Abstract
We investigated the effects of excipients in solutions of keratinocyte growth factor 2 (KGF-2) on protein aggregation during agitation as well as on interfacial shear rheology at the air-water interface. Samples were incubated with or without agitation, and in the presence or absence of the excipients heparin, sucrose or polysorbate 80 (PS80). The effect of excipients on the extent of protein aggregation was determined by UV spectroscopy and microflow imaging (MFI). Interfacial shear rheology was used to detect the gelation time and strength of protein gels at the air-water interface. During incubation, protein particles of size ≥ 1 μm and insoluble aggregates formed faster for KGF-2 solutions subjected to agitation. Addition of either heparin or sucrose promoted protein aggregation during agitation. In contrast, PS 80 substantially inhibited agitation-induced KGF-2 aggregation but facilitated protein particulate formation in quiescent solutions. The combination of PS 80 and heparin or sucrose completely prevented protein aggregation during both non-agitated and agitated incubations. Interfacial rheological measurements showed that KGF-2 in buffer alone formed an interfacial gel within a few minutes. In the presence of heparin, KGF-2 interfacial gels formed too quickly for gelation time to be determined. KGF-2 formed gels in about 10 minutes in the presence of sucrose. The presence of PS80 in the formulation inhibited gelation of KGF-2. Furthermore, the interfacial gels formed by the protein in the absence of PS80 were reversible when PS80 was added to the samples after gelation. Therefore, there is a correspondence between formulations that exhibited interfacial gelation and formulations that exhibited agitation-induced aggregation.
Introduction
Agitation can damage therapeutic proteins in liquid formulations during mixing, pumping, filtering, filling, shipping and administration to patients.1–3 The pathways and mechanisms of agitation-induced protein aggregation and the role(s) of excipients -- which sometimes are empirically found to protect proteins against aggregation during agitation -- are not well understood. It is thought that agitation-induced protein aggregation is mainly due to the adsorption of protein at air-water interfaces formed.4–8 Because the air-water interface is relatively hydrophobic, it is hypothesized that the structure of adsorbed proteins may be perturbed2,9,10 and that these structurally-perturbed protein molecules in turn may be prone to aggregate.2
Surfactants are often included as stabilizers against aggregation in therapeutic protein formulations, and they generally are thought to inhibit aggregation by reducing protein adsorption to various interfaces.5–8,11 Other protection mechanisms may be protein-specific.4–6,12,13 For example, polysorbate 20 (PS20) protected recombinant human growth hormone (rhGH) against agitation-induced aggregation by binding to hydrophobic sites on the protein’ surface and sterically hindering intermolecular contacts leading to aggregation.4 The protection of a recombinant fusion protein of human growth hormone and albumin against agitation-induced aggregation was ascribed to an increase in the free energy of unfolding of the protein caused by PS20 binding to the native state.12 Another study employing isothermal titration calorimetry (ITC) and differential scanning calorimetry (DSC) indicated that polysorbates bind to human serum albumin but not to immunoglobulins.14 In contrast, no specific binding of either polysorbate 80 (PS80) to recombinant hemoglobin or PS20 to recombinant human factor XIII was observed by electron paramagnetic resonance spectroscopy, and protection against aggregation was ascribed to inhibition of protein adsorption to interfaces.6,13 Finally, surfactants may also function as chemical chaperones by transiently binding to structurally-perturbed protein molecules, thus preventing aggregation.6,7
Surfactants and other excipients that are used to reduce protein aggregation may sometimes cause unexpected deleterious effects. For example, non-ionic surfactants may reduce agitation-induced protein aggregation, but they may also decrease the unfolding free energy of protein in bulk solution, thereby promoting protein aggregation in bulk solutions.15 In contrast, it is well known that preferentially excluded excipients such as sucrose increase the protein’s free energy of unfolding.16,17 It might be expected that these excipients would reduce aggregation by reducing the equilibrium populations of reactive, partially unfolded protein species 15,18 However, a recent study showed that sucrose accelerated the rate of aggregation of an IgG monoclonal antibody during agitation.8 In that case, sucrose increased the free energy of unfolding of protein molecules in the bulk solution but apparently decreased the protein’s conformational stability at the air-water interface.8,15
In the food industry, surfactants and milk proteins are widely used for the formation and stabilization of emulsions and foams.19,20 The mechanisms of the formation and stabilization of emulsions and foams have been investigated extensively in Food Sciences field, which may shed light on understanding the interaction of surfactants and therapeutic proteins at air-water interface. Proteins alone stabilize an interface by forming a strong viscoelastic gel-like protein network while surfactants alone stabilize an interface by a high degree of lateral mobility (the Gibbs-Marangoni mechanism).21 These two mechanisms are different and compete when surfactant is added into a protein stabilized interface. Mackie et al. found nonionic surfactant Polysorbate 20 (PS20) displaced β-casein, β-lactoglobulin and α-lactalbumin from the air-water interface where protein formed a gel-like network.21 PS20 adsorbed competitively at defects in the protein network, grew and compressed the protein network, which finally failed and desorbed from the interface. In another study of competitive adsorption of milk protein and surfactant at oil-water interface, Dickinson et al found it was difficult for PS20 to displace the protein gel-like network with higher interfacial shear viscosities due to heating or aging. 22
The aim of the current study is to better understand the effects of excipients on aggregation of therapeutic proteins during agitation. Our hypothesis was that the effects of a given excipient during agitation would depend on the balance between the excipient’s effects on the conformational stability of the native protein in the bulk solution and on the gelation of protein adsorbed to air-water interfaces.
Polyanions such as heparin can bind to and stabilize the native state of some proteins such as fibroblast growth factors (FGF)23–28. For example, it was found that heparin stabilized acidic FGF, increasing its melting temperature (Tm) by 20°C and inhibiting protein aggregation during isothermal incubation.24 Similarly, heparin stabilized basic FGF, raising the Tm by up to 31°C.25,26 In another study, heparin increased the Tm of FGF-20 by more than 10 °C.29 We used keratinocyte growth factor 2 (KGF-2, FGF-10) as a model protein. This protein belongs to the FGF family, to which heparin and other polyanions may bind.28–30 The KGF-2 used in this study, Repifermin®, is a truncated form (140 amino acids, 16 kDa) of full-length KGF-2. However, its physiological and biological activity is intact.31 It is relatively unstable in aqueous solution (onset temperature for thermally-induced unfolding is about 32 °C), but its onset temperature for thermal unfolding increases by 9–15°C when it binds to heparin28. The effects of heparin on agitation-induced aggregation of KGF-2 have not been reported.
