Abstract
Using a high throughput screening (HTS) approach, we have identified and validated several small molecule Mcl-1 inhibitors (SMIs). Here we describe a novel selective Mcl-1 SMI inhibitor, 2 (UMI-77), developed by structure-based chemical modifications of the lead compound 1 (UMI-59). We have characterized the binding of UMI-77 to Mcl-1 by using complementary biochemical, biophysical and computational methods, and determined its antitumor activity against panel of pancreatic cancer (PC) cells and in vivo xenograft model. UMI-77 binds to the BH3 binding groove of Mcl-1 with Ki of 490 nM, showing selectivity over other members of anti-apoptotic Bcl-2 members. UMI-77 inhibits cell growth and induces apoptosis in PC cells in a time and dose-dependent manner, accompanied by cytochrome c release and caspase-3 activation. Co-immunoprecipitation experiments revealed that UMI-77 blocks the heterodimerization of Mcl-1/Bax and Mcl-1/Bak in cells, thus antagonizing the Mcl-1 function. The Bax/Bak-dependent induction of apoptosis was further confirmed by using murine embryonic fibroblasts that are Bax and Bak deficient. In an in vivo BxPC-3 xenograft model, UMI-77 effectively inhibited tumor growth. Western blot analysis in tumor remnants revealed enhancement of pro-apoptotic markers and significant decrease of survivin. Collectively, these promising findings demonstrate the therapeutic potential of Mcl-1 inhibitors against PC and warrant further preclinical investigations.
Keywords: Mcl-1, Apoptosis, Small molecule inhibitor, Pancreatic cancer, Xenograft model
Introduction
Pancreatic cancer (PC) is among the most therapy resistant cancers with poor prognosis and high mortality rate (1). Primary or acquired resistance of PC to chemotherapy and radiation therapy as a consequence of apoptosis defects is a major cause of treatment failure and poor outcome (2, 3). Hence, future efforts toward development of novel therapies to improve survival and quality of life of PC patients should include new strategies that specifically target resistance of cancer cells to apoptosis (4).
Myeloid cell leukemia-1 (Mcl-1) is a potent anti-apoptotic protein, a member of the prosurvival Bcl-2 family, and its role is emerging as a critical survival factor in a broad range of human cancers, including PC (5, 6). Functional studies have confirmed that Mcl-1 is capable of blocking apoptosis induced by various apoptotic stimuli, including chemotherapy and radiation (7). Mcl-1 is highly expressed at the protein level in PC cells and is associated with resistance to chemotherapeutic agents (8–13). It has been demonstrated that down-regulation of Mcl-1 enhances the induction of apoptosis and sensitivity of PC to gemcitabine and radiation (11, 12). Thus, Mcl-1 represents attractive molecular target for development of a new class of cancer therapy for treatment of PC.
The most potent small molecule inhibitors of the Bcl-2 subfamily described to date are the Bad-like BH3 mimetics (14–16). ABT-737, one of these mimetics, binds with high affinity (Ki ≤1 nM) to Bcl-2, Bcl-xL and Bcl-w but fails to bind to Mcl-1 (14). Studies have demonstrated that resistance to ABT-737 is linked to high expression levels of Mcl-1 and in many instances this resistance can be overcome by treatment with agents that down-regulate, destabilize, or inactivate Mcl-1 (17, 18). It was shown that knockdown of Mcl-1 sensitizes human PC cancer cells to ABT-737-induced apoptosis, indicating that Mcl-1 is a relevant therapeutic target in these cancer cells (13).
Using a high throughput screening (HTS) approach, we have identified and validated several small molecule Mcl-1 inhibitors. Here we describe a novel selective small molecule Mcl-1 inhibitor, 2 (UMI-77), an analog of the lead compound 1 (UMI-59). 2 (UMI-77) selectively binds Mcl-1, induce Bax/Bak dependent apoptosis in PC cells and exhibits single-agent antitumor activity in a BxPC-3 xenograft model, providing promising evidence for therapeutic potential of Mcl-1 inhibitors against PC.
Materials and Methods
Chemistry Information
Synthesis and characterization of compounds 1 (UMI-59) and 2 (UMI-77) are provided in the Supplementary Methods. Compound 3 (UMI-101) was purchased from Princeton BioMolecular Research.
Protein purification
Five recombinant anti-apoptotic Bcl-2 proteins were used in the binding studies: Mcl-1, Bcl-2, Bcl-xL, Bcl-w and A1/Bfl-1 (for details see Supplementary Methods).
Fluorescence polarization (FP) based binding assays
IC50 and Ki values of Mcl-1 inhibitors to anti-apoptotic proteins from Bcl-2 family were determined in FP-based competitive binding assays provided in the Supplementary Methods. The Ki values were calculated as described previously (19).
Surface plasmon resonance (SPR) binding assays
SPR experiments were performed on Biacore 2000 optical biosensor using immobilized recombinant Bax (Novus Biologicals) and biotin-labeled Bid BH3 peptide in competitive solution binding experiments (for details see Supplementary Methods).
Induced fit docking (IFD)
Crystal structure of Mcl-1 with mouse Noxa BH3 peptide (PDB ID: 2NLA) was used to model the binding pose of 2 (UMI-77) (20). The Schrödinger’s IFD protocol (21), provided in the Supplementary Methods, was employed for the docking studies and the docking pose was refined with molecular dynamic simulation program of Schrödinger’s MacroModel (22).
Biotin Streptavidin Pull-Down Experiment
Human breast cancer 2LMP cells, a subclone of the MDA-MB-231 cell line, were lysed in CHAPS buffer (10 mM HEPES (pH 7.4), 2.5 mM EDTA, 150 mM NaCl, 1.0% CHAP). Pre-cleared cell lysates were incubated with different concentrations of compounds followed by incubation with biotinylated Noxa BH3 peptide (18–43) and streptavidin agarose beads to pull-down Mcl-1 protein bound to Noxa peptide. Beads were washed with CHAPS buffer, and Mcl-1 protein was eluted by boiling in SDS-PAGE sample buffer and analyzed by Western blotting using Mcl-1 antibody (Santa Cruz).
