Abstract
The effects of reduced intensity conditioning (RIC) on human leucocyte antigen (HLA)-alloimmunization and platelet transfusion refractoriness (PTR) following allogeneic haematopoietic stem cell transplantation (Allo-HSCT) are unknown. We studied HLA-alloantibodies in a cohort of 16 patients (8 HLA-alloimmunized with pre-transplant histories of PTR and 8 non-alloimmunized controls) undergoing Allo-HSCT using fludarabine/cyclophosphamide-based RIC. Pre- and post-transplant serum samples were analysed for HLA-antibodies and compared to myeloid, T-cell and bone marrow plasma cell chimaerism. Among alloimmunized patients, the duration that HLA-antibodies persisted post-transplant correlated strongly with pre-transplant HLA-antibody mean fluorescence intensity (MFI) and PRA levels (Spearman’s rank correlation = 0.954 (p=0.0048) and 0.865 (p=0.0083) respectively). Pre-transplant MFI >10,000 was associated with post-transplant HLA antibody persistence >100 days (p=0.029). HLA-antibodies persisted ≥100 days in 3/8 patients despite recipient chimaerism being undetectable in all lympho-haematopoietic lineages including plasma cells. Post-transplant de-novo HLA-antibodies developed in 3 control patients with 2 developing PTR; the donors for 2 of these patients demonstrated pre-existing HLA-antibodies of equivalent specificity to those in the patient, confirming donor origin. These data show HLA-antibodies may persist for prolonged periods following RIC. Further study is needed to determine the incidence of post-transplant PTR as a consequence of donor–derived HLA alloimmunization before recommendations on donor HLA-antibody screening can be made.
Keywords: HLA antibodies, platelets, allogeneic bone marrow transplantation
INTRODUCTION
Platelet transfusion refractoriness (PTR), defined as a post-transfusion platelet increment that is less than expected, can result from immune and/or non-immune causes. Immune causes include alloimmunization to human leucocyte antigens (HLA) and/or human platelet antigens (HPA) due to prior exposure to allo-antigens from pregnancy, transfusions and/or transplantation. HLA alloimmunization is more common than alloimmunization to HPA and is the primary cause of immune-mediated PTR (Kickler et al, 1990). The incidence of PTR from HLA alloimmunization has decreased as a result of the increased use of leucoreduced blood products. Nevertheless, chronic platelet transfusion support using leucoreduced blood products still results in approximately 20% of patients becoming HLA alloimmunized (The Trial to Reduce Alloimmunization to Platelets Study Group, 1997). A number of approaches have been explored to overcome HLA alloimmunization, including plasmapheresis, intravenous immunoglobulin, rituximab and bortezomib (Claas & Doxiadis, 2009; Gloor & Stegall, 2010; Warren & Montgomery, 2010; Zachary & Eng al, 2011). These therapies, utilized mostly in HLA alloimmunized solid organ transplant recipients, are successful for a minority of patients, typically resulting in only transient decreases in HLA-antibody titres (Warren & Montgomery, 2010; Kupin et al, 1991; Perry et al, 2008).
Patients who have undergone an allogeneic haematopoietic stem cell transplantation (HSCT) often demonstrate poor increments to platelet transfusions due to a combination of immune and non-immune causes, including pre-existing HLA alloimmunization, medications, viral infections and sepsis (Balduini et al, 2001; Klumpp et al, 1996). Although HLA antigen-sensitized patients are likely to benefit from platelets that are closely HLA-matched, the routine provision of 4-antigen HLA-matched platelets is not always feasible, which can lead to PTR, resulting in an increased risk of haemorrhage-associated morbidity and mortality.
The presence of donor-specific HLA-antibodies (DSAs) in the recipient has been shown to be associated with higher incidences of graft rejection in both solid organ transplantation and HLA-mismatched HSCT, most notably unrelated cord blood and haploidentical stem cell transplants (Ciurea et al, 2009; Ciurea et al, 2011; Cutler et al, 2011; Eng et al, 2011; Gutman et al, 2009; Reinsmoen et al, 2004; Spellman et al, 2010; Takanashi et al, 2010; Yoshihara et al, 2012). Furthermore, the presence of DSAs in unrelated cord blood transplant (UCBT) recipients has been implicated in delayed engraftment following myeloablative conditioning regimens (Takanashi et al, 2008). Recently, donor-derived HLA-antibody production has been described in patients undergoing HSCT from HLA antibody-positive donors (Lapierre et al, 2002; Taniguchi et al, 2012), with a few reports implicating donor-derived HLA-antibodies as a cause for PTR occurring in the post-transplant setting (Hatakeyama et al, 2011; Nakazawa et al, 2007). Based on these observations, some have advocated screening both patients and donors for HLA antibodies before HSCT to identify patients who would be at an increased risk for graft rejection as well as HLA alloimmune-mediated PTR after transplantation (Balduini et al, 2001; Hatakeyama et al, 2011).
Previously, we and others have shown that recipient plasma cells can survive reduced intensity conditioning (RIC) regimens and are relatively resistant to donor immune-mediated graft-versus-host lympho-haematopoietic effects. The persistence of recipient plasma cells in the post-transplant setting that produce anti-A and/or anti-B isoantibodies is thought to play a dominant role in the pathophysiology of delayed donor erythropoiesis and pure red cell aplasia (PRCA), which complicate major-ABO incompatible HSCT (Griffith et al, 2005). Recipient plasma cells involved in HLA-antibody production that survive transplant conditioning and donor T-cell mediated graft-vs-host lympho-haematopoietic effects could similarly lead to the protracted production of HLA-antibodies after reduced intensity transplants which could cause PTR. Likewise, PTR could also occur in the post-transplant setting in patients who were HLA-antibody-negative prior to transplantation as a consequence of B lymphocytes being transferred in grafts from HLA-antibody-positive donors that differentiate into HLA-antibody producing plasma cells (Taniguchi et al, 2012). At present, little is known regarding the effects of using RIC on the persistence HLA alloantibodies and the risk for developing HLA alloimmune-mediated PTR after transplantation. Therefore, to better understand this effect, we measured HLA-antibodies pre- and multiple times post-transplant and investigated the temporal relationship between HLA-antibody persistence and donor-host lympho-haematopoietic chimaerism in a cohort of patients undergoing fludarabine/cyclophosphamide-based RIC.
