypes. Besides PMA activation of macropinocytosis in certain types of macrophages, interestingly, modified forms of LDL such as acetylated LDL and oxidized LDL have been shown to trigger macropinocytosis and fluid-phase pinocytosis in pigeon monocyte-derived macrophages, THP-1 human macrophages, resident mouse peritoneal macrophages, and J774 mouse macrophages [38••, 39••]. Induction of fluid-phase pinocytosis in mouse peritoneal macrophages by minimally oxidized 15-lipoxygenase-treated LDL is mediated by toll-like receptor 4 and spleen tyrosine kinase [39••, 40], similar to toll-like receptor ligand-induced fluid-phase pinocytosis in mouse bone marrow- and spleen-derived dendritic cells [41]. Fluid-phase pinocytosis can be stimulated by 15-lipoxygenase treated cholesteryl arachidonate suggesting that cholesteryl ester hydroperoxides likely mediate the minimally oxidized LDL triggering of fluid-phase pinocytosis. However, some caution must be taken in the interpretation of the signaling studies reported in [39••–41] because FITC-dextran was used to monitor fluid-phase uptake, and as discussed below, dextran also can be internalized through receptors. Acetylated LDL and minimally oxidized LDL both stimulate macrophage uptake of co-incubated native LDL, and it was suggested but not demonstrated that fluid-phase pinocytosis rather than LDL bound to macrophages mediated the native LDL uptake [39••, 42•]. Lastly, many viruses have been shown to trigger macropinocytosis (reviewed in [43]), and thus, viruses could be another trigger for fluid-phase pinocytosis of LDL and foam cell formation that should be examined in future research.
Fluid-phase pinocytosis due to macropinocytosis is phosphoinositide (PI) 3-kinase dependent [44]. We have recently shown that PI3-kinase gamma mediates fluid-phase macropinocytosis of LDL by murine bone marrow-derived macrophages differentiated with GM-CSF [29]. This finding helps explain why genetic deletion or pharmacologic inhibition of PI3-kinase gamma substantially decreases development of atherosclerotic plaques in mice [45].
Fluid-phase pinocytosis occurs within atherosclerotic plaques and other tissues
LDL receptor-independent uptake of LDL by tissues occurs in all species examined and ranges between 22–28% of total LDL uptake in animals, but is higher in humans reaching 42% of total tissue uptake of LDL [46]. Approximately 70% of this LDL receptor-independent LDL uptake occurs in extra-hepatic tissues. The observation that LDL receptor-independent uptake of LDL in vivo shows linear dependence on plasma LDL concentration is consistent with this process being mediated by fluid-phase pinocytosis. In this regard, it has been shown that the levels of fluid-phase pinocytosis in mouse spleen and liver account for the levels of LDL receptor-independent uptake of LDL in these organs [47••]. In mutant NPC1 mice, which show a defect in cholesterol trafficking that causes lysosomal unesterified cholesterol accumulation, fluid-phase pinocytosis contributes to increased cholesterol accumulation in extrahepatic organs. These organs show infiltration with macrophage foam cells suggesting that macrophages fluid-phase pinocytosis contributes to the increased fluid-phase pinocytosis of LDL in the organs [47••]. Interestingly, enrichment of mouse peritoneal macrophages with unesterified cholesterol stimulates increased M-CSF secretion [48••], an inducer of macropinocytosis in some mouse macrophage phenotypes. Thus, a possible amplification mechanism may exist for macrophage foam cells to induce other macrophages to macropinocytose lipoproteins.
M-CSF administered in vivo decreases plasma cholesterol levels in non-human primates, humans, and rabbits including WHHL rabbits that lack the LDL receptor [49–51]. On the other hand, both hypercholesterolemic apoE- and LDL receptor-deficient mice show a further increase in plasma cholesterol when these mice are additionally made genetically deficient in M-CSF [52, 53]. Studies show that the cholesterol lowering effect of M-CSF treatment is due in part to M-CSF stimulation of LDL clearance by an LDL receptor-independent mechanism [49, 50]. Thus, M-CSF stimulation of macrophage number and macrophage fluid-phase pinocytosis of LDL could be how M-CSF regulates plasma cholesterol levels.
