Abstract
Cardiovascular disease (CVD) is the leading cause of mortality in older adults, however, in the elderly accurate stratification of CVD risk to guide management decisions is challenging due to the heterogeneity of the population. Conventional assessment of CVD and therapeutic risk is based on extrapolation of guidelines developed from evidence demonstrated in younger individuals and fails to weight the increased burden of complications and multimorbidity. Using a comprehensive geriatric based assessment of older adults with CVD that includes an estimation of complexity of multimorbidity as well as traditional risk assessment provides a patient centered approach that allows for management decisions congruent with patient preferences. This review examines the complexity of risk stratification in adults over 80, assessment methods to augment current tools and the basis of management decisions to optimize patient and family centered goals.
Keywords: Multimorbidity, polypharamacy, cardiovascular disease, risk stratification, elderly
Introduction
Currently, 13% of the United States population is over the age of 65 years. However, as survival rates increase, adults aged 65 and older will increase to comprise 19% of the population by 2030, with nearly 19 million adults aged over 85.1 Cardiovascular (CV) disease (CVD) affects approximately 40 million individuals over the age of 65 and is the leading cause of morbidity and mortality in that population.2 For individuals over the age of 80 the prevalence rises to encompass 83% of men and 87% of women. 2 Heterogeneity among very old adults is vast and current risk stratification methods to predict an individual’s health trajectory is challenging when compared to younger individuals. The majority of cardiovascular practitioners stratify risk based on standardized scores that have been developed and validated in middle aged adults; however, in the very elderly their value in discrimination of who will benefit rather than be harmed by a specific management strategy or intervention is unreliable. Further, standardized guidelines that are frequently used and promoted for common CV conditions do not accommodate for the very old adult with significant multimorbidity, polypharmacy, and goals of care not focused on mortality.3,4 For the patient over 80 that has already reached their anticipated life expectancy the relevance and utility of predicting 10 year mortality is shadowed by the importance of quality of life and maintaining independence. This article will focus on the challenges of stratifying risk in very old adults, assessment methods to augment current practices, and management decision processes for optimum patient centered care.
Traditional Risk Stratification
Conventional assessment of CV and therapeutic risk is frequently based on validated instruments that are optimal for differentiation in patients in their 50’s and 60’s without significant comorbidities.5–7 Although some do encompass well defined factors such as hypertension (HTN), diabetes mellitus (DM), increasing age (up to 79), cholesterol, and smoking, they lack the ability to distinguish attributed risk from the complexity of multiple contributing disease processes. Furthermore, they do not allow for the addition of sensitive predictors of outcomes in very elderly patients such as pulse pressure, cognitive impairment, disability, functional status and frailty.
Current Clinical Practice Guidelines
Despite the high prevalence of CVD in older adults, their corresponding under representation in randomized controlled trials of disease modifying therapies is significant. 8,9 The resulting effect is that clinicians are required to extrapolate standard practice guidelines based on trial data derived from younger adults.10 Patients over 80 are essentially absent from most pivotal randomized clinical trials which can result in either the omission of evidence based beneficial therapies or the underestimate of risk of procedures without survival benefit for that patient cohort.11,12 Recent updates to American College of Cardiology/American Heart Association (AHA/ACC) guidelines have expanded the content related to elderly patients and highlighted the difficulty in evaluating and managing adults >75 due to specificity of symptoms and limitations of diagnostics.10,13,14 The guidelines also recognize the presence of discordant outcomes when stratified by age and the higher risk of complications experienced by older adults.10,13,14 Population based recommendations however remain non-specific for the very elderly suggesting that decisions should be undertaken after incorporating age-related issues, careful consideration of patient and family preferences, functional capacity, quality-of-life and end-of-life issues, as well as alternative management strategies.4
Role of traditional risk factors in very elderly
Hypertension
Hypertension in the elderly individual is frequently combined with other significant physiological hemodynamic changes such as low renal blood flow, lower intra vascular volume, reduced cardiac output (particularly during activity), increased pulse pressure and marked vascular stiffness and resistance. 15 The result is that many will require a combination of medications to control their blood pressure (BP) and hence be exposed to significant adverse effects. The overwhelming evidence documents that treating significant HTN at any age, including the very elderly, reduces CV and cerebrovascular events.16,17 Previous guidelines based on expert opinion recommended a goal BP of less than 140/90 mmHg for older adults with an acceptable systolic BP of 140–145 mmHg in individuals over 80 years of age if tolerated, and particular caution to the aggressive reduction of BP (to below 130/70 mmHg) in octogenarians with comorbidites due to the increase in adverse events.18 Recent updates have now suggested an even higher goal of less than 150/90 mmHg; however, the changes remain controversial. 19 Practitioners should plan on starting each therapy at a low dose and slowly but consistently increasing medication with frequent contact to monitor for adverse drug events.