MATERIALS & METHODS
Study Population
We retrospectively studied 16 patients who received an allogeneic peripheral blood stem cell (PBSC) transplant from an HLA-matched sibling on National Heart, Lung and Blood Institute (NHLBI) Institutional Review Board approved protocol 99-H-0050, which investigated a fludarabine/cyclophosphamide-based RIC regimen for patients with malignant and non-malignant haematological disorders (ClinicalTrials.gov: NCT00003838).
Patients who had a clinical history of pre-transplant PTR, defined as a 1-h post-transfusion corrected count increment of < 5 × 109 platelets /l on two separate occasions, and who had HLA class I antibodies detected prior to HSCT (N = 8), and all additional patients treated on the protocol 99-H-0050 who had no history of PTR prior to HSCT (controls, N = 8) were included for analysis if they had stored serum available for analysis that was collected pre-transplant and multiple time-points post-transplant. All received an allogeneic granulocyte colony-stimulating factor (G-CSF) mobilized, lymphocyte-replete PBSC allograft from an HLA-identical sibling following cyclophosphamide (120 mg/kg) and fludarabine (125 mg/m2) (+/− equine anti-thymocyte globulin [ATG] 40 mg/kg × 4 days), with ciclosporin (CSA; beginning on day −4) and methotrexate (5 mg/m2 IV days +1, +3, and +6) used as prophylaxis for graft-versus-host disease. All 8 patients who had pre-transplant PTR (and Patients 11, 16) received ATG as part of the conditioning.
Chimaerism Analysis
Peripheral blood lineage-specific chimaerism analysis was performed on days +14, +30, +45, +60 and ≥100 post-transplant. Ficoll–Hypaque fractionated mononuclear cells were sorted into CD14+/CD15+ myeloid and CD3+ T-cell lineages using immunomagnetic beads (Dynal A.S., Oslo, Norway) as previously described (Childs et al, 1999). Bone marrow aspirate samples collected post-transplant were available for plasma cell chimaerism analysis on 6 of 16 patients (Patients 4, 5, 7, 8, 9, 16). Plasma cells chimaerism analysis was performed on DNA obtained from CD38+ bright, CD138+ bright, CD19− bone marrow cells isolated by flow cytometry from day +100 and day +180 as previously described (Antin et al, 2001). Polymerase chain reaction (PCR)-based analysis of short tandem repeats (STRs) polymorphic between patient and donor was used to quantify donor/recipient chimaerism in lympho-haematopoietic lineages including plasma cells (Griffith et al, 2005; Antin et al, 2001) as previously described (sensitivity for minor populations: 1–2%).
HLA-Antibody Analysis
Stored patient serum collected pre-transplant (≤ 30 days from initiation of transplant conditioning) and at multiple post-transplant intervals (days +30, +60, +100, +180 and ≥ 365) was analysed for HLA class I antibodies using a membrane-independent solid phase assay and a flow analyser (LABScreen and LABScan 100 flow analyser, One Lambda Inc., Canoga Park, CA). Samples with mean fluorescence intensity (MFI) > 500 were further tested for antibody specificity using LABScreen Single Antigen kits (One Lambda Inc.). A background-adjusted MFI > 2000 was determined to be positive based on previous reports (Taniguchi et al, 2012). Panel reactive antibody (PRA) and MFI were analysed to measure HLA-antibody strength and specificity, respectively.
Statistical Analysis
Spearman’s rank correlation coefficient was used to test for correlation between the intensity of pre-transplant HLA-antibodies (measured by MFI and PRA) and the time duration of HLA antibody persistence following allogeneic transplant in the 8 patients with pre-transplant PTR. Fisher’s exact test was used to examine whether MFI >10,000 was associated with persistence of HLA-antibodies ≥100 days post-transplant. To evaluate the differences between the patient groups who received or did not receive ATG as part of the conditioning, the log-rank test and the unpaired t-test assuming unequal variances were carried out to compare lymphoid and myeloid engraftment and lymphoid recovery, respectively. Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC) and a two-tailed P-value of less than 0.05 was considered significant.
RESULTS
Patient demographics and post-transplant outcomes are shown in Tables I and II. The mean time until chimaerism converted from mixed to full donor in myeloid and T lymphocyte lineages was 48 days (range: 15 to 161 days) and 44 days (range: 15 to 141 days), respectively. All of the 8 patients who had PTR prior to transplantation were placed on HLA-matched platelet transfusion restrictions post-transplant using a previously validated HLA Matchmaker program (Nambiar et al, 2006).
Table I.
Patient demographic information
| Patient | Diagnosis | Age (years) |
Sex (donor/recipient) |
Blood type (donor/recipient) |
Pre-transplant platelet refractoriness |
Pre-transplant HLA Ab screen (PRA) |
CD34+ dose (×106/kg)‡ |
CD3+ dose (×106/kg)‡ |
|---|---|---|---|---|---|---|---|---|
| 1 | SAA | 20 | M/M | O+/A+ | Yes | 22% | 9.12 | 209 |
| 2 | MDS | 34 | M/M | O+/O+ | Yes | 10%* | 6.18 | 155 |
| 3 | MDS | 22 | F/M | B−/B− | Yes | 55% | 14.84 | 154 |
| 4 | SAA | 37 | F/F | AB−/AB− | Yes | 15% | 7.74 | 333 |
| 5 | SAA | 13 | F/M | O+/O+ | Yes | 47% | 5.16 | 312 |
| 6 | SAA | 8 | F/M | A+/O+ | Yes | 9% | 4.98 | 357 |
| 7 | SAA | 28 | M/F | B+/AB+ | Yes | 85% | 6.18 | 144 |
| 8 | MDS | 39 | F/M | AB+/A+ | Yes | 95% | 4.08 | 152 |
| 9 | MDS | 52 | F/F | A+/A+ | No | 3%* | 3.79 | 639 |
| 10 | NHL | 51 | M/M | O+/B+ | No | 0% | 7.88 | 159 |
| 11 | SAA | 16 | M/F | B+/O+ | No | 29%§ | 6.81 | 321 |
| 12 | NHL | 47 | F/M | O+/A+ | No | 2% | 7.28 | 207 |
| 13 | MM | 64 | M/M | B−/A+ | No | 0% | 6.70 | 114 |
| 14 | NHL | 55 | F/F | O+/O+ | No | 0% | 7.20 | 129 |
| 15 | HL | 40 | F/M | B+/B+ | No | 2% | 4.70 | 297 |
| 16 | MDS | 51 | F/M | O+/A+ | No† | 0% | 5.68 | 166 |
HLA-antibody testing done by solid phase enzyme immunoassay (One Lambda Inc., Lambda Antigen Tray).