Recently, it has been shown that macrophages in mouse atherosclerotic lesions carry out fluid-phase pinocytosis [17••]. This was demonstrated with the use of fluorescent pegylated nanoparticles (similar in size to LDL) that do not bind to cells, but are taken up by cultured macrophages through fluid-phase pinocytosis. Thus, the pegylated nanoparticles are useful in vivo tracers of LDL fluid-phase pinocytosis. The pegylated nanoparticles injected into apoE-deficient mice accumulated rather selectively within the macrophage foam cells of atherosclerotic lesions. This demonstrates that foam cells within atherosclerotic lesions show active fluid-phase pinocytosis and this can be a mechanism for uptake of lipoproteins such as LDL within plaques.
Considerations when measuring fluid-phase pinocytosis
The ideal probe for quantifying fluid-phase pinocytosis should reflect uptake exclusively in the fluid-phase and thus, the chosen probe should not bind to cells or extracellular matrix. If it is desired to measure total fluid-phase pinocytosis, the probe should be of small enough size to enter cells by both micropinocytosis and macropinocytosis. Also, once taken up by the cell, the probe should not be excreted by the cell during the period of probe uptake. Of course it must be possible to quantify the cell accumulated probe. Unfortunately, few probes satisfy all of these requirements. Commonly used probes such as radiolabeled sucrose or polyvinylpyrrolidone, the fluorescent dye lucifer yellow, and fluorescently labeled dextrans typically underestimate fluid-phase pinocytosis due to their concurrent cellular excretion during cellular uptake [54]. 125I-labeled albumin is an especially useful fluid-phase tracer for studies of LDL uptake because, similar to studies of 125I- LDL uptake, cellular metabolism of albumin provides a means to account for all cellular uptake including retained albumin and excreted albumin degradation products [15, 31••, 33•].
Another commonly used probe type, fluorescently labeled dextrans, potentially bind to pattern recognition receptors and this should be checked before their use as fluid-phase probes [25, 37, 55–57]. Thus, it is imperative to check concentration-dependent linearity of probe uptake before determining fluid-phase pinocytosis in the cell type under investigation. Another problem with the application of fluorescently labeled dextrans relates to the assumption by many investigators that all sized-dextrans selectively measure uptake by macropinocytosis rather than micropinocytosis. 150 kilodalton dextran has been shown to selectively label macropinosomes in mouse bone-marrow derived macrophages, while smaller sized-dextrans label micropinosomes in addition to macropinosomes [44, 58].
Conclusion
Fluid-phase pinocytosis is a mechanism that can contribute to receptor-independent uptake of LDL leading to macrophage foam cell formation. Macrophage LDL uptake by fluid-phase pinocytosis is linearly related to LDL concentration consistent with the risk of developing cardiovascular disease also being linearly related to LDL levels. To the extent that it is desirable to inhibit macrophage accumulation of cholesterol in atherosclerotic plaques, it will be necessary to pharmacologically target fluid-phase pinocytosis in plaques. Macrophage fluid-phase macropinocytosis of LDL depends on Rho GTPase and PI3-kinase function [18•, 28•, 29, 59] suggesting that these are useful signaling molecules to target for inhibition. On the other hand, stimulating fluid-phase pinocytosis of LDL in organs such as the spleen and liver potentially provides a new approach to lowering plasma cholesterol levels. In any case, recent research has shown clearly that fluid-phase pinocytosis of LDL should be considered in any attempt to further investigate the decades-old research problem of macrophage foam cell formation.
Supplementary Material
Supplemental Online Material: Figure S1. Movie of constitutive macropinocytosis in M-CSF differentiated human monocyte-derived macrophages (from [18•])
Key Points.
Uptake of LDL by receptor-independent, fluid-phase pinocytosis produces massive storage of LDL-derived cholesterol in macrophages.
Fluid-phase pinocytosis occurs within atherosclerotic lesions.
PI-3 kinase, RhoGTPase, and LXR transcription factor regulate fluid-phase pinocytosis of LDL.
Fluid-phase pinocytosis of LDL is a novel mechanism of macrophage cholesterol accumulation demonstrating that neither modification of LDL nor receptors are necessary for macrophage foam cell formation.
Macrophage fluid-phase pinocytosis of LDL is a relevant pathway to target for modulating macrophage cholesterol accumulation in atherosclerosis.
Acknowledgments
The Author acknowledges the intramural research program of the National Institutes of Health that has funded the Author’s research, and the many research colleagues who have contributed to the Author’s investigation of fluid-phase pinocytosis of lipoproteins.