Cells and growth inhibition
All PC cell lines were obtained from American Type Culture Collection (ATCC) (Manassas, VA USA). The cells have been tested and authenticated in the Applied Genomics Technology Center at Wayne State University in March 2011. The method used for testing was short tandem repeat (STR) profiling using the PowerPlex(r) 16 System from Promega (Madison, WI). Human PC cell lines AsPC-1, BxPC-3 and Capan-2 were cultured in RPMI 1640 medium, while Panc-1 and MiaPaCa were cultured in DMEM medium (Life Technologies), all supplemented with 10% fetal bovine serum (Thermo Scientific HyClone). The cell growth inhibition after treatment with increasing concentrations of the compounds was determined by WST-8 assay (Dojindo Molecular Technologies Inc.).
Quantification of Apoptosis
An Annexin-V-FLUOS/Propidium iodide staining kit and ELISA kit (Roche Applied Science) were used to detect apoptosis in PC cells. Cells were treated with Mcl-1 inhibitors for different time points, harvested, washed with PBS and apoptosis was quantified according to manufacturer’s protocol.
Immunofluorescence microscopy
Cells (3–4 × 105) were seeded on glass coverslips in six-well cell culture dishes, allowed to attach overnight, and treated with UMI-77 for 24 hours. Cells mounted on glass slides were permeabilized with PBS containing 0.3% Triton X-100, and blocked with 1% bovine serum albumin in PBS for 30 min at room temperature, followed with overnight incubation with anti-Bax 6A7 (Calbiochem) at 4°C. After washing with PBS, a secondary antibody labeled with DyLight 488 (Thermo Scientific) was added and incubated for 2 h at room temperature. Nuclei were visualized with DAPI. Samples were analyzed with a FluoView 500 Confocal Laser Scanning Microscope (Olympus). The fluorescent intensity of the active Bax was quantified using image processing program ImageJ 1.47 (NIH).
RNA interference
Human PC cells were transfected with Mcl-1 siRNA and control siRNA respectively (both from Santa Cruz), using Lipofectamine 2000 as described in the manufacturers protocol (Cell Signaling).
Western Blot Analysis
The cells were treated and harvested at the indicated time points. Total cell lysates were subjected to electrophoresis, transferred to PVDF membranes and incubated with a specific primary antibody, followed by visualization with chemiluminescent detection reagent (Roche Molecular Biochemicals). Primary antibodies included: caspase-3 (Enzo Life Sciences), Mcl-1 and actin (Santa Cruz), Bcl-xL (BD Transduction Laboratories); Bcl-2, Bax, pro-PARP and cytochrome-c (Cell Signaling), Bak (Calbiochem), and Smac (Abgent).
Immunoprecipitation
Cell lysate (500 μg) was subjected to immunoprecipitation by adding 2.5 – 5 μg of anti-Mcl-1 antibody and incubation overnight at 4 ºC. After adding 30 μl of Protein G-agarose (Immunoprecipitation Kit, Sigma) and incubation for 4 h, the samples were centrifuged. The agarose pellet was washed, resuspended in Laemmli buffer (Santa Cruz), boiled and supernatant was used for Western blot analysis.
Metabolic Stability Assay
Metabolic stability of UMI-77 was determined using the pooled mice liver microsomes (XenoTech, LLC). The conditions of the assay and quantification of UMI-77 in different time points are provided in SI.
Animal Preclinical Efficacy Trail Design
For BxPC-3 subcutaneous model, 10×106 cells were subcutaneously injected into the flanks of 4–5 week old female severe combined immune deficient mice (ICR-SCID) (Taconic Farms). Palpable tumors started to appear in 3–5 weeks (23). Tumors were measured twice weekly. To prevent any pain or discomfort, mice were euthanized and their tumors removed once they reached ~1800 mg burden. Tumors were then dissected into 50 mg pieces and re-transplanted into naïve ICR-SCID for serial propagation. Animals were treated with either vehicle or UMI-77 given i.v. (60 mg/kg) on day three post BxPC-3 transplantation for two weeks (5 days a week). Tumor weight was recorded throughout the treatment period. At the end of the treatment period, animals were euthanized and their tumors harvested for protein isolation and western blot analysis for apoptotic markers.
Statistical analysis
Statistics was evaluated using GraphPad StatMate software (GraphPad Software, Inc.). P < 0.05 or P < 0.01 was used to indicate statistical significance.
Results
Compound 2 (UMI-77) selectively binds Mcl-1
Applying a HTS approach we have screened a library of 53,000 synthetic small molecules available at the Center for Chemical Genomics, University of Michigan using a FP based binding assay. Compound 1 (UMI-59) (Fig. 1A) is one of the validated hits, which was re-synthesized and confirmed its binding to Mcl-1 protein (Supplemental Scheme 1). In this paper, we report compound 2 (UMI-77), an analog of the lead compound UMI-59 with improved binding affinity to Mcl-1.
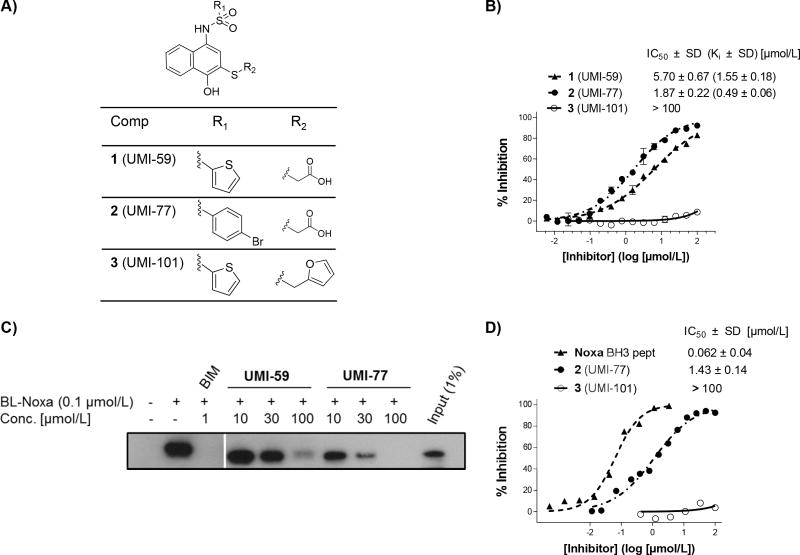
Fig. 1. Biochemical characterization of 2 (UMI-77) binding to Mcl-1.
