Abstract
BACKGROUND
The study of autoinflammatory diseases has uncovered mechanisms underlying cytokine dysregulation and inflammation.
METHODS
We analyzed the DNA of an index patient with early-onset systemic inflammation, cutaneous vasculopathy, and pulmonary inflammation. We sequenced a candidate gene, TMEM173, encoding the stimulator of interferon genes (STING), in this patient and in five unrelated children with similar clinical phenotypes. Four children were evaluated clinically and immunologically. With the STING ligand cyclic guanosine monophosphate–adenosine monophosphate (cGAMP), we stimulated peripheral-blood mononuclear cells and fibroblasts from patients and controls, as well as commercially obtained endothelial cells, and then assayed transcription of IFNB1, the gene encoding interferon-β, in the stimulated cells. We analyzed IFNB1 reporter levels in HEK293T cells cotransfected with mutant or nonmutant STING constructs. Mutant STING leads to increased phosphorylation of signal transducer and activator of transcription 1 (STAT1), so we tested the effect of Janus kinase (JAK) inhibitors on STAT1 phosphorylation in lymphocytes from the affected children and controls.
RESULTS
We identified three mutations in exon 5 of TMEM173 in the six patients. Elevated transcription of IFNB1 and other gene targets of STING in peripheral-blood mono-nuclear cells from the patients indicated constitutive activation of the pathway that cannot be further up-regulated with stimulation. On stimulation with cGAMP, fibro-blasts from the patients showed increased transcription of IFNB1 but not of the genes encoding interleukin-1 (IL1), interleukin-6 (IL6), or tumor necrosis factor (TNF). HEK293T cells transfected with mutant constructs show elevated IFNB1 reporter levels. STING is expressed in endothelial cells, and exposure of these cells to cGAMP resulted in endothelial activation and apoptosis. Constitutive up-regulation of phosphorylated STAT1 in patients’ lymphocytes was reduced by JAK inhibitors.
CONCLUSIONS
STING-associated vasculopathy with onset in infancy (SAVI) is an autoinflammatory disease caused by gain-of-function mutations in TMEM173.
Studies involving children with mono-genic autoinflammatory disease have provided insights into the regulation of key proinflammatory cytokine pathways and their role in causing systemic and organ-specific inflammation.1,2 We studied six patients who presented in early infancy with systemic inflammation and violaceous, scaling lesions of fingers, toes, nose, cheeks, and ears that progressed to acral necrosis in most of the patients and did not respond to therapy. A mixed histologic pattern was observed, consisting of a prominent dermal inflammatory infiltrate with features of leukocytoclastic vasculitis and microthrombotic angiopathy of small dermal vessels. Three of the patients had severe interstitial lung disease.
Although many of the genetically defined auto-inflammatory diseases are associated with increased interleukin-1 signaling and have a clinical response to interleukin-1 inhibition,2 interleukin-1–inhibiting therapy was ineffective in one of the patients. A prominent interferon-response-gene signature in the peripheral blood of this participant suggested interferon dysregulation similar to that seen in other genetically defined inflammatory syndromes, including the Aicardi–Goutières syndrome,3,4 an early-onset inflammatory encephalopathy, and chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE),5,6 a proteasome-associated autoinflammatory syndrome with proposed interferon-mediated pathologic features.7,8 We detected autosomal dominant mutations in TMEM173, the gene encoding the stimulator of interferon genes (STING), in the six children we assessed, who presented with a clinical syndrome we have called STING-associated vasculopathy with onset in infancy (SAVI).
METHODS
PATIENTS
Written informed consent was provided for all four living study participants; for three participants, one or both parents provided written informed consent, and the fourth provided written informed consent himself. These patients were enrolled in an ongoing, institutional review board–approved study of the pathogenesis and natural history of their systemic inflammatory disease at the National Institutes of Health.
GENETIC STUDIES
We obtained samples of genomic DNA from Patient 1 and her parents for whole-exome sequenc ing (performed by Otogenetics and Beijing Genomics Institute). We used Sanger sequencing to analyze the introns and exons of TMEM173, the candidate gene revealed by means of whole-exome sequencing, in five unrelated patients who presented with a clinically similar phenotype.
FUNCTIONAL STUDIES
Quantitative reverse-transcriptase–polymerase-chain-reaction, cytokine, protein, and gene-expression analyses were performed according to standard procedures and are described in the Supplementary Appendix, available with the full text of this article at NEJM.org. Constructs of mutated TMEM173 (V147L, N154S, V155M, and V155R) and nonmutated TMEM173 were transfected into a STING-negative cell line (HEK293T cells) and stimulated with the STING ligand cyclic guanosine monophosphate–adenosine monophosphate (cGAMP [3′3′-cGAMP, Invivogen]).
When possible, we obtained blood and tissue samples from the study participants to assess activation and cell death of peripheral-blood cells. Tissue blocks from skin biopsies (in five patients), samples from lung biopsies (in two), and slides of a sample from a previous muscle biopsy (in one) were obtained and analyzed. Dermal fibroblast lines were obtained from two patients, four healthy controls, and three controls with the CANDLE syndrome. Primary endothelial cells were stimulated with the STING ligand cGAMP.
CD4 T cells and CD19 B cells from Patients 4 and 6 were treated for 4 hours with one of three Janus kinase (JAK) inhibitors — tofacitinib (1 μM), ruxolitinib (100 nM), or baricitinib (200 nM) — to assess their ability to block phosphorylation of the signal transducers and activators of transcription 1 (STAT1) and 3 (STAT3). Fibroblasts from Patient 1 and healthy controls were stimulated with 500 ng of cGAMP per milliliter and were also treated with 0.1 or 1.0 μM tofacitinib. We assayed the suppression of the gene encoding interferon-β (IFNB1) and other interferon-induced genes (CXCL10, MX1, and OAS3). Additional details are provided in the Supplementary Appendix.