Abbreviations
- LDL
low density lipoprotein
- GM-CSF
granulocyte macrophage-colony stimulating factor
- M-CSF
macrophage-colony stimulating factor
- PMA
phorbol 12-myristate 13-acetate
- apo
apolipoprotein
- ABCA1
ATP-binding cassette transporter A1
- LXR
liver X receptors
- ORP2
oxysterol-binding protein-related protein 2
- PI
phosphoinositide
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Kruth HS. Cholesterol deposition in atherosclerotic lesions. Subcell Biochem. 1997;28:319–362. doi: 10.1007/978-1-4615-5901-6_12. [DOI] [PubMed] [Google Scholar]
- 2.Hoff HF, Gaubatz JW, Gotto AM., Jr Apo B concentration in the normal human aorta. Biochem Biophys Res Commun. 1978;85:1424–1430. doi: 10.1016/0006-291x(78)91162-2. [DOI] [PubMed] [Google Scholar]
- 3.Smith EB. Transport, interactions and retention of plasma proteins in the intima: the barrier function of the internal elastic lamina. Eur Heart J. 1990;11(Suppl E):72–81. doi: 10.1093/eurheartj/11.suppl_e.72. [DOI] [PubMed] [Google Scholar]
- 4.Galis ZS, Sukhova GK, Kranzhofer R, et al. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc Natl Acad Sci U S A. 1995;92:402–406. doi: 10.1073/pnas.92.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesnik P, Rouis M, Skarlatos S, et al. Uptake of exogenous free cholesterol induces upregulation of tissue factor expression in human monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1992;89:10370–10374. doi: 10.1073/pnas.89.21.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rouis M, Nigon F, Lafuma C, et al. Expression of elastase activity by human monocyte-macrophages is modulated by cellular cholesterol content, inflammatory mediators, and phorbol myristate acetate. Arteriosclerosis. 1990;10:246–255. doi: 10.1161/01.atv.10.2.246. [DOI] [PubMed] [Google Scholar]
- 7.Brown MS, Basu SK, Falck JR, et al. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 1980;13:67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- 8.Yla-Herttuala S, Rosenfeld ME, Parthasarathy S, et al. Gene expression in macrophage-rich human atherosclerotic lesions. 15- lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991;87:1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruth HS. Macrophage foam cells and atherosclerosis. Front Biosci. 2001;6:D429–455. doi: 10.2741/kruth. [DOI] [PubMed] [Google Scholar]
- 10.Steinbrecher UP, Lougheed M. Scavenger receptor-independent stimulation of cholesterol esterification in macrophages by low density lipoprotein extracted from human aortic intima. Arterioscler Thromb. 1992;12:608–625. doi: 10.1161/01.atv.12.5.608. [DOI] [PubMed] [Google Scholar]
- 11••.Manning-Tobin JJ, Moore KJ, Seimon TA, et al. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. This study shows that macrophage foam cells form in mouse atherosclerotic lesions even with genetic deletion of macrophage scavenger receptors. This finding suggests that alternative mechanisms such as fluid-phase pinocytosis of lipoproteins predominate in macrophage foam cell formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoppe G, O’Neil J, Hoff HF. Inactivation of lysosomal proteases by oxidized low density lipoprotein is partially responsible for its poor degradation by mouse peritoneal macrophages. J Clin Invest. 1994;94:1506–1512. doi: 10.1172/JCI117490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lougheed M, Zhang HF, Steinbrecher UP. Oxidized low density lipoprotein is resistant to cathepsins and accumulates within macrophages. J Biol Chem. 1991;266:14519–14525. [PubMed] [Google Scholar]
- 14.Roma P, Catapano AL, Bertulli SM, et al. Oxidized LDL increase free cholesterol and fail to stimulate cholesterol esterification in murine macrophages. Biochem Biophys Res Commun. 1990;171:123–131. doi: 10.1016/0006-291x(90)91365-y. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein JL, Brown MS. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974;249:5153–5162. [PubMed] [Google Scholar]
- 16.Goldstein JL, Brown MS. The LDL pathway in human fibroblasts: a receptor-mediated mechanism for the regulation of cholesterol metabolism. Curr Top Cell Regul. 1976;11:147–181. doi: 10.1016/b978-0-12-152811-9.50011-0. [DOI] [PubMed] [Google Scholar]