A) Chemical structures of the lead compound 1 (UMI-59) and its two analogs 2 (UMI-77) and 3 (UMI-101). B) Competitive binding curves of small-molecule inhibitors against Mcl-1 obtained by FP based binding assay using fluorescent labeled Bid BH3 peptide. C) Probing the interaction of 2 (UMI-77) to cellular Mcl-1 by a pull-down assay using biotin labeled Noxa (BL-Noxa). D) Solution competitive SPR based binding assay. Recombinant Bax protein (residues 1–100) was immobilized on the CM5 chip and increasing concentrations of 2 (UMI-77) pre-incubated with Mcl-1 were injected over the surface. *All binding studies were performed minimum three times and the average values ± standard deviations are reported.
The binding affinity and selectivity of 2 (UMI-77) against five members of Bcl-2 family of proteins was determined using FP-based binding assays (Fig. 1B and Table 1). The obtained results showed that UMI-77 selectively and potently displaced fluorescent labeled BID-BH3 peptide from Mcl-1 protein with a Ki = 0.49 μM, showing three times higher potency for binding to Mcl-1 than 1 (UMI-59) (Ki = 1.55 μM). Compound 3 (UMI-101), an analog of UMI-77, did not show binding to Mcl-1 up to 100 μM and therefore was used as a negative control in cell based assays. The binding profile studies showed that UMI-77 displayed significantly decreased binding affinities to the rest of the anti-apoptotic proteins and its binding selectivity was consistent with the structural similarities between Mcl-1 and the anti-apoptotic members of Bcl-2 family. UMI-77 bound to A1/Bfl-1 with 11 fold lower affinity than to Mcl-1 (Ki = 5.33 μM), followed by Bcl-w with Ki = 8.19 μM (17 fold decreased), and more than 50-fold reduced binding to Bcl-2 (Ki = 23.83 μM) and Bcl-xL (Ki = 32.99 μM). To confirm the binding selectivity of UMI-77 to anti-apoptotic proteins, a solution competitive SPR based binding assay using a biotin-labeled Bid peptide immobilized on streptavidin chip was performed. Consistent with FP results, UMI-77 showed 4-fold improved binding affinity to Mcl-1 (IC50 = 0.31 μM) in comparison with UMI-59 (IC50 = 1.23 μM) (Fig. S1A) and more than 30 fold decreased binding to Bcl-2 and Bcl-xL (Fig. S1B).
Table 1.
Binding affinities of 1 (UMI-59) and 2 (UMI-77) to five members of Bcl-2 family of proteins in competitive FP assay using fluorescent labeled Bid BH3 peptide.
| Protein | Ki ± SD [μM] | |
|---|---|---|
| 1 (UMI-59) | 2 (UMI-77) | |
|
| ||
| Mcl-1 | 1.55 ± 0.18 | 0.49 ± 0.06 |
|
| ||
| A1/Bfl-1 | 6.14 ± 1.0 | 5.33 ± 1.0 |
|
| ||
| Bcl-2 | 54.65 ± 9.56 | 23.83 ± 1.81 |
|
| ||
| Bcl-xL | 99.0 ± 22.63 | 32.99 ± 4.33 |
|
| ||
| Bcl-w | 37.53 ± 7.96 | 8.19 ± 1.91 |
To extend these findings to a cellular context, we employed a pull-down assay using a biotin-labeled Noxa BH3 peptide (BL-Noxa) to probe whether UMI-77 interacts with cellular Mcl-1 protein. BL-Noxa selectively pulls down cellular Mcl-1 from 2LMP cell lysate and both compounds, UMI-59 and UMI-77, effectively disrupt the interactions between BL-Noxa and cellular Mcl-1 in a dose dependent manner (Fig. 1C). Consistent with our binding results, UMI-77 is more potent than UMI-59 and blocks this interaction starting from 10 μM. These data demonstrate that UMI-77 binds the endogenous, cellular Mcl-1 protein and blocks the binding of BL-Noxa to Mcl-1.
It was reported that Mcl-1 regulates pro-apoptotic Bax and Bak proteins through binding to their BH3 domains and preventing their pro-apoptotic activity (24, 25). Accordingly, we have developed an SPR based binding assay to tests the ability of Mcl-1 inhibitors to block the Mcl-1/Bax protein-protein interactions. Recombinant Bax protein (residues 1–100) was immobilized and it was determined that Mcl-1 binds to Bax with a Kd value of 217 nM (Fig. S2). Pre-incubation of the Mcl-1 protein with increasing concentrations of UMI-77 blocked the binding of Mcl-1 to Bax in a dose-dependent manner with an IC50 = 1.43 μM (Fig. 1D). Noxa BH3 peptide inhibits the binding of Mcl-1 to Bax with an IC50 = 0.062 μM, 23 fold more potent than UMI-77. Consistent with the results obtained from the FP based assay, UMI-101 failed to disrupt the Mcl-1/Bax protein-protein interactions. These results confirmed that UMI-77 binds to the Mcl-1 protein and is capable of disrupting Mcl-1/Bax protein-protein interactions.
2 (UMI-77) binds to the BH3 binding pocket of Mcl-1 protein
To confirm the binding of 2 to the BH3 groove of Mcl-1 protein and to explore the interaction between the UMI-77 and Mcl-1, in silico docking analysis and heteronuclear single quantum correlation (HSQC) NMR spectroscopy studies were performed. The interactions between helical BH3 domain of pro-apoptotic and the BH3 binding groove in anti-apoptotic proteins are well characterized (Fig. S3). They involve hydrophobic interactions through four conserved hydrophobic residues of the BH3 domain in pro-apoptotic proteins and a salt bridge between conserved aspartic acid and arginine on the anti-apoptotic proteins. Mimicking these interactions is the main strategy towards developing small-molecule BH3 mimetic Mcl-1 inhibitors (26).
