Abstract
Objective
To evaluate the effect of lymphovascular space invasion on survival of early-stage epithelial ovarian cancer patients.
Methods
A multicenter retrospective study was conducted for patients with stage IA-C epithelial ovarian cancer who underwent primary comprehensive surgery including lymphadenectomy. Histopathology slides for ovarian tumors were examined by gynecologic pathologists for presence or absence of lymphovascular space invasion. Survival analysis was performed examining tumoral factors.
Results
A total of 434 cases were included in the analysis. Lymphovascular space invasion was detected in 76 (17.5%) cases, associated with histology (p=0.042) and stage (p=0.044). Lymphovascular space invasion was significantly associated with decreased survival outcomes (disease-free survival [DFS], 5-year rate 78.4% versus 90.7%, p=0.024, and overall survival [OS], 84.9% versus 93.2%, p=0.031) in univariate analysis. In multivariate analysis, lymphovascular space invasion did not remain a significant variable for DFS (hazard ratio [HR] 1.98, 95%CI 0.97-3.97, p=0.059) or OS (HR 2.41, 95%CI 0.99-5.85, p=0.052). Lymphovascular space invasion was associated with increased risk of hematogenous and lymphatic metastasis (HR 4.79, 95%CI 1.75-13.2, p=0.002) but not peritoneal metastasis (p=0.33) in multivariate analysis. Among lymphovascular space invasion-expressing tumors, patients who received fewer than 6 cycles of postoperative chemotherapy had significantly poorer DFS than those who received six or more cycles (HR 4.59, 95%CI 1.20-17.5, p=0.015).
Conclusion
Lymphovascular space invasion is an important histological feature to identify a subgroup of patients with increased risk of recurrence in stage I epithelial ovarian cancer.
Introduction
Direct peritoneal spread is recognized as a common metastatic pattern of ovarian cancer where the majority of patients present with advanced-stage disease including peritoneal carcinomatosis and ascites.1-3 Despite extensive treatment, disease-related mortality for advanced ovarian cancer remains considerably high.4-6 Unlike advanced-stage disease, stage I ovarian cancer is associated with good prognosis, with a 5-year overall survival rate of approximately 80-90%.7-9 However, approximately 10% of stage I ovarian cancer patients develop recurrent disease. Therefore, identifying biomarkers that could lead to reliable prediction of recurrence could have implications for management of ovarian cancer.
Recently, lymphovascular space invasion was identified as an important biomarker in the progression of ovarian cancer.10 Specifically, tumoral lymphovascular space invasion is commonly seen in high-grade serous histology, the most common histology type of ovarian cancer, and is independently associated with poor survival outcome of advanced-stage ovarian cancer patients. Lymphovascular space invasion refers to tumor cells present within the lymphatic or microvascular capillaries in ovarian tumors. Thus, lymphovascular space invasion could be histopathologic evidence of early tumor spread through hematogenous and lymphatic drainage. Nevertheless, the exact mechanism of lymphovascular space invasion-driven cancer progression and metastasis is not yet clearly known in ovarian cancer. The aim of this study was to evaluate the effect of lymphovascular space invasion on survival of stage I epithelial ovarian cancer patients.
Patients and Methods
A multicenter retrospective study was conducted by utilizing institutional databases for consecutive ovarian cancer cases. Participating institutions were Osaka University (2000-2012), Niigata University (2002-2011), Saitama Medical University International Medical Center (2007-2012), Tokushima University (1986-2009), Osaka Rosai Hospital (2000-2006), and Mercy Medical Center in Baltimore (1994-2009). In addition to the six institutions, an archived database from Gynecologic Oncology Group of Osaka (GOGO, hosted by Osaka University, 1997-2004) was utilized. Institutional Review Board (IRB) approval was obtained at each participating institution.
Inclusion criteria for the study were stage I epithelial ovarian cancer cases that underwent primary comprehensive surgical staging and postoperative care at participating institutions. Standard surgical treatment of ovarian cancer included total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and lymphadenectomy. Stage I ovarian cancer refers to histology-confirmed ovarian cancer apparently confined to the ovary (pT1N0M0) upon complete surgical staging; presence of lymph node involvement or extra-ovarian metastasis were not defined as stage I disease. Cases that underwent incomplete staging, received neoadjuvant chemotherapy, or had no histology slides to evaluate lymphovascular space invasion were excluded from analysis.
Among the eligible cases identified in the database for the analysis, medical records were examined to abstract the following variables: (i) patient demographics including age at diagnosis and race; (ii) histopathology results for histology subtype, grade, stage, lymphovascular space invasion status per records, peritoneal cytology, nodal metastasis, and distant metastasis; (iii) type of treatment including surgical procedure and intraoperative capsule rupture, residual disease at closure, and type and cycle of postoperative chemotherapy; and (iv) survival outcomes for disease-free survival (DFS) and overall survival (OS). Among recurrent cases, location of recurrence was recorded.
