Abstract
A computerized visual search task was presented to 18 guinea baboons (Papio papio) ranging from 2.7 to 14.3 years of age. The task, inspired from Hick’s (1952) task, required detection of a target among a variable number of distractors equidistant to a start button. The reaction times (RTs) and movement times both increased with the number of distractors expressed in bits of information. However, the slope of RT per bit function correlated positively with age, whereas a negative correlation was found for the movement time slopes. In Experiment 2, the same baboons were required to inhibit an ongoing manual pointing toward a target stimulus, to reengage in a new point as a consequence of a change in target location. Results revealed a more accurate performance in the adults, suggesting that differences in behavioral strategies in Experiment 1 can be accounted for by a greater inhibitory control of the adult participants. Implications of these results are discussed regarding the relation between attention, inhibitory control, and behavioral strategies in monkeys, and the general significance of RT slopes in visual search tasks.
Keywords: excecutive function, executive control, development, nonhuman primate, behavior
To be better adapted, animals must possess the ability to direct their behavior toward objects in the environment that are relevant to their needs. Achieving this success in real life therefore requires that relevant objects are selected and processed as distinct from the other objects. This amazing capacity to selectively filter some dimensions of the environment, while neglecting others, is known as a process of selective attention (e.g., Posner, 1980).
Selective attention has been investigated in a very large number of behavioral animal studies, probably because of its critical importance in improving our understanding of the organism’s perceptual systems, cognition, and behavior. A number of studies have revealed similar selective attention processes in humans and other animals, suggesting that common psychological mechanisms are involved in selective attention. For instance, pigeons (Blough, 1979), baboons (Deruelle & Fagot, 1998), cynomolgus monkeys (Azzato & Butter, 1984), chimpanzees (Fagot & Tomonaga, 1999), and humans (Treisman & Gelade, 1980) all show increased response times to detect a target object in visual displays when the displays contain distractors. Moreover, perceptual similarity between the target stimulus and the surrounding distractors significantly increases target detection time in various species (pigeons: Cook, Cavoto, Katz, & Cavoto, 1997; monkeys: Buračas & Albright, 1999; humans: Treisman & Gelade, 1980). Just like humans, animals may also purposively and flexibly shift their attention across dimensions of the visual display (Fremouw, Herbranson, & Shimp, 2002).
One interesting aspect of selective attention in humans is its interindividual variability. First, performance in tasks of selective attention may vary with age. Performance on selective attention tasks are slowed early and late in life (Hommel, Li, & Li, 2004), in particular when the target and distractors share common features (conjunction search; e.g., Plude & Doussard-Roosevelt, 1989). Second, peculiarities in selective attention also characterize clinical human groups, such as those with autistic spectrum disorders (e.g., Remington, Swettenham, Campbell, & Coleman, 2009), Alzheimer diseases (Levinoff, Li, Murtha, & Chertkow, 2004), or children with attention-deficit/hyperactivity disorders (Chan, Mattingley, Huang-Pollock, Vance, & Bellgrove, 2009). Finally, detectable differences may emerge between human groups after an extensive practice in tasks with strong attentional demands, such as action-video game playing, which improves performance in attention tasks (Green & Bavelier, 2003).
To our knowledge, no published reports have attempted to investigate individual variability in selective attention in nonhuman primates. Lack of consideration of this issue might be explained, in part, by the need to test a sufficiently large number of subjects in well-controlled tasks, which is very difficult if not impossible in many laboratories. Demonstration that attentional abilities differ in individual monkeys would be important for several reasons, however. First, it would be important for neuroscientists looking for the most appropriate animal subjects to study the neural bases of attention. It would also be of strong heuristic value, allowing a better understanding of individual differences in behaviors recruiting attentional processes, either in laboratories or in more natural settings.
In that context, the first aim of our research was to investigate interindividual differences in selective attention in a group of baboons. For this purpose, we used an adapted version of the Hick task (Hick, 1952). The Hick task is a particularly well-controlled visual search task requiring identification of a target among a variable number of distractors that are all equidistant from a “start stimulus.” According to Hick (1952), a linear relation with a positive slope should be found in that task between reaction time (RT) and the amount of information (scaled in bits) available in the display. This relation is referred to in the literature as Hick’s law. It provides information on the ability to selectively attend to components of the visual scenes while ignoring others. The ability for selective attention may be critical for a broad range of cognitive activities and may have broader implications in measures of intelligence. For example, suggestive evidence in humans reveals negative correlations between the slope of the linear function relating the RTs and the number of bits of information available in the display in the Hick task (i.e., RT slopes) and performance on tests of intelligence (e.g., Raven’s advanced progressive matrices; Jensen & Munro, 1979; Neubauer, 1990).
Earlier studies on pigeons (Vickrey & Neuringer, 2000) and monkeys (Laursen, 1977) have shown that these animals perform in accordance with Hick’s law, that is, they show a linear relation between RTs and display size, suggesting that the processes of selective attention might be highly comparable in humans and nonhuman primates. However, use of the small number of animals in these two studies did not allow conclusions on their interindividual variability across participants. The main goal of Experiment 1 was therefore to present the Hick task in a large number of baboons to study individual variations in search slopes and their perceptual, motor, or cognitive origins.
Experiment 1: The Hick Task
Experiment 1 presented the Hick task to 18 baboons (Papio papio) of different ages and sex, and analyzed interindividual variations in processing speed.