In the current study, we investigated the effects of the excipients heparin, sucrose and PS80 on protein particle formation and precipitation during agitation. Also, we determined the effects of the excipients on the interfacial shear rheology of KGF-2 adsorbed to the air-water interface. We studied the frequency-dependent deformation of interfacial layers as a result of an applied force.32,33 Interfacial shear rheology measurements are sensitive to the properties of adsorbed proteins at the air-water interface.34 A thin viscoelastic layer (i.e., a quasi-two-dimensional gel) can form after sufficient protein molecules adsorb to the air-water interface.34,35 The interfacial gel transition can be determined by comparing the interfacial shear elastic (storage) and viscous (loss) moduli.34 The gelation time and mechanical strength of the interfacial gel can be obtained and used to compare the effects of various solution conditions on protein behavior at the air-water interface. Finally, we used micro flow imaging (MFI) to count sub-visible protein particles in the size range of 1 to 400 μm. MFI is a valuable tool to monitor protein aggregation, especially in early phase of agitation-induced aggregation when techniques such as UV spectroscopy and size exclusion high performance liquid chromatography (SE-HPLC) may not be sensitive enough to detect minute losses of monomeric protein.9,36
Materials and Methods
Materials
KGF-2, provided in a surfactant-free formulation, was a generous gift from Human Genome Sciences, Inc. (Rockville, MD). Heparin sodium salt from porcine intestinal mucosa (Catalogue number: H3393, average molecular weight MW 18kDa) was purchased from Sigma-Aldrich (St.Louis, MO). Sucrose was obtained from Pfanstiehl Laboratories (Waukegan, IL). PS80 (low carbonyl and peroxide), sulfuric acid and hydrogen peroxide (30% vol./vol.) were purchased from Thermo Fisher Scientific (Rockford, IL). All chemicals used were of reagent grade or higher. Ultra-pure water (0.22 μm filtered) acquired from a Millipore Synergy Ultrapure Water Systems (Millipore, Billerica, MA) was used throughout the studies.
Preparation of KGF-2 Solutions
KGF-2 solutions were prepared prior to each experiment by dialyzing the protein solution against 10 mM sodium citrate buffer, pH 6.2 (hereafter referred as “buffer”) at 2–8°C. Excipient stock solutions were prepared in the buffer and mixed with the KGF-2 to give the final desired protein and excipient concentrations. It should be pointed out that the order of addition was critical for the preparation of solutions containing heparin and KGF-2. First, heparin was weighed and dissolved in buffer. Then, stock KGF-2 solution was gradually added to heparin solution to reach the target concentrations. If, in contrast, heparin was added into KGF-2 solution, a precipitate formed immediately.
Interfacial shear rheology measurement
A custom-built interfacial shear rheometer was used as previously described.35,37 Briefly, protein solutions were placed in a glass channel (length × width = 15 cm × 1 cm), which was placed in a glass container. A magnetic rod (diameter × length = 0.06 cm × 2.54 cm) -- with anodized black and white stripes to aid in visualization -- was inserted in the middle of a 5 cm polytetrafluoethylene (PTFE) tube (ID = 0.0635 cm; part number SLTT-22-12, SmallParts.com). Both ends of the PTFE tube were sealed with paraffin wax. This magnetic rod assembly could remain suspended on the surface of the protein solution, even in the presence of PS80. The magnetic rod assembly was aligned in the middle of the glass channel due to gravitational forces and the curvature of the meniscus. Oscillatory forces were applied on the rod by electromagnetic coils placed on each side of the glass channel. As a result, the magnetic rod assembly moved backward and forward, and sheared the air-water interface. The applied forces were proportional to the difference in currents between the two electromagnetic coils. These forces were then used to determine the applied stress. A charge-coupled-device (CCD) camera was employed to track the rod’s motion. This motion was later used to determine the resulting strain. The rheological parameters were calculated and reported34,37 in terms of the complex shear modulus
| Equation 1 |
where
| Equation 2 |
and
| Equation 3 |
are the elastic (solid-like) and viscous (liquid-like) moduli, respectively. In these equations, σ, γ, and φ represent stress, strain, and phase angle (the difference between the rod response and the applied force), respectively. The strain (γ) is equal to the amplitude of the rod displacement divided by the half width of the channel. The stress (σ) may be calculated from:
| Equation 4 |
where I is the difference between currents in the two electromagnetic coils in amperes, L is the length of magnetic rod (5 cm), and K is a calibration constant relating the difference in current to the force applied to the rod. K is obtained by analyzing solutions of each excipient in the absence of KGF-2.
Specifically, the rod can be described by a damped harmonic oscillator:33,37
| Equation 5 |
where f is the force applied on the rod, k is the effective elastic constant which is similar to a spring constant, m and x are the mass and the position of the rod, respectively, and t is time. If an external oscillatory force
| Equation 6 |
is applied on the rod, where f0 is the maximum force, ω is the frequency, the position of the rod will be
| Equation 7 |
where x0 is the maximum displacement. We did not measure the force directly. However, the force and the current passing through the coil are expected to be linearly related:
| Equation 8 |
where K is a calibration constant and I0 is the maximum difference in current. Equations 5–8 may be combined to yield
| Equation 9 |
which can be rearranged as
| Equation 10 |
where .
The ratio of the amplitude of the rod displacement to the current amplitude and the phase angle φ were extracted from the frequency spectrum by taking the fast Fourier transform (FFT) of the applied current and the response of the rod. Values of kf, mf, and df were then fit to Equation 10, using input frequencies ranging from 0.011 to 4 Hz. As the actual mass of the rod was measured using a balance, we calculated the K value.
The bulk solution contribution complex modulus G*(bulk) can be measured in the absence of protein.35 With the assumption of simple additivity, the interfacial modulus is then determined by:
| Equation 11 |
For buffer in the absence or presence of added excipients, the elastic modulus (G′) is smaller than viscous modulus (G″) and the magnitude of shear modulus is small. If the added protein molecules formed a gel at the interface, the initially smaller G′ will surpass G″34. Therefore, the interfacial gel transition time can be determined by the crossover time.