- 17••.Buono C, Anzinger JJ, Amar M, Kruth HS. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J Clin Invest. 2009;119:1373–1381. doi: 10.1172/JCI35548. This study reports a method for monitoring fluid-phase pinocytosis in vivo using fluorescent nanoparticles (of similar size to LDL) that do not bind cells. With this methodology, the Authors have demonstrated very active fluid-phase pinocytosis by macrophage foam cells within mouse atherosclerotic lesions. This finding substantiates the idea that macrophages actively take up LDL with atherosclerotic lesions by fluid-phase pinocytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Anzinger JJ, Chang J, Xu Q, et al. Native low-density lipoprotein uptake by macrophage colony-stimulating factor-differentiated human macrophages is mediated by macropinocytosis and micropinocytosis. Arterioscler Thromb Vasc Biol. 2010;30:2022–2031. doi: 10.1161/ATVBAHA.110.210849. Reference [29] shows that M-CSF differentiated human monocyte-derived macrophages convert to foam cells due to fluid-phase pinocytosis of LDL This study shows that fluid-phase pinocytosis is mediated equally by micropinocytosis and macropinocytosis. Thus, phamacologic inhibition of either pathway only partially decreases fluid-phase pinocytosis of LDL and macrophage cholesterol accumulation. This shows that limiting macrophage foam cell formation due to fluid-phase pinocytosis in vivo may require multiple pharmacologic agents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahley RW, Innerarity TL, Brown MS, et al. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res. 1980;21:970–980. [PubMed] [Google Scholar]
- 20.Knight BL, Soutar AK, Patel DD. Non-saturable degradation of LDL by monocyte-derived macrophages leads to a reduction in HMG-CoA reductase activity with little synthesis of cholesteryl esters. Atherosclerosis. 1987;64:131–138. doi: 10.1016/0021-9150(87)90238-3. [DOI] [PubMed] [Google Scholar]
- 21.Knight BL, Soutar AK. Changes in the metabolism of modified and unmodified low-density lipoproteins during the maturation of cultured blood monocyte- macrophages from normal and homozygous familial hypercholesterolaemic subjects. Eur J Biochem. 1982;125:407–413. doi: 10.1111/j.1432-1033.1982.tb06698.x. [DOI] [PubMed] [Google Scholar]
- 22.Tabas I, Weiland DA, Tall AR. Unmodified low density lipoprotein causes cholesteryl ester accumulation in J774 macrophages. Proc Natl Acad Sci U S A. 1985;82:416–420. doi: 10.1073/pnas.82.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akagawa KS. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Int J Hematol. 2002;76:27–34. doi: 10.1007/BF02982715. [DOI] [PubMed] [Google Scholar]
- 24.Waldo SW, Li Y, Buono C, et al. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norbury CC. Drinking a lot is good for dendritic cells. Immunology. 2006;117:443–451. doi: 10.1111/j.1365-2567.2006.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- 27••.Kruth HS, Huang W, Ishii I, Zhang WY. Macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2002;277:34573–34580. doi: 10.1074/jbc.M205059200. This investigation shows that protein kinase C-dependent activation of human monocyte-derived macrophages differentiated with human serum stimulates fluid-phase pinocytosis of LDL This converts the macrophages into cholesterol-rich foam cells. The findings show that macrophage foam cell formation can occur with receptor-independent, fluid-phase pinocytosis of native LDL. [DOI] [PubMed] [Google Scholar]
- 28•.Kruth HS, Jones NL, Huang W, et al. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2005;280:2352–2360. doi: 10.1074/jbc.M407167200. This report shows that the fluid-phase uptake pathway macropinocytosis, in which certain cells such as macrophages engulf droplets of extracellular fluid, mediates fluid-phase pinocytosis of LDL by human monocyte-derived macrophages differentiated with human serum. [DOI] [PubMed] [Google Scholar]
- 29.Anzinger JJ, Chang J, Xu Q, et al. Arteriosclerosis, Thrombosis and Vascular Biology 2011 Scientific Sessions. Chicago, Illinois: 2011. Murine Bone Marrow-Derived Macrophages Differentiated with GM-CSF Become Foam Cells by PI 3-Kinase Gamma-Dependent Fluid-Phase Pinocytosis of Native LDL. [Google Scholar]