Patient became PTR, with positive HLA-alloantibody screen within 1 month of post-peripheral blood stem cell transplant.
Cell dose based on weight of recipient
HLA-antibody testing done by solid phase microbead assay revealed a mean fluorescence intensity of only 300.
HLA, human leucocyte antigen; Ab, antibody; PTR, platelet transfusion refractoriness; PRA, Panel reactive antibody; SAA, Severe Aplastic Anaemia; MDS, Myelodysplastic Syndrome; NHL, Non-Hodgkin Lymphoma; HL, Hodgkin Lymphoma; MM, Multiple Myeloma; M, male; F, female.
Table II.
Post-transplant data
| Patient | PTR pre- transplant |
PTR post- transplant |
T cell engraftment* (days) |
Myeloid engraftment* (days) |
Persistence of HLA Ab† (days) |
|---|---|---|---|---|---|
| 1 | Yes | Yes | +28 | +15 | +60 |
| 2 | Yes | Yes | +29 | +29 | 0 |
| 3 | Yes | Yes | +30 | +14 | +60 |
| 4 | Yes | Yes | +15 | +15 | +60 |
| 5 | Yes | Yes | +14 | +14 | +100 |
| 6 | Yes | Yes | +15 | +15 | 0 |
| 7 | Yes | Yes | +30 | +30 | > +365 |
| 8 | Yes | Yes | +45 | +96 | +180 |
| 9 | No | Yes | +17 | +17 | +100 |
| 10 | No | No | +114 | +114 | 0 |
| 11 | No | No | +28 | +28 | 0 |
| 12 | No | No | +15 | +29 | 0 |
| 13 | No | No | +15 | +15 | 0 |
| 14 | No | No | +31 | +130 | 0 |
| 15 | No | No | +30 | +100 | > +365 |
| 16 | No | Yes | +16 | +16 | +100 |
| Median (days) | 8/16 (50%) | 10/16 (63%) | +28 | +23 | +60 |
Engraftment represents the time at which complete donor engraftment (≥ 95% donor) was documented
Persistence of HLA-antibodies is defined as day the last HLA-antibody screen showed a mean fluorescence intensity >2000 (two consecutive tests).
HLA, human leucocyte antigen; Ab, antibody; PTR, platelet transfusion refractoriness
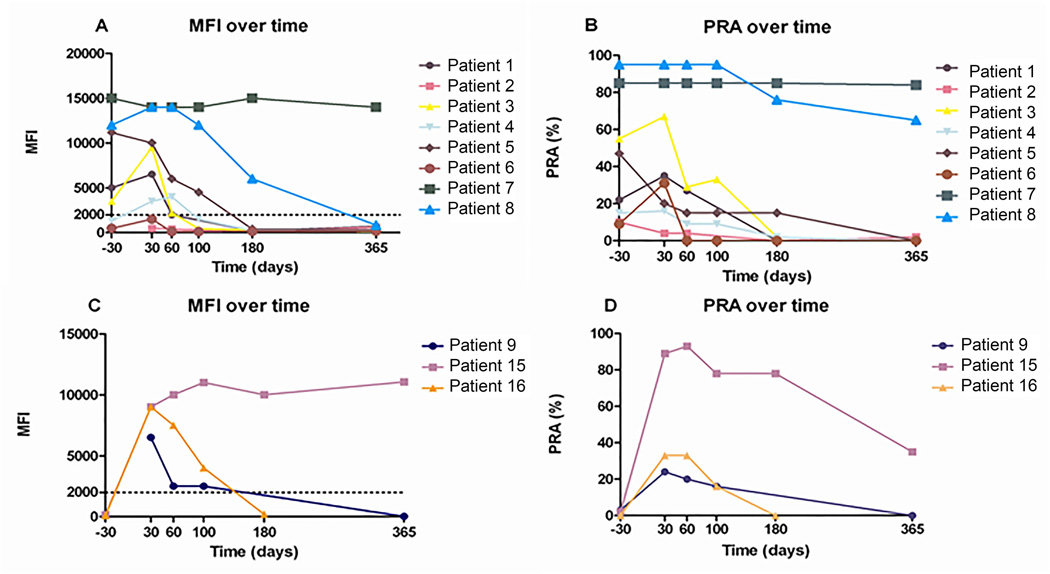
Peripheral blood lineage-specific myeloid and T cell chimaerism were available on all patients at 2-week intervals up to day +60 and until full donor chimaerism was achieved. Bone marrow aspirates collected after transplantation to evaluate plasma cell chimaerism were available on 6 subjects (Patients 4, 5, 7, 8, 9, 16) and revealed no evidence for any residual recipient plasma cells populations (i.e. 100% donor plasma cells) at day +100 and day +180 (data not shown). HLA class I allo-reactivity, assessed in serum samples collected after transplantation, using PRA and MFI analysis is shown in Figure 1. Among the 8 patients who had pre-transplant HLA alloimmunization, HLA-antibodies remained detectable for more than 100 days post-transplant in 3 patients (38%).
Figure 1. MFI and percentage of PRA positivity.
MFI and percentage of PRA positivity in 8 patients with HLA-antibodies prior to HSCT (A, B) and in 3 patients with de novo HLA antibodies post-HSCT (C, D). Pre-transplant MFI values not illustrated in Patients 2 and 9 (A, C) because HLA-antibody testing was done by solid phase enzyme immunoassay. Dotted line in panels A and C denotes MFI detection cut-off threshold for positive antibody reactivity.
MFI, mean fluorescence intensity; PRA, Panel Reactive antibody; HLA, human leucocyte antigen; HSCT, haematopoietic stem cell transplantation.