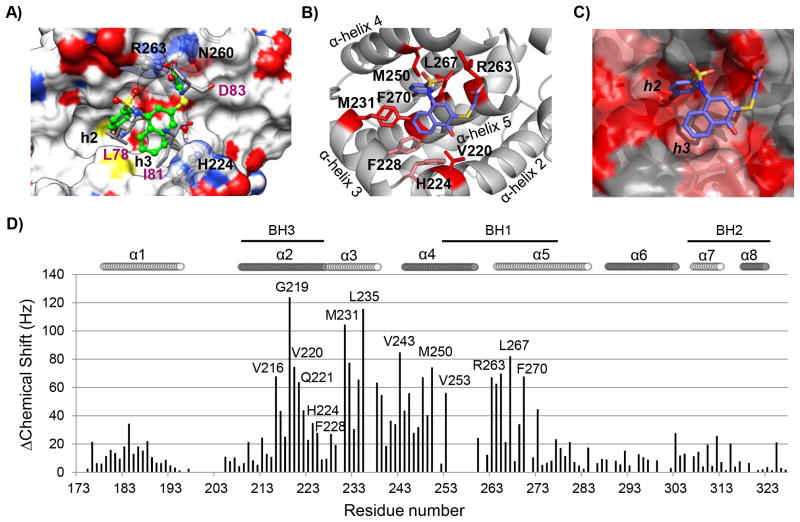
The predicted binding model of UMI-77 in the complex with Mcl-1 revealed that UMI-77 occupies two hydrophobic pockets in Mcl-1, h2 and h3, mimicking two conserved hydrophobic residues from mNoxaB (PDB ID:2NLA), Leu78 and Ile81, respectively (Fig. 2A and S3). Specifically, the p-bromophenyl group inserts into the h2 pocket and has hydrophobic interactions with Met231, Met250, Val253, Leu267 and Phe270. The proposed interactions were confirmed with the HSQC NMR experiments in which these residues showed significant chemical shift perturbations (≥60 Hz) (Fig. 2B and 2D). The naphthalenyl ring of UMI-77 occupies the h3 pocket and makes hydrophobic interactions with Phe228, while the 4-hydroxyl group forms a hydrogen bond with His224. This residue was identified as an acidic hotspot in the h3 site of Mcl-1 (27), supporting our predicted electrostatic interaction in this region of Mcl-1 (Fig. 2A). Consistent with the computational model, NMR experiments showed that Met231 and Val220 from the h3 pocket, have significant chemical shift perturbations (> 60 Hz), as well as His224 and Phe228 (between 30 Hz and 60 Hz), confirming the predicted interactions of the UMI-77 in this region of the Mcl-1 protein (Fig. 2B and 2D). The docking model revealed that the carboxylic group of UMI-77 forms hydrogen bonding network with Arg263 and Asn260, mimicking the conserved aspartate in pro-apoptotic proteins. Indeed, the HCQS NMR spectrum of UMI-77/Mcl-1 complex showed that Arg263 has a significant chemical shift (Fig. 2B–2D). Overall analysis of the chemical shifts of the Mcl-1/UMI-77 and Mcl-1/Bim BH3 peptide complexes showed that UMI-77 affected the same residues as Bim BH3 peptide (Fig. S4). Taken together, in silico docking and HSQC NMR studies provided conclusive evidence that UMI-77 binds to the BH3-binding groove of Mcl-1 protein. To understand the selective binding of UMI-77 to Mcl-1, we compared its binding model to the reported selective Mcl-1 SMI, maritoclax (28), as well as with the Bims2A, a selective Mcl-1 BH3-like peptide derived from Bim peptide (29). Interestingly, the two SMI have different binding modes, UMI-77 occupies the h2 and h3 hydrophobic pockets, while maritoclax binds near the h4 hydrophobic pocket. However, structural studies of Bims2A demonstrated that the hydrophobic residue in position h3, Ile65 (Fig. S4) is the most critical for the selective and high affinity binding to Mcl-1 (29). Consistent with this study, the naphthalenyl ring of UMI-77 occupies h3 pocket and mimics this hydrophobic residue. During the revision of this paper the first complex structure between small-molecule inhibitor and Mcl-1 was reported (30) confirming the importance of the h2 and h3 hydrophobic pockets for selective targeting of Mcl-1 and providing insights for understanding the structural determinants for selective targeting of Mcl-1 protein.
Fig. 2. Computational and structural binding studies of 2 (UMI-77) with Mcl-1.
A) Computational predicted binding pose of 2 (UMI-77) with Mcl-1 using the mNoxa BH3 peptide-bound Mcl-1 structure (PDB ID 2NLA). Superposition of the predicted binding pose of 2 (UMI-77) onto the crystal structure of mNoxa BH3 peptide with Mcl-1 (conserved residues from mNoxa, L78, I81 and D83, are presented in pink color). Hydrogen bonds with R263 and H224 from Mcl-1 protein are shown in magenta dotted lines. B) Chemical shift mapping on the predicted binding model of 2 (UMI-77) to Mcl-1. The side chains of the residues involved in the interactions with UMI-77 and confirmed with the HSQC NMR studies are shown and labeled. C) Chemical shift mapping using a surface representation of the predicted binding model between UMI-77 and Mcl-1 (chemical shifts ≥60 Hz are labeled in red, shifts between 30 and 60 Hz are labeled in pink). D) Chemical shift differences for Mcl-1 in presence of 2 (UMI-77) (2 equivalents) against residue number.
2 (UMI-77) inhibits growth of PC cells and induces intrinsic apoptotic pathway
Cytotoxic effect of 2 was evaluated using a panel of five PC cell lines with different expression level of anti- and pro-apoptotic proteins (Fig. S5). A dose-response analysis revealed that UMI-77 most potently inhibits the cell growth of BxPC-3 and Panc-1 cell lines with IC50 values of 3.4 μM and 4.4 μM respectively, and shows 3 to 5 times less potency in inhibition of the cell growth of two other tested cell lines MiaPaCa-2 (12.5 μM) and AsPC-1 (16.1 μM) (Fig. 3A). The cell growth inhibition potency of UMI-77 correlates with the highest expression of Mcl-1 and Bak, and lowest expression of Bcl-xL in the sensitive cell lines, BxPC-3 and Panc-1. Consistent with this, less sensitive PC cells, MiaPaCa and AsPC-1, express low level of Mcl-1 and Bak, and high expression of Bcl-xL which may contribute to their less sensitivity since UMI-77 selectively binds Mcl-1 and shows more than 50-fold reduced binding to Bcl-xL. Interestingly, Capan-2 cells are showing similar sensitivity to UMI-77 (IC50 of 5.5 μM) as BxPC-3 and Panc-1, although has low Mcl-1 levels. One of the potential explanations might be the p53 status since only Capan-2 cells express wild type p53, while all other tested PC cell lines express mutated p53. Differences in p53 status might influence the efficacy of UMI-77 in Capan-2 cell line as it is known that p53 activates the transcription of Puma and Noxa, thus shifting the balance of the Bcl-2 family towards pro-apoptotic members (31–33). p53-mediated induction of Puma might enhances the sensitivity of Capan-2 cells by lowering the threshold set by Bcl-xL, which is highly expressed in these cells. However, further mechanistic studies are needed, which is beyond the scope of this paper. Importantly, the specificity of cell growth inhibition by UMI-77 was confirmed by experiments with compound 3 which showed no binding to Mcl-1 at concentrations up to 100 μM and failed to show inhibition of the cell growth in all tested PC cells.