Archived histopathology slides for hematoxylin and eosin staining were pulled and examined by gynecologic pathologists or gynecologic oncologists certified for gynecologic pathology at each institution. These evaluators for lymphovascular space invasion were completely blinded from clinical information, as described previously.10 Briefly, slides representing the primary ovarian tumors were examined and cluster of tumor cells within lymphovascular spaces except for the area for potential artifact or tumor cell contamination (torn tissue, free tumor fragments along the edge of the tissue) was determined as tumoral lymphovascular space invasion assessed as presence or absence. Based on our prior study, quantity of lymphovascular space invasion did not have an effect on survival outcome of epithelial ovarian cancer, and thus, qualification of lymphovascular space invasion was scored in a dichotomized fashion.10 Total number of slides for ovarian tumor was also recorded. Data entry was performed by the investigators at each of the participating institutions. A second investigator randomly picked selected medical records for assessing accuracy. The de-identified data sheet was reviewed by the principal investigator: all the data were carefully examined for accuracy, consistency, and quality. When there was a disagreement in the data set, the principal investigator and each participating institution discussed for adjudication.
Definition
Stage I ovarian cancer cases were classified into stage IA (unilateral ovarian involvement), IB (bilateral ovarian involvement), and IC. Stage IC was further sub-classified into: (i) intraoperative capsule rupture, (ii) malignant cells in cytology, or (iii) capsule tumor involvement. High-grade serous ovarian carcinoma was defined as grade 2-3 serous histology type whereas low-grade serous ovarian carcinoma was defined as grade 1 serous histology type based on a previous study.10 Clear cell carcinoma type is not routinely graded per World Health Organization (WHO) recommendation.11 The date of recurrence was determined by clinical examination, imaging studies, and CA-125 levels. DFS was defined as the time interval from the date of primary surgery to the date of documented first recurrence of disease. If there was no recurrence, DFS was determined as the date of last follow-up. OS was defined as the interval between the primary cytoreductive surgery and the date of death related to ovarian cancer or last follow-up. Postoperative chemotherapy type was divided into paclitaxel and carboplatin versus others, and its administered cycles were assessed per cycles (≥6 versus <6 cycles). Location of recurrence was grouped into: (i) hematogenous and lymphatic metastasis (recurrent site for: lymph nodes, liver or spleen parenchyma, lung, bone, or brain), (ii) peritoneal spread (peritoneum, mesentery, omentum, or carcinomatosis), and (iii) others.
Statistical analysis
Primary outcome of interest was survival outcomes (DFS and OS) of stage I epithelial ovarian cancer based on tumoral lymphovascular space invasion expression. Secondary outcomes of interest were risk of hematogenous and lymphatic metastasis stratified by tumoral lymphovascular space invasion status as well as effects of postoperative chemotherapy among the lymphovascular space invasion-expressing tumors. Continuous variables were assessed for normality (Kolmogorov–Smirnov test) and expressed as appropriate (mean with standard deviation [SD] or median with range). Student's t test or Mann–Whitney U test was performed for continuous variables as appropriate. Categorical variables were evaluated with Fisher's exact test or chi-square test as appropriate, expressed with odds ratio (OR) and 95% confidence interval (CI). For survival data analysis, to determine the significance of variables for the survival outcomes for DFS and OS, univariate (Log-rank test) and multivariate (Cox proportional hazard regression test) analyses were performed as appropriate expressed with hazard ratio (HR) and 95%CI. Because recurrence is a time-dependent event, cumulative risks for organ site-specific recurrence (hematogenous and lymphatic versus peritoneal metastasis) were also evaluated in survival analysis. Survival curves were constructed with Kaplan-Meier method. P-values of less than 0.05 were considered statistically significant (all, 2-tailed). The Statistical Package for Social Science software (SPSS, version 21.0, IL) was used for all analyses.
Results
Selection criteria for the analysis are shown in Figure 1. A total 1,978 stage I-IV epithelial ovarian cancer cases were screened, which included 693 (35.0%) cases of stage I disease. Among those with stage I disease, 237 (34.2%) cases did not undergo primary comprehensive surgical staging including lymphadenectomy and were excluded from the study. The remaining 456 cases were examined for the availability of archived histology slides. There were 22 (4.8%) cases that had no slides to examine, and the remaining 434 cases were evaluated for tumoral lymphovascular space invasion and statistical analysis.
Figure 1. Selection criteria of stage I ovarian cancer.