There was a strong positive correlation between both the pre-transplant HLA-antibody MFI and the pre-transplant PFA, and the duration of post-transplant HLA-antibody persistence (Spearman’s rank correlation of 0.954 (exact two sided p-value = 0.0048) and 0.865 (exact two sided p-value = 0.0083), respectively. All 3 patients with persistence of antibody reactivity ≥ 100 days post-transplant exhibited a pre-transplant MFI >10,000 whereas HLA-antibodies had disappeared completely by this time point in alloimmunized patients who had a lower pre-transplant MFI. The Fisher’s exact test for the association between pre-transplant MFI >10,000 and persistence of HLA antibodies for >100 days was significant (p-value = 0.029). One patient (Patient 7) continued to express high levels of HLA-antibody reactivity (PRA 84%; MFI 14,000) for over 1 year post-transplant despite myeloid and T cell lineages in the blood and the plasma cell lineage in the bone marrow being 100% donor in origin. Interestingly, this patient had previously been infected with hepatitis B virus prior to HSCT and continued to express a native anti-HBc antibody that persisted for more than 2 years after HSCT. Because the donor was found to have no immunity to the hepatitis B virus (i.e. donor serum was negative for any antibodies against hepatitis B virus), persistence of anti-HBc antibody production in this patient in the post-transplant setting was also probably mediated by recipient plasma cells. Although Patient 8 had a persistently elevated PRA (65%) at 1 year post-transplant, his MFI had decreased, from 14,000 to 850, suggesting the production of HLA-antibodies was in the process of resolving.
Three additional patients (Patients 9, 15, 16) developed de novo HLA-antibodies post-HSCT, including two (Patients 9 and 16) who developed clinically significant PTR in the post-transplant setting. Patient 9 demonstrated a negative HLA-antibody screen pre-transplant (Table II), but was found to be refractory to platelet transfusions within the first month after HSCT with a PRA of 24% and an MFI of 6500 on a day +30 HLA-antibody screen. Her course was complicated by fungal sinusitis requiring granulocyte transfusions and frequent (≥ 1 daily) platelet transfusions (Table III). Although her HLA-antibody screen reverted to negative at 1 year post-transplant, she returned 3 years later while being treated for chronic hepatitis C infection, with severe interferon-induced thrombocytopenia and a life-threatening intracranial bleed. At that time she demonstrated severe HLA-alloimmune PTR (PRA 98%; MFI 11,000) necessitating treatment with HLA-matched platelet transfusions and the thrombopoietin mimetic, eltrombopag. Patients 15 and 16, who were also negative for HLA-antibodies pre-transplant, developed HLA-antibodies by day +30 post-transplant (Figure 1).
Table III.
Post-transplant transfusion support
| Patient | Transfusion Restriction |
Day 0–30 | Day 30–100 | Day 100–180 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLT† | RBC† | Gran† | PLT | RBC | Gran | PLT | RBC | Gran | ||
| 1 | HLAM PLTs | 11 | 4 | 0 | 2 | 3 | 0 | 0 | 0 | 0 |
| 2 | HLAM PLTs | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | HLAM PLTs | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | HLAM PLTs | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | HLAM PLTs | 7 | 4 | 0 | 23 | 13 | 0 | 50 | 14 | 0 |
| 6 | HLAM PLTs | 2 | 2 | 0 | 34 | 19 | 0 | 4 | 4 | 0 |
| 7 | HLAM PLTs | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | HLAM PLTs | 11 | 10 | 0 | 0 | 10 | 0 | 0 | 0 | 0 |
| 9 | none | 45 | 13 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | none | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | none | 6 | 4 | 0 | 0 | 9 | 0 | 0 | 3 | 0 |
| 12 | none | 4 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | none | 2 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| 14 | none | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | none | 6 | 0 | 0 | 0 | 2 | 0 | 3 | 5 | 0 |
| 16 | HLAM PLTs* | 18 | 7 | 0 | 4 | 2 | 0 | 0 | 0 | 0 |
| Mean | 8/16 (50%)* | 8.1 | 3.9 | 0.5 | 3.9 | 3.8 | 0 | 3.6 | 1.6 | 0 |
Patient 16 was placed HLAM platelets on day +30 due to PTR and a positive HLA-antibody screen.
Number of units (All platelet products were single donor platelets).
PLT, platelets; RBC, red blood cells; Gran, granulocytes; HLA, human leucocyte antigen; HLAM, HLA-matched; PTR, platelet transfusion refractoriness.
Following the observance of de novo HLA-antibody production in three patients (Patients 9, 15, 16), we retrospectively analysed archived serum collected prior to transplantation from all 8 donors for control patients who did not have HLA-antibodies detected pre-transplant. Two of 8 of these donors were found to have HLA antibodies. Remarkably, the recipients for both of these donors developed de novo HLA antibody production post-transplant with HLA antibody specificity being similar to their donor’s, confirming HLA-antibodies in these patients were probably donor in origin (Table IV). Patient 16 exhibited PTR in the post-transplant setting, which eventually resolved after being placed on HLA-matched platelet restrictions. HLA-antibodies in this patient were no longer detectable by day 180 post-transplant. In contrast, Patient 15 had limited transfusion requirements and demonstrated satisfactory increments to the few platelet transfusions he received within the first 30 days of HSCT, although his HLA-antibody MFI and PRA were noted to have increased by day +60 and were still detectable at high levels at 1 year post-transplant (Table III). In contrast to Patients 15 and 16, Patient 9 demonstrated de novo HLA-antibodies on day +30 post-transplant despite her donor having no HLA antibodies. These antibodies had disappeared by 1 year post-transplantation.
Table IV.
Donor and patient HLA-antibody specificities.
| Donor/Patient | MFI | PRA | HLA antibody specificities¥ |
|---|---|---|---|
| Donor 15 | Class 1 = 6500 | 60% | A:1*, 11*, 25†, 26†, 34†, 36*, 43†, 66†, 80*; B:8, 73‡; C: 6, 7, 18 |
| Patient 15 | Class 1 = 9000–11055 | 93% | A:1* 3* 11* 23* 24*, 25†, 26†, 32†, 34†, 36*, 43†, 66†, 80* B:49‡, 51‡, 52‡, 53‡, 59, 63‡, 73‡, 76‡ |
| Donor 16 | Class 1 = 2500 | 31% | B:13, 35‡, 46‡, 49‡, 50‡, 51‡, 52‡, 53‡, 56, 57‡, 62‡, 63‡, 71‡, 72‡, 75‡, 77‡, 78‡ |
| Patient 16 | Class 1 = 4000–9000 | 16%–33% | B:13,35‡,49‡,53‡,71‡,75‡; A 23*,24* |
| Donor 9 | Class 1 = <200 | 0% | None |
| Patient 9 | Class 1 = 2500–6000 | 16–24% | A2+ |
A1 CREG,
A10 CREG,
B5 CREG,
Shared HLA-antibody specificities between donor and patient are in bold.