Method
Subjects and housing
The subjects were 18 baboons (six males and 12 females) ranging in age from 2.7 to 14.3 years (M = 7.23 years, SD = 4.11; see Table 1). The baboons belong to a social group of 27 individuals living in a 670 m2 outdoor enclosure connected by tunnels to a 6 × 4 m indoor housing in the CNRS primate facility, Rousset-sur-Arc, France. They were already familiar with test procedures involving touch screens (see Fagot & Bonté, 2010) and participated in the task at their own will. The baboons were neither food nor water deprived at any point in the study. The daily ration of monkey chow, vegetables, and fruits was delivered once a day in the housing area, usually at 5 p.m., but in the morning during the weekend. Water was also permanently available in the enclosure and housing quarters. Baboons were marked by two biocompatible 1.2 × 0.2 cm RFID subcutaneous implanted microchips in each forearm. These microchips served the self-identification procedure (see below). All baboons were already familiar with the Hick task from previous unpublished studies and other visual search tasks (e.g., Barbet & Fagot, 2011), but have never received the Hick task using the +-shaped and ×-shaped stimuli involved in the current research.
Table 1.
Data Set on Which the Analyses Were Conducted
| Baboon | Sex | Age (years) | % correct | Response time (ms) | RT (ms) | MT (ms) | RT slope (ms/bit) | MT slope (ms/bit) |
|---|---|---|---|---|---|---|---|---|
| Ang | F | 4.6 | 93.3 | 581 | 336 | 245 | 48 | 89 |
| Ari | F | 4.2 | 93.2 | 516 | 227 | 289 | 31 | 87 |
| Art | M | 4.2 | 91.4 | 533 | 278 | 255 | 40 | 71 |
| Atm | F | 11.8 | 80.5 | 760 | 507 | 253 | 128 | 54 |
| Bar | M | 3.5 | 84.7 | 620 | 424 | 196 | 82 | 33 |
| Bob | M | 3.3 | 95.7 | 531 | 338 | 192 | 65 | 51 |
| Bri | F | 13.8 | 54.6 | 473 | 354 | 119 | 77 | 12 |
| Cau | M | 2.7 | 90.6 | 611 | 308 | 302 | 8 | 101 |
| Clo | M | 2.8 | 93.2 | 639 | 319 | 321 | 52 | 113 |
| Kal | F | 14.3 | 82.6 | 649 | 414 | 235 | 77 | 49 |
| Mic | F | 13.8 | 66.9 | 414 | 316 | 99 | 83 | 17 |
| Mon | F | 12.8 | 80.4 | 571 | 431 | 140 | 67 | 29 |
| Rom | F | 9.7 | 83.0 | 590 | 244 | 346 | 23 | 108 |
| Tar | F | 7.5 | 62.8 | 560 | 314 | 246 | 76 | 61 |
| Ura | F | 6.0 | 90.9 | 536 | 315 | 221 | 56 | 66 |
| Van | F | 5.1 | 87.4 | 612 | 299 | 313 | 19 | 115 |
| Vio | F | 5.0 | 95.7 | 528 | 249 | 279 | 19 | 66 |
| Viv | M | 5.2 | 92.2 | 560 | 264 | 296 | 23 | 96 |
| Mean | 7.2 | 84.4 | 571 | 330 | 241 | 54 | 68 | |
| SD | 4.2 | 11.9 | 75 | 74 | 70 | 31 | 32 |
Note. RT = reaction time; MT = movement time.
Automated learning device for monkeys test systems
The experiments described here employed a new test system, called the Automated Learning Device for Monkeys (ALDM), described in detail in Fagot and Paleressompoulle (2009) and Fagot and Bonté (2010). A unique feature of ALDM is to identify the baboons automatically in the test cage, allowing self-testing on a voluntary basis, while being maintained in a social group and on a 24-hr schedule. Each ALDM test system has a freely accessible test chamber (0.7 × 0.7 × 0.8 m) with the back end open and connected to the outdoor enclosure. The test chamber is fitted in its inner most front side with a 7 × 7 cm view port and two 8 × 5 cm hand ports with access to a 19-in. 1,024 × 768 pixel definition LCD touch monitor installed at eye level 25 cm from the view port. An antenna reads the ID number of each monkey when it inserts its microchipped forearm through one of the hand ports. Identification signals from the chips trigger the computer-controlled presentation of the stimuli and serve to assign behavioral measures (stimulus choices and response times) to each participant. Each ALDM test system is also fitted with a homemade food dispenser delivering grains of dry wheat. The equipment is controlled by a test program developed by the first author using E-Prime language (V 2.0 professional; Psychology Software Tools, Pittsburgh, PA). Ten ALDM systems were accessible to the monkeys during the research. They were installed inside 4 × 8 m2 test rooms connected to the enclosure by holes made in the wire mesh (for more details, see Fagot & Bonté, 2010).
Stimuli and test procedure for ALDM testing
The task was designed to simulate the Hick task. Figure 1 illustrates the trial procedure. The computer displayed a 100 × 100 pixel +-shaped start button on a black background in the bottom of the screen (see Figure 1a) immediately after the self-identification procedure. Touching the start button triggered the probe display, which contained one, two, four, or eight white 80 × 80 pixel squares each containing a black × shape (see Figure 1b) called potential targets. These potential targets were randomly located on the screen considering a set of eight possible predefined locations with an equal radial (25.7°) and absolute distance (i.e., 400 pixels) from the start button. The duration of the probe display was 200 ms, during which the baboon had to maintain contact with the start button. Releasing this button during the probe display aborted the trial. Aborted trials were not counted and were therefore represented at a later point in training and testing. If the baboon maintained contact on the start button during the requested duration of 200 ms, a +-shaped stimulus was presented in the center of one of the squares (see Figure 1c). That shape defined the target. The other squares, which remained unchanged and therefore continued to contain a × shape, served as distractors. The task for the subject was then to release the start button to touch the target. The baboon was required to touch one of the shapes on the screen within an 8-s window, after which the trial was aborted. Correct responses were defined as the baboon touching the square containing the + shape, and these responses were food rewarded. Incorrect selections were defined as touching one of the distractors and produced a 3-s time-out during which the screen turned green. All trials were followed by a 3-s intertrial interval during which the screen was black. The subject could then proceed to the next test trial immediately after the intertrial interval had elapsed. During the test period, the baboons were presented with six randomly ordered test sessions of 200 trials, each session containing 50 trials for each condition of display size (i.e., one, two, four, and eight shapes on the screen).