Before each measurement, the glass channel was soaked for 1 hour in a mixture of sulfuric acid and hydrogen peroxide (vol:vol = 2:1) to remove any surface contaminants.34 Also, this procedure helped to maximize the hydrophilicity of the glass surface and thus ensured that the magnetic rod assembly remained at the center of the glass channel. The surface of magnetic rod assembly was rinsed with ultra-pure water and wiped with 100 % isopropanol to remove protein or other residue. Then the assembly was placed in a magnetic coil (0.06 Tesla) for 1 hour to magnetize the rod.
For solutions without PS80, the rheometer was calibrated by varying the frequency of the sinusoidal current applied to the coils (from 0.011 to 4 Hz), resulting in a sinusoidal motion of the magnetic rod that was suspended at the air-water interface above 40 mL of buffer or excipient solutions in the absence of KGF-2. After a calibration, 20–30 mL of solution was removed, taking care not to disturb the suspended magnetic rod assembly. This aliquot was replaced by solution containing KGF-2 with the same excipient concentration. Maintaining a constant volume of solution assured that the interface on which the magnetic rod was suspended would return to its previous height, thus avoiding the need to refocus the CCD camera. For solutions containing PS80, the average calibration constant from other solutions was used.
Then, the rheology measurement was started immediately. Three different current amplitudes were analyzed at a constant frequency of 0.125 Hz. The measurement was typically run overnight, although in some cases it was stopped after gel formation immobilized the magnetic rod at the air-water interface. After gelation, 40 μL of PS80 (10%, w/v) was added at the bottom of glass channel to give a final PS80 concentration of 0.01% (w/v). The rheology measurement was restarted and run until the magnetic rod moved freely again. The de-gelation time was determined by the time required after addition of surfactant for G″ to become greater than G′.
Circular Dichroism Spectroscopy (CD)
Dialyzed KGF-2 samples were centrifuged at 14,000g for 10 min to remove particles, and protein concentrations were determined by UV absorption at 280 nm. Solutions were prepared in buffer to final concentrations of 0.5 mg/mL KGF-2, 1 mg/mL heparin, 0.5 M sucrose and 0.01% (w/v) PS80. Approximately 400 μL KGF-2 were transferred into a 1 mm path-length cuvette and scanned from 200 nm to 280 nm at 1 nm increments using Chirascan Plus circular dichroism spectrometer from Applied Photophysics (Leatherhead, UK). To measure the thermal transition temperature for KGF-2, the sample was heated from 20 to 70°C in 2°C increments. The heating rate was 1°C/min, and samples were held for 120 s at each temperature before recording CD spectra. In order to determine the mid-point transition temperature the intensity at 230 nm was fitted with the sigmoidal model in Chirascan software. Analyses of each sample solution were conducted in triplicate.
Isothermal Titration Calorimetry (ITC)
A MicroCal VP-ITC (Northampton, MA) was employed to determine the number of binding sites between heparin and KGF-2 as well as potential binding between PS80 and KGF-2. All samples and water were degassed for 5 minutes without stirring by ThermoVac. Water (1.7 mL) was loaded to the reference cell. Heparin solution (0.005 mM, 1.7 mL) was loaded into the ITC cell. KGF2 solution (0.2 mM, 305 μL) was loaded in the injection syringe. Thirty injections of KGF-2 solution at 5 minute intervals were used during each run. The volume of the first injection was 2 μL. Subsequent injections were 10 μL. Control experiments using buffer in the injection syringe were performed in order determine the heat of dilution of heparin. In turn, the thermograms resulting from injections of KGF-2 solutions were corrected for this heat of dilution. To investigate potential binding of PS80 to KGF-2 and PS80, KGF2 (0.05 mM, 1.7 mL) was loaded into the ITC cell, and PS80 (5 mM, 305 μL) was loaded in the injection syringe. The volume of the first injection was 2 μL, and the following 29 injection volumes were 5 μL, with an injection spacing time of 4 minutes. To control for the heat of dilution of PS80, buffer was loaded into the ITC cell and PS80 solution was injected as described above. The heats of PS80 dilution obtained from these experiments were subtracted from the corresponding experimental data for each injection of PS80 solution into the KGF-2 solution. Data were analyzed using the calorimetric fitting routines imbedded in the Origin (OriginLab Corp., Northampton, MA) for ITC packages (MicroCal, Northampton, MA).
Incubation of KGF-2 formulations with and without agitation
Freshly dialyzed KGF-2 solution in buffer was centrifuged at 14,000g for 10 minutes to remove any insoluble aggregates, and the supernatant was used as the stock solution for incubation experiments. KGF-2 samples (0.5 mg/mL) were prepared in a laminar flow hood by diluting the KGF-2 stock solution into buffer. In addition, KGF-2 stock solution was added to each of the excipient solutions. The final concentration for heparin, sucrose, and PS80 were 1 mg/mL, 0.5M, and 0.01% (w/v), respectively. Sample solutions (1.0 mL) for agitation studies were pipetted into 1.5 mL polypropylene microcentrifuge tubes that were fixed horizontally on plastic racks attached to an orbital shaker (Model No. 3500, VWR international, West Chester, PA). KGF2 samples were incubated with or without agitation (350 rpm) for 24 hours at room temperature. Triplicate sample tubes were prepared and tested for each formulation.
Micro-Flow Imaging (MFI)
Counts and size distributions for particles of size ≥1 μm in KGF-2 solutions were determined using a Brightwell Micro-Flow Imaging™ (MFI) instrument (DPA4100 Flow Microscope, Series B, Brightwell Technologies, Ottawa, Canada). The flow cell was used in SP3 mode after calibrating using 10 μm polystyrene microsphere standard (Cat. No. 4210A, Duke Scientific Corporation, Palo Alto, CA). Ultra-pure Millipore water was used to check the background counts during MFI measurements. The total volume of sample dispensed into the flow cell was 0.45 mL, and 0.15 mL was allowed to flow through the cell prior to data acquisition.