Fig. 3.
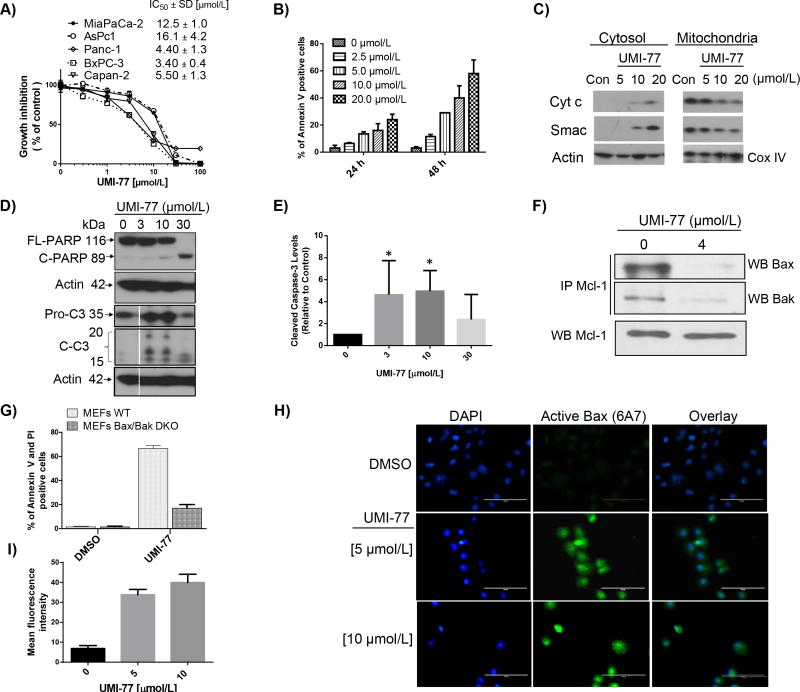
Fig. 3. 2 (UMI-77) effect on pancreatic cancer cell growth and induction of apoptosis
A) IC50 values of cell growth inhibition of 2 (UMI-77) in panel of PC cell lines after 4 days treatment. B) Time and dose-dependent induction of apoptosis in Panc-1 cells after treatment with 2 (UMI-77). Cells were treated for different time points and apoptosis was determined with Annexin V/PI double staining. C) Release of cytochrome c and Smac from mitochondria in Panc-1 cells. Cells were treated for 24 h, mitochondria were isolated and cytochrome c and Smac were probed by Western blotting. D) Full length PARP (FL-PARP), cleaved PARP and activation of caspase-3 in BxPC-3 cells after 24 h treatment with 2 (UMI-77). Cells were treated for 24 h, and caspase-3 and PARP were probed by Western blotting. E) Cleaved caspase-3 was quantified by densitometric analysis and presented in graphical form. The statistical significance was calculated with minimum of three values for all tested concentrations (n=3; P < 0.05). F) Immunoprecipitation on 2 (UMI-77) treated BxPC-3 cell lysate was performed using Mcl-1 antibody followed by western blot analysis with Bax or Bak. G) Induction of apoptosis in wild type (WT) and Bax/Bak-deficient (DKO) MEFs cells after 24 h treatment with 10 μM of 2 (UMI-77). H) and I) 2 (UMI-77) induces Bax activation in Panc-1 cells. Immunocytochemistry analysis (H) and quantification of the fluorescence intensity (I) demonstrated the increased number of positive cells stained with anti-Bax(6A7) antibody, which specifically detect the active form of Bax, 24 h after 2 (UMI-77) treatment in Panc-1 cells. Conversely, DMSO control did not induce the active form of Bax (green: anti-Bax(6A7) antibody, blue: DAPI). Scale bar, 100 μm. Forty cells were used for quantification of the fluorescence intensity. *All experiments were performed minimum three times and the most representative results are presented.
To gain insights into the underlying mechanism of action for the cell growth inhibition of UMI-77, we selected BxPC-3 and Panc-1 cell lines for further investigation. To determine if apoptosis contributes to the antiproliferative effect of UMI-77, BxPC-3 and Panc-1 cells were treated with increasing concentrations of this compound for different times. Induction of apoptosis was monitored by flow cytometry using Annexin V and propidium iodide double staining. UMI-77 was effective in induction of apoptosis in a time-dependent and dose-dependent manner in Panc-1 cells (Fig. 3B). Treatment of the Panc-1 cells with 5 and 10 μM concentrations of UMI-77 resulted in 15% and 21%, respectively, of early apoptotic cells after 24 hours treatment, and 21% and 49% after 48 hours treatment. Similar results were also obtained in BxPC-3 cells (Fig. S6). The inactive compound 3 even at concentration of 100 μM failed to induce apoptosis in both tested PC cell lines. AsPC-1 cells which are less sensitive to UMI-77, as was expected, showed minimum induction of early apoptosis (8%) in the highest tested concentration (20 μM) (Fig. S6). Next we examined whether UMI-77 could directly induce apoptosis through activation of the intrinsic mitochondrial pathway. Since cytochrome c release from mitochondria to the cytosol and subsequent activation of caspases represent key steps during intrinsic apoptosis, we first determined whether UMI-77 could affect this process in Panc-1 cells. Treatment for 24 h of Panc-1 cells with UMI-77 resulted in dose-dependent release of cytochrome c and Smac from mitochondria, starting at a concentration of 10 μM (Fig. 3C). Similar results were obtained after treatment of BxPC-3 cells (Fig. S7). The induction of apoptosis and release of cytochrome c was accompanied by PARP cleavage (Fig. 3D) and activation of caspase-3 quantified by densitometric analysis, where two concentrations of UMI-77 (3 and 10 μM) showed statistically significant cleaved caspase-3 versus DMSO control (Fig. 3E). These results demonstrated that UMI-77 induced apoptosis in PC through activation of the intrinsic apoptotic pathway.