Figure 1.
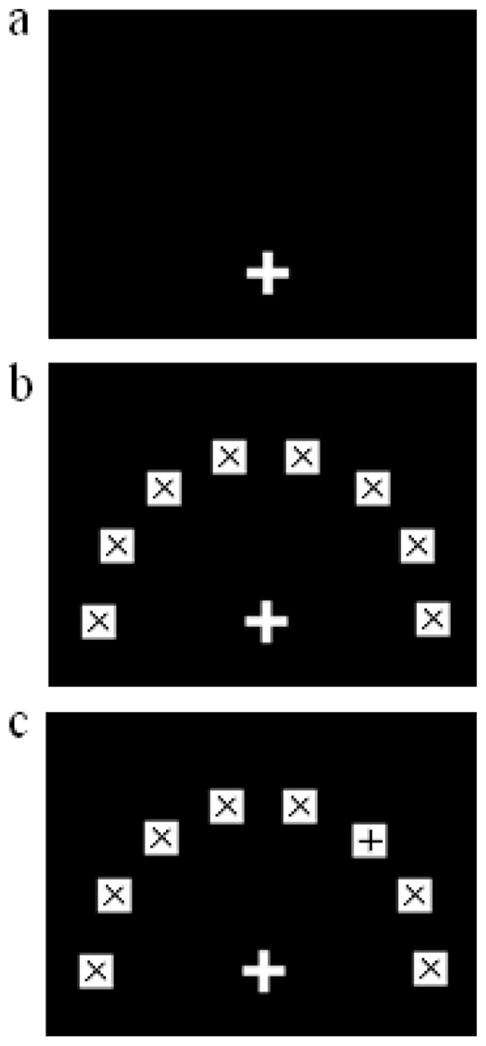
Diagram of the test procedure. (1a) Display of the start button. A 3-bit display before (1b) and after (1c) the appearance of the target. The target is indicated by a + sign and the distractors by × signs.
Training procedure
The general procedure used in the Hick task, which is a special case of visual search task, was mastered by the baboons prior to testing. However, subjects were unfamiliar with the +-shaped (target) and ×-shaped stimuli (distractors) and therefore required training to learn which stimulus was the target to be touched. A training procedure was thus proposed to achieve this discrimination prior to testing. During the training phase, the baboons had to touch the start button during the 200 ms to display the + stimulus (the target), which appeared on the screen in any of the eight possible locations shown in Figure 1, but without distractors. Repeated sessions of 100 trials were run until the baboons responded correctly without aborting the trial during its execution. That phase required only a few training sessions per subject (M = 8, SD = 7). The test phase was presented once the baboons achieved a performance correct of 90% or higher in three consecutive training sessions.
Dependent variables
Accuracy, response time, reaction time (RT), and movement time were recorded as dependent variables. A correct response was recorded when a hand contact was made on the appropriate target stimulus. The RTs were defined as the time (in ms) elapsed between the onset of the target and the release of the start button. The movement times corresponded to the delay (in ms) between the release of the start button and the hand contact on the target. The response time was defined as the sum of RT and movement time, and therefore corresponded to the total duration of the behavioral response. Statistical RT, movement time, and response time analyses considered only the correct trials for which neither the RT nor the movement time exceeded 2 standard deviations from the individual mean. This outlier screening procedure, which was aimed at discarding the trials on which the subjects likely paid little or no attention to the task, removed only 5.3% of the total data set.
Results
Accuracy
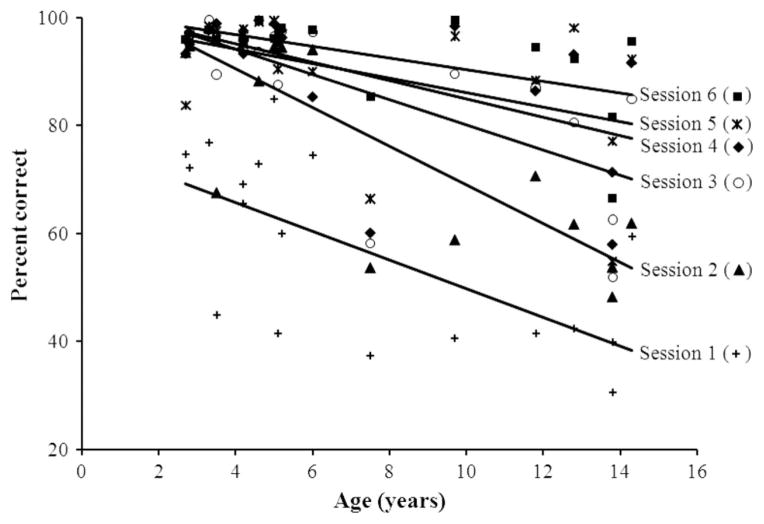
Table 1 lists the individual monkeys, their sex and age, and provides information on their accuracy and response speed in the Hick task. Performance was very high on average. The average percentages correct were equal to 83% (SD = 37.6), 78.34% (SD = 41.2), 80.17% (SD = 40) for the one-, two-, and three-bit conditions, respectively. It was necessarily 100% correct for the zero-bit condition, as there were no distractors on these trials. Accuracy data were submitted to an analysis of covariance (ANCOVA) considering the factors of age, bit, and test session. This analysis omitted the zero-bit condition as there were no possible errors in that condition. The sex factor was similarly omitted from this analysis and the next ones because that factor had no detectable effect on either dependent variable. The three-way ANCOVA showed a main effect of age, F(1, 288) = 178.54, p < .001, η2 = .25, corresponding to a reduced performance in the older baboons mostly accounted for by two adults (Bri and Tar; see Table 1). The main effect of bit was also reliable, F(2, 288) = 4.57, p < .05, η2 = .01. Similarly, there was a main effect of test session, indicating an increased accuracy with repeated testing, F(5, 288) = 38.87, p < .001, η2 = .27. The three-way interaction was not significant (p > .05), but the above three factors were involved in several significant two-way interactions. First, the significant Age × Bit interaction showed that the oldest baboons tended to have reduced scores with the most complex displays containing the largest numbers of bits, F(2, 288) = 4.23, p < .05, η2 = .01. Finally, the Age × Test Session interaction was also significant, F(5, 288) = 5.90, p < .001, η2 = .04. As illustrated in Figure 2, the youngest baboons had a maximal performance as early as Test Session 2 and during the next sessions. The oldest baboons more progressively improved their performance from Test Sessions 1 to 6. That difference explains the overall better performance of the youngest baboons in comparison to the older ones.