UV-visible spectroscopy (UV) to determine protein concentration
After centrifuging incubated KGF-2 samples at 14,000g for 10 minutes to remove insoluble aggregates, concentrations of soluble KGF-2 were determined using an Agilent 8453 diode array UV-visible spectrophotometer (Santa Clara, CA) by absorbance at 280 nm, using an extinction coefficient of 1.18 mL/mg/cm. The effect of light scattering at 280 nm on the measured signal was corrected with the instrument software (Chemstation) using measurements recorded from 320 nm to 400 nm.
Statistical analysis
A two tailed unpaired Student’s t-test with 95% confidence interval was performed to determine the significance of differences for the means of two samples.
Results
Incubation study of KGF-2 formulations
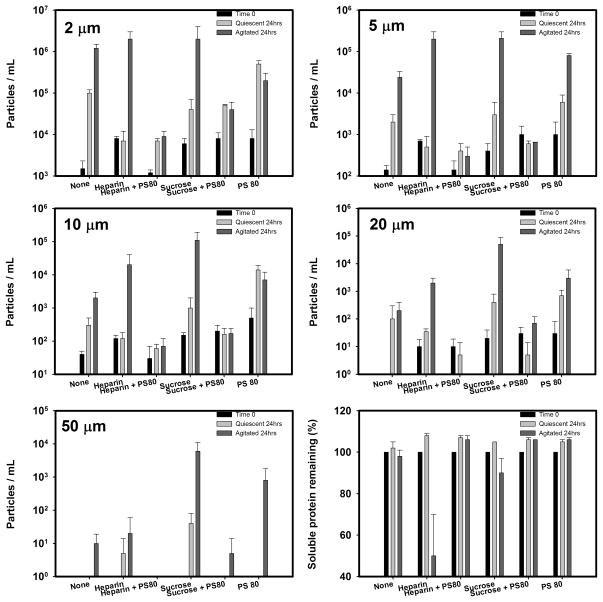
Results at time 0 and at the end of 24 hrs of incubation are shown for particle counts and percent recovery of soluble KGF-2 in sample supernatants after centrifugation in Figure 1. In quiescent samples, loss of soluble protein was not detected in any of the formulations tested (assay variability = ± 5%). However, in buffer alone there was a small increase in particle counts (≥ 1 μm) during quiescent incubation, suggesting that there was aggregation of the protein but the mass converted to aggregates was too low to be detected by measurement of total protein concentration. In formulations with heparin or sucrose, alone or in combination with PS80, the increase in particle counts during quiescent incubation was substantially reduced. Interestingly, in the presence of only PS80 there was a modest increase in particle counts relative to that observed in buffer alone.
Figure 1.
Particle concentrations and soluble KGF-2 remaining during non-agitated and agitated incubation
Agitation in buffer alone did not measurably decrease the amount of soluble protein (p-value > 0.05) but there was a large increase in particle counts compared to that noted during quiescent incubation. With heparin alone there was both a large loss of soluble protein and an increase in particle counts. In the sucrose formulation, there was not a detectable loss of soluble protein (p-value > 0.05) but there was a much larger increase in particle levels than observed in buffer alone. The presence of PS80 in both heparin and sucrose formulations resulted in no significant loss of soluble protein and greatly reduced particle counts. Finally, in the formulation with PS80 alone there was no detectable loss of soluble protein, but there was an increase in particle counts to levels similar to those noted in buffer alone.
Interfacial shear rheology
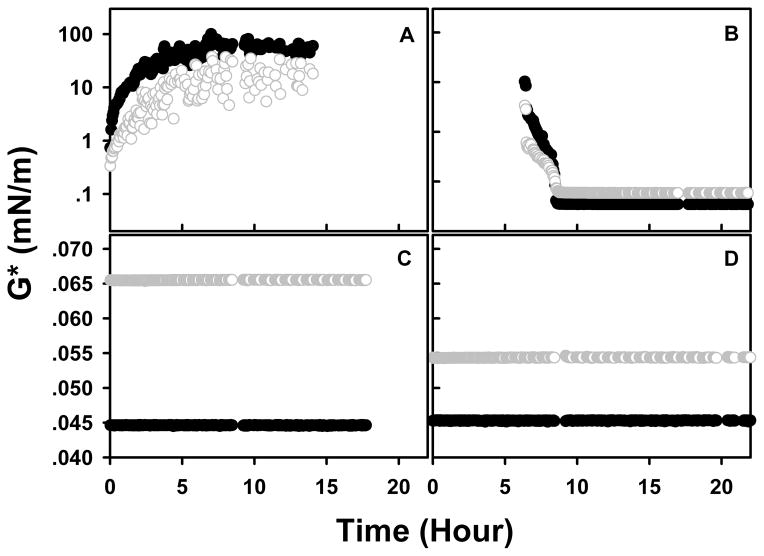
Representative interfacial shear moduli as a function of aging time are shown in Figure 2. KGF-2 at 0.5 mg/mL in buffer gelled almost instantly at the air-water interface (Figure 2A) as evidenced by the observation that G′ > G″ starting from the second time point, which was only approximately two minutes after the initial measurement. After the gel was aged overnight, 40 μL of PS80 (10%, w/v) were pipetted under the gel layer to achieve a final concentration of PS80 of 0.01% (w/v). After about 10 hours in the presence of PS80 gelation was completely reversed (Figure 2B). Solutions containing PS80 alone observed for 18 hours (Figure 2C) or PS80 with KGF-2 observed for 56 hours (Figure 2D; data shown for first 24 hours) did not exhibit interfacial gel formation.
Figure 2.
Representative dynamic interfacial shear moduli as a function of aging time. Solid black cycle: Elastic modulus (G′); Open gray cycle: Viscous modulus (G″); Panel A: KGF2 (0.5 mg/mL) alone; B: After gel formed at air-liquid interface of the sample in panel A, PS80 was added to make the final concentration of PS80 to 0.01%, w/v; C: PS80 (0.01%, w/v) in buffer; D: Premixed KGF2 (0.5 mg/mL) and PS80 (0.01%, w/v).