Dyslipidemia(DYS)
Numerous trials have documented the beneficial effects of statin therapy on CVD and mortality.20–22 The age range, however, for the majority has not included adults over 80 years and hence the benefits have not been extrapolated to managing octogenarians. The single randomized controlled trial that recruited older adults specifically showed a benefit in statin therapy for secondary prevention but not for primary prevention of DYS.23 The gap in management strategy lies in the continued treatment of DYS in the octogenarian and nonagenarian that has been taking and tolerating medications for several years and/or has a significantly low future life expectancy. Recent developments of Pooled Cohort Risk Equations may allow for more accurate mortality risk estimations.24 Currently, guidelines suggest caution in using high intensity statin therapy in individuals over 75 years of age and that therapy decisions should be balanced between benefits, life expectancy, comorbidities and treatment priorities.25
Smoking
The prevalence of smoking decreases with age but remains a significant risk factor contributing to 30% of attributable risk in strokes and 36% in first myocardial infarction (MI). Smoking also aggravates angina symptoms and can precipitate silent MI seen in older adults. Although the relative risk for MI or death attributed to smoking in an individual over 70 is twice that of an individual 55–60,26 older adults are 20% less likely to be advised to quit smoking by health care providers. Very elderly individuals may be resistant to changing long-term lifestyle habits and providers may minimize anticipated benefits, however the impact of smoking and the positive effects of cessation continue to be demonstrated irrelevant of age.
Diabetes
Epidemiological evidence has demonstrated the association between uncontrolled DM and an increased of CVD.27 For the older adult, the burden of DM is linked to significant morbidity, reduced functional status, and mortality secondary to CVD events.28 Despite this, the goal of tight glycemic control in reducing CVD in adults over 80 has not been established due to the dearth of evidence from previous randomized control trials and the occurrence of significantly higher occurrences of hypoglycemia and mortality.29 Currently, opinion suggests that for older adults who are functional, cognitively intact and have a significant future life expectancy, risk and management of DM should be consistent with goals developed for younger adults. 28 Management and goals for older adults with comorbid conditions or life expectancy < 10 years should balance glycemic control with risk of adverse events and for those with life expectancy < 5 years glucose lowering management should be established on an individual basis.28
Multimorbidity
The conceptual approach to disease-centered care with consideration of comorbid conditions has not been a main focus of prior management strategies. Evidence based clinical practice guidelines continue to focus on single disease entities and therefore create barriers to their application in adults with numerous interacting comorbid disease processes.30 As a more patient-centered approach has evolved the concept of multimorbidity allows not only the consideration of how each comorbid condition interacts with the primary disease but the changing and varying interaction of all conditions.31 The prevalence of multimorbidity (the coexistence of 2 or more chronic conditions) rapidly increases with age such that it occurs in over 70% of 75 or older. 32 In Medicare beneficiaries with heart failure the burden of multimorbidity is significant with over 50% having 5 or more coexisting chronic conditions. 32 The health care burden of multimorbidity is marked with individuals costing 3 times the national average per capita spending of Medicare beneficiaries and the highest 30 day readmission rates. Evaluation of multimorbidity should include not only the contributing effects of chronic and acute diagnoses but also the prevalence of common and frequently under-recognized geriatric syndromes such as dementia, falls, delirium, incontinence, weight loss, depressive symptoms and functional status decline.