Figure 2.
Percentage correct in Experiment 1 depending on the age of the participant and the test session. Each line represents a linear fit for the considered session.
Response time analyses
An Age × Bit × Test Session ANCOVA on response times showed a main effect of bit, F(3, 384) = 258.90, p < .001, η2 = .64. As revealed by a regression analysis, responses times increased linearly with bit (0 bit: M = 369 ms; 1 bit = 524 ms; 2 bits = 664 ms; 3 bits = 729 ms), F(1, 70) = 177.9, p < .001, and this relation accounted for 71.36% of the variance. The ANCOVA also revealed a main effect of test session, F(5, 384) = 3.52, p < .01, η2 = .01, but no reliable effect of age, F(1, 384) = 1.13, p > .1. The lack of a main effect of age is important because it shows that the older baboons were not slower on average for responding to the task than the younger baboons, suggesting equal motivation in these subjects. In addition, several two-way interactions were significant in the analysis, but the three-way interaction was not. First, there is a significant Age × Bit interaction, which is unfortunately hardly amenable to interpretation from its graphical representation, F(3, 384) = 3.07, p < .05, η2 = .01. Second, there was also significant interaction between age and test session, F(5, 384) = 3.03, p < .05, η2 = .01. This interaction suggests a reduction of response times with repeated testing, which was more pronounced in the youngest baboons.
RT analyses
The Age × Bit × Test Session ANCOVA on RTs showed a main effect of age, indicating longer RTs for the older participants, F(1, 384) = 54.79, p < .001, η2 = .08. There was also a significant main effect of test session, indicating reduced RTs with repeated testing, F(5, 384) = 32.53, p < .05, η2 =.02, and a main effect of bit, showing that the RTs increased with the number of bits, F(3, 384) = 67.96, p < .001, η2 = .29. This effect of bit validates our procedure for RT recording because it demonstrates that the monkeys did not simply learn to release the start button after a fixed duration of 200 ms, but released it after some processing at least of the stimulus display. It is interesting that linearity accounted for a significant proportion of the variance (32.91%) in this data set, as demonstrated by a linear regression analysis of the RT per bit function, F(1, 70) = 35.83, p < .001. This finding supports the predictions of Hick’s law.
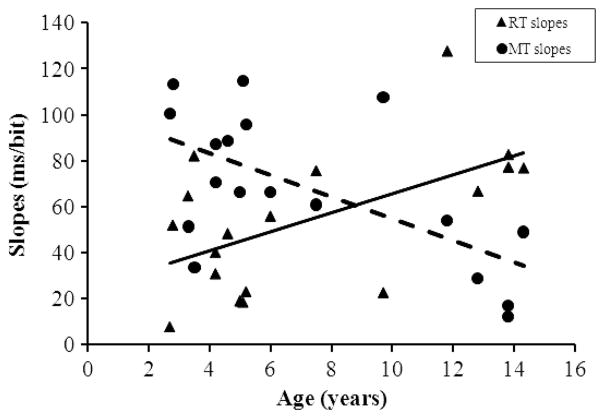
It is important to note that there was a reliable two-way Age × Bit interaction, F(3, 384) = 9.41, p < .001, η2 = .04. To analyze this effect in the context of Hick’s law, we computed the slope of RT/bit linear function for each subject and submitted these slope values to a linear regression using age as the unique predictor. There was a significant effect of age, accounting for 27.57% of the variance, F(1, 16) = 7.47, p < .05. It indicated steeper RT slopes with increasing age, as illustrated in Figure 3. None of the other interactions were significant.
Figure 3.
Each symbol indicates the slope of the reaction time (RT; triangles) or movement time (MT; circle) per bit function for each individual. These slopes are plotted on the graph as a function of the age of the participants. The two lines indicate the linear fits for the RTs (continuous line) and MTs (dashed line) slopes as a function of age.
Movement time analyses
The Bit × Age × Test Session ANCOVA on movement times revealed two main effects. First, the significant age factor demonstrated shorter movement times with increasing age, F(1, 384) = 102, p < .001, η2 < .1. Second, the significant effect of bit showed longer movement times on average for the largest numbers of bits, F(3, 384) = 135.50, p < .001, η2 = .43. These two factors moreover interacted reliably, F(3, 384) = 11.63, p < .001, η2 = .04. To analyze their interaction, we computed the slope of movement time/bit linear functions for each subject and submitted their values to a linear regression using the age factor as the unique predictor. There was a significant effect of age, accounting for 34.91% of the variance, F(1, 16) = 10.12, p < .01. The movement time slopes became flatter with increasing age (see Figure 3). No other interactions emerged as significant from statistical analyses.