RESULTS
CLINICAL PHENOTYPE
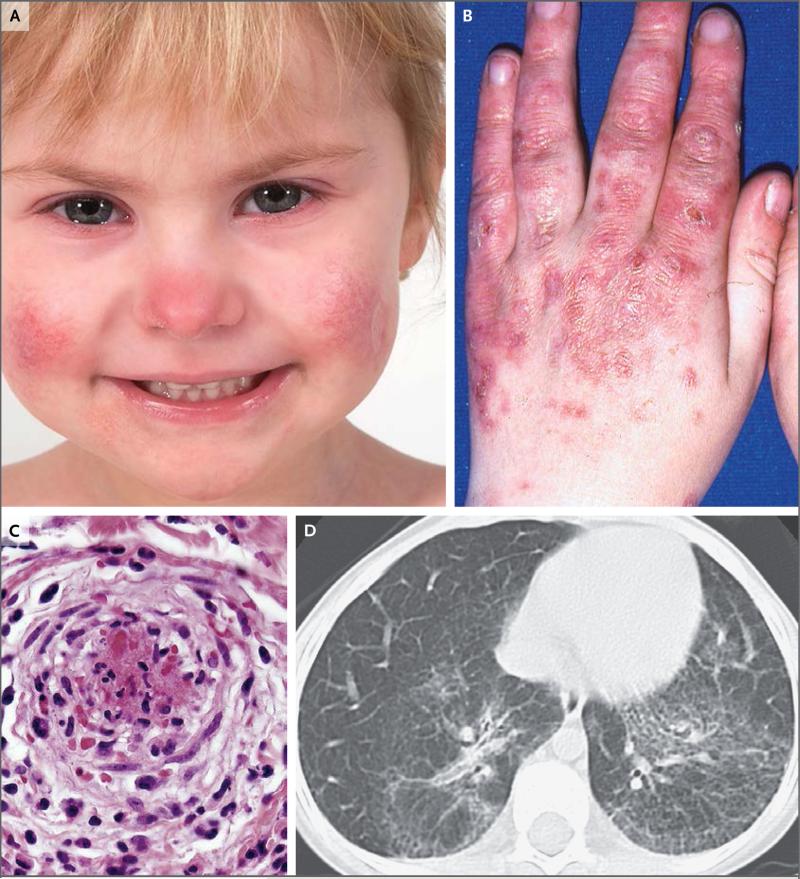
Demographic data and details of the clinical presentation of the six participants are provided in Table 1, and Table S1 in the Supplementary Appendix. All the patients were symptomatic within the first 8 weeks of life. Two patients, Patients 1 and 5, presented initially with tachypnea. Between 2 weeks and 6 months of age, telangiectatic, pustular, or blistering rashes (or a combination of these types) developed on the cheeks, nose, fingers, toes, and soles of all the patients (Fig. 1A and 1B, and Fig. S1A through S1E in the Supplementary Appendix), with evidence of systemic inflammation (elevations of the erythrocyte sedimentation rate and C-reactive protein level) (Table S2 in the Supplementary Appendix). Five patients had recurrent low-grade fever flares. Skin lesions, which worsened in cold weather, extended to the pinnae of the ears and scattered sites on the extremities. Eschar and secondary painful crusts covered ulcerated skin lesions (Fig. S1A through S1E in the Supplementary Appendix).
Table 1.
Demographic and Clinical Characteristics of Six Patients with SAVI.*
| Characteristic | No. of Patients |
|---|---|
| Age at presentation 1 day to 8 wk | 6 |
| Sex | |
| Female | 3 |
| Male | 3 |
| Symptom at initial presentation | |
| Rash | 4 |
| Tachypnea | 2 |
| Features of systemic inflammation | |
| Increased acute-phase reactant levels† | 6 |
| Fever | 6 |
| Features of peripheral vascular inflammation | |
| Acral violaceous plaques | 6 |
| Nodules on face, nose, or ears | 6 |
| Distal ulcerative lesions with infarcts | 6 |
| Manifestations of vascular and tissue damage | |
| Nail dystrophy or loss | 6 |
| Gangrene of fingers or toes | 4 |
| Nasal-septum perforation | 4 |
| Pulmonary manifestations | |
| Paratracheal adenopathy | 6 |
| Abnormal pulmonary-function test | 5 |
| Interstitial lung disease observed on CT | 5 |
| Lung fibrosis | 3 |
| Low-titer autoantibodies | |
| Antinuclear antibody | 3 |
| Antiphospholipid antibodies‡ | 5 |
| c-ANCA | 1 |
| No response or incomplete response to treatment | |
| Glucocorticoid | 6 |
| DMARD | 6 |
| Biologic agent§ | 6 |
The term c-ANCA denotes cytoplasmic antineutrophil antibody, CT computed tomography, DMARD disease-modifying antirheumatic drug, and SAVI stimulator of interferon genes (STING)–associated vasculopathy with onset in infancy.
Measurements for acute-phase reactants included the C-reactive protein level and the erythrocyte sedimentation rate.
Autoantibody titers were only transiently positive (Table S1 in the Supplementary Appendix).
Biologic agents that elicited minimal clinical responses are listed in Table S1 in the Supplementary Appendix.
Figure 1. Clinical Features of Stimulator of Interferon Genes (STING)–Associated Vasculopathy with Onset in Infancy (SAVI).
Panel A shows the typical facial distribution of telangiectatic lesions on the nose and cheeks of Patient 1, who has SAVI. Panel B shows violaceous, scaling, atrophic plaques on the hands of Patient 6. Panel C shows histologic features of vascular inflammation in a skin-biopsy sample from a clinically involved area depicting a dense neutrophilic infiltrate with karyorrhexis throughout the vessel wall (hematoxylin and eosin); fibrin deposits are seen in the lumen of a severely damaged vessel. In Panel D, a high-resolution computed tomographic image of the lung of Patient 5 shows interstitial lung disease.
Over time, clinical features of small-vessel damage developed in all the patients, including nailfold capillary tortuosity and capillary-loop loss (in three patients) (Fig. S1F and Table S1 in the Supplementary Appendix), and telangiectasia of the extremities and hard palate (in all six). Vascular occlusions caused scarring of the ear cartilage and perforation of the nasal septum (Fig. S1A, S1G, S1H, and S1I in the Supplementary Appendix), dystrophic nail changes, resorption of distal phalanges of fingers and toes, and gangrenous digits necessitating surgical amputation (Table 1; and Table S1 and Fig. S1C, S1D, S1J, S1K, and S1L in the Supplementary Appendix).