Polypharmacy
Older adults with multimorbidity are frequently seen by many general and specialist providers and as a result receive multiple therapies. Often the standard of care for individual chronic illnesses leads to polypharmacy and potential drug-drug interactions for individuals with multiple chronic illnesses.30 Polypharmacy, defined as taking ≥4 chronic medications, is associated with non-adherence33 as well as inappropriate prescribing, drug interactions, adverse drug events, hospitalization, and mortality. Adverse consequences including poor adherence are not only related to the number of medications but also to the regimen complexity.34 Studies have estimated that 50% of older adults are taking at least one medication with no ongoing indication, and many of these drugs are initiated during hospitalization, such as stress ulcer prophylaxis and antipsychotics for delirium.35 Paradoxically, a further consequence of polypharmacy is therapeutic omissions by patient or provider of potentially beneficial medications. 36 Ensuring patients are on as few medicines as necessary to manage their diseases and provide evidence based mortality benefit should be the optimal goal. This also leads to improvement in adherence and patient safety, and reduce medication costs for the patient.
Frailty
Frailty is an emerging and important geriatric syndrome affecting approximately 20% of community dwelling adults over 80.37 It encompasses a biological decline across multiple interrelated organ systems and a subsequent loss of reserve in response to stressors, such as acute illness.38,39 In older patients hospitalized with CVD it is estimated that the prevalence is over 50%.40 Frailty is an independent predictor of subclinical CVD,41 coronary artery disease,42 and congestive heart failure mortality.43 It confers an increased risk of hospitalization, and institutionalization, and ≥ 2 fold mortality.37,44,45 Currently, there is no widespread standard for assessment of frailty in the CV setting despite the recognition as a significant factor in the evaluation of an older adult with CVD. There are numerous multidimensional assessment tools that have been developed to measure physical frailty46 and range from a composite score of clinical deficits to physical performance based criteria.37,47,48 As a prognostic tool, assessment of frailty in combination with other risk factors is an invaluable addition to assist providers in defining the optimum management strategy.
Cognitive Impairment
The presence of dementia significantly increases the financial cost, management complexity and mortality rates for an older adult.49,50 Hypertension and frailty are now recognized in association with and may well be significant risk factors for the development of mild cognitive impairment, a prodromal phase of Alzheimer’s type dementia. 51,52 For an individual that has both HTN and mild cognitive impairment, institutionalization and mortality rates are similar to those with dementia and significantly higher than those with HTN alone.53 In older adults who are classified as frail the prevalence of cognitive impairment is 5 times greater than those who are not frail and the coalescing of these factors causes a more rapid decline in cognition and disability.54 High rates of unidentified cognitive impairment in older adults suggest that periodic screening is necessary to accurately establish future risk and determine benefits of simplification of treatment strategies.
Evaluation methods
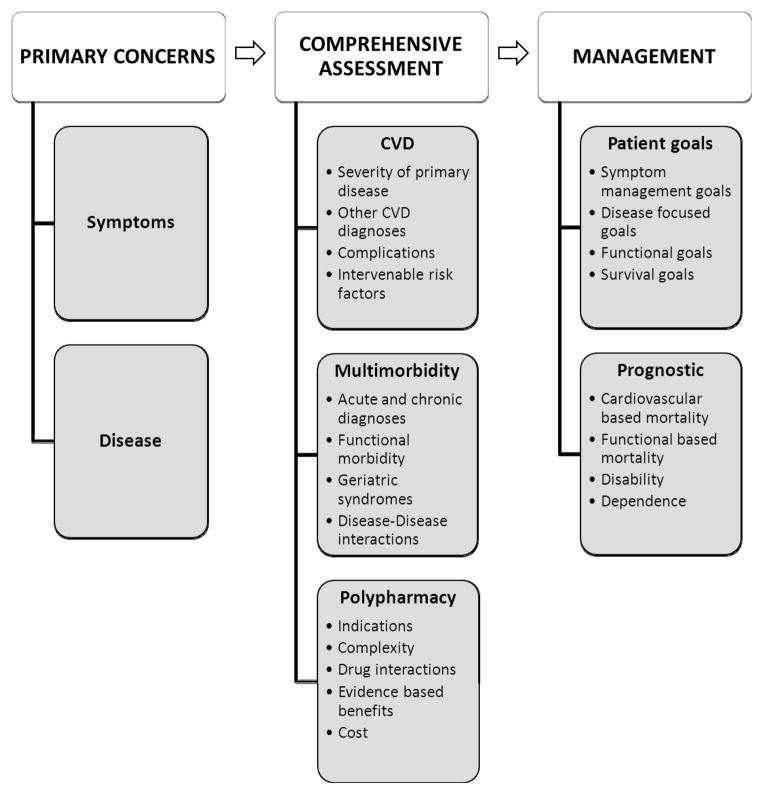