KGF-2 at a concentration of 0.005 mg/mL in buffer alone formed an interfacial gel within minutes, compared to the 0.5 mg/ml solution, which gelled within seconds (Table 1). In the presence of 1 mg/mL heparin, KGF-2 at 0.5 mg/mL formed an interfacial gel before the first measurement could be made with the rheometer. In contrast, at 0.005 mg/mL KGF-2, the presence of 0.01 mg/mL heparin greatly slowed gelation (Table 1). In 0.5 M sucrose, the mean gelation times were slightly longer but not significantly different than those observed in buffer alone for either 0.5 or 0.005 mg/mL KGF-2 (p-values > 0.05). Finally, no interfacial gels were observed when PS80 was present in heparin or sucrose formulations of KGF-2.
Table 1.
Interfacial shear rheological values of KGF2 with or without excipients.
| Sample | G′ (mN/m) | G″ (mN/m) | Gelation time (Hour) | Degelation time (Hour) |
|---|---|---|---|---|
| KGF2 (0.5 mg/mL) in buffer | 11.4 ± 13.4 | 2.0 ± 1.7 | 0.01 ± 0.02 | 9.88 ± 5.85 |
| KGF2 (0.005 mg/mL) in buffer | 4.1 ± 1.2 | 0.6 ± 0.2 | 0.09 ± 0.01 | 2.71 ± 0.43 |
| KGF2 (0.5 mg/mL) + heparin (1 mg/mL) | 19.2 ± 1.5 | 1.4 ± 0.4 | NA | 11.51 ± 6.06 |
| KGF2 (0.005 mg/mL) + heparin (0.01 mg/mL) | 1.1 ± 1.8 | 0.2 ± 0.2 | 3.42 ± 2.00 | 0.81 ± 0.44 |
| KGF2 (0.5 mg/mL) + sucrose (0.5M) | 12.3 ± 14.0 | 4.6 ± 6.6 | 0.19 ± 0.26 | 2.13 ± 0.37 |
| KGF2 (0.005 mg/mL) + sucrose (0.5M) | 3.0 ± 1.9 | 0.3 ± 0.1 | 0.16 ± 0.14 | 1.31 ± 1.60 |
The degelation time of samples was obtained after gently pipetting 40 μL of 10% PS80 under the interfacial gel without mixing. The final concentration of PS80 for all samples is 0.01%.
The elastic modulus (G′) and viscous modulus (G″) are values at aging time of 3 hours.
NA: Not applicable (gelation time was too fast to be measured).
Data is the average of triplicate samples ± standard deviation.
In the presence of heparin, the magnitudes of the interfacial shear moduli were greater for formulations containing 0.5 mg/mL KGF-2 as compared to formulations containing 0.005 mg/mL (p-values < 0.05). In the absence of heparin, the magnitudes of the interfacial shear moduli were independent of KGF-2 concentration. Similarly, in the absence of heparin, the mean degelation times were independent of KGF-2 concentration, but the degelation times were significantly different for the two protein concentrations when heparin was present.
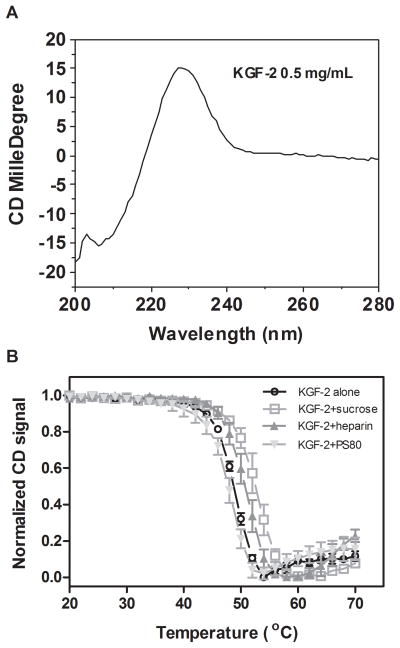
Circular Dichroism Spectroscopy (CD)
A typical far UV CD spectrum was observed for KGF-2 in buffer (Figure 3A).28 There was a positive peak near 230 nm with a sharp decrease into a negative peak close to 200 nm. The 230 nm peak reflects β-trefoil structure, as previously reported for KFG-2 and interleukin α and β.28 To determine the effects of excipients on the thermal stability of KGF-2, the intensity of the 230 nm peak was monitored as a function of temperature during warming. The midpoint of thermal transition temperatures of KGF-2 were 48.4 ± 0.2, 50.9 ± 0.4, 52.4 ± 0.7 and 47.3 ± 0.5°C in buffer alone, 1 mg/mL heparin, 0.5 M sucrose and 0.01% PS80, respectively (Figure 3B). Therefore, as expected, sucrose and heparin stabilized the protein, and PS80 slightly reduced stability against thermally-induced unfolding.
Figure 3.
(A) Far UV CD spectrum of KGF-2 alone; (B) The effect of temperature on the CD signal of KGF-2 at 230 nm in the absence and presence of excipient.
Isothermal Titration Calorimetry (ITC)
A large exotherm was observed during the initial injection of KGF-2 into the heparin solution, suggesting enthalpically-driven binding (Figure 4A, Table 2). There was an apparent transition at a 4:1 molar ratio of KGF-2 to heparin, at which point titration of additional KGF-2 into the heparin solution caused a weak endothermic heat signal, suggesting entropy-driving binding (Table 2). The endothermic reaction gradually decreased until a molar ratio for KGF-2 and heparin of 5:1. Results of fitting of a two-site binding model to the ITC data were consistent with at least five KGF-2 molecules binding to one heparin molecule. In contrast, a very weak exotherm, due to the heat of dilution of PS80 micelle, was observed during the titration of PS80 into KGF-2 solutions. Therefore, the results for titration of PS80 into KGF-2 solution did not show evidence of enthalpy or entropy-driven binding (Figure 4B).
Figure 4.