Samples from biopsies of lesional skin in all the patients revealed marked vascular inflammation limited to capillaries (Fig. 1C, and Fig. S2A through S2L in the Supplementary Appendix). Microthrombotic vascular changes were seen predominantly in biopsy samples from chronic skin lesions. We did not see vasculitis of medium-size or large vessels or deep venous thrombosis. Skin-biopsy samples from four patients showed evidence of IgM deposition in two patients and C3 deposition in scattered vessels with fibrin deposits in three, suggesting the presence of trapped immune complexes (Fig. S2E through S2H in the Supplementary Appendix). Three patients had low-titer autoantibodies that are seen in vasculitis (cytoplasmic and perinuclear anti-neutrophil cytoplasmic antibodies [c-ANCA and p-ANCA, respectively]) and the antiphospholipid syndrome (anticardiolipin antibodies and antibodies against B2-glycoprotein), but these antibodies disappeared over time (Table S1 in the Supplementary Appendix).
Five of the six patients (all except for Patient 4) had evidence of interstitial lung disease, and all had hilar or paratracheal lymphadenopathy as observed on computed tomography of the chest (Fig. 1D). However, Patients 3, 4, and 6 had no respiratory symptoms. Lung-biopsy samples from two patients (Patients 1 and 5) showed a scattered mixed lymphocytic inflammatory infiltrate (Fig. S3A through S3D in the Supplementary Appendix), interstitial fibrosis, and emphysematous changes (Fig. S3A in the Supplementary Appendix). All the patients had failure to thrive (Table S1 in the Supplementary Appendix).
By 7 years of age, Patient 5 had symmetric seropositive polyarthritis with carpal-bone erosions and progressive ulnar deviation (Fig. S1M in the Supplementary Appendix). Two patients (Patients 2 and 5) had histologically confirmed myositis and muscle atrophy (Fig. S3E and S3F in the Supplementary Appendix). These two children died from pulmonary complications and secondary infection. An autopsy performed on Patient 2 showed a widespread vasculopathy of the systemic and pulmonary vasculature (Table S3 in the Supplementary Appendix).
GENETIC ANALYSIS OF TMEM173 MUTATIONS
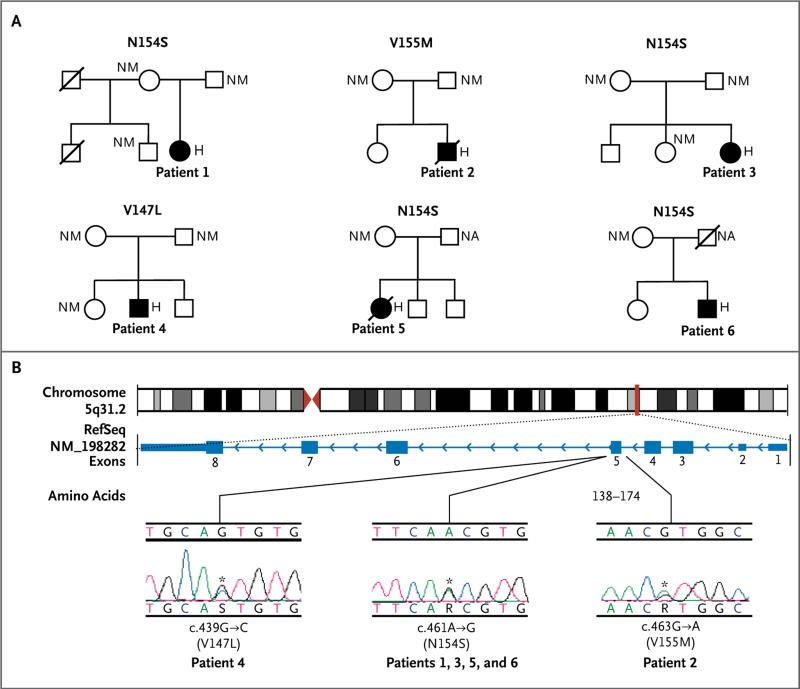
We performed whole-exome sequencing on samples from Patient 1 and her parents, and we filtered coding variants against allele frequencies from public and local databases and variants found in her parents’ samples. We identified a de novo germline mutation in a coding region of TMEM173, c.461A→G, p.N154S (Fig. 2, and Fig. S4A in the Supplementary Appendix). We used targeted Sanger sequencing of the candidate gene in five unrelated children with phenotypically similar disease. Three unrelated children, one of Turkish ancestry (Patient 3) and two of European ancestry (Patients 5 and 6), had the same mutation (p.N154S) as Patient 1, who is of French-Canadian ancestry. Samples from the parents of Patients 1 through 4 and maternal samples available for Patients 5 and 6 were negative for the mutation.
Figure 2. Mutations in TMEM173, the Gene Encoding STING.
Panel A shows the family pedigrees of the six children with the mutations N154S, V155M, and V147L in TMEM173. Solid symbols indicate Patients 1 through 6, open symbols unaffected relatives, squares male persons, circles female persons, and slashes deceased persons. For the persons for whom the TMEM173 genotype was determined, H denotes heterozygous mutated gene, NA not available, and NM nonmutated gene. Panel B shows the genomic structure with the centromere in red triangles and the location of the TMEM173 locus shown by a red line. Also shown is the gene structure (National Center for Biotechnology Information Reference Sequence [RefSeq] number, NM_198282) with the exons shown as blue boxes. The mutations were clustered in a small region of exon 5. Electropherograms of the three de novo mutations are shown (which are named under the plots, along with the predicted amino acid substitutions) for Patients 1, 2, and 4; Patients 3, 5, and 6 had the same mutation as Patient 1. The mutation detected in Patient 6 is probably somatic.
Other de novo mutations were detected in Patient 2 (c.463G→A, p.V155M), who was of European ancestry, and Patient 4 (c.439G→C, p.V147L), who was of Chilean ancestry (Fig. 2B, and Fig. S4B and Table S4 in the Supplementary Appendix). Sanger sequencing of DNA from Patient 6 showed a variable prevalence of the mutation c.461A→G, p.N154S across different cell types (whole blood, neutrophils, buccal cells, dermal fibroblasts, and keratinocytes), suggesting somatic mosaicism of the mutation (Fig. S4B and S5 in the Supplementary Appendix). Amino acids at positions 154 and 155 were absolutely conserved across the STING orthologues (across a broad range of species) that we aligned (Fig. S6 in the Supplementary Appendix). The amino acid at position 147 was either valine or isoleucine in most of the STING orthologues we aligned, except for the chicken (Gallus gallus), which carries a leucine at amino acid position 147 (Fig. S6 in the Supplementary Appendix).