In the indirect activation model (34) the activity of the apoptosis effectors Bak and Bax can be suppressed by multidomain anti-apoptotic proteins such as Mcl-1. If cell death induction is specifically mediated by Mcl-1 protein, Bax or Bak would be required for release of cytochrome c and subsequent cell death. Therefore, to investigate if UMI-77-induced apoptosis involved the disruption of Mcl-1/Bax and/or Mcl-1/Bak complexes in PC, imminoprecipitation studies were performed. Treatment of BxPC-3 cells with UMI-77 at 4 μM for 24 h resulted in inhibition of the endogenous protein-protein interactions of Bax and Bak with Mcl-1, indicating that UMI-77 induced cell death and apoptosis is through attenuating the ability of Mcl-1 to sequester pro-apoptotic proteins such as Bax and Bak (Fig. 3F). To further elucidate the role of Bak and Bax, wild type (WT) murine embryonic fibroblasts (MEF) and double knockout (DKO) cells, deficient in both Bax and Bak, were used. UMI-77 at a concentration of 10 μM induced more than 60% apoptosis in the MEF WT cells, while at the same concentration the induction of apoptosis in MEF DKO cells was significantly reduced showing only 16% apoptotic cells (Fig. 3G). These results demonstrated that UMI-77 induced apoptosis in a Bax/Bak-dependent manner. To further interrogate the mechanism of action, we examined the activation of Bax in Panc-1 and BxPC-3 cells after treatment with UMI-77 using the 6A7 anti-Bax antibody, which specifically recognizes the conformationally active form of Bax (35). The quantification of the fluorescent intensity of the active Bax in the cells treated with UMI-77 showed a substantial increase in activated Bax in both tested pancreatic cell lines starting from 5 μM (Fig. 3H and 3I and S8). These data suggest that UMI-77 treatment leads to Bax conformational change, consistent with our co-immunoprecipitation and functional studies.
Knocking down Mcl-1 expression abrogates growth inhibition and apoptosis by UMI-77
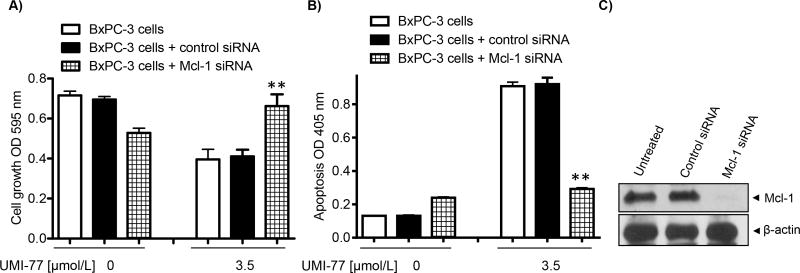
To further confirm the functional role of Mcl-1 in UMI-77-mediated cell growth inhibition and induction of apoptosis, we examined whether siRNA-mediated silencing of Mcl-1 expression would affect UMI-77 induced apoptosis. Cells were treated for 72 h with the 3.5 μM of UMI-77, after 24 hours siRNA transfection. UMI-77 induced 50% cell growth inhibition and 7-fold induction of apoptosis in BxPC-3 cells transfected with control siRNA (Fig. 4A and 4B). However when BxPC-3 cells were treated with UMI-77 in the presence of Mcl-1 siRNA, sensitivity of BxPC-3 cells to UMI-77-induced cell growth and apoptosis were significantly reduced by Mcl-1 silencing (p < 0.05) in comparison with cells transfected with control siRNA. In addition, the level of induced apoptosis in BxPC-3 cells treated with UMI-77 and Mcl-1 siRNA was similar as in the cells treated only with Mcl-1 siRNA, clearly suggesting that UMI-77 induced apoptosis is mediated by Mcl-1 (Fig. 4B). Immunoblot analysis indicated that Mcl-1 siRNA completely and specifically inhibits Mcl-1 protein expression under the treatment condition and not in control siRNA samples, confirming efficient silencing (Fig. 4C). Collectively these data are emphasizing the critical role of Mcl-1 in UMI-77 cellular efficacy in PC.
Fig. 4. Down-regulation of Mcl-1 by siRNA in BxPC-3 cells blocks growth inhibition and apoptosis induced by 2 (UMI-77).
A) Cell growth inhibition determined with MTT assay. B) Induction of apoptosis detected using Histone/DNA ELISA assay. C) Mcl-1 protein expression in non transfected Bx-PC3 cells and transfected with control siRNA and Mcl-1 siRNA. Cells were transiently transfected with siRNA against Mcl-1 or control siRNA for 24 h and then treated with the indicated concentration of UMI-77 for 72 h. *p<0.05 vs control siRNA and UMI-77 3.5 μM. The experiment was replicated two times independently and gave consistent results.