Discussion
Experiment 1 calls for several conclusions. First, performances in the Hick task were very high on average but varied as a function of age, with the youngest subjects outperforming their older counterparts. It is interesting that this effect of age was mostly evident in the earliest sessions (see Figure 2), suggesting that the discrimination between the target and distractor stimuli was acquired faster by the young baboons than by the old ones. In spite of that difference in learning speed as a function of age, the overall response time was not significantly affected by the baboons’ age. This lack of reliable relation between age and response time suggests that the participants had similar perceptual or motor abilities in our task, regardless of their age difference. This finding is not surprising because our oldest participants were less than 15 years old for a species with a life span of approximately 30 years. The age of our baboons therefore ranged from childhood to mid-adulthood, and we had no extremely old participants with presumed deficient perceptual or motor abilities as subjects. The second important conclusion of Experiment 1 is that linearity accounted for an important proportion of the variance of the RT per bit function. This effect demonstrates Hick’s law in baboons. It is interesting, however, that the slope of the RT per bit functions was positively affected by the age of the subjects, with the older baboons having steeper slopes than the young ones. Because the RT slope correlates negatively with IQ in humans (Jensen, 1982), our finding could be interpreted as demonstrating lower cognitive abilities in our older participants. This explanation appears inappropriate, however, because the prerequisite of flat movement time slopes was not fulfilled in our experiment. Indeed, the slope of the movement time per bit function decreases with age in Experiment 1, suggesting a trade-off between the RT and movement time slopes (see Figure 3). Confirmation of this trade-off is provided by a partial correlation analysis considering age, RT slopes, and movement time slopes as factors. This analysis indicates a significant partial r of −.54 (p < .05) for the correlation between the RT and movement time slopes.
To account for the RT/movement time trade-off, we propose that two different response behaviors were used to solve the task, and that the production of these two behaviors was age dependent. The first possible behavior would imply that the target is both detected and identified during the RT period, before the onset of the pointing movement. This behavior is supported in our task by an effect of display size during the RT period and therefore by steep RT slopes. The alternative behavior would imply that the subject releases the start button before the target is identified, which would delay the process of target search until the start button has been released. That behavior would be demonstrated by a positive relation between the movement time and display size. Because Experiment 1 showed that the slope was steeper in the RT period for the older participants and in the movement time period for the young baboons, it is suspected that the older participants adopt the first behavior, whereas the youngest baboons adopt the second one.
At this point, two different but nonmutually exclusive hypotheses can be proposed to account for the selection of either behavior in Experiment 1. The first one implies that the younger monkeys have reduced inhibitory control compared with their older counterparts, leading to an anticipatory release of the start button prior to the identification of the target. For those subjects, the early release of the start button would impose that the target is searched for during the execution of the pointing, therefore leading to steep movement time slopes. Several reports on either monkeys (e.g., Fairbanks et al., 1999) or humans (e.g., Rubia, Hyde, Halari, Giampietro, & Smith, 2010) suggest that the inhibitory control system gains in efficiency from infancy to mid-adulthood. This effect is particularly well demonstrated in a stop task, in which the participants have to inhibit a go response toward a target on a screen after a stop signal has been perceived (Williams, Ponesse, Schachar, Logan, & Tannock, 1999). The alternative hypothesis is that the older baboons have a relative deficiency in visuomotor actions (e.g., Buch, Young, & Contreras-Vidal, 2003; Pratt, Chasteen, & Abrams, 1994), which would promote a behavioral strategy favoring identification of the target prior to the initiation of the point and thus steep RT slopes. Experiment 2 was aimed at testing the validity of these two hypotheses.
Experiment 2: Test of Inhibitory Control
Experiment 2 mixed baseline and test (“shift”) trials. The baseline trials required that the baboons select a red square on the screen while avoiding a white square, their left–right location being counterbalanced between trials. This task has been regularly used in the laboratory in the past as it serves as the standard fill-in task proposed to baboons in between two tests (see Fagot & Paleressompoulle, 2009). The shift trials were identical to the baseline trials, except that the white and red (target) squares unexpectedly switched their location during the execution of the pointing. To be rewarded in these shift trials, the baboon had to stop its ongoing pointing to reengage in a new point toward the new target location. We considered the shift task interesting in our context because it taxes the process of inhibitory control, even more so because the monkey has to stop and adapt its behavior in an extremely well-known context while also requesting abilities for an online visuomotor control of the gesture to reorient the new point. Considering that Experiment 1 suggested a greater inhibitory control of the older participants (first hypothesis) and/or deficit for controlling visuo-guided action (second hypothesis), it would be particularly informative to demonstrate that the old baboons outperform the young ones in the shift trials. First, that performance would confirm the greater ability of the old participants to inhibit their action, in comparison to the less inhibited young baboons. Second, a high performance in this task would moreover invalidate the hypothesis of a reduced ability for visuo-motor controls in the oldest participants.
Method
Subjects and apparatus
They were the same as in Experiment 1.
Test procedure
This task also employed the ALDM systems. The baboons were initially required to point and maintain contact during 200 ms on a start button identical to that in Experiment 1. At the end of 200 ms, a red and a white 400 × 400 pixel square stimuli appeared on the screen, with their left–right location balanced across trials. The red stimulus served as the positive stimulus (S+), and touching it triggered the delivery of a food reward. The white stimulus served as the negative stimulus (S−), and touching it triggered a 3-s green screen indicating a time-out period. We administered baseline (no-shift) and probe (shift) trials. In the no-shift trials (two thirds of the trials), the location of both S− and S+ remained constant once these two stimuli appeared on the screen. In the shift trials (one third of the trials), these two stimuli interchanged their locations on the screen immediately after the baboon released the start button. Thus, for instance, when red and white squares were initially located on the left and right hemiscreen, respectively, at the onset of the trial, the red square suddenly appeared on the right hemiscreen and the white square on the left hemiscreen after release of the start button. These probe trials required that the subject recognize a change in S+/S− location after the start button was released. All participants received three series of 150 trials each in the task, each series containing 50 probe trials intermixed with 100 baseline trials. Scores, RTs, and movement times were all recorded on each trial. There was no pretraining in Experiment 2 as the no-shift trials are part of the regular experimental regimen of the baboons and were thus already well known. Experiment 2 was run immediately after Experiment 1.