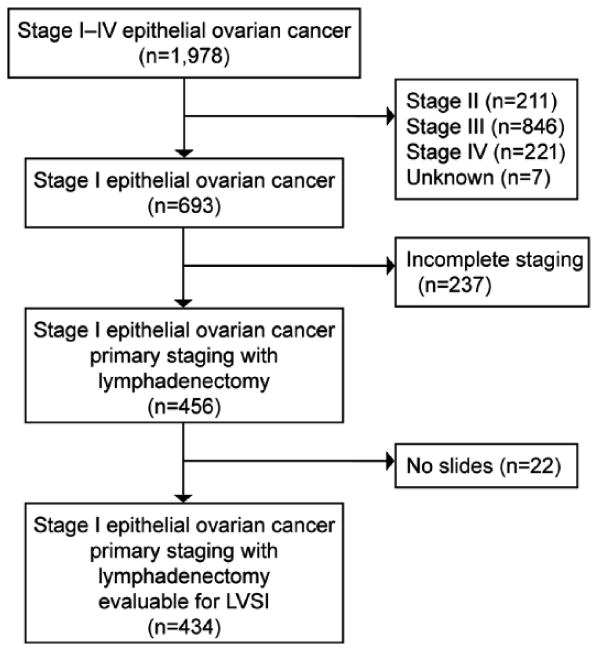
Inclusion and exclusion of the study is shown. The study was conducted per 6 institutions and 1 gynecologic oncology group. Abbreviation: w/, with.
Patient demographics are shown in Table 1. Mean patient age was 53.9 (SD±11.9) years. The majority of the cases were Asian (97.0%), had clear cell histology (41.2%) and stage IC disease (67.3%). The majority (74.1%) of stage I epithelial ovarian cancer patients received postoperative chemotherapy, and combination therapy of carboplatin and paclitaxel (63.5%) was the most common regimen with the median cycle being 6. None of the cases had residual disease at the end of surgery. Median follow-up time was 45.3 months with cumulative 5-year DFS and OS rates to be 88.4% and 91.9% in the entire cohort, respectively. Median follow-up times for lymphovascular space invasion-positive and lymphovascular space invasion–negative cases were 45.9 and 45.2 months, respectively (p=0.43). There were total 48 organ sites for the first recurrence. Of those, peritoneal recurrence (peritoneum, mesentery, or omentum) was seen in 14 sites, and hematogenous and lymphatic metastasis was recorded in 19 sites (lymph nodes 10 sites, liver parenchyma or lung 8 sites). Vaginal cuff recurrence was recorded in 7 sites.
Table 1. Demographics of stage I ovarian cancer.
| All | LVSI (+) | LVSI (-) | P-value | |
|---|---|---|---|---|
| Cases | n=434 | n=76 (17.5%) | n=358 (82.5%) | |
|
| ||||
| Age | 53.9 (±11.9) | 55.7 (±12.0) | 53.5 (±11.8) | 0.15 |
|
| ||||
| Race | 0.75 | |||
| Caucasian | 10 (2.3%) | 2 (2.6%) | 8 (2.2%) | |
| African American | 3 (0.7%) | 1 (1.3%) | 2 (0.6%) | |
| Asian | 421 (97.0%) | 73 (96.1%) | 348 (94.2%) | |
|
| ||||
| Histology | 0.042 | |||
| High-grade serous | 36 (8.3%) | 8 (10.5%) | 28 (7.8%) | |
| Low grade serous | 21 (4.8%) | 3 (3.9%) | 18 (5.0%) | |
| Clear cell | 179 (41.2%) | 41 (53.9%) | 138 (38.5%) | |
| Endometrioid | 112 (25.8%) | 9 (11.8%) | 103 (28.8%) | |
| Mucinous | 67 (15.4%) | 11 (14.5%) | 56 (15.6%) | |
| Mixed | 16 (3.7%) | 4 (5.3%) | 12 (3.4%) | |
| Other | 3 (0.7%) | 0 | 3 (0.8%) | |
|
| ||||
| Stage | 0.044 | |||
| IA | 136 (31.3%) | 16 (21.1%) | 120 (33.5%) | |
| IB | 6 (1.4%) | 0 | 6 (1.7%) | |
| IC | 292 (67.3%) | 60 (78.9%) | 232 (64.8%) | |
|
| ||||
| Stage IC sub-category | 0.24 | |||
| Capsule involvement | 86 (29.5%) | 18 (30.0%) | 68 (29.3%) | |
| Intraoperative capsule rupture | 163 (55.8%) | 34 (56.7%) | 130 (56.0%) | |
| Malignant cytology† | 29 (9.9%) | 8 (13.3%) | 21 (9.1%) | |
| Rupture + cytology | 14 (4.8%) | 0 | 13 (5.6%) | |
|
| ||||
| Grade‡ | 0.17 | |||
| 1 | 155 (61.5%) | 17 (48.6%) | 138 (63.6%) | |
| 2 | 63 (25.0%) | 13 (37.1%) | 50 (23.0%) | |
| 3 | 34 (13.5%) | 5 (14.3%) | 29 (13.4%) | |
|
| ||||
| Postoperative chemotherapy | 0.006 | |||
| No | 110 (25.9%) | 10 (13.2%) | 100 (28.7%) | |
| Yes | 315 (74.1%) | 66 (86.8%) | 249 (71.3%) | |
|
| ||||
| Chemotherapy cycle | 6 (1-12) | 6 (1-8) | 6 (1-12) | 0.27 |
|
| ||||
| Chemotherapy type | 0.67* | |||
| Carboplatin + paclitaxel | 200 (63.5%) | 40 (60.6%) | 160 (60.6%) | |
| Carboplatin + docetaxel | 42 (13.3%) | 5 (7.6%) | 37 (14.9%) | |
| Irrinotecan + mitomycin C | 16 (5.1%) | 2 (3.0%) | 14 (5.6%) | |
| Irrinotecan + cisplatin | 15 (4.8%) | 2 (3.0%) | 13 (5.2%) | |
| CB-EC | 10 (3.2%) | 7 (10.6%) | 3 (1.2%) | |
| Others** | 32 (10.2%) | 10 (15.2%) | 22 (8.8%) | |
|
| ||||
| Slide No. for ovarian tumor | 5 (1-19) | 6 (1-19) | 5 (1-17) | 0.009 |