UMI-77 exhibits single-agent antitumor activity in BxPC-3 xenograft model
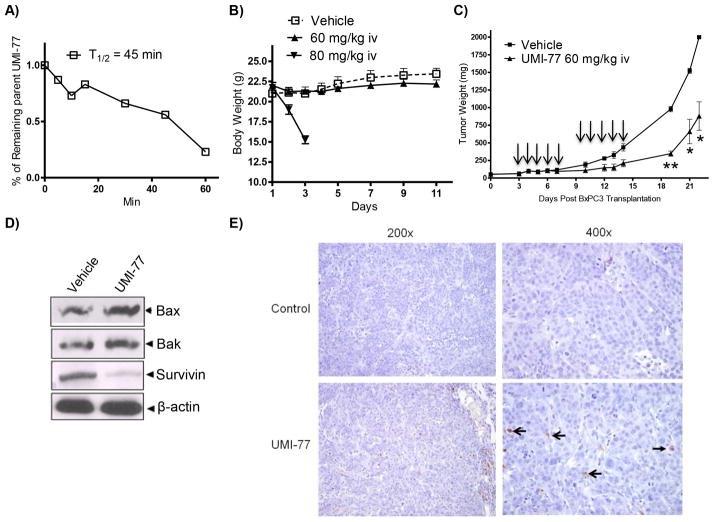
The in vitro data described earlier prompted us to extend these observations and test the in vivo efficacy of UMI-77 in a BxPC-3 xenograft model in SCID mice. First, we tested its in vitro microsomal stability by incubating UMI-77 with pooled mice liver microsomes. UMI-77 exhibited moderate metabolic stability with a half-life of 45 minutes (Fig. 5A). This result was promising for continuation with in vivo efficacy studies in view of the fact that in vitro microsomal stability correlates with the in vivo plasma clearance. Second we determined the maximum tolerated dose (MTD) of UMI-77 in SCID mice. Administered 60 mg/kg i.v. for 5 consecutive days per week for two weeks did not cause any loss in the animal weight and there was no obvious sign of toxicity during the course of the treatment (Fig. 5B). Increasing the dose to 80 mg/kg showed severe animal weight loss (>20%), therefore 60 mg/kg was used as a therapeutic dose for the in vivo efficacy studies. Daily treatment with UMI-77 for 5 consecutive days a week for two weeks resulted in statistically significant tumor growth inhibition by 65% and 56% in comparison with the controls in day 19 (p < 0.0001) and day 22 (p < 0.003) respectively (Fig. 5C). To elucidate the molecular mechanism of UMI-77 mediated tumor growth inhibition, western blot and immunohistochemistry on tumor tissue were performed. The western blots of the tumor tissue lysates showed slightly elevated levels of pro-apoptotic proteins, Bax and Bak, and significant decrease of survivin, one of the Inhibitors of Apoptosis Proteins (IAPs) which potently inhibits apoptosis by antagonizing caspase activity (Fig. 5D). The apoptotic cells in tumor tissue were determined by TUNEL-based in situ method and the obtained results showed that positive apoptotic cells of tumor sections were significantly increased in UMI-77-treated BxPC-3 xenograft mice as compared with the control group (Fig. 5E). The toxicity of UMI-77 on normal tissues was examined by H&E analyses (Fig. S9). Histopathology revealed that treatment of mice with UMI-77 did not cause damage to tested tissues from kidney, liver and pancreas, demonstrating that it is not toxic to normal mouse tissues. These findings provide in vivo support of the involvement of Mcl-1 regulated pathway in PC, implicating the potential of Mcl-1 inhibitors as novel antitumor agents for treatment of PC.
Fig. 5. In vivo characterization of 2 (UMI-77).
A) Determination of the microsome stability of UMI-77 (expressed as T1/2). B) Evaluation of the effect of UMI-77 on weight loss of SCID mice. UMI-77 was administrated two cycles i.v. for 5 days per week in two tested concentrations 60 and 80 mg/kg. C) In vivo efficacy of UMI-77 in BxPC-3 xenograft animal model. BxPC-3 xenografts were inoculated subcutaneously in SCID mice. Once transplanted, fragments developed into palpable tumors, about 60 mg, groups of 4 animals with bi-lateral tumors were removed randomly and assigned to two treatment groups. Mice were administered UMI-77 i.v 60 mg/kg for 5 consecutive days a week for two weeks. [■] vehicle treated group and [▲] UMI-77 treated groups. UMI-77 treated groups showed significant reduction of the tumor growth when compared to treatments with vehicle on day 19 (p < 0.0001), 20 (p < 0.003) and 21 (p < 0.003). D) Western blots analysis for different pro-apoptotic and survival markers on lysates isolated from tumours harvested from mice of different treatment groups showing enhancement of pro-apoptotic Bax and Bak and down-regulation of survivin compared with control. E) Immunohistochemical staining of BxPC-3 tumor xenografts using Apoptag Kit. No positively-staining nuclei are present in the control samples. Several positively-staining cells (open arrows) are present, as is apoptotic debris (closed arrows) in presented field (400× original magnification).
Discussion
The present study is the first report demonstrating the in vitro and in vivo efficacy of novel, selective small molecule Mcl-1 inhibitor 2 (UMI-77) in PC. A hallmark of cancer cells is defects in the apoptotic cell death program (36) and research on anti-apoptotic resistance mechanisms has shown that Mcl-1 represents an important survival factor for PC (8–10). Therefore, the obtained results provide significant proof-of-concept that SMIs that can bind Mcl-1 and block its interactions with pro-apoptotic proteins may have potential as a new targeted treatment for PC by overcoming the apoptosis resistance mechanism.
Mcl-1 is a homologous protein related to other anti-apoptotic proteins such as Bcl-2 and Bcl-xL, but there are subtle differences in the hydrophobic binding groove in Mcl-1 that translate into selective binding to the pro-apoptotic BH3-only proteins (37, 38). Using high throughput screening we have identified and validated a novel, selective Mcl-1 inhibitor, 1 (UMI-59) that binds to the BH3 binding groove and antagonizes its function. Chemical modifications of this compound led to 2 (UMI-77) with improved binding affinity to Mcl-1.
In vitro FP based binding studies showed that UMI-77 selectively binds to Mcl-1 with Ki of 490 nM, and has significantly decreased binding affinity to the additional four members of anti-apoptotic proteins. Docking and HSQC NMR binding studies provide conclusive evidence that UMI-77 binds to the BH3-binding groove of Mcl-1 protein and the interactions are mediated by two conserved hydrophobic pockets, h2 and h3, and the hydrogen bonding network including the conserved hydrogen bond interaction with Arg263.
From a functional standpoint, UMI-77 effectively targets endogenous Mcl-1, and induces apoptosis in a time and dose-dependent manner. Apoptosis induction occurs at low micro-molar doses correlating well with the in vitro binding affinity of this compound to Mcl-1 as well as its potency in inhibition of the PC cell growth. Mechanistically, apoptosis induction by UMI-77 is Bax/Bak dependent (Fig. 3F), preceded by disrupting the Mcl-1/Bak and Mcl-1/Bax complexes (Fig. 1D and 3E), followed by Bax activation (Fig. 3G), which results to a mitochondria-mediated cell death (Fig. 3C and 3D) (39–41). These results suggest that UMI-77 functions as BH3 mimetic and exhibits specific and mechanism-based cell growth inhibition. Using siRNA interference approach, knocking down the Mcl-1 expression significantly decreased induction of apoptosis and protected PC cells from killing induced by UMI-77 in comparison with control siRNA transfected cells (Fig. 4), demonstrating that Mcl-1 is a mediator of cell sensitivity to this compound and the effect is Mcl-1 dependent.