Results
Analysis of the shift and no-shift trials
Table 2 reports a summary of the data set. The first analyses focused on no-shift trials. Because of very high scores (99.64% correct on average) leading to ceiling effects, we omitted the analysis of scores for those trials. Sex was neglected for these analysis and the next ones, as preliminary analyses showed that it had no reliable effect on either performance or speed. Following the same rationale as in Experiment 1, the RTs and the movement times of the correct no-shift trials were analyzed with a linear regression using age as the unique predictor. The effect of age on RT was not reliable, F(1, 16) = .67, p > .1, and there was similarly no reliable effect of age on movement time, F(1, 16) = 3.95, p > .05. This lack of significant effect of age confirmed that the older baboons had no particular visuomotor deficits.
Table 2.
Data Set of Experiment 2
| Baboon | Shift trial
|
No-shift trial
|
||||
|---|---|---|---|---|---|---|
| RT (ms) | MT (ms) | % correct | RT (ms) | MT (ms) | % correct | |
| Ang | 304 | 332 | 21.1 | 314 | 258 | 100 |
| Ari | 396 | 296 | 36.7 | 424 | 287 | 100 |
| Art | 283 | 297 | 33.6 | 321 | 251 | 100 |
| Atm | 371 | 278 | 30.4 | 385 | 215 | 99.7 |
| Bar | 250 | 291 | 31.2 | 280 | 239 | 100 |
| Bob | 312 | 289 | 29.1 | 279 | 254 | 100 |
| Bri | 216 | 386 | 55.4 | 247 | 355 | 100 |
| Cau | 362 | 404 | 35.8 | 377 | 281 | 100 |
| Clo | 334 | 261 | 25.7 | 363 | 235 | 99.7 |
| Kal | 467 | 178 | 12.9 | 473 | 216 | 99.3 |
| Mic | 208 | 650 | 66.7 | 174 | 453 | 96.9 |
| Mon | 368 | 439 | 54.7 | 203 | 314 | 98.2 |
| Rom | 384 | 331 | 36.5 | 477 | 293 | 99.7 |
| Tar | 288 | 390 | 44.5 | 287 | 403 | 98.6 |
| Ura | 341 | 262 | 27.8 | 357 | 235 | 99.7 |
| Van | 388 | 208 | 4.7 | 400 | 187 | 99.7 |
| Vio | 342 | 157 | 10.8 | 353 | 154 | 100 |
| Viv | 331 | 256 | 26.1 | 393 | 199 | 100 |
| Mean | 330 | 316 | 32.0 | 339 | 268 | 100 |
| SD | 65 | 112 | 16.0 | 84 | 75 | 1 |
Note. RT = reaction time; MT = movement time.
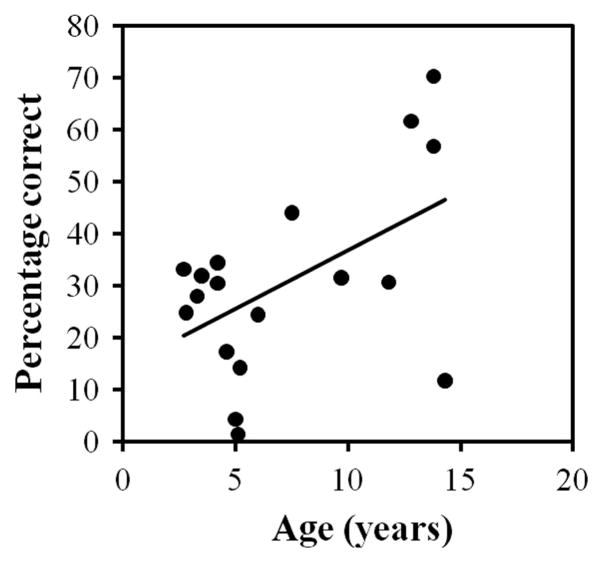
Inspection of the data in the shift trials revealed important interindividual variability in the baboons’ performance. At the group level, only 32.42% (SD = 15.83) of the pointing responses were correct during these trials. The percentage of correct responses on the target in spite of a location change varied between individuals from 5% to 67% correct. Computation of a linear regression using age as the unique predictor showed that the performance in the shift trials increased with the age of the participants, with 16.95% of the variance explained, F(1, 16) = 4.47, p = .05; see Figure 4.
Figure 4.
Proportion of correct responses in the probe (shift) trials as a function of age of participants.
Regarding the RT of the correct shift trials, there were absolutely no significant effects emerging from the linear regression with age as the factor, F(1, 16) = .001, p > .1. This latter result is not surprising given the above finding on the no-shift trials and the lack of perceptual cue distinguishing the shift and no-shift trials during the RT period. The same result was observed for the movement time of the shift trials, F(1, 16) = .07, p > .1, showing that the older baboons were no slower in the successful shift trials than the younger ones, in spite of their higher performance in these trials.
Association between scores in the shift and Hick tasks
Pearson product–moment correlations were computed between the RT and movement time slopes obtained in the Hick task (Experiment 1) and the proportion of correct responses obtained in the shift trials (Experiment 2). A positive but nonsignificant association was obtained between the Hick RT slope and the proportion of correct shift trials (r = .36). Those individuals with steeper RT slopes in the Hick task (i.e., the oldest participants) showed a tendency to have higher proportion of correct shift trials. There was, by contrast, a significant negative correlation between the movement time slope and the proportion of correct shifts, showing that baboons with steeper movement time slopes in the Hick task (i.e., the youngest subjects) were the least successful in the shift task (r = −.59, p < .05).