Number (%) per group [all, LVSI (+) or LVSI (-)], mean (±SD), or mean (range) is shown. P-values for comparison of LVSI (+) and LVSI (-).
P-value for carboplatin + paclitaxel versus others.
cytology for ascites or washing.
No routine grading for clear cell type, and 3 cases from other histology types missed grading.
15 different regimens.
Abbreviations: LVSI, lymphovascular space invasion; and intraope, intraoperative; CB-EC, carboplatin + epirubicin + cyclophosphamide.
Lymphovascular space invasion was detected in 76 (17.5%, 95%CI 13.9-21.1) of the 434 cases evaluated for analysis (Table 1). Lymphovascular space invasion was significantly associated with histology. Specifically, there were more high-grade serous (10.5% versus 7.8%) and clear cell (53.9% versus 38.5%) cases while there were fewer with endometrioid histology (11.8% versus 28.8%) in lymphovascular space invasion cases when compared to no lymphovascular space invasion cases (p=0.042). Across the histological subtypes, incidence of lymphovascular space invasion was as follows: clear cell (22.9%), high-grade serous (22.2%), mucinous (16.4%), low-grade serous (14.3%), and endometrioid (8.0%). There were more stage IB-C disease in lymphovascular space invasion-expressing cases when compared to cases with no lymphovascular space invasion (78.9% versus 66.5%, p=0.044). There was no statistical association between lymphovascular space invasion and grade (p=0.17). Presence of lymphovascular space invasion did not affect malignant cytology for ascites or washing (13.3% versus 9.1%, p=0.24). Patients with lymphovascular space invasion-positive tumors were more likely to receive postoperative chemotherapy (86.8% versus 71.3%, p=0.006) than those without lymphovascular space invasion. In the original pathology reports, none of participating institution routinely evaluated lymphovascular space invasion, as it was documented in only 12.2% of the cases. Of those reported lymphovascular space invasion status in the original pathology results, lymphovascular space invasion was positive in 13.2% of cases.
Survival outcome of lymphovascular space invasion-expressing tumors in stage I ovarian cancer was examined. In univariate analysis, tumoral lymphovascular space invasion was significantly associated with decreased DFS (5-year rate, 78.4% versus 90.7%, p=0.024, Figure 2A) and decreased OS (84.9% versus 93.2%, p=0.031, Figure 2B). High-grade serous type was distinctively associated with poorer DFS than other types (5-year DFS rate, high-grade serous, low-grade serous, clear cell, mucinous, and endometrioid, 70.9%, 94.4%, 86.6%, 87.5%, and 95.6%, p=0.01). Stage was also associated with survival outcome (5-year DFS rate for IA, IB, and IC: 94.9%, 100%, and 85.3%, p=0.03). In multivariate analysis, tumoral lymphovascular space invasion did not remain a prognostic indicator associated with decreased DFS (HR 1.98, 95%CI 0.97-3.97, p=0.059, Table 2) after controlling for age (p=0.85), histology (p=0.036), stage (p=0.075), and postoperative chemotherapy (p=0.29) although it pointed toward significance. In addition to tumoral lymphovascular space invasion, High-grade serous histology (HR 2.47, p=0.036) associated with increased risk of recurrence in multivariate analysis. Similarly, tumoral lymphovascular space invasion did not remain an independent prognosticator for decreased OS (HR 2.41, 95%CI 0.99-5.85, p=0.052, Table 2) after controlling for age (p=0.54), histology (p=0.91), stage (p=0.11), and postoperative chemotherapy (p=0.13) although it pointed toward significance.