HLA-antibody screen of serum collected from the donors of Patients 10–14 revealed class 1 MFI < 200 and 0% PRA.
HLA, human leucocyte antigen; MFI, mean fluorescence intensity; PRA, panel reactive antibody.
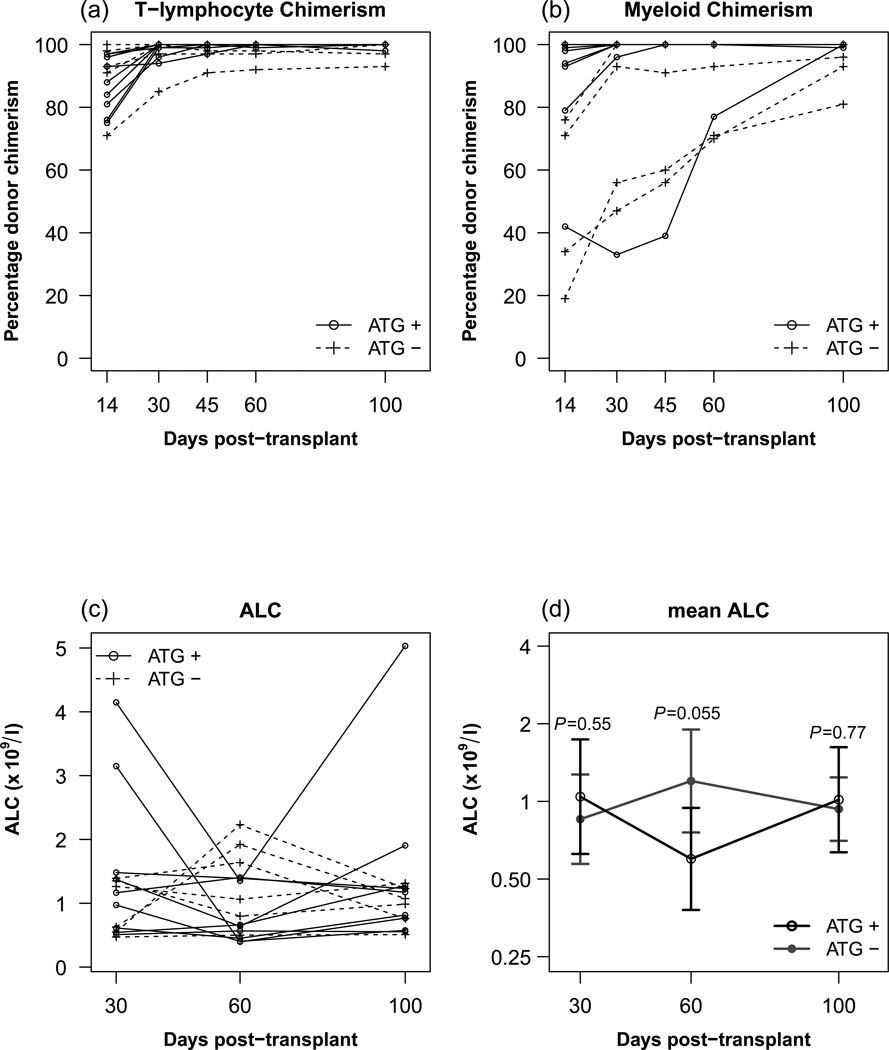
The persistence of HLA-antibodies did not appear to be influenced by the use of ATG in the conditioning regimen, as the engraftment kinetics (T-Lymphocyte chimaerism) and lymphoid recovery (Figure 2) were similar in the10 patients who received ATG (Patients 1–8, 11, 16) compared those who did not.
Figure 2. Donor T-Cell and Myeloid Engraftment kinetics and lymphoid recovery.
A, B: Post-transplant percentage donor T-Lymphocyte chimerism (A) and percentage donor myeloid chimerism (B) in patients who received ATG versus those who did not receive ATG in the conditioning regimen. Comparisons of median donor chimerism at days 30, 60, 100 post-transplant were not statistically significant between groups. C, D: ALC (C) and mean ALC (D) in patients who received ATG versus those who did not receive ATG in the condition regimen.
ATG, anti-thymocyte globulin; ALC, absolute lymphocyte count
DISCUSSION
Little is known regarding the effects of using RIC on HLA alloimmunization and PTR following HSCT. Although sporadic reports have described HLA alloimmunization as a risk factor for post-transplant PTR, none of these studies have followed the temporal course of HLA-antibodies beyond 60 days of the transplant (Balduini et al, 2001; Klumpp et al, 2006). Here, we show that prolonged persistence of HLA-antibodies leading to post-transplant PTR can occur with the use of RIC regimens. Among the patients who were alloimmunized pre-transplant, 3 demonstrated persistence of HLA-antibodies more than 100 days post-transplant. Remarkably, 1 patient had clinically significant PTR and extremely protracted production of HLA-antibodies lasting > 1 year after RIC HSCT. Although several investigators have recently reported that HLA antibodies can persist for up to 60 days after HSCT (Lapierre et al, 2002), this is the first report to show that these antibody populations can persist for extended periods following RIC HSCT, leading to an unexpected prolongation in time until PTR resolves in the post-transplant setting.
The persistence of HLA-antibodies more than 100 days after HSCT was probably impacted by the use of RIC, which would allow recipient plasma cells producing HLA-antibodies to have a better chance of surviving compared to transplant regimens using myeloablative conditioning. Furthermore, the previously established temporal delay in the eradication of recipient antibody-producing plasma cell populations by engrafting donor T-cells relative to other recipient lympho-haematopoietic populations could also account for the protracted persistence of HLA-antibodies observed in this study. This phenomenon of plasma cell resistance to donor T-cells mediating graft-vs-marrow effects was previously observed to occur in recipients of major ABO incompatible transplants and was associated with the protracted production of anti-A and/or anti-B isoantibodies of recipient origin leading to extended periods of pure red cell aplasia after RIC HSCT (Griffith et al, 2005). Although we don’t have data on patients receiving myeloablative conditioning, one may surmize from prior studies on isohaemagluttinins persistence in the context of major ABO incompatibility (Griffith et al 2005) that HLA-antibodies would recede earlier after a fully myeloablative transplant. Additionally, our results apply to RIC approaches that utilize agents that have minimal anti-plasma cell activity against plasma cells and may not apply to transplants that utilize RIC that have agents that are more toxic to plasma cells. It is unlikely that the administration of ATG influenced the HLA-antibody kinetics given that there was no significant difference in either the speed of engraftment or lymphoid recovery in those patients who received ATG versus those that did not (Figure 2).