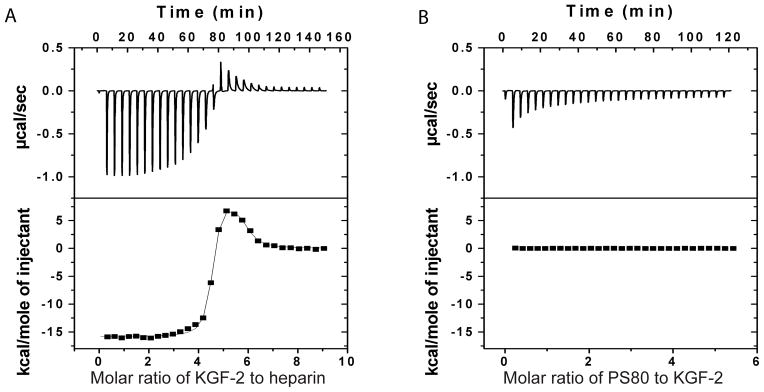
Representative isothermal titration calorimetric (ITC) measurements of KGF2 and heparin (A) or KGF2 and PS80 (B). In panel A, heparin solution (0.005 mM, 1.7 mL) was loaded into the ITC cell, whereas KGF-2 solution (0.2 mM, 305 μL) was loaded in syringe. In panel B, KGF-2 (0.05 mM, 1.7 mL) was loaded into the ITC cell, whereas PS80 (5 mM, 305 μL) was loaded in syringe. The upper panels in A and B are raw data without subtracting the heat of dilution in the control experiments. The lower panels in A and B are the heat per injection from the integrations of data in the upper panels after subtraction of the heat of dilution in the control experiments.
Table 2.
Binding and thermodynamic parameters of KGF2 and heparin.
| Exothermic | Endothermic | |
|---|---|---|
| Number of binding sites | 3.8 ± 0.1 | 1.0 ± 0.1 |
| Binding constant (M−1) | (4.8 ± 1.7) ×108 | (3.2 ± 0.3) ×106 |
| ΔH (cal/mol) | (−1.5 ± 0.1) ×104 | (1.2 ± 0.1) ×104 |
| ΔS (cal/mol/deg) | −12.4 ± 0.9 | 68.7 ± 3.9 |
Discussion
Interfacial shear rheology and incubation studies provide insights into the mechanisms by which excipients, especially PS80, impact the degree of agitation-induced protein aggregation. Interfacial gels were not observed in any of the KGF-2 formulations containing PS80. Furthermore, the interfacial gels formed by the protein in the absence of PS80 were reversible when PS80 was added to the samples after gelation. During agitation of KGF-2 solutions, the presence of PS80 was an effective means to reduce protein aggregation and particle formation. Therefore, there is a correspondence between formulations that exhibited interfacial gelation and formulations that exhibited agitation-induced aggregation. It does not appear that PS80 reduced agitation-induced aggregation or interfacial gelation of KGF-2 by binding to the protein, because ITC measurement did not show evidence of this interaction. Instead, as shown previously11, surfactants can compete with proteins for adsorption onto the air-water interface, which could be an important factor in inhibition of interfacial gelation of the proteins.
In KGF-2 formulations that did not contain PS80, relatively high levels of protein particles were formed during agitation; even in the presence of sucrose and heparin, which increase the thermodynamic stability of the protein’s native state in bulk solutions. We suggest that the presence of an interfacial gel contributes to the formation of aggregates/particles during agitation. Agitation creates cycles wherein the interfacial area alternately increases and decreases.38 When the surface area increases, protein molecules and aggregates rapidly adsorb and accumulate; in principle this process could be reversed when the surface area is decreased. However, the presence of an interfacial gel would presumably slow the dissociation kinetics dramatically so that rapid changes in the interfacial area would result in buckling of the interfacial gel.39 In contrast, in the presence of PS80, there was no interfacial gel formation and particle formation was greatly reduced.
A recent study provides additional evidence to support this mechanism. Bee et.al examined the effect of interfacial compression and dilation cycles on the aggregation of a monoclonal antibody. They found that above a critical interfacial compression ratio of ~5, protein aggregates and particles were rapidly generated.38 Smaller compression ratios resulted in a significant decrease in the rate of particle formation.
PS80 appeared to facilitate KGF-2 particle formation in the quiescent state. Serno et al also found that PS80 and hydroxypropyl-β-cyclodextran can increase the protein aggregation rate in bulk solution and ascribed this observation to a reduced free energy of unfolding in the presence of PS80 in bulk solution.8 For our case, the Tm for KGF-2 decreased about 1 °C in the presence of PS80. The level of particle formation in the presence of PS80 during agitation was comparable to that measured in the presence of PS80 without agitation.
In the quiescent state, KGF-2 aggregation was inhibited when KGF-2 bound to heparin. In contrast, heparin appeared to facilitate KGF-2 aggregation during agitated incubation. Our Far UV CD, ITC and interfacial shear rheology results provide insight into these findings. In particular, heating studies of KGF-2 with heparin show that: 1) At a 2:1 ratio of heparin to KGF-2, the apparent melting temperature of KGF-2 heparin complex (Tm = 50.9 ± 0.4°C) increased 2.5 °C compared to that of KGF-2 alone (Tm = 48.4 ± 0.2°C); and 2) heparin prevented KGF-2 precipitation during the heating process. ITC results show there were up to 5 KGF-2 binding sites on each heparin molecule. Therefore, at a 2:1 ratio of heparin and KGF-2, free heparins were still available in the solution. Heparin is a linear molecule with multiple binding sites, where as a KGF-2 is a globular molecule. Binding to heparin not only reduced the equilibrium populations of unfolded or partially unfolded KGF-2 molecules, but also dramatically reduced the intermolecular association kinetics of KGF-2 molecules in the quiescent state due to relative immobilization of KGF-2 molecules. However, heparin promoted interfacial gel formation at a higher KGF-2 concentration (0.5 mg/mL), probably due to the neutralization of electrostatic charge on the protein; i.e. the reduction of electrostatic repulsion facilitated adsorption of protein molecules at the interface. Again, interfacial gelation would slow the dissociation kinetics of KGF-2 and heparin complex dramatically so that rapid changes in the interfacial area could result in the formation of macroscopic aggregates of protein/heparin complexes.
The magnitudes of the interfacial shear moduli in the presence of heparin were greater for formulations containing higher protein and heparin concentrations as compared to formulations containing lower protein and heparin concentration. At a higher concentration, protein-heparin complex could interact closely due to the neutralization of electrostatic charge on the protein. The reduction of electrostatic repulsion likely facilitated adsorption of multi-layer of protein-heparin molecules at the interface, which showed higher interfacial shear moduli. In contrast, at a lower concentration, the quantity of protein-heparin complex was probably only enough to form a monolayer, which showed lower interfacial shear moduli. In the absence of heparin, the magnitudes of the interfacial shear moduli were independent of KGF-2 concentration, most likely due to the electrostatic repulsion preventing formation of a strong multi-layer network.