General Discussion
Our study marks the first examination of interindividual differences in a visual attention task in monkeys. Summarized briefly, the overall results of this study are threefold. First, baboons performing the Hick task produced results that followed the prediction of Hick’s (1952) law. That is, there was a positive association between RT and the bits of information processing demand. This finding converges with previous reports also showing that RTs increased in visual search tasks with display size in baboons (Barbet & Fagot, 2011; Deruelle & Fagot, 1998) and chimpanzees (Fagot & Tomonaga, 1999), and that the RTs also follow Hick’s law in similar test conditions in pigeons (Vickrey & Neuringer, 2000). Noticeably, the RT slope of the baboons (54 ms/bit) is close to that initially reported in humans (e.g., from 34 to 44 ms/bit in Vickrey & Neuringer, 2000), suggesting similar modes of processing in the two species. Second, the underlying factors that best explained interindividual variation in the RT search slope was the age of the participant. In our research, the older baboons showed steeper RT search slopes than the younger participants. Third, a negative correlation emerged in our study between the movement time slopes obtained in the Hick task (Experiment 1) and performance in a task of inhibitory control, in which the participants inhibit an ongoing pointing to adjust to a stimulus change in location (Experiment 2).
In line with Treisman and Gelade (1980), the visual search task literature traditionally interprets steep search slopes as demonstrating the involvement of attentional (serial) search operations, whereas flat search slopes would reflect preattentive (parallel) stages of processing. A strict application of this theoretical framework to our results would suggest that the target was detected more preattentively in the younger baboons than in their older counterparts. That explanation would appear hazardous in our research because of the reported trade-off between the RT/movement time slopes. A more parsimonious explanation for our result is that two different strategies were used to solve the task in our research, and that the selection of either strategy was age dependent.
The older baboons had the highest scores in the shift trials of Experiment 2. That finding rules out the hypothesis of deficiency for visuomotor controls for these baboons that might, for instance, be due to early aging. We therefore favor the hypothesis that the selection of one response strategy in Experiment 1 was largely determined by the inhibitory control of the subjects. The shift task implies the inhibition of an ongoing action to adapt to a new perceptual constraint. When processing the Hick task, the animal must refrain from touching the distractors. The Hick task therefore shares with the shift task an important inhibitory component, and we propose that the development of the central executive system, in particular, inhibition, is the main factor affecting the behavior in our two tasks. Observation of a more developed inhibitory control in adults, in comparison to the young baboons, is consistent with cross-sectional data on developing children who generally show poorer performance on inhibitory control tasks (Barkley, 1997; Kagan, Reznick, & Snidman, 1987; Mostofsky, Cooper, Kates, Denckla, & Kaufmann, 2002). Developmental changes in executive functions in children are often attributed to maturational changes in the organization and connectivity of the central nervous system, notably prefrontal cortex (Tsujimoto, 2008). Unfortunately, although there have been several recent studies using in vivo imaging in baboons (Kaufman, Phillips-Conroy, Black, & Perlmutter, 2003; Kochunov et al., 2010; McBride, Arnold, & Gur, 1999; Rogers et al., 2007), none has explicitly focused on the development of the prefrontal cortex, leaving the neural process of this developmental trend uncertain in monkeys.
In a different perspective, the current study provides some clues on why performance in the Hick task correlates positively with psychometric intelligence in humans (e.g., Jensen & Munro, 1979; Neubauer, 1990). Our study suggests that the common factor between the Hick task and the test of psychometric intelligence accounting for this correlation is executive control. In the near future, we plan to further investigate whether such a relation can also be found in monkeys between performance in the Hick task and that obtained in other cognitive tasks with strong executive components.
Acknowledgments
We acknowledge the technical contribution of Jean Christophe Marin, as well as the animal keepers from the Rousset-sur-Arc Primate Research Station. Joël Fagot was supported by a grant from the PACA Region (Project “Ethique”; Volet Exploratoire 2008) and the ANR-2010-BLANC-1908 – 01. Elodie Bonté was supported by a PhD grant from the PACA Region. William D. Hopkins was supported by National Institutes of Health Grants NS-42867, HD-56232, and HD-60563.
Contributor Information
Joël Fagot, CNRS and Aix-Marseille University.
Elodie Bonté, Aix Marseille University.
William D. Hopkins, Agnes Scott College and Yerkes National Primate Research Center
References
- Azzato MC, Butter CM. Visual search in cynomolgus monkeys: Stimulus parameters affecting two stages of visual search. Perception & Psychophysics. 1984;36:169–176. doi: 10.3758/BF03202677. [DOI] [PubMed] [Google Scholar]
- Barbet I, Fagot J. Processing of contour closure by baboons. Journal of Experimental Psychology: Animal Behavior Processes. 2011;37:407– 419. doi: 10.1037/a0025365. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Blough DS. Effects of the number and form of stimuli on visual search in the pigeon. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:211–223. doi: 10.1037/0097-7403.5.3.211. [DOI] [PubMed] [Google Scholar]
- Buch ER, Young S, Contreras-Vidal JL. Visuomotor adaptation in normal aging. Learning & Memory. 2003;10:55–63. doi: 10.1101/lm.50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buračas GT, Albright TD. Covert visual search: A comparison of performance by humans and macaques (Macaca mulatta) Behavioral Neuroscience. 1999;113:451– 464. doi: 10.1037/0735-7044.113.3.451. [DOI] [PubMed] [Google Scholar]