It is unclear from our data what role continued alloantigen exposure from platelet transfusions played in maintaining plasma cell production of HLA-antibodies. Patients who demonstrated prolonged HLA alloreactivity (Patients 5, 7, 8, 9, 15, 16), compared to those who displayed extinction of HLA-antibodies by day +100, did receive slightly more transfusion support with HLA-unmatched platelets between days 0–30 (Table III). Furthermore, in Patient 16, although the HLA antibody specificities were similar to his donor, he also developed antibodies to the A1 CREG, which were not evident in his donor, and, by day 30, was noted to have a > three-fold higher MFI than was observed in his donor. Likewise, Patient 9 demonstrated de novo HLA-antibodies despite her donor having no HLA antibodies. Taken altogether, these data suggest antigen exposure from platelet transfusions may play a contributory role in the development of new and/or increasing titres of HLA antibodies in the post-transplant setting.
Furthermore, Patient 5 demonstrated a decreasing intensity of HLA-antibodies (MFI 4500 to 400) from day +100 to day +180 despite having increased antigen exposure from multiple transfusions during this period (albeit he was receiving HLA-matched platelet transfusions during that time). Indeed, one could postulate that, had HLA-matched restrictions not been placed on these patients, the time until HLA alloimmunity was eradicated may have been further prolonged, as was observed in Patient 15, who received non-HLA-matched platelets and demonstrated persistently high HLA alloreactivity for more than 1 year after HSCT.
Only recently have a few reports provided evidence that passive transfer of donor HLA-antibodies to recipient can occur post HSCT (Taniguchi et al, 2012; Hatakeyama et al, 2011). De-novo HLA alloimmunization has been observed in up to 4.6% of paediatric patients (Balduini et al, 2001), and was implicated in a fatal case of PTR after HSCT in which HLA-antibodies were transferred from a maternal donor to her child (Nakazawa et al, 2007). Similarly to these reports, we observed de novo HLA-antibody production after HSCT in 3 patients. Given similar specificities of HLA-antibodies in the donor and patient in 2 of these patients, it is highly likely that HLA-antibody producing cells in the donor were transferred to the patient at the time of transplantation and led to de novo HLA-antibody production in the post-transplant setting. The data presented here are unique in that we are the first to show that this de novo antibody production can persist for extended periods, in some patients persisting for more than 1 year after transplant, leading to unexpected and clinically significant PTR in the post-transplant setting. Although speculative at this point, there may a potential benefit to screen patients with a history of prolonged post-HSCT PTR for HLA-antibodies should a subsequent requirement for platelet transfusions develop, to establish the necessity for HLA-matched platelets.
Unexpectedly, in several patients who were HLA alloimmunuzed pre-transplant, we observed persistence of detectable HLA-antibodies in the post-transplant setting even after all recipient myeloid, T-cell, and bone marrow plasma cell populations became undetectable by our chimaerism analysis. Two possibilities exist to explain this observation. The first is that there may be non-bone marrow niches where host anti-HLA-producing plasma cells find sanctuary. The second possibility is that our chimaerism assay was not sufficiently sensitive to detect these plasma cell populations in the bone marrow; the limit of detection for minor cellular populations using our STRbased chimaerism analysis is only 1–2%. Therefore, it is possible that only a small quantity (i.e. < 2%) of surviving recipient HLA-antibody producing cells is necessary to produce HLA-antibodies at a level that can lead to PTR in the post-transplant setting.
We also observed that the duration of HLA-antibody persistence post-transplant was strongly associated with pre-transplant HLA-antibody level of intensity, as measured by MFI and PRA. HLA-antibodies disappeared more quickly in patients who had lower levels of pre-transplant HLA-antibodies, in contrast to those who had pre-transplant HLA-antibody MFIs >10,000 where HLA-antibodies remained detectable 100 days or more after HSCT (Patients 5, 7, 8). This may be partly explained by the kinetics of immunoglobulins in vivo with the half-life of IgG ranging between 26 and 36 days (Mankarious et al, 1988; Zhou et al, 2008). This finding has important implications for the post-transplant transfusion management of highly HLA allosensitized patients (especially those with MFI >10,000) in terms of maintaining an inventory of HLA-matched platelets for a prolonged period of time after transplant (> 100 days) in those patients who continue to require platelet transfusion support.
This series brings to light interesting considerations in terms of the evaluation and selection of donors for HSCT. All 3 patients (Patient 8, 15, 16) with de novo HLA alloimmunization received grafts from female siblings, of whom 2 were HLA alloimmunized. The donors for Patients 15 and 16 were multiparous females with no history of transfusions, but the donor of Patient 8 (who had no detectable HLA-antibodies) was nulliparous and had no history of transfusions. It has been recently reported that approximately 4% of healthy non-transfused men, 8% of nulliparous women and up to 26% of multiparous, non-transfused women may possess HLA class I antibodies (Triulzi et al, 2009; Densmore et al, 1999). Taniguchi et al. (2012) reported donor-derived HLA-antibody production in four out of seven patients who received either a bone marrow transplant (n=2) or a PBSCT transplant (n=5) from HLA-antibody-positive donors. These data, as well as observations from our patient series, suggest that screening patients and their donors for HLA-antibodies before transplant would assist in peri-transplant transfusion support and would identify the majority of subjects at risk for the development of post-transplant alloimmune-mediated PTR. Although the development of HLA-antibody-dependent PTR as a consequence of transplanting stem cell grafts from HLA alloimmunized donors tempts one to propose that the donor’s HLA-antibody status should be considered in the decision analysis for selecting optimal stem cell donors, the small numbers of subjects in our study prevent us from making any definitive conclusions in this regard.