Figure 2. Survival outcome and pattern of metastasis in stage I ovarian cancer.

Univariate analysis with Log-rank test for p-values, and Kaplan-Meier method for survival curve analysis. Survival outcomes of 434 patients with stage I epithelial ovarian cancer are shown based on lymphovascular space invasion status: A) disease-free survival and B) overall survival. Cumulative risks for C) hematogenous and lymphatic metastasis and D) peritoneal metastasis. There were 4 patients who had no survival data, and 1 patient with no recurrence information. Abbreviation: LVSI, lymphovascular space invasion.
Table 2. Survival effect of lymphovascular space invasion on stage I ovarian cancer.
| Disease-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. | 5-year (%) | HR (95% CI) | P-value | 5-year (%) | HR (95% CI) | P-value | |
| LVSI | 0.059 | 0.052 | |||||
| No | 358 | 90.7% | 1 | 93.7% | 1 | ||
| Yes | 76 | 78.4% | 1.98 (0.97-3.97) | 84.1% | 2.41 (0.99-5.85) | ||
|
| |||||||
| Age | 0.85 | 0.54 | |||||
| < 65 | 357 | 88.6% | 1 | 91.8% | 1 | ||
| ≥ 65 | 77 | 87.5% | 0.92 (0.38-2.24) | 92.5% | 0.68 (0.20-2.33) | ||
|
| |||||||
| High-grade serous | 0.036 | 0.91 | |||||
| No | 398 | 90.1% | 1 | 92.4% | 1 | ||
| Yes | 36 | 71.4% | 2.47 (1.06-5.77) | 88.2% | 0.92 (0.21-4.04) | ||
|
| |||||||
| Stage | 0.075 | 0.11 | |||||
| IA | 136 | 94.9% | 1 | 95.5% | 1 | ||
| IB-C | 298 | 85.6% | 2.37 (0.92-6.12) | 90.3% | 2.64 (0.81-8.60) | ||
|
| |||||||
| Chemotherapy* | 0.29 | 0.13 | |||||
| No | 110 | 91.0% | 1 | 92.2% | 1 | ||
| Yes | 315 | 87.5% | 0.63 (0.26-1.50) | 91.8% | 0.45 (0.16-1.27) | ||
Multivariate analysis with Cox proportional hazard regression test (grade was not entered in the model due to 179 clear cell histology cases that had no grading).
postoperative chemotherapy.
Abbreviations: No., number; HR, 5-year (%), 5-year survival rate; hazard ratio; 95%CI, 95% confidence interval; and LVSI, lymphovascular space invasion.
Because tumoral lymphovascular space invasion refers to the presence of tumor cells within the microvasculature and lymphatic drainage system in the ovarian tumor, pattern and risk of recurrence after primary surgery was examined based on the tumoral lymphovascular space invasion status (Table 3). In univariate analysis, when a tumor expresses lymphovascular space invasion, there is a significant increased risk of hematogenous and lymphatic metastasis during the course of follow-up after surgery (5-year cumulative risk, lymphovascular space invasion-positive versus lymphovascular space invasion-negative tumor, 16.2% versus 3.2%, p=0.001, Figure 2C). In multivariate analysis, lymphovascular space invasion remained a statistically significant predictive indicator for increased risk of hematogenous and lymphatic metastasis (HR 4.79, 95%CI 1.75-13.2, p=0.002) after controlling for age (p=0.28), histology (p=0.20), stage (p=1.0), and postoperative chemotherapy (p=0.74). On the contrary, presence of tumoral lymphovascular space invasion in stage I ovarian cancer did not increase risk of peritoneal metastasis (5-year cumulative rate, 1.7% versus 4.3%, p=0.33, Figure 2D). Multivariate analysis re-demonstrated the insignificant result for lymphovascular space invasion for peritoneal metastasis (p=0.29), but stage IB-C disease (5.2% versus 0.9%, HR 8.20, 95%CI 1.00-67.1, p=0.05) showed an increased risk of peritoneal metastasis.