A comprehensive evaluation incorporating all contributing risks is essential to provide the most accurate assessment of absolute risk and benefit of treatment strategies. Inquiring about primary concerns and symptoms provides an initial framework to direct interpretation of further findings. Although disease focused evaluation of symptoms may allow for assessment of the primary CVD, they may well not be congruent with the most significant or problematic symptoms of the patient. Allowing the older adult to prioritize concerns and functional limitations can allow for a more patient centered assessment of risk and hence care plan. Figure 1 highlights essential elements of the process of evaluation. After establishing primary concerns, a comprehensive assessment should allow for interpretation of the severity and acuity of the primary CV problem as well as other CV disease processes, complications and the presence of traditional CVD risk factors amenable to intervention. Assessment of multimorbidity should include contributions of chronic and acute disease processes as well as an evaluation for coexisting geriatric syndromes and functional status. Table 1 provides an overview of commonly used valuable tools for assessment of the geriatric population.
Figure 1.
Overview of proposed comprehensive assessment for risk stratification in adult over 80
Table 1.
Summary of Assessment Tools for Screening for Common Geriatric Conditions
| Assessment Tool | |
|---|---|
| Frailty | Fried Frailty Scale: Grip strength, gait speed, exhaustion, weight loss and activity questionnaire37 Short Physical Performance Battery56 |
| Functional Status | Katz Activities of Daily Living57 Lawton Instrument of Activities of Daily Living58 Timed up and Go59 Functional Reach60 |
| Cognition | Montreal Cognitive Assessment (www.mocatest.org) Mini-COG61 |
| Weight loss/Sarcopenia | Grip strength Body Mass Index change loss > 5% |
| Depression | Geriatric Depression Scale62 Patient Health Questionnaire63 |
Prognostication and Management Decision Making
Contributing factors to management choices involves both prognosis and patient preferences and goals. Prognosis may not only include mortality but also disability and dependence. Although median future life expectancy for a person aged 80 is approximately 7 years the range can vary significantly depending on functional status, and the presence of significant comorbid disease.55 For example, the presence of Alzheimer’s disease is associated with a 2–4 times risk of mortality at any age.49 By evaluating the contributing risk of all factors the provider can gauge whether an individual has a higher, average or lower risk of the potential outcome. Patient preferences and priority of goals should also be balanced with prognostic evaluation. All interactions within and among current conditions, therapies and future therapies should be considered before communicating a detailed plan to the patient and significant family involved in the care. This should include the discussion of all benefits and harms to the patient without influence of the provider’s personal priorities and values. Finally, for the very elderly the importance of frequent reassessment of symptom priority, benefit and risk, adherence and alignment with patient centered goals is essential.
Conclusions
The challenges of stratifying risk in the very elderly and hence balancing patient centered management strategies are immense due to the vast hetereogenity seen in this population. Although CV clinicians are adept at calculating disease centered risk in younger populations, their sensitivity of risk stratification and judgment for detecting overall risk burden from multimorbidity is imperfect. A comprehensive geriatric based evaluation incorporating all contributing factors is essential to provide the most accurate assessment of absolute risk and benefit of treatment strategies to guide management decisions in older adults. Assessment of geriatric syndromes, complexity of multimorbidity and polypharmacy along with patient preferences and goals into management plans can lead to optimum patient centered care.