- Chan E, Mattingley JB, Huang-Pollock C, Vance A, Bellgrove MA. Abnormal spatial asymmetry of selective attention in ADHD. Journal of Child Psychology and Psychiatry. 2009;50:1064–1072. doi: 10.1111/j.1469-7610.2009.02096.x. [DOI] [PubMed] [Google Scholar]
- Cook RG, Cavoto BR, Katz JS, Cavoto KK. Pigeon perception and discrimination of rapidly changing texture stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:390–400. doi: 10.1037/0097-7403.23.4.390. [DOI] [PubMed] [Google Scholar]
- Deruelle C, Fagot J. Visual search for global/local stimulus features in humans and baboons. Psychonomic Bulletin & Review. 1998;5:476– 481. doi: 10.3758/BF03208825. [DOI] [Google Scholar]
- Fagot J, Bonté E. Automated testing of cognitive performance in monkeys: Use of a battery of computerized test systems by a troop of semi-free ranging baboons. Behavior Research Methods. 2010;42:507–516. doi: 10.3758/BRM.42.2.507. [DOI] [PubMed] [Google Scholar]
- Fagot J, Paleressompoulle D. Automatic testing of cognitive performance in baboons maintained in social groups. Behavior Research Methods. 2009;41:396– 404. doi: 10.3758/BRM.41.2.396. [DOI] [PubMed] [Google Scholar]
- Fagot J, Tomonaga M. Global–local processing in humans (Homo sapiens) and chimpanzees (Pan troglodytes): Use of a visual search task with compound stimuli. Journal of Comparative Psychology. 1999;113:3–12. doi: 10.1037/0735-7036.113.1.3. [DOI] [Google Scholar]
- Fairbanks LA, Fontenot MB, Phillips-Conroy JE, Jolly CJ, Kaplane JR, Mann JJ. CSF monoamines, age and impulsivity in wild grivet monkeys (Cercopithecus aethiops aethiops) Brain, Behavior and Evolution. 1999;53:305–312. doi: 10.1159/000006601. [DOI] [PubMed] [Google Scholar]
- Fremouw T, Herbranson WT, Shimp CP. Dynamic shifts of pigeon local/global attention. Animal Cognition. 2002;5:233–243. doi: 10.1007/s10071-002-0152-9. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier B. Action video game modifies visual selective attention. Nature. 2003 May 29;423:534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- Hick WE. On the rate gain of information. Quarterly Journal of Experimental Psychology. 1952;4:11–26. doi: 10.1080/17470215208416600. [DOI] [Google Scholar]
- Hommel B, Li KZH, Li S. Visual search across the life span. Developmental Psychology. 2004;40:545–558. doi: 10.1037/0012-1649.40.4.545. [DOI] [PubMed] [Google Scholar]
- Jensen AR. Reaction time and psychometricg. In: Eysenck HJ, editor. A model for intelligence. New York, NY: Springer; 1982. pp. 93–132. [Google Scholar]
- Jensen AR, Munro E. Reaction time, movement time, and intelligence. Intelligence. 1979;3:121–126. doi: 10.1016/0160-2896(79)90010-2. [DOI] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–1473. doi: 10.2307/1130685. [DOI] [PubMed] [Google Scholar]
- Kaufman JA, Phillips-Conroy JE, Black KJ, Perlmutter JS. Asymmetric regional cerebral blood flow in sedated baboons measured by positron emission tomography (PET) American Journal of Physical Anthropology. 2003;121:369–377. doi: 10.1002/ajpa.10181. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Fox PT, Lancaster JL, Saleem KS, Shelledy W, Rogers J. Genetics of primary cerebral gyrification: Heritability of length, depth and area of primary sulci in an extended pedigree of Papio baboons. NeuroImage. 2010;53:1126–1134. doi: 10.1016/j.neuroimage.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen AM. Task dependence of slowing after pyramidal lesions in monkeys. Journal of Comparative and Physiological Psychology. 1977;91:897–906. doi: 10.1037/h0077375. [DOI] [PubMed] [Google Scholar]
- Levinoff EJ, Li KZH, Murtha S, Chertkow H. Selective attention impairments in Alzheimer’s disease: Evidence for dissociable components. Neuropsychology. 2004;18:580–588. doi: 10.1037/0894-4105.18.3.580. [DOI] [PubMed] [Google Scholar]
- McBride T, Arnold SE, Gur RC. A comparative volumetric analysis of the prefrontal cortex in human and baboon MRI. Brain, Behavior and Evolution. 1999;54:159–166. doi: 10.1159/000006620. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla SB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2002;52:785–794. doi: 10.1016/S0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Neubauer AC. Speed of information processing in the Hick paradigm and response latencies in a psychometric intelligence test. Personality and Individual Differences. 1990;11:147–152. doi: 10.1016/0191-8869(90)90007-E. [DOI] [Google Scholar]
- Plude DJ, Doussard-Roosevelt JA. Aging, selective attention, and feature integration. Psychology and Aging. 1989;4:98–105. doi: 10.1037/0882-7974.4.1.98. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Pratt J, Chasteen AL, Abrams RA. Rapid aimed limb movements: Age differences and practice effects in component sub-movements. Psychology and Aging. 1994;9:325–334. doi: 10.1037/0882-7974.9.2.325. [DOI] [PubMed] [Google Scholar]
- Remington A, Swettenham J, Campbell R, Coleman M. Selective attention and perceptual load in autism spectrum disorder. Psychological Science. 2009;20:1388–1393. doi: 10.1111/j.1467-9280.2009.02454.x. [DOI] [PubMed] [Google Scholar]
- Rogers J, Kochunov PV, Lancaster JL, Sheeledy W, Glahn D, Blangero J, Fox P. Heritability of brain volume, surface area and shape: An MRI study in an extended pedigree of baboons. Human Brain Mapping. 2007;28:576–583. doi: 10.1002/hbm.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Hyde Z, Halari R, Giampietro V, Smith A. Effects of age and sex on developmental neural networks of visual–spatial attention allocation. NeuroImage. 2010;51:817– 827. doi: 10.1016/j.neuroimage.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature integration theory of attention. Cognitive Psychology. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S. The prefrontal cortex: Functional neural development during early childhood. The Neuroscientist. 2008;14:345–358. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- Vickrey C, Neuringer A. Pigeon reaction time, Hick’s law, and intelligence. Psychonomic Bulletin & Review. 2000;7:284–291. doi: 10.3758/BF03212983. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35:205–213. doi: 10.1037/0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]