Table 3. Type of recurrence and lymphovascular space invasion in stage I ovarian cancer.
| Hematogenous and lymphatic | Peritoneal | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. | 5-year (%) | HR (95% CI) | P-value | 5-year (%) | HR (95% CI) | P-value | |
| LVSI | 0.002 | 0.29 | |||||
| No | 358 | 3.2% | 1 | 4.3% | 1 | ||
| Yes | 76 | 16.2% | 4.79 (1.75-13.2) | 1.7% | 0.33 (0.04-2.56) | ||
|
| |||||||
| Age | 0.28 | 0.75 | |||||
| < 65 | 357 | 5.8% | 1 | 4.0% | 1 | ||
| ≥ 65 | 77 | 4.8% | 0.33 (0.04-2.50) | 2.8% | 0.78 (0.17-3.67) | ||
|
| |||||||
| High-grade serous | 0.20 | 0.086 | |||||
| No | 398 | 4.8% | 1 | 3.2% | 1 | ||
| Yes | 36 | 12.0% | 2.34 (0.63-8.64) | 12.0% | 3.26 (0.85-12.6) | ||
|
| |||||||
| Stage | 1.0 | 0.05 | |||||
| IA | 136 | 3.3% | 1 | 0.9% | 1 | ||
| IB-C | 298 | 6.8% | 1.00 (0.27-3.68) | 5.2% | 8.20 (1.00-67.1) | ||
|
| |||||||
| Chemotherapy* | 0.74 | 0.14 | |||||
| No | 110 | 3.9% | 1 | 4.3% | 1 | ||
| Yes | 315 | 6.3% | 0.78 (0.19-3.27) | 3.8% | 0.37 (0.10-1.36) | ||
Multivariate analysis with Cox proportional hazard regression test (grade was not entered in the model due to 179 clear cell histology cases that had no grading).
postoperative chemotherapy.
Abbreviations: No., number; 5-year (%), 5-year cumulative rate; HR, hazard ratio; 95%CI, 95% confidence interval; and LVSI, lymphovascular space invasion.
To evaluate the clinical and treatment implications of tumoral lymphovascular space invasion in stage I ovarian cancer, the effect of postoperative chemotherapy on survival outcome was examined in exploratory analysis. Among 76 cases with lymphovascular space invasion-expressing tumors in stage I epithelial ovarian cancer, 65 (85.5%) cases received postoperative chemotherapy. The cases that received less than 6 cycles of postoperative chemotherapy showed a statistically significantly poorer DFS (<6 versus ≥6 cycles, 5-year rate, 57.6% versus 90.5%, HR 4.59, 95%CI 1.20-17.5, p=0.015, Figure 3A) and a borderline significance for decreased OS (72.5% versus 92.2%, HR 4.53, 95%CI 0.87-23.6, p=0.05, Figure 3B) than those who received 6 or more cycles. Among the 358 cases with no tumoral lymphovascular space invasion, postoperative chemotherapy cycles did not affect survival outcomes (5-year DFS, <6 versus ≥6 cycles, 89.6% versus 91.0%, p=0.86; and 5-year OS rate, 90.8% versus 98.0%, p=0.12). When type of postoperative chemotherapy was compared, there was no statistical difference in survival outcomes across the five most common regimens shown in Table 1 (DFS p=0.61, and OS p=0.59).
Figure 3. Effect of chemotherapy cycle and lymphovascular space invasion.
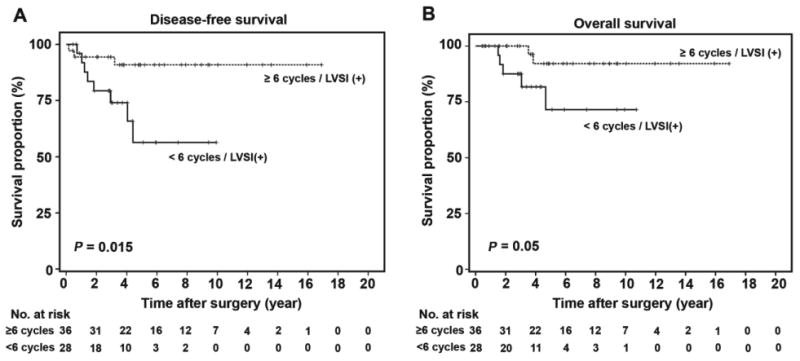
Univariate analysis with Log-rank test for p-values, and Kaplan-Meier method for survival curve analysis. Survival outcomes of 65 patients with LVSI-expressing tumors are shown based on number of postoperative chemotherapy cycle: A) disease-free survival and B) overall survival. There was 1 patient who had no survival and recurrence information. Abbreviation: LVSI, lymphovascular space invasion.