- 30.Ma HT, Lin WW, Zhao B, et al. Protein kinase C beta and delta isoenzymes mediate cholesterol accumulation in PMA-activated macrophages. Biochem Biophys Res Commun. 2006;349:214–220. doi: 10.1016/j.bbrc.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 31••.Zhao B, Li Y, Buono C, et al. Constitutive receptor-independent low density lipoprotein uptake and cholesterol accumulation by macrophages differentiated from human monocytes with macrophage-colony-stimulating factor (M-CSF) J Biol Chem. 2006;281:15757–15762. doi: 10.1074/jbc.M510714200. Human monocyte-derived macrophages differentiated with fetal bovine serum containing M-CSF are a distinct macrophage phenotype when compared to human monocyte-derived macrophages differentiated with human serum. Fluid-phase pinocytosis of LDL must be activated with human serum differentiated macrophages as shown in [26]. This report shows that fluid-phase pinocytosis of LDL is constitutive in M-CSF differentiated human monocytes and can convert this macrophage phenotype into cholesterol-rich foam cells. [DOI] [PubMed] [Google Scholar]
- 32••.Wang MD, Kiss RS, Franklin V, et al. Different cellular traffic of LDL-cholesterol and acetylated LDL-cholesterol leads to distinct reverse cholesterol transport pathways. J Lipid Res. 2007;48:633–645. doi: 10.1194/jlr.M600470-JLR200. This interesting report shows that how cholesterol enters macrophages influences how it effluxes from macrophages. ABCA1-mediated cholesterol efflux to apoA-I was much greater for AcLDL-loaded macrophages (receptor-mediated uptake) compared with LDL-loaded macrophages (fluid-phase uptake). In contrast, LDL-derived cholesterol was preferentially effluxed to HDL, presumably mediated by ABCG1. [DOI] [PubMed] [Google Scholar]
- 33••.Buono C, Li Y, Waldo SW, Kruth HS. Liver X receptors inhibit human monocyte-derived macrophage foam cell formation by inhibiting fluid-phase pinocytosis of LDL. J Lipid Res. 2007 doi: 10.1194/jlr.M700170-JLR200. Liver X receptors are well known to be anti-atherogenic through their promotion of macrophage cholesterol efflux. This study reports the surprising finding that activation of liver X receptors also inhibits fluid-phase pinocytosis of LDL Thus, activation of liver X receptors results in limiting macrophage cholesterol accumulation by both decreasing cholesterol uptake and enhancing cholesterol efflux. [DOI] [PubMed] [Google Scholar]
- 34.Zha X, Genest J, Jr, McPherson R. Endocytosis is enhanced in Tangier fibroblasts: possible role of ATP-binding cassette protein A1 in endosomal vesicular transport. J Biol Chem. 2001;276:39476–39483. doi: 10.1074/jbc.M105067200. [DOI] [PubMed] [Google Scholar]
- 35.Alder-Baerens N, Muller P, Pohl A, et al. Headgroup-specific exposure of phospholipids in ABCA1-expressing cells. J Biol Chem. 2005;280:26321–26329. doi: 10.1074/jbc.M413993200. [DOI] [PubMed] [Google Scholar]
- 36.Hynynen R, Laitinen S, Kakela R, et al. Overexpression of OSBP-related protein 2 (ORP2) induces changes in cellular cholesterol metabolism and enhances endocytosis. Biochem J. 2005;390:273–283. doi: 10.1042/BJ20042082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Jones NL, Willingham MC. Modified LDLs are internalized by macrophages in part via macropinocytosis. Anat Rec. 1999;255:57–68. doi: 10.1002/(SICI)1097-0185(19990501)255:1<57::AID-AR7>3.0.CO;2-Z. This study shows that modified lipoproteins such as acetylated LDL and oxidized LDL stimulate fluid-phase pinocytosis mediated by macropinocytosis. This finding suggests a means by which macrophage phenotypes that lack constitutive fluid-phase pinocytosis nevertheless can show induced fluid-phase pinocytosis. [DOI] [PubMed] [Google Scholar]
- 39••.Choi SH, Harkewicz R, Lee JH, et al. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. This report confirms the finding in [38••] that oxidized LDL stimulates fluid-phase pinocytosis and implicates TLR4/spleen tyrosine kinase signaling in the stimulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller YI, Choi SH, Fang L, Harkewicz R. Toll-like receptor-4 and lipoprotein accumulation in macrophages. Trends Cardiovasc Med. 2009;19:227–232. doi: 10.1016/j.tcm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.West MA, Wallin RP, Matthews SP, et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 42•.Jones NL, Reagan JW, Willingham MC. The Pathogenesis of Foam Cell Formation : Modified LDL Stimulates Uptake of Co-Incubated LDL Via Macropinocytosis. Arterioscler Thromb Vasc Biol. 2000;20:773–781. doi: 10.1161/01.atv.20.3.773. This report shows that the modified LDLs, acetylated LDL and oxidized LDL, stimulate degradation of coincubated LDL The authors suggest but do not demonstrate that the increased LDL degradation possibly occurrs through the modified lipoprotein stimulation of fluid-phase pinocytosis. [DOI] [PubMed] [Google Scholar]