FUNCTIONAL ANALYSES
Constitutive Activation of the STING–Interferon-β Pathway
TMEM173 encodes the adaptor protein STING, which functions as a homodimer. On binding its ligand, cGAMP, it mediates the production of interferon-β by means of a pathway involving the phosphorylation of TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF-3) (Fig. 3).9 The finding that all three mutations are predicted to result in the substitution of amino acid residues close to the STING dimerization site suggested that they might interfere with dimerization, but two recombinant mutant STING proteins (N154S and V155M) each formed a stable dimer (Fig. S7 in the Supplementary Appendix) (we did not carry out this experiment using the third mutation, V147L).
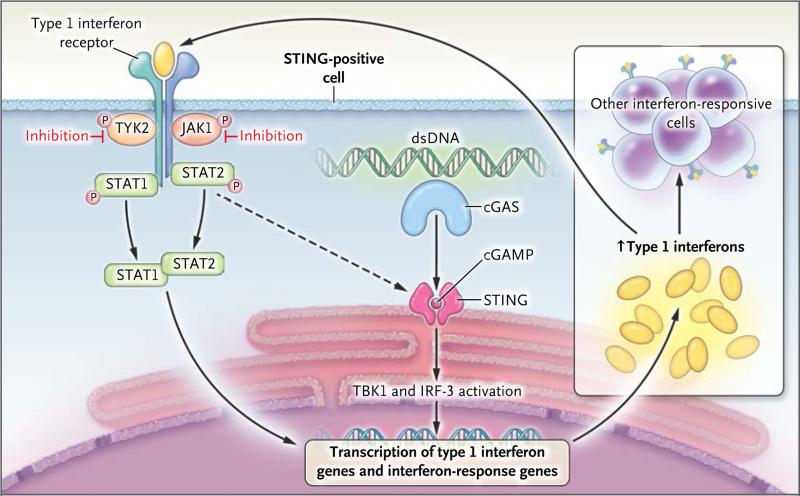
Figure 3. The STING–Interferon-β Pathway.
STING, an endoplasmic reticulum transmembrane protein, forms homodimers and functions as an adaptor for cytosolic DNA sensing. STING is activated by the binding of cyclic guanosine monophosphate–adenosine monophosphate (cGAMP), a second messenger that is synthesized by cyclic GMP–AMP synthase (cGAS), a family member of nucleotidyltransferases that is activated on its recognition and binding of double-stranded DNA (dsDNA). Binding of cGAMP to the STING homodimer activates interferon regulatory factor 3 (IRF-3) through TANK-binding kinase 1 (TBK1) and leads to the induction of interferon-β. In patients with SAVI, constitutively activated STING leads to increased transcription of the type 1 interferon gene, IFNB1, which encodes interferon-β. Binding of inter feron-β to its receptor activates Janus kinases (JAKs), including JAK1 and tyrosine kinase 2 (TYK2), which in turn phosphory-late the receptor. This process allows the binding of the DNA-binding proteins signal transducers and activators of transcription 1 (STAT1) and 2 (STAT2) to the receptor, whereupon they become phosphorylated (P). Phosphorylation allows them to dimerize, and the dimer translocates to the nucleus, where it up-regulates transcription of interferon-response genes, including interferon regulatory factor 7–dependent transcription of type 1 interferon genes. The synthesis and release of interferons and their binding to interferon receptor further up-regulate STING and the transcription of other proinflammatory cytokine genes in a positive feedback loop. JAK inhibition blocks the loop, resulting in a decrease in STAT1 phosphorylation and transcription of its target genes in vitro.
We observed a strong transcriptional interferon-response-gene signature and elevated levels of interferon-inducible protein 10 (IP-10) and other interferon-induced cytokines in the peripheral whole blood of four of the patients (Fig. 4A, and Table S5 in the Supplementary Appendix), suggesting that their TMEM173 mutations effected a gain of function in STING, leading to overproduction of interferon. We used cGAMP to stimulate STING and then analyzed downstream events in the STING–interferon-β pathway (IRF-3 phosphorylation and transcription of the gene encoding interferon-β [IFNB1]), interferon-induced STAT1 phosphorylation, and interferon-response-gene induction in participants’ peripheral-blood mono-nuclear cells (PBMCs) and fibroblasts.
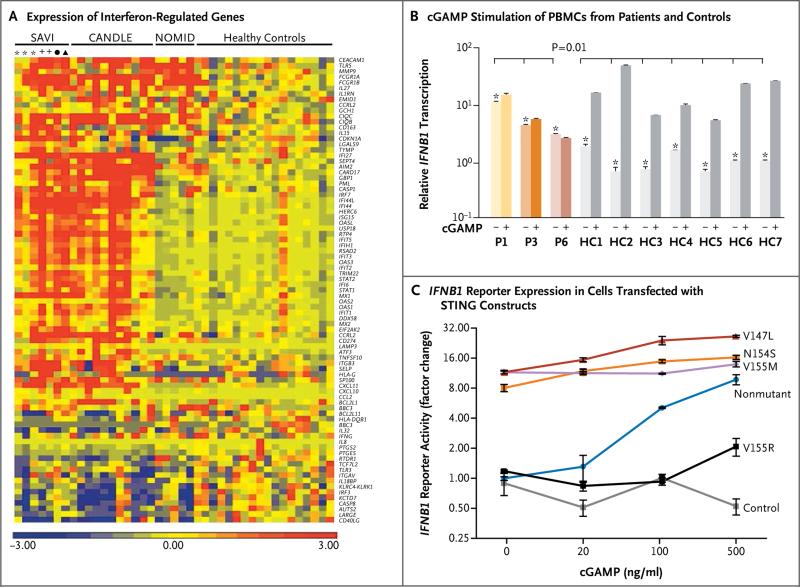
Figure 4. Functional Data Showing Gain-of-Function Mutation in TMEM173 in Patients with SAVI.