Discussion
Our study shows that tumoral lymphovascular space invasion plays a pivotal role in the progression and metastasis of stage I ovarian cancer. Key findings are that (i) tumoral lymphovascular space invasion is infrequently seen in stage I disease, (ii) presence of tumoral lymphovascular space invasion increases risk of recurrence and death related to ovarian cancer, (iii) tumoral lymphovascular space invasion increases risk of hematogenous and lymphatic metastasis but not peritoneal metastasis, and (iv) number of postoperative chemotherapy cycles affects survival outcomes of patients with lymphovascular space invasion-expressing tumors. The implications of our results merit further discussion.
Ovarian cancer is historically recognized to spread mainly through direct spread to the peritoneal cavity rather than hematogenously or lymphatically as demonstrated in an autopsy study.12 However, there is accumulating evidence demonstrating that considerable proportions of ovarian cancer cases actually have hematogenous metastasis such as liver parenchyma and lung.13 In some studies, hematogenous dissemination of ovarian cancer was proposed to happen as a relatively early event even in early-stage ovarian cancer which resulted in significantly poor survival outcomes as shown in the study evaluating bone marrow biopsy obtained from ovarian cancer patients (occult dissemination rate, 30%).14 Our current study partly supports such insight of the ovarian cancer metastasis route since a subset of stage I ovarian cancer with tumoral lymphovascular space invasion eventually resulted in a significantly increased risk of hematogenous and lymphatic spread. Although other studies suggested that increased incidence of hematogenous spread may be the consequence of the use of chemotherapy,15-16 our study showed that tumoral lymphovascular space invasion remained a significant predictor of hematogenous and lymphatic metastasis after controlling for the use of postoperative chemotherapy (Table 3). Due to its common peritoneal tumor seeding in ovarian cancer, intraperitoneal chemotherapy is recommended for stage III optimally debulked ovarian cancer per the National Comprehensive Cancer Network (NCCN) guidelines.17 However, our results suggest the importance of intravenous chemotherapy to potentially control hematogenous and lymphatic metastasis in ovarian cancer.
In stage I ovarian cancer, postoperative chemotherapy is generally recommended with carboplatin and paclitaxel for stage IC disease, grade 3 tumor, and incompletely staged patients.7 A recent clinical trial demonstrated that the number of chemotherapy cycles for carboplatin and paclitaxel did not alter the survival outcome in stage I ovarian cancer but resulted in an increased risk of grade 3-4 neurotoxicity in patients who received 6 cycles.18 Postoperative chemotherapy was even associated with a significantly increased risk of long-term morbidity in early-stage ovarian cancer patients.19 In this setting, the 6-cycle administration of postoperative chemotherapy would be beneficial when its use is limited to certain indications. Indeed, recent literature showed that serous histology had a survival benefit associated with 6-cycle administration in early-stage ovarian cancer.20 Our data showed that patients with tumor expressing lymphovascular space invasion who received 6 or more cycles of postoperative chemotherapy had better survival outcomes than those who received less than 6 cycles (Figure 3A-B). Therefore, our data may at least suggest that lymphovascular space invasion-expressing tumor is indicated for 6 or more cycles of postoperative chemotherapy.
A strength of the study was that this was a multi-center study with strict enrolling criteria limited to comprehensively staged early ovarian cancer. A possible weakness of the study is the retrospective design, which may include potential confounding factors such as variability in chemotherapy regimens at multiple different institutions and unknown exact indications for chemotherapy cycles among lymphovascular space invasion-positive tumor cases. Central pathology review was also not performed in our study. Although corrected in the multivariate model, histology type and stage associated with lymphovascular space invasion are important potential confounders for survival outcomes. A limitation of our study may include sample size that was underpowered for survival analysis. Although we utilized a large study sample, our results for survival outcomes based on tumoral lymphovascular space invasion status did not reach statistical significance in multivariate analysis. This is likely due to the high survival rate of stage I ovarian cancer patients and the relatively low incidence of lymphovascular space invasion in early stage tumors. Post-hoc power analysis by using alpha level of 5% showed that the power for sample size for 5-year DFS was adequate at 81.3%; however, the power for sample size for OS was 74.5% which is not adequate (below 80%). In order to have an 80% power for a 5-year OS rate, the sample size is estimated to be 510.