In conclusion, the protracted persistence of HLA-antibodies leading to continued PTR can occur after HSCT and may be exacerbated by the use of RIC. Furthermore, non-HLA alloimmunized patients may develop de novo HLA alloimmunization as a consequence of the transfer of HLA-antibody producing cells from the donor to the patient at the time of transplantation, which can lead to clinically significant post-transplant PTR. Screening patients (and possibly donors) for HLA-antibodies before HSCT would assist in post-transplant transfusion support, as it would identify the majority of subjects at risk for developing alloimmune-mediated PTR after transplantation. Further study is needed in a larger series of donor/patient pairs to determine the effect of donor-derived de novo HLA-antibodies on post-transplant PTR prior to making any definitive conclusions on the need for donor HLA-antibody screening.
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Programs of the National Heart, Lung and Blood Institute and the Department of Transfusion Medicine at the Clinical Center, National Institutes of Health. The authors would like to acknowledge the patients who participated in this trial, and Lisa Cook, Catalina Ramos, Patty Prince, Rob Reger, Maria Berg and Rose Goodwin for their critical support of the clinical study described in this manuscript.
Footnotes
Authorship contribution
R.M.F. initiated the study, analysed and interpreted data, and wrote the manuscript. E.M. assisted with analysing and interpreting data, and writing the manuscript. S.A. performed and analysed HLA antibody data. T.D. participated in the patient care, collected patient samples and patient care information. K.S performed and analysed plasma cell chimaerism data. X.T. performed statistical analysis. W.A.F. interpreted data and co-wrote the manuscript. R.W.C. participated in the patient care, collected samples, contributed to the interpretation of data and to writing.
Statement of disclaimer
The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
Conflict-of-interest disclosure
All authors do not declare any conflict-of-interest.
REFERENCES
- Antin JH, Childs R, Filipovich AH, Giralt S, Mackinnon S, Spitzer T, Weisdorf D. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings of the International Bone Marrow Transplant Registry and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2001;7(9):473–485. doi: 10.1053/bbmt.2001.v7.pm11669214. [DOI] [PubMed] [Google Scholar]
- Balduini CL, Salvaneschi L, Klersy C, Noris P, Mazzucco M, Rizzuto F, Giorgiani G, Perotti C, Stroppa P, Pumpo MD, Nobili B, Locatelli F. Factors influencing post-transfusional platelet increment in pediatric patients given hematopoietic stem cell transplantation. Leukemia. 2001 Dec;15(12):1885–1891. doi: 10.1038/sj.leu.2402307. [DOI] [PubMed] [Google Scholar]
- Childs R, Clave E, Contentin N, Jayasekera D, Hensel N, Leitman S, Read EJ, Carter C, Bahceci E, Young NS, Barrett AJ. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999 Nov 1;94(9):3234–3241. [PubMed] [Google Scholar]
- Ciurea SO, de Lima M, Cano P, Korbling M, Giralt S, Shpall EJ, Wang X, Thall PF, Champlin RE, Fernandez-Vina M. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009 Oct 27;88(8):1019–1024. doi: 10.1097/TP.0b013e3181b9d710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciurea SO, Thall PF, Wang X, Wang SA, Hu Y, Cano P, Aung F, Rondon G, Molldrem JJ, Korbling M, Shpall EJ, de Lima M, Champlin RE, Fernandez-Vina M. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011 Nov 24;118(22):5957–5964. doi: 10.1182/blood-2011-06-362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas FH, Doxiadis II. Management of the highly sensitized patient. Current opinion in immunology. 2009 Oct;21(5):569–572. doi: 10.1016/j.coi.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Cutler C, Kim HT, Sun L, Sese D, Glotzbecker B, Armand P, Koreth J, Ho V, Alyea E, Ballen K, Ritz J, Soiffer RJ, Milford E, Antin JH. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011 Dec 15;118(25):6691–6697. doi: 10.1182/blood-2011-05-355263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densmore TL, Goodnough LT, Ali S, Dynis M, Chaplin H. Prevalence of HLA sensitization in female apheresis donors. Transfusion. 1999 Jan;39(1):103–106. doi: 10.1046/j.1537-2995.1999.39199116901.x. [DOI] [PubMed] [Google Scholar]
- Eng HS, Bennett G, Chang SH, Dent H, McDonald SP, Bardy P, Coghlan P, Russ GR, Coates PT. Donor human leukocyte antigen specific antibodies predict development and define prognosis in transplant glomerulopathy. Human immunology. 2011 May;72(5):386–391. doi: 10.1016/j.humimm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Gloor J, Stegall MD. Sensitized renal transplant recipients: current protocols and future directions. Nat Rev Nephrol. 2010 May;6(5):297–306. doi: 10.1038/nrneph.2010.34. [DOI] [PubMed] [Google Scholar]
- Griffith LM, McCoy JP, Jr, Bolan CD, Stroncek DF, Pickett AC, Linton GF, Lundqvist A, Srinivasan R, Leitman SF, Childs RW. Persistence of recipient plasma cells and anti-donor isohaemagglutinins in patients with delayed donor erythropoiesis after major ABO incompatible non-myeloablative haematopoietic cell transplantation. British journal of haematology. 2005 Mar;128(5):668–675. doi: 10.1111/j.1365-2141.2005.05364.x. [DOI] [PubMed] [Google Scholar]