Acknowledgments
Dr. Bell is supported in part by K12HD043483-11, Mentored Clinical Scientist Development Award from NICHD and the Eisenstein Women’s Heart Service Fund
Abbreviations
- BP
Blood pressure
- CV
Cardiovascular
- CVD
Cardiovascular disease
- DM
Diabetes mellitus
- DYS
Dyslipidemia
- HTN
Hypertension
- MI
Myocardial infarction
References
- 1. [Accessed January 15th, 2014];Projected Future Growth of the Older Population. 2008 http://www.aoa.gov/AOARoot/Aging.
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013 Jan 1;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman DE, Rich MW, Alexander KP, et al. Cardiac care for older adults. Time for a new paradigm. J Am Coll Cardiol. 2011 May 3;57(18):1801–1810. doi: 10.1016/j.jacc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman D, Wenger NK. What do the recent American Heart Association/American College of Cardiology Foundation Clinical Practice Guidelines tell us about the evolving management of coronary heart disease in older adults? Journal of geriatric cardiology : JGC. 2013 Jun;10(2):123–128. doi: 10.3969/j.issn.1671-5411.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000 Aug 16;284(7):835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 6.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999 Jun 14;159(11):1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 7.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998 May 12;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 8.Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001 Aug 8;286(6):708–713. doi: 10.1001/jama.286.6.708. [DOI] [PubMed] [Google Scholar]
- 9.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002 Aug 12–26;162(15):1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 10.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012 Dec 18;126(25):e354–471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 11.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. The New England journal of medicine. 1999 Aug 26;341(9):625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 12.Krumholz HM, Murillo JE, Chen J, et al. Thrombolytic therapy for eligible elderly patients with acute myocardial infarction. JAMA. 1997 Jun 4;277(21):1683–1688. [PubMed] [Google Scholar]
- 13.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 14.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009 Apr 14;119(14):e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 15.Messerli FH, Sundgaard-Riise K, Ventura HO, Dunn FG, Glade LB, Frohlich ED. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet. 1983 Oct 29;2(8357):983–986. doi: 10.1016/s0140-6736(83)90977-7. [DOI] [PubMed] [Google Scholar]
- 16.Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet. 2000 Mar 11;355(9207):865–872. doi: 10.1016/s0140-6736(99)07330-4. [DOI] [PubMed] [Google Scholar]
- 17.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. The New England journal of medicine. 2008 May 1;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 18.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. Journal of the American Society of Hypertension : JASH. 2011 Jul-Aug;5(4):259–352. doi: 10.1016/j.jash.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 19.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014 Feb 5;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 20.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002 Jul 6;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen TA, Pyorala K, Olsson AG, et al. Cholesterol-lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Survival Study (4S) Circulation. 1997 Dec 16;96(12):4211–4218. doi: 10.1161/01.cir.96.12.4211. [DOI] [PubMed] [Google Scholar]
- 22.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. The New England journal of medicine. 1996 Oct 3;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002 Nov 23;360(9346):1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 24.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12; [Google Scholar]
- 25.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12; [Google Scholar]
- 26.Hermanson B, Omenn GS, Kronmal RA, Gersh BJ. Beneficial six-year outcome of smoking cessation in older men and women with coronary artery disease. Results from the CASS registry. The New England journal of medicine. 1988 Nov 24;319(21):1365–1369. doi: 10.1056/NEJM198811243192101. [DOI] [PubMed] [Google Scholar]
- 27.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004 Sep 21;141(6):421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 28.Sue Kirkman M, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012 Dec;60(12):2342–2356. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Group AS, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. The New England journal of medicine. 2011 Mar 3;364(9):818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005 Aug 10;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 31.Guiding principles for the care of older adults with multimorbidity: an approach for c. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2012 Oct;60(10):E1–E25. doi: 10.1111/j.1532-5415.2012.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009 Jun 30;54(1):25–33. doi: 10.1016/j.jacc.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 33.Wilson IB, Schoen C, Neuman P, et al. Physician-patient communication about prescription medication nonadherence: a 50-state study of America’s seniors. Journal of general internal medicine. 2007 Jan;22(1):6–12. doi: 10.1007/s11606-006-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001 Aug;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 35.Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc. 2013 Jul;61(7):1128–1134. doi: 10.1111/jgs.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wehling M. Multimorbidity and polypharmacy: how to reduce the harmful drug load and yet add needed drugs in the elderly? Proposal of a new drug classification: fit for the aged. J Am Geriatr Soc. 2009 Mar;57(3):560–561. doi: 10.1111/j.1532-5415.2009.02131.x. [DOI] [PubMed] [Google Scholar]