In Panel A, a heat map shows increased expression of interferon-regulated genes in 4 patients with SAVI, as compared with another interferon-mediated autoinflammatory disease, the chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome (11 patients), the interleukin-1 mediated neonatal-onset multisystem inflammatory disease (NOMID; 5 patients), and 18 healthy controls. Up-regulated genes are shown in red, and down-regulated genes in blue. Asterisks indicate samples obtained at different time points from Patient 1, and plus signs indicate samples obtained at different time points from Patient 3. The circle indicates a sample from Patient 4, and the triangle a sample from Patient 6. Panel B shows the result of a quantitative reverse-transcriptase–polymerase-chain-reaction assay of IFNB1 messenger RNA (mRNA) in peripheral-blood mononuclear cells (PBMCs) from 3 patients with SAVI and 7 relatives or controls, with (+) and without (−) cGAMP stimulation. Shown are normalized IFNB1 mRNA levels against the average value for healthy controls in the absence of stimulation. P1, P3, and P6 denote Patients 1, 3, and 6, respectively. The healthy controls included Patient 1's sibling, father, and mother (HC1, HC2, and HC3, respectively), Patient 3's female sibling and mother (HC4 and HC5, respectively), and two persons who were unrelated to the patients (HC6 and HC7). Errors bars indicate the standard error for triplicates in the assay. Asterisks denote P = 0.01 for the comparison between unstimulated samples from patients and unstimulated samples from controls; no significant increase in IFNB1 mRNA levels was seen after cGAMP stimulation in the patients, as compared with controls. Panel C shows cotransfection of HEK293T cells with a TMEM173 construct (2 ng of plasmid DNA), either the nonmutated gene or a gene with a disease-associated mutation (N154S, V155M, or V147L) or loss-of-function mutation (V155R), in addition to an IFNB1 luciferase reporter construct, performed to assess IFNB1 reporter activity with and without cGAMP stimulation. A green fluorescent protein expressing plasmid was also contransfected as control. The reporter activity was normalized to the result of nonmutant construct without cGAMP stimulation. The three constructs carrying the putative disease-causing mutations, but not the nonmutated constructs, constitutively activated IFNB1 transcription, which was further up-regulated with cGAMP stimulation. The cells with the nonmutated construct responded to cGAMP stimulation in a dose-dependent manner, whereas the cells with the loss-of-function mutation V155R had minimal responses only at the highest cGAMP concentrations. The cells with the control transfection did not respond to all tested cGAMP concentrations. I bars indicate standard errors.
STING was expressed in T cells, monocytes, natural killer cells, and dermal fibroblasts but was absent in neutrophils and resting B cells (Fig. S8A and S8B in the Supplementary Appendix). Transcription of IFNB1 and the induction of interferon-response genes were maximally up-regulated in PBMCs from the patients; exposure to cGAMP brought about no change (Fig. 4B, and Fig. S8C in the Supplementary Appendix). In contrast, expression of IFNB1 and interferon-response genes in control cells was low at baseline and increased on exposure to cGAMP. Baseline and induced transcription levels of the genes encoding inter feron-α4 (IFNA4), interferon-γ (IFNG), and the proinflammatory cytokine interleukin-1β (IL1B) were similar in patients and controls, whereas transcription of the genes encoding tumor necrosis factor (TNF) and interleukin-6 (IL6) was elevated in unstimulated PBMCs from the patients and was augmented on exposure to cGAMP (Fig. S8C in the Supplementary Appendix).
STAT1 was constitutively phosphorylated in T and B lymphocytes from the three patients tested and could not be further increased with interferon-α stimulation. Phosphorylation of other STATs was also up-regulated either by interferon-β directly10 or by other induced cytokines (Fig. S9A and S9B in the Supplementary Appendix). In dermal fibroblasts from the patients, basal IFNB1 transcription was not up-regulated (in Patient 1), but a set of IRF-3–dependent genes that were induced by cGAMP in controls was constitutively up-regulated in fibroblasts from Patient 1 (Fig. S10A and S10B in the Supplementary Appendix). Fibro-blasts from the patients were more responsive to low-dose stimulation with cGAMP with up-regulation of IRF-3 phosphorylation (Fig. S10C in the Supplementary Appendix) and IFNB1 transcription than were fibroblasts from healthy controls or from patients with the CANDLE syndrome6 (Fig. S10D and S10E in the Supplementary Appendix).
Transfection Experiments and Confirmation of Gain of Function
We transfected HEK293T cells that do not express endogenous STING with nonmutant and mutant constructs encoding STING (N154S, V155M, and V147L, and a previously reported loss-of-function mutant, V155R).11 IFNB1 reporter activity was highly elevated in cells transfected with the three mutant constructs and was barely boosted on stimulation with the STING ligand cGAMP, a finding that was similar to our observations in PBMCs. In contrast, cells transfected with nonmutated TMEM173, TMEM173 with the loss-of-function mutation, or control plasmid had no substantial baseline activation. Cells transfected with nonmutated TMEM173 had a response to cGAMP stimulation in a dose-dependent manner, and the sample expressing the loss-of-function mutant had a minimal response only at the highest cGAMP concentration (Fig. 4C, and Fig. S11 in the Supplementary Appendix).
STING in Vasculitic Lesions and Its Activation in Endothelial Cells
STING was expressed in endothelial cells from biopsy samples of nonlesional skin from healthy adults and lesional skin from patients with SAVI and in commercially available endothelial-cell lines (Fig. S12A, S12B, and S13A in the Supplementary Appendix). It was also expressed in alveolar type 2 pneumocytes, bronchial epithelium, and alveolar macrophages in lung tissue (Fig. S12C through S12E in the Supplementary Appendix).
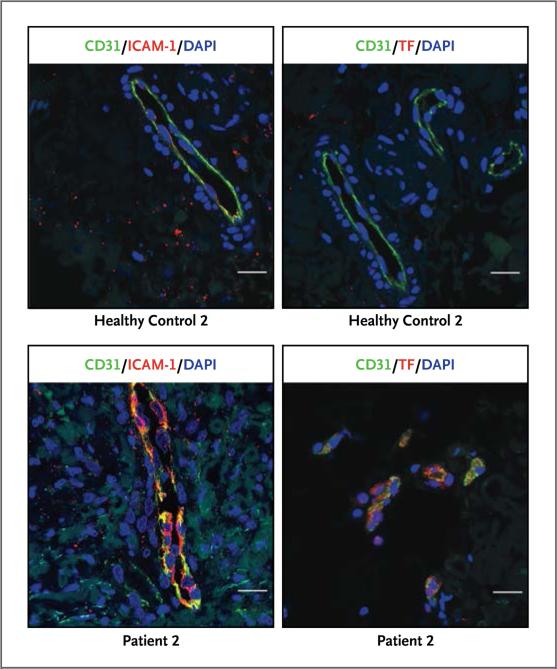
Vascular endothelial cells in biopsy samples from lesional skin (from Patients 2 and 3) expressed selected markers of endothelial inflammation (inducible nitric oxide synthase), coagula tion (tissue factor), and endothelial-cell adhesion and activation (E-selectin and intercellular adhesion molecule 1); we detected no up-regulation of these markers in the endothelial cells of healthy controls. The endothelial-cell layer in affected vessels was damaged in some areas and was preserved in healthy controls (Fig. 5, and Fig. S13A in the Supplementary Appendix).