Conclusions
Our data support our hypothesis, i.e., the effects of a given excipient during agitation depend on the balance between the excipient’s effects on the conformational stability of the native protein in the bulk solution and the extent of protein gelation at air-water interfaces. Addition of heparin and sucrose increased the apparent melting temperature of KGF-2 in bulk solution. However, a strong interfacial gel formed at the air-water interface within minutes, with the net overall result that agitation-induced aggregation increased in the presence of these excipients. In contrast, PS80 reduced the apparent melting temperature of KGF-2, leading to particle formation in the bulk solution. However, since no interfacial gels were formed in the presence of PS80, agitation-induced aggregation was inhibited. The combination of PS 80 with either heparin or sucrose completely prevented protein aggregation at both bulk and air-water interface during both incubations. Presumably, in this case addition of PS80 inhibited gel formation at the air-water interface, whereas the added sucrose or heparin served to overcome the destabilizing effects of PS80 in the bulk solution.
Acknowledgments
We thank NIH (5R01 EB006006) for financial support and Human Genome Sciences, Inc. for providing KGF-2.
References
- 1.Carpenter JF, Kendrick BS, Chang BS, Manning MC, Randolph TW. Inhibition of stress-induced aggregation of protein therapeutics. Methods Enzymol. 1999;309:236–255. doi: 10.1016/s0076-6879(99)09018-7. [DOI] [PubMed] [Google Scholar]
- 2.Mahler HC, Muller R, Friess W, Delille A, Matheus S. Induction and analysis of aggregates in a liquid IgG1-antibody formulation. Eur J Pharm Biopharm. 2005;59(3):407–417. doi: 10.1016/j.ejpb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Patro SY, Freund E, Chang BS. Protein formulation and fill-finish operations. Biotechnol Annu Rev. 2002;8:55–84. doi: 10.1016/s1387-2656(02)08004-3. [DOI] [PubMed] [Google Scholar]
- 4.Bam NB, Cleland JL, Yang J, Manning MC, Carpenter JF, Kelley RF, Randolph TW. Tween protects recombinant human growth hormone against agitation-induced damage via hydrophobic interactions. J Pharm Sci. 1998;87(12):1554–1559. doi: 10.1021/js980175v. [DOI] [PubMed] [Google Scholar]
- 5.Kerwin BA, Heller MC, Levin SH, Randolph TW. Effects of Tween 80 and sucrose on acute short-term stability and long-term storage at −20 degrees C of a recombinant hemoglobin. J Pharm Sci. 1998;87(9):1062–1068. doi: 10.1021/js980140v. [DOI] [PubMed] [Google Scholar]
- 6.Kreilgaard L, Jones LS, Randolph TW, Frokjaer S, Flink JM, Manning MC, Carpenter JF. Effect of Tween 20 on freeze-thawing- and agitation-induced aggregation of recombinant human factor XIII. J Pharm Sci. 1998;87(12):1597–1603. doi: 10.1021/js980126i. [DOI] [PubMed] [Google Scholar]
- 7.Randolph TW, Jones LS. Surfactant-protein interactions. Pharm Biotechnol. 2002;13:159–175. doi: 10.1007/978-1-4615-0557-0_7. [DOI] [PubMed] [Google Scholar]
- 8.Serno T, Carpenter JF, Randolph TW, Winter G. Inhibition of agitation-induced aggregation of an IgG-antibody by hydroxypropyl-beta-cyclodextrin. J Pharm Sci. 2010;99(3):1193–1206. doi: 10.1002/jps.21931. [DOI] [PubMed] [Google Scholar]
- 9.Mahler HC, Senner F, Maeder K, Mueller R. Surface activity of a monoclonal antibody. J Pharm Sci. 2009;98(12):4525–4533. doi: 10.1002/jps.21776. [DOI] [PubMed] [Google Scholar]
- 10.Katakam M, Bell LN, Banga AK. Effect of surfactants on the physical stability of recombinant human growth hormone. J Pharm Sci. 1995;84(6):713–716. doi: 10.1002/jps.2600840609. [DOI] [PubMed] [Google Scholar]
- 11.Serno T, Hartl E, Besheer A, Miller R, Winter G. The Role of Polysorbate 80 and HPbetaCD at the Air-Water Interface of IgG Solutions. Pharm Res. 2013;30(1):117–130. doi: 10.1007/s11095-012-0854-x. [DOI] [PubMed] [Google Scholar]
- 12.Chou DK, Krishnamurthy R, Randolph TW, Carpenter JF, Manning MC. Effects of Tween 20 and Tween 80 on the stability of Albutropin during agitation. J Pharm Sci. 2005;94(6):1368–1381. doi: 10.1002/jps.20365. [DOI] [PubMed] [Google Scholar]
- 13.Jones LS, Randolph TW, Kohnert U, Papadimitriou A, Winter G, Hagmann ML, Manning MC, Carpenter JF. The effects of Tween 20 and sucrose on the stability of anti-L-selectin during lyophilization and reconstitution. J Pharm Sci. 2001;90(10):1466–1477. doi: 10.1002/jps.1098. [DOI] [PubMed] [Google Scholar]
- 14.Garidel P, Hoffmann C, Blume A. A thermodynamic analysis of the binding interaction between polysorbate 20 and 80 with human serum albumins and immunoglobulins: a contribution to understand colloidal protein stabilisation. Biophys Chem. 2009;143(1–2):70–78. doi: 10.1016/j.bpc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Mahler H-C, Fischer S, Randolph TW, Carpenter JF. Protein Aggregation and Particle Formation: Effects of Formulation, Interfaces and Drug Product Manufacturing Operations. Hoboken, N.J: Wiley; 2010. [Google Scholar]
- 16.Arakawa T, Timasheff SN. Stabilization of protein structure by sugars. Biochemistry. 1982;21(25):6536–6544. doi: 10.1021/bi00268a033. [DOI] [PubMed] [Google Scholar]