- Gutman JA, McKinney SK, Pereira S, Warnock SL, Smith AG, Woolfrey AE, Hansen JA, Delaney C. Prospective monitoring for alloimmunization in cord blood transplantation: "virtual crossmatch" can be used to demonstrate donor-directed antibodies. Transplantation. 2009 Feb 15;87(3):415–418. doi: 10.1097/TP.0b013e3181943ba3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama N, Hori T, Yamamoto M, Inazawa N, Iesato K, Miyazaki T, Ikeda H, Tsutsumi H, Suzuki N. Platelet transfusion refractoriness attributable to HLA antibodies produced by donor-derived cells after allogeneic bone marrow transplantation from one HLA-antigen-mismatched mother. Pediatr Transplant. 2011 Dec;15(8):E177–E182. doi: 10.1111/j.1399-3046.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- Kickler T, Kennedy SD, Braine HG. Alloimmunization to platelet-specific antigens on glycoproteins IIb-IIIa and Ib/IX in multiply transfused thrombocytopenic patients. Transfusion. 1990 Sep;30(7):622–625. doi: 10.1046/j.1537-2995.1990.30790385520.x. [DOI] [PubMed] [Google Scholar]
- Klumpp TR, Herman JH, Innis S, Pearlman E, Culling N, Kotz KW, Slachta C, Goldberg SL, Mangan KF. Factors associated with response to platelet transfusion following hematopoietic stem cell transplantation. Bone marrow transplantation. 1996 Jun;17(6):1035–1041. [PubMed] [Google Scholar]
- Kupin WL, Venkat KK, Hayashi H, Mozes MF, Oh HK, Watt R. Removal of lymphocytotoxic antibodies by pretransplant immunoadsorption therapy in highly sensitized renal transplant recipients. Transplantation. 1991 Feb;51(2):324–329. doi: 10.1097/00007890-199102000-00010. [DOI] [PubMed] [Google Scholar]
- Lapierre V, Auperin A, Tayebi H, Chabod J, Saas P, Michalet M, Francois S, Garban F, Giraud C, Tramalloni D, Oubouzar N, Blaise D, Kuentz M, Robinet E, Tiberghien P. Increased presence of anti-HLA antibodies early after allogeneic granulocyte colony-stimulating factor-mobilized peripheral blood hematopoietic stem cell transplantation compared with bone marrow transplantation. Blood. 2002 Aug 15;100(4):1484–1489. doi: 10.1182/blood-2001-11-0039. [DOI] [PubMed] [Google Scholar]
- Mankarious S, Lee M, Fischer S, Pyun KH, Ochs HD, Oxelius VA, Wedgwood RJ. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. The Journal of laboratory and clinical medicine. 1988 Nov;112(5):634–640. [PubMed] [Google Scholar]
- Nakazawa Y, Saito S, Hasegawa Y, Yanagisawa R, Sakashita K, Kamijo T, Miyazaki T, Sato S, Ikeda H, Ikebuchi K, Koike K. A possible role for the production of multiple HLA antibodies in fatal platelet transfusion refractoriness after peripheral blood progenitor cell transplantation from the mother in a patient with relapsed leukemia. Transfusion. 2007 Feb;47(2):326–334. doi: 10.1111/j.1537-2995.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- Nambiar A, Duquesnoy RJ, Adams S, Zhao Y, Oblitas J, Leitman S, Stroncek D, Marincola F. HLAMatchmaker-driven analysis of responses to HLA-typed platelet transfusions in alloimmunized thrombocytopenic patients. Blood. 2006 Feb 15;107(4):1680–1687. doi: 10.1182/blood-2004-10-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DK, Pollinger HS, Burns JM, Rea D, Ramos E, Platt JL, Gloor JM, Stegall MD. Two novel assays of alloantibody-secreting cells demonstrating resistance to desensitization with IVIG and rATG. Am J Transplant. 2008 Jan;8(1):133–143. doi: 10.1111/j.1600-6143.2007.02039.x. [DOI] [PubMed] [Google Scholar]
- Reinsmoen NL, Nelson K, Zeevi A. Anti-HLA antibody analysis and crossmatching in heart and lung transplantation. Transplant immunology. 2004 Jun-Jul;13(1):63–71. doi: 10.1016/j.trim.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, Vierra-Green C, Anasetti C. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010 Apr 1;115(13):2704–2708. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanashi M, Fujiwara K, Tanaka H, Satake M, Nakajima K. The impact of HLA antibodies on engraftment of unrelated cord blood transplants. Transfusion. 2008 Apr;48(4):791–793. doi: 10.1111/j.1537-2995.2008.01678.x. [DOI] [PubMed] [Google Scholar]
- Takanashi M, Atsuta Y, Fujiwara K, Kodo H, Kai S, Sato H, Kohsaki M, Azuma H, Tanaka H, Ogawa A, Nakajima K, Kato S. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116(15):2839–2846. doi: 10.1182/blood-2009-10-249219. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Yoshihara S, Maruya E, Ikegame K, Kaida K, Hayashi K, Kato R, Inoue T, Fujioka T, Tamaki H, Okada M, Onuma T, Fujii N, Kusunoki Y, Soma T, Saji H, Ogawa H. Donor-derived HLA antibody production in patients undergoing SCT from HLA antibody-positive donors. Bone marrow transplantation. 2012 Oct;47(10):1338–1342. doi: 10.1038/bmt.2012.28. [DOI] [PubMed] [Google Scholar]
- The Trial to Reduce Alloimmunization to Platelets Study Group. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The New England journal of medicine. 1997 Dec 25;337(26):1861–1869. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- Triulzi DJ, Kleinman S, Kakaiya RM, Busch MP, Norris PJ, Steele WR, Glynn SA, Hillyer CD, Carey P, Gottschall JL, Murphy EL, Rios JA, Ness PM, Wright DJ, Carrick D, Schreiber GB. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009 Sep;49(9):1825–1835. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DS, Montgomery RA. Incompatible kidney transplantation: lessons from a decade of desensitization and paired kidney exchange. Immunologic research. 2010 Jul;47(1–3):257–264. doi: 10.1007/s12026-009-8157-y. [DOI] [PubMed] [Google Scholar]
- Yoshihara S, Taniguchi K, Ogawa H, Saji H. The role of HLA antibodies in allogeneic SCT: is the 'type-and-screen' strategy necessary not only for blood type but also for HLA? Bone marrow transplantation. 2012 Jan 9; doi: 10.1038/bmt.2011.249. [DOI] [PubMed] [Google Scholar]
- Zachary AA, Eng HS. Desensitization: achieving immune detente. Tissue antigens. 2011 Jan;77(1):3–8. doi: 10.1111/j.1399-0039.2010.01596.x. [DOI] [PubMed] [Google Scholar]
- Zhou B, Saito S, Nakazawa Y, Kobayashi N, Matsuda M, Matsumoto Y, Hosoyama T, Koike K. Existence of an immunoglobulin G component of naturally occurring HLA class I antibodies that are not directed against self-antigens in human serum. Tissue antigens. 2008 Aug;72(2):98–104. doi: 10.1111/j.1399-0039.2008.01074.x. [DOI] [PubMed] [Google Scholar]