Figure 5. Analysis of Dermal Vascular Endothelial-Cell Activation.
The images show tissue immunofluorescence staining of skin-biopsy samples from Patient 2 and a healthy control. Endothelial-cell marker CD31 (green) was costained with intercellular adhesion molecule 1 (ICAM-1, red) and coagulation marker tissue factor (TF, red). Shown is a damaged endothelial-cell layer in Patient 2 with loss of continuous staining of CD31 in the endothelial lining (lower panels) and a preserved endothelial-cell layer in healthy controls (upper panels). Both ICAM-1 and TF were absent in control cells but were up-regulated in the endothelial cells from Patient 2. Nuclei were stained blue with 4′,6-diamidine-2-phenylidole dihydrochloride (DAPI). The scale bars represent 20 μm.
The skin-biopsy samples showed features of substantial cutaneous small-vessel inflammation with leukocytoclasia, which was present at an early stage of life in all the participants, leading to tissue loss on hands and feet (Fig. S1 and S2 in the Supplementary Appendix). Vasculopathic changes without evidence of thrombosis in larger vessels suggested a widespread vasculopathy (Table S3 in the Supplementary Appendix). To explore the effect of STING in endothelial-cell activation, we assessed the transcription profile of cGAMP-stimulated endothelial cells from human umbilical veins. The genes that showed the greatest increase in expression were interferon-response genes or other genes that mediate inflammation, as well as genes that control apoptosis, cell adhesion, and coagulation pathways (Fig. S13B and S13C in the Supplementary Appendix). Stimulation of endothelial cells with cGAMP led to increased expression of inducible nitric oxide synthase, E-selectin, and tissue factor and induced endothelial-cell death (Fig. S13D and S13E in the Supplementary Appendix). Spontaneous cell death was also observed in T cells and monocytes from the patients but not in B cells, which lack STING expression (Fig. S14 in the Supplementary Appendix).
Down-Regulation of STING–Interferon-β Pathway with JAK Inhibitors
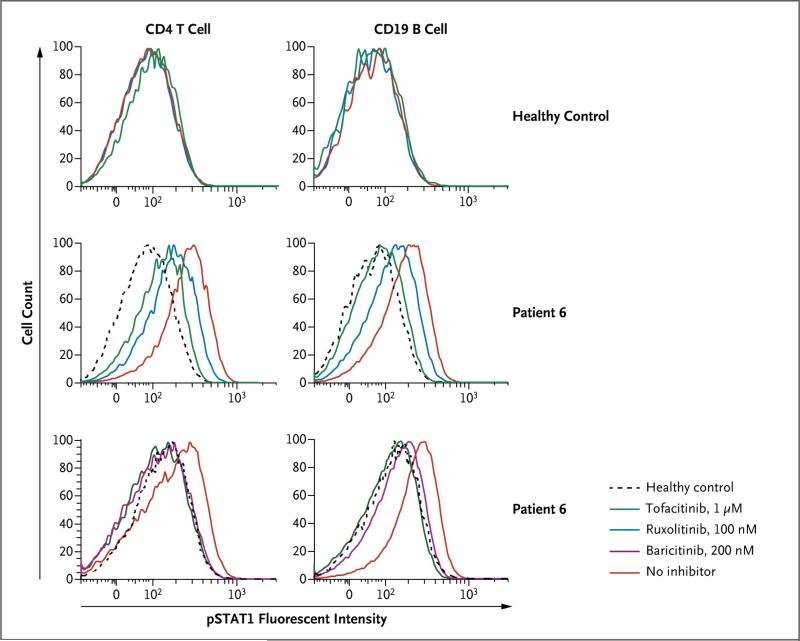
There are currently no effective therapies for patients with this disease. We performed in vitro experiments to determine whether three JAK inhibitors — tofacitinib, ruxolitinib, and baricitinib — could suppress the expression of STING-induced targets and interferon-response genes.12,13 The JAKs are activated by interferon receptors (on binding of interferon to the receptor) and phosphorylate the potent transcription factors STAT1 and STAT2, leading to the expression of interferon-response genes (Fig. 3).14 Each of the JAK inhibitors blocked the constitutive phosphorylation of STAT1 in T cells and B cells from Patients 4 and 6 (Fig. 6, and Fig. S15A in the Supplementary Appendix). We observed a similar effect of tofacitinib on STAT3 phosphorylation in T cells from Patients 4 and 6 (Fig. S15B in the Supplementary Appendix). Treatment with a JAK inhibitor of fibroblasts from patients and controls that were then maximally activated with cGAMP led to a 30 to 50% reduction in IFNB1 transcription and a 20 to 40% reduction in the transcription of IL6 and IL1B. However, some interferon-response genes were blocked by 60 to 90% in a dose- dependent fashion (Fig. S16 in the Supplementary Appendix).
Figure 6. Suppression of Constitutively Phosphorylated STAT1 (pSTAT1) with JAK Inhibitors in a Patient with SAVI.
The upper panel shows data from a healthy control, and the middle and lower panels data from Patient 6. Peripheral-blood mononuclear cells from the patient with SAVI and from the healthy control were treated with and without the indicated JAK inhibitors for 4 hours. STAT1 phosphorylation in CD4 T cells and CD19 B cells was analyzed by means of intracellular staining of pSTAT1 and the respective cell-surface marker. The healthy-control data are representative of experiments with samples from five healthy controls.
DISCUSSION
We describe a new autoinflammatory syndrome, which is caused by de novo mutations in TMEM173, the gene that encodes the STING protein Our data suggest that the three STING mutations that we identified confer a gain of function and constitutive activation of STING and hyper-sensitivity to ligand stimulation, resulting in chronic activation of the STING–interferon pathway.