- 43.Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 44.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fougerat A, Gayral S, Gourdy P, et al. Genetic and pharmacological targeting of phosphoinositide 3-kinase-gamma reduces atherosclerosis and favors plaque stability by modulating inflammatory processes. Circulation. 2008;117:1310–1317. doi: 10.1161/CIRCULATIONAHA.107.720466. [DOI] [PubMed] [Google Scholar]
- 46.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 47••.Liu B, Xie C, Richardson JA, et al. Receptor-mediated and bulk-phase endocytosis cause macrophage and cholesterol accumulation in Niemann-Pick C disease. J Lipid Res. 2007;48:1710–1723. doi: 10.1194/jlr.M700125-JLR200. This report extends to the NPC mouse model of abnormal cholesterol accumulation, the pioneering work of this group’s earlier studies of receptor- and bulk-phase-mediated (i.e., fluid-phase) uptake of LDL by various tissues in vivo. This study shows that fluid-phase pinocytosis of LDL contributes substantially to the pathologic accumulation of cholesterol that occurs in macrophages and parenchymal cells in the organs of NPC mice. [DOI] [PubMed] [Google Scholar]
- 48••.Li Y, Tabas I. The inflammatory cytokine response of cholesterol-enriched macrophages is dampened by stimulated pinocytosis. J Leukoc Biol. 2007;81:483–491. doi: 10.1189/jlb.0806518. This investigation reports that macrophages enriched with free cholesterol secrete factors including M-CSF that function to induce pinocytosis in other non-free cholesterol-enriched macrophages. Thus, given this finding, it is possible that macrophage cholesterol accumulation mediated by fluid-phase pinocytosis could be perpetuated in other macrophages by a paracrine mechanism. [DOI] [PubMed] [Google Scholar]
- 49.Stoudemire JB, Garnick MB. Effects of recombinant human macrophage colony-stimulating factor on plasma cholesterol levels. Blood. 1991;77:750–755. [PubMed] [Google Scholar]
- 50.Shimano H, Yamada N, Ishibashi S, et al. Human monocyte colony-stimulating factor enhances the clearance of lipoproteins containing apolipoprotein B-100 via both low density lipoprotein receptor-dependent and -independent pathways in rabbits. J Biol Chem. 1990;265:12869–12875. [PubMed] [Google Scholar]
- 51.Schaub RG, Bree MP, Hayes LL, et al. Recombinant human macrophage colony-stimulating factor reduces plasma cholesterol and carrageenan granuloma foam cell formation in Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb. 1994;14:70–76. doi: 10.1161/01.atv.14.1.70. [DOI] [PubMed] [Google Scholar]
- 52.Rajavashisth T, Qiao JH, Tripathi S, et al. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor- deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith JD, Trogan E, Ginsberg M, et al. Decreased atherosclerosis in mice deficient in both macrophage colony- stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berthiaume EP, Medina C, Swanson JA. Molecular size-fractionation during endocytosis in macrophages. J Cell Biol. 1995;129:989–998. doi: 10.1083/jcb.129.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kato M, Neil TK, Fearnley DB, et al. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int Immunol. 2000;12:1511–1519. doi: 10.1093/intimm/12.11.1511. [DOI] [PubMed] [Google Scholar]
- 56.Yanagawa Y, Onoe K. CCR7 ligands induce rapid endocytosis in mature dendritic cells with concomitant up-regulation of Cdc42 and Rac activities. Blood. 2003;101:4923–4929. doi: 10.1182/blood-2002-11-3474. [DOI] [PubMed] [Google Scholar]
- 57.Kang YS, Yamazaki S, Iyoda T, et al. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int Immunol. 2003;15:177–186. doi: 10.1093/intimm/dxg019. [DOI] [PubMed] [Google Scholar]
- 58.Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao W, Li K, Liao K. Macropinocytosis contributes to the macrophage foam cell formation in RAW264.7 cells. Acta Biochim Biophys Sin (Shanghai) 2009;41:773–780. doi: 10.1093/abbs/gmp066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Online Material: Figure S1. Movie of constitutive macropinocytosis in M-CSF differentiated human monocyte-derived macrophages (from [18•])