In conclusion, tumoral lymphovascular space invasion is an important histologic finding in stage I epithelial ovarian cancer, and routine evaluation of tumoral lymphovascular space invasion is highly recommended in daily practice. In addition, standardization of examination and therapeutic implication of lymphovascular space invasion in ovarian cancer will merit further development.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Lim MC, Kang S, Lee KS, et al. The clinical significance of hepatic parenchymal metastasis in patients with primary epithelial ovarian cancer. Gynecol Oncol. 2009;112:28–34. doi: 10.1016/j.ygyno.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Pignata S, Cannella L, Leopardo D, Pisano C, Bruni GS, Facchini G. Chemotherapy in epithelial ovarian cancer. Cancer Lett. 2011;303:73–83. doi: 10.1016/j.canlet.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Roett MA, Evans P. Ovarian cancer: an overview. Am Fam Physician. 2009;80:609–16. [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo K, Lin YG, Roman LD, Sood AK. Overcoming platinum resistance in ovarian carcinoma. Expert Opin Investig Drugs. 2010;19:1339–54. doi: 10.1517/13543784.2010.515585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuo K, Bond VK, Eno ML, Im DD, Rosenshein NB. Low drug resistance to both platinum and taxane chemotherapy on an in vitro drug resistance assay predicts improved survival in patients with advanced epithelial ovarian, fallopian and peritoneal cancer. Int J Cancer. 2009;125:2721–7. doi: 10.1002/ijc.24654. [DOI] [PubMed] [Google Scholar]
- 7.Sood A, Matsuo K, Gershenson D. Management of early-stage ovarian cancer. In: Bristow RE, Karlan BY, editors. Surgery for ovarian cancer: Principles and practice. Chapter 3. 2nd. Vol. 3. Informa Healthcare; New York: 2010. pp. 37–60. [Google Scholar]
- 8.Berek JS, Crum C, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2012;119(Suppl 2):S118–29. doi: 10.1016/S0020-7292(12)60025-3. [DOI] [PubMed] [Google Scholar]
- 9.Young RC, Pecorelli S. Management of early ovarian cancer. Semin Oncol. 1998;25:335–9. [PubMed] [Google Scholar]
- 10.Matsuo K, Sheridan TB, Yoshino K, et al. Significance of lymphovascular space invasion in epithelial ovarian cancer. Cancer Med. 2012;1:156–64. doi: 10.1002/cam4.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malpica A. Grading of ovarian cancer: a histotype-specific approach. Int J Gynecol Pathol. 2008;27:175–81. doi: 10.1097/PGP.0b013e31816085e0. [DOI] [PubMed] [Google Scholar]
- 12.Rose PG, Piver MS, Tsukada Y, Lau TS. Metastatic patterns in histologic variants of ovarian cancer. An autopsy study Cancer. 1989;64:1508–13. doi: 10.1002/1097-0142(19891001)64:7<1508::aid-cncr2820640725>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Reed E, Zerbe CS, Brawley OW, Bicher A, Steinberg SM. Analysis of autopsy evaluations of ovarian cancer patients treated at the National Cancer Institute, 1972-1988. Am J Clin Oncol. 2000;23:107–16. doi: 10.1097/00000421-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Braun S, Schindlbeck C, Hepp F, et al. Occult tumor cells in bone marrow of patients with locoregionally restricted ovarian cancer predict early distant metastatic relapse. J Clin Oncol. 2001;19:368–75. doi: 10.1200/JCO.2001.19.2.368. [DOI] [PubMed] [Google Scholar]
- 15.Guth U, Huang DJ, Bauer G, Stieger M, Wight E, Singer G. Metastatic patterns at autopsy in patients with ovarian carcinoma. Cancer. 2007;110:1272–80. doi: 10.1002/cncr.22919. [DOI] [PubMed] [Google Scholar]
- 16.Tyndale R, Aoyama T, Broly F, et al. Identification of a new variant CYP2D6 allele lacking the codon encoding Lys-281: possible association with the poor metabolizer phenotype. Pharmacogenetics. 1991;1:26–32. doi: 10.1097/00008571-199110000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Morgan RJ, Jr, Alvarez RD, Armstrong DK, et al. Ovarian cancer, version 2.2013. J Natl Compr Canc Netw. 2013;11:1199–209. doi: 10.6004/jnccn.2013.0142. [DOI] [PubMed] [Google Scholar]
- 18.Bell J, Brady MF, Young RC, et al. Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;102:432–9. doi: 10.1016/j.ygyno.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Engelen MJ, Snel BJ, Schaapveld M, et al. Long-term morbidity of adjuvant whole abdominal radiotherapy (WART) or chemotherapy for early stage ovarian cancer. Eur J Cancer. 2009;45:1193–200. doi: 10.1016/j.ejca.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Chan JK, Tian C, Fleming GF, et al. The potential benefit of 6 vs. 3 cycles of chemotherapy in subsets of women with early-stage high-risk epithelial ovarian cancer: an exploratory analysis of a Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:301–6. doi: 10.1016/j.ygyno.2009.10.073. [DOI] [PubMed] [Google Scholar]