STING is a key adaptor molecule that links the sensing of cytosolic DNA, derived from foreign triggers (i.e., viral and bacterial) or from self, to the production of interferons,15-19 which are potent cytokines with antiviral and antitumor activity.20-22 Recently, the characterization of cyclic GMP–AMP synthase (cGAS) as a DNA sensor, which on binding double-stranded DNA releases the second messenger 2′3′-cGAMP (a high-affinity ligand of STING), has shed light on the mechanism of STING-induced interferon activation by means of TBK1 and IRF-3 phosphorylation.23,24
Interferons modulate innate (autoinflammatory) and adaptive (autoimmune) pathologic immune responses in addition to the cell-intrinsic and antiviral effect.25,26 Patients with SAVI share clinical, histologic, and functional features with patients who have the proteasome defect–associated autoinflammatory disease CANDLE,5,6,27,28 the Aicardi–Goutières syndrome, or TREX1-mediated familial chilblain lupus.29,30 Patients with these conditions have a vasculopathy early in life, have granulocyte precursors in the inflammatory skin infiltrate, and present with a prominent interferon-response-gene signature in the peripheral blood and with variable B-cell activation (Fig. S17 in the Supplementary Appendix), suggesting that different defects in the interferon pathway may result in overlapping clinical features.
The administration of recombinant inter-feron-α and interferon-β for the treatment of chronic hepatitis and multiple sclerosis can, in rare cases, result in vasculitis,31,32 and interferon-induced endothelial-cell dysfunction has been implicated as a cause of premature vascular damage and atherosclerosis in patients with systemic lupus erythematosus.33 Nevertheless, the presentation of SAVI as an inflammatory vasculopathy was unexpected. The characteristic early onset and localized involvement of cheeks, ears, nose, and digits distinguish SAVI from other known vasculitis syndromes.34 These features, antibodies associated with the antiphospholipid syndrome that were initially present at low titers and then disappeared, and the absence of thrombocytopenia also differentiate SAVI from typical presentations of the antiphospholipid syndrome.35
Stimulation of the STING pathway can activate endothelial cells directly and induces up-regulation of interferon-response genes, apoptosis-pathway genes, and endothelial-cell death in culture, as well as tissue-factor expression, which is a potent initiator of the coagulation cascade. Our data suggest that STING-induced endothelial-cell dysfunction may instigate an inflammatory and vaso-occlusive process and localize it to the vessels. In fact, the development of gangrene early in infancy in areas with low-flow circulation, such as the fingers, toes, nose, and ears, resembles other vaso-occlusive conditions characterized by activation of intravascular coagulation, including symmetric peripheral gangrene (a rare complication of sepsis),36 the antiphospholipid syndrome,37 and levamisole-induced vasculopathy,38,39 raising questions about the role of STING-pathway dysregulation in these conditions.
Some studies have suggested that STING acts as a gatekeeper by streamlining the inter-feron signaling triggered by upstream double-stranded DNA sensors, such as cGAS.15,40,41 For example, in a Trex1-deficient mouse model of the Aicardi–Goutières syndrome, the development of disease features depends on STING,42 which suggests that the production of interferon in patients with the Aicardi–Goutières syndrome may depend on STING. Blocking STING may therefore represent an experimental therapeutic strategy in interferon-mediated diseases other than SAVI.30
Options for treating patients with SAVI are currently limited, but our study suggests that the blockade of interferon signaling with a JAK inhibitor may offer a therapeutic strategy. We observed that, at the right dose, JAK inhibitors blocked in vitro IFNB1 transcription by 30 to 50% and the induction of interferon-response genes by 60 to 90%, suggesting that JAK inhibition may break a positive feedback loop that fuels continuous interferon signaling. A recently approved clinical protocol for the treatment of patients with SAVI will assess the clinical response to JAK inhibition (ClinicalTrials.gov number, NCT01724580).
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
We thank Dr. Richard Siegel for suggestions and a critical review of an earlier version of the manuscript, Dr. John O'shea for early project discussions, Dr. Robert Wesley for statistical advice, Mr. Joshua Kaufman and Mr. Stephen Stahl for assistance with bacterial expression of stimulator of interferon genes (STING) proteins, Ms. Jackie Pidduck and Ms. Lucille Cormier for emotional and travel support for Patient 1 and the family, and Ms. Kimberley Lemberg and Ms. Jacqueline Cleary for help with cell preparations.
APPENDIX
The authors’ full names and academic degrees are as follows: Yin Liu, M.D., Ph.D., Adriana A. Jesus, M.D., Ph.D., Bernadette Marrero, Ph.D., Dan Yang, M.D., Ph.D., Suzanne E. Ramsey, M.D., Gina A. Montealegre Sanchez, M.D., Klaus Tenbrock, M.D., Helmut Wittkowski, M.D., Olcay Y. Jones, M.D., Ph.D., Hye Sun Kuehn, Ph.D., Chyi-Chia R. Lee, M.D., Ph.D., Michael A. DiMattia, Ph.D., Edward W. Cowen, M.D., Benito Gonzalez, M.D., Ira Palmer, M.S., John J. DiGiovanna, M.D., Angelique Biancotto, Ph.D., Hanna Kim, M.D., Wanxia L. Tsai, M.S., Anna M. Trier, B.A., Yan Huang, M.D., Deborah L. Stone, M.D., Suvimol Hill, M.D., H. Jeffery Kim, M.D., Cynthia St. Hilaire, Ph.D., Shakuntala Gurprasad, M.T., Nicole Plass, R.N., Dawn Chapelle, R.N., Iren Horkayne-Szakaly, M.D., Dirk Foell, M.D., Andrei Barysenka, Dipl.Phys., Fabio Candotti, M.D., Ph.D., Steven M. Holland, M.D., Jason D. Hughes, Ph.D., Huseyin Mehmet, Ph.D., Andrew C. Issekutz, M.D., Mark Raffeld, M.D., Joshua McElwee, Ph.D., Joseph R. Fontana, M.D., Caterina P. Minniti, M.D., Susan Moir, Ph.D., Daniel L. Kastner, M.D., Ph.D., Massimo Gadina, Ph.D., Alasdair C. Steven, Ph.D., Paul T. Wingfield, Ph.D., Stephen R. Brooks, Ph.D., Sergio D. Rosenzweig, M.D., Ph.D., Thomas A. Fleisher, M.D., Zuoming Deng, Ph.D., Manfred Boehm, M.D., Amy S. Paller, M.D., and Raphaela Goldbach-Mansky, M.D.