- 37.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 38.Fried L, editor. Frailty. 6. McGraw-Hill; 2009. [Google Scholar]; Hazzard W, Halter J, Ouslander J, Tinetti M, Studenski S, High K, Asthana S, editors. Hazzard’s Geriatric Medicine and Gerontology. [Google Scholar]
- 39.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009 Oct;64(10):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009 Jun 1;103(11):1616–1621. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 41.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. The journals of gerontology. Series A, Biological sciences and medical sciences. 2001 Mar;56(3):M158–166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 42.Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005 Jun;60(6):729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 43.Cacciatore F, Abete P, Mazzella F, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. European journal of clinical investigation. 2005 Dec;35(12):723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 44.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. Journal of the American Geriatrics Society. 2005 Aug;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 45.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. Journal of the American Geriatrics Society. 2006 Nov;54(11):1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 46.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing research reviews. 2011 Jan;10(1):104–114. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999 Jan 16;353(9148):205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 48.Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mechanisms of ageing and development. 2002 Sep;123(11):1457–1460. doi: 10.1016/s0047-6374(02)00082-9. [DOI] [PubMed] [Google Scholar]
- 49.Xie J, Brayne C, Matthews FE Medical Research Council Cognitive F, Ageing Study c. Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. Bmj. 2008 Feb 2;336(7638):258–262. doi: 10.1136/bmj.39433.616678.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill JW, Futterman R, Duttagupta S, Mastey V, Lloyd JR, Fillit H. Alzheimer’s disease and related dementias increase costs of comorbidities in managed Medicare. Neurology. 2002 Jan 8;58(1):62–70. doi: 10.1212/wnl.58.1.62. [DOI] [PubMed] [Google Scholar]
- 51.Wilkins CH, Roe CM, Morris JC, Galvin JE. Mild physical impairment predicts future diagnosis of dementia of the Alzheimer’s type. J Am Geriatr Soc. 2013 Jul;61(7):1055–1059. doi: 10.1111/jgs.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hajjar I, Quach L, Yang F, et al. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the Cardiovascular Health Study. Circulation. 2011 Mar 1;123(8):858–865. doi: 10.1161/CIRCULATIONAHA.110.978114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rockwood K, Wentzel C, Hachinski V, Hogan DB, MacKnight C, McDowell I. Prevalence and outcomes of vascular cognitive impairment. Vascular Cognitive Impairment Investigators of the Canadian Study of Health and Aging. Neurology. 2000 Jan 25;54(2):447–451. doi: 10.1212/wnl.54.2.447. [DOI] [PubMed] [Google Scholar]
- 54.Dumont C, Voisin T, Nourhashemi F, Andrieu S, Koning M, Vellas B. Predictive factors for rapid loss on the mini-mental state examination in Alzheimer’s disease. J Nutr Health Aging. 2005;9(3):163–167. [PubMed] [Google Scholar]
- 55.Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001 Jun 20;285(23):2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 56.Volpato S, Cavalieri M, Guerra G, et al. Performance-based functional assessment in older hospitalized patients: feasibility and clinical correlates. J Gerontol A Biol Sci Med Sci. 2008 Dec;63(12):1393–1398. doi: 10.1093/gerona/63.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. The Gerontologist. 1970 Spring;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 58.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969 Autumn;9(3):179–186. [PubMed] [Google Scholar]
- 59.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991 Feb;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 60.Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. Journal of gerontology. 1990 Nov;45(6):M192–197. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]
- 61.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000 Nov;15(11):1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 62.Hoyl MT, Alessi CA, Harker JO, et al. Development and testing of a five-item version of the Geriatric Depression Scale. J Am Geriatr Soc. 1999 Jul;47(7):873–878. doi: 10.1111/j.1532-5415.1999.tb03848.x. [DOI] [PubMed] [Google Scholar]
- 63.Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. General hospital psychiatry. 2010 Jul-Aug;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]