- 17.Lee JC, Timasheff SN. The stabilization of proteins by sucrose. J Biol Chem. 1981;256(14):7193–7201. [PubMed] [Google Scholar]
- 18.Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm Res. 2003;20(9):1325–1336. doi: 10.1023/a:1025771421906. [DOI] [PubMed] [Google Scholar]
- 19.Bos MA, van Vliet T. Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv Colloid Interface Sci. 2001;91(3):437–471. doi: 10.1016/s0001-8686(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson E. Adsorbed protein layers at fluid interfaces: interactions, structure and surface rheology. Colloids and Surfaces B: Biointerfaces. 1999;15(2):161–176. [Google Scholar]
- 21.Mackie AR, Gunning AP, Wilde PJ, Morris VJ. Orogenic Displacement of Protein from the Air/Water Interface by Competitive Adsorption. J Colloid Interface Sci. 1999;210(1):157–166. doi: 10.1006/jcis.1998.5941. [DOI] [PubMed] [Google Scholar]
- 22.Dickinson E, Soon-Taek H. Surface Coverage of beta-Lactoglobulin at the Oil-Water Interface: Influence of Protein Heat Treatment and Various Emulsifiers. Journal of Agricultural and Food Chemistry. 1994;42(8):1602–1606. [Google Scholar]
- 23.Chen BL, Arakawa T, Hsu E, Narhi LO, Tressel TJ, Chien SL. Strategies to suppress aggregation of recombinant keratinocyte growth factor during liquid formulation development. J Pharm Sci. 1994;83(12):1657–1661. doi: 10.1002/jps.2600831204. [DOI] [PubMed] [Google Scholar]
- 24.Copeland RA, Ji H, Halfpenny AJ, Williams RW, Thompson KC, Herber WK, Thomas KA, Bruner MW, Ryan JA, Marquis-Omer D, et al. The structure of human acidic fibroblast growth factor and its interaction with heparin. Arch Biochem Biophys. 1991;289(1):53–61. doi: 10.1016/0003-9861(91)90441-k. [DOI] [PubMed] [Google Scholar]
- 25.Vemuri S, Beylin I, Sluzky V, Stratton P, Eberlein G, Wang YJ. The stability of bFGF against thermal denaturation. J Pharm Pharmacol. 1994;46(6):481–486. doi: 10.1111/j.2042-7158.1994.tb03831.x. [DOI] [PubMed] [Google Scholar]
- 26.Prestrelski SJ, Fox GM, Arakawa T. Binding of heparin to basic fibroblast growth factor induces a conformational change. Arch Biochem Biophys. 1992;293(2):314–319. doi: 10.1016/0003-9861(92)90401-h. [DOI] [PubMed] [Google Scholar]
- 27.Fan H, Li H, Zhang M, Middaugh CR. Effects of solutes on empirical phase diagrams of human fibroblast growth factor 1. J Pharm Sci. 2007;96(6):1490–1503. doi: 10.1002/jps.20796. [DOI] [PubMed] [Google Scholar]
- 28.Derrick T, Grillo AO, Vitharana SN, Jones L, Rexroad J, Shah A, Perkins M, Spitznagel TM, Middaugh CR. Effect of polyanions on the structure and stability of repifermin (keratinocyte growth factor-2) J Pharm Sci. 2007;96(4):761–776. doi: 10.1002/jps.20797. [DOI] [PubMed] [Google Scholar]
- 29.Fan H, Vitharana SN, Chen T, O’Keefe D, Middaugh CR. Effects of pH and polyanions on the thermal stability of fibroblast growth factor 20. Mol Pharm. 2007;4(2):232–240. doi: 10.1021/mp060097h. [DOI] [PubMed] [Google Scholar]
- 30.Burke CJ, Volkin DB, Mach H, Middaugh CR. Effect of polyanions on the unfolding of acidic fibroblast growth factor. Biochemistry. 1993;32(25):6419–6426. doi: 10.1021/bi00076a015. [DOI] [PubMed] [Google Scholar]
- 31.Huang M, Berkland C. Controlled release of repifermin from polyelectrolyte complexes stimulates endothelial cell proliferation. J Pharm Sci. 2009;98(1):268–280. doi: 10.1002/jps.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahin GT. The Stress Deformation Interfacial Rheometer. University of Pennsylvania; 1986. [Google Scholar]
- 33.Bantchev G Chemical and Biological Engineering, editor. An Interfacial Protein Gel of β-Casein: Study of the Rheology and Nanostructure. Boulder: University of Colorado at Boulder; 2003. p. 196. [Google Scholar]
- 34.Vessely CR, Carpenter JF, Schwartz DK. Calcium-induced changes to the molecular conformation and aggregate structure of beta-casein at the air-water interface. Biomacromolecules. 2005;6(6):3334–3344. doi: 10.1021/bm050353w. [DOI] [PubMed] [Google Scholar]
- 35.Bantchev GB, Schwartz DK. Surface shear rheology of beta-casein layers at the air/solution interface: Formation of a two-dimensional physical gel. Langmuir. 2003;19(7):2673–2682. [Google Scholar]
- 36.Barnard JG, Singh S, Randolph TW, Carpenter JF. Subvisible particle counting provides a sensitive method of detecting and quantifying aggregation of monoclonal antibody caused by freeze-thawing: insights into the roles of particles in the protein aggregation pathway. J Pharm Sci. 100(2):492–503. doi: 10.1002/jps.22305. [DOI] [PubMed] [Google Scholar]
- 37.Brooks CF, Fuller GG, Frank CW, Robertson CR. An interfacial stress rheometer to study rheological transitions in monolayers at the air-water interface. Langmuir. 1999;15(7):2450–2459. [Google Scholar]
- 38.Bee JS, Schwartz DK, Trabelsi S, Freund E, Stevenson JL, Carpenter JF, Randolph TW. Production of particles of therapeutic proteins at the air-water interface during compression/dilation cycles. Soft Matter 2012 [Google Scholar]
- 39.Pocivavsek L, Dellsy R, Kern A, Johnson S, Lin B, Lee KY, Cerda E. Stress and fold localization in thin elastic membranes. Science. 2008;320(5878):912–916. doi: 10.1126/science.1154069. [DOI] [PubMed] [Google Scholar]