Footnotes
(Funded by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases; ClinicalTrials.gov number, NCT00059748.)
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol. 2009;27:621–68. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez GA, de Jesus AA, Goldbach-Mansky R. Monogenic autoinflammatory diseases: disorders of amplified danger sensing and cytokine dysregulation. Rheum Dis Clin North Am. 2013;39:701–34. doi: 10.1016/j.rdc.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crow YJ. Aicardi-Goutières syndrome. Handb Clin Neurol. 2013;113:1629–35. doi: 10.1016/B978-0-444-59565-2.00031-9. [DOI] [PubMed] [Google Scholar]
- 4.Rice GI, Kasher PR, Forte GM, et al. Mutations in ADAR1 cause Aicardi-Gout-ières syndrome associated with a type I interferon signature. Nat Genet. 2012;44:1243–8. doi: 10.1038/ng.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal AK, Xing C, DeMartino GN, et al. PSMB8 encoding the β5i protea-some subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipo dys trophy syndrome. Am J Hum Genet. 2010;87:866–72. doi: 10.1016/j.ajhg.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Ramot Y, Torrelo A, et al. Mutations in proteasome subunit β type 8 cause CANDLE syndrome with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 2011;64:895–907. doi: 10.1002/art.33368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crow YJ. Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci. 2011;1238:91–8. doi: 10.1111/j.1749-6632.2011.06220.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldbach-Mansky R. Immunology in clinic review series: focus on autoinflammatory diseases: update on monogenic autoinflammatory diseases: the role of interleukin (IL)-1 and an emerging role for cytokines beyond IL-1. Clin Exp Immunol. 2012;167:391–404. doi: 10.1111/j.1365-2249.2011.04533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rani MR, Shrock J, Appachi S, Rudick RA, Williams BR, Ransohoff RM. Novel interferon-beta-induced gene expression in peripheral blood cells. J Leukoc Biol. 2007;82:1353–60. doi: 10.1189/jlb.0507273. [DOI] [PubMed] [Google Scholar]
- 10.van Boxel-Dezaire AH, Zula JA, Xu Y, Ransohoff RM, Jacobberger JW, Stark GR. Major differences in the responses of primary human leukocyte subsets to IFN-beta. J Immunol. 2010;185:5888–99. doi: 10.4049/jimmunol.0902314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouyang S, Song X, Wang Y, et al. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity. 2012;36:1073–86. doi: 10.1016/j.immuni.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosengren S, Corr M, Firestein GS, Boyle DL. The JAK inhibitor CP-690,550 (tofacitinib) inhibits TNF-induced chemokine expression in fibroblast-like synoviocytes: autocrine role of type I interferon. Ann Rheum Dis. 2012;71:440–7. doi: 10.1136/ard.2011.150284. [DOI] [PubMed] [Google Scholar]
- 13.Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014 Jan 23; doi: 10.1021/jm401490p. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–70. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. The helicase DDX41 senses intra-cellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12:959–65. doi: 10.1038/ni.2091. [Erratum, Nat Immunol 2012;13:196.]
- 16.Keating SE, Baran M, Bowie AG. Cytosolic DNA sensors regulating type I inter-feron induction. Trends Immunol. 2011;32:574–81. doi: 10.1016/j.it.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Burdette DL, Vance RE. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013;14:19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- 18.Gall A, Treuting P, Elkon KB, et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36:120–31. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A. 2012;109:19386–91. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–67. [PubMed] [Google Scholar]
- 21.Stetson DB, Medzhitov R. Type I inter-ferons in host defense. Immunity. 2006;25:373–81. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Sun L, Chen X, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–30. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I inter-feron pathway. Science. 2013;339:786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–63. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36:515–28. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arima K, Kinoshita A, Mishima H, et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc Natl Acad Sci U S A. 2011;108:14914–9. doi: 10.1073/pnas.1106015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitamura A, Maekawa Y, Uehara H, et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest. 2011;121:4150–60. doi: 10.1172/JCI58414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee-Kirsch MA, Gong M, Schulz H, et al. Familial chilblain lupus, a monogenic form of cutaneous lupus erythematosus, maps to chromosome 3p. Am J Hum Genet. 2006;79:731–7. doi: 10.1086/507848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice G, Newman WG, Dean J, et al. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome. Am J Hum Genet. 2007;80:811–5. doi: 10.1086/513443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beuthien W, Mellinghoff HU, Kempis J. Vasculitic complications of interferon-alpha treatment for chronic hepatitis C virus infection: case report and review of the literature. Clin Rheumatol. 2005;24:507–15. doi: 10.1007/s10067-005-1093-x. [DOI] [PubMed] [Google Scholar]
- 32.Szilasiová J, Gdovinová Z, Jautová J, Baloghová J, Ficová M, Bohus P. Cutaneous vasculitis associated with interferon beta-1b treatment for multiple sclerosis. Clin Neuropharmacol. 2009;32:301–3. doi: 10.1097/WNF.0b013e3181a2b5fd. [DOI] [PubMed] [Google Scholar]
- 33.Denny MF, Yalavarthi S, Zhao W, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284–97. doi: 10.4049/jimmunol.0902199. [Erratum, J Immunol 2010;185:3779.]
- 34.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010;376:1498–509. doi: 10.1016/S0140-6736(10)60709-X. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin JN. Symmetrical peripheral gangrene. Arch Surg. 1974;108:780–4. doi: 10.1001/archsurg.1974.01350300022006. [DOI] [PubMed] [Google Scholar]
- 37.Thornsberry LA, LoSicco KI, English JC., III The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450–62. doi: 10.1016/j.jaad.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 38.Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948–51. doi: 10.1046/j.1365-2133.1999.02833.x. [DOI] [PubMed] [Google Scholar]
- 39.Graf J. Rheumatic manifestations of cocaine use. Curr Opin Rheumatol. 2013;25:50–5. doi: 10.1097/BOR.0b013e32835b4449. [DOI] [PubMed] [Google Scholar]
- 40.Kondo T, Kobayashi J, Saitoh T, et al. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci U S A. 2013;110:2969–74. doi: 10.1073/pnas.1222694110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unterholzner L, Keating SE, Baran M, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–98. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.