UMI-77 demonstrated robust anti-tumor efficacy in a resistant PC xenograft model with no toxicity to the surrounding tissue and minimal discomfort to the host. Molecular analysis of tumor remnants showed a significant decrease of the anti-apoptotic protein survivin, which potently inhibits apoptosis through antagonizing caspase activity. In situ apoptosis detection assays confirmed that UMI-77 induces apoptosis in the tumor tissue, but not in the control treated tumors.
In summary, we have shown that UMI-77, a novel selective small molecule Mcl-1 inhibitor, potently inhibits PC growth in vitro and in vivo. Our results provide insights into the potential role of Mcl-1 as a therapeutic target in PC treatment and suggest that antagonizing Mcl-1 function with small molecules as UMI-77 warrants further investigation as a therapeutic strategy against PC. In particular, future studies are necessary for testing UMI-77 in combination with chemotherapy and radiotherapy for the development of new therapeutic strategies to overcome the intrinsic and acquired resistance of PC cells and to enhance the treatment efficacy.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by grants from the National Cancer Institute, National Institutes of Health R21NS056915, R01CA149442 and R21CA0158976 to Z. Nikolovska-Coleska.
The authors thank M. Larsen from the Center for Chemical Genomics, University of Michigan, for technical expertise during HTS. The authors greatly appreciate support from Dr. S. Wang (University of Michigan) for providing the research environment for carrying out the HTS study and providing the murine embryonic fibroblast cells. The authors thank Dr. G.W.A. Milne for his critical reading of the manuscript.
Footnotes
Conflicts of Interest: The authors disclose no potential conflicts of interest.
References
- 1.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 2.Hamacher R, Schmid RM, Saur D, Schneider G. Apoptotic pathways in pancreatic ductal adenocarcinoma. Mol Cancer. 2008;7:64. doi: 10.1186/1476-4598-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi X, Liu S, Kleeff J, Friess H, Buchler MW. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology. 2002;62:354–62. doi: 10.1159/000065068. [DOI] [PubMed] [Google Scholar]
- 4.Fischer U, Schulze-Osthoff K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005;12 (Suppl 1):942–61. doi: 10.1038/sj.cdd.4401556. [DOI] [PubMed] [Google Scholar]
- 5.Day CL, Chen L, Richardson SJ, Harrison PJ, Huang DC, Hinds MG. Solution structure of prosurvival Mcl-1 and characterization of its binding by proapoptotic BH3-only ligands. J Biol Chem. 2005;280:4738–44. doi: 10.1074/jbc.M411434200. [DOI] [PubMed] [Google Scholar]
- 6.Day CL, Smits C, Fan FC, Lee EF, Fairlie WD, Hinds MG. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380:958–71. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 7.Zhou P, Qian L, Kozopas KM, Craig RW. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood. 1997;89:630–43. [PubMed] [Google Scholar]
- 8.Miyamoto Y, Hosotani R, Wada M, Lee JU, Koshiba T, Fujimoto K, et al. Immunohistochemical analysis of Bcl-2, Bax, Bcl-X, and Mcl-1 expression in pancreatic cancers. Oncology. 1999;56:73–82. doi: 10.1159/000011933. [DOI] [PubMed] [Google Scholar]
- 9.Schniewind B, Christgen M, Kurdow R, Haye S, Kremer B, Kalthoff H, et al. Resistance of pancreatic cancer to gemcitabine treatment is dependent on mitochondria-mediated apoptosis. Int J Cancer. 2004;109:182–8. doi: 10.1002/ijc.11679. [DOI] [PubMed] [Google Scholar]
- 10.Ren LN, Li QF, Xiao FJ, Yan J, Yang YF, Wang LS, et al. Endocrine glands-derived vascular endothelial growth factor protects pancreatic cancer cells from apoptosis via upregulation of the myeloid cell leukemia-1 protein. Biochem Biophys Res Commun. 2009;386:35–9. doi: 10.1016/j.bbrc.2009.05.149. [DOI] [PubMed] [Google Scholar]
- 11.Wei SH, Dong K, Lin F, Wang X, Li B, Shen JJ, et al. Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemother Pharmacol. 2008;62:1055–64. doi: 10.1007/s00280-008-0697-7. [DOI] [PubMed] [Google Scholar]
- 12.Guoan X, Hanning W, Kaiyun C, Hao L. Adenovirus-mediated siRNA targeting Mcl-1 gene increases radiosensitivity of pancreatic carcinoma cells in vitro and in vivo. Surgery. 2010;147:553–61. doi: 10.1016/j.surg.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 13.Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–51. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 15.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, Chen J, Meagher JL, Yang CY, Aguilar A, Liu L, et al. Design of Bcl-2 and Bcl-xL Inhibitors with Subnanomolar Binding Affinities Based upon a New Scaffold. J Med Chem. 2012;55:4664–82. doi: 10.1021/jm300178u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 19.Nikolovska-Coleska Z, Wang R, Fang X, Pan H, Tomita Y, Li P, et al. Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal Biochem. 2004;332:261–73. doi: 10.1016/j.ab.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 20.Czabotar PE, Lee EF, van Delft MF, Day CL, Smith BJ, Huang DC, et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci U S A. 2007;104:6217–22. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrödinger Suite 2010 Induced Fit Docking protocol; Glide version 5.6. Schrödinger, LLC; New York, NY: 2009. [Google Scholar]; Prime version 2.2. Schrödinger, LLC; New York, NY: 2010. [Google Scholar]
- 22.Souza-Fagundes EM, Frank AO, Feldkamp MD, Dorset DC, Chazin WJ, Rossanese OW, et al. A high-throughput fluorescence polarization anisotropy assay for the 70N domain of replication protein A. Anal Biochem. 2012;421:742–9. doi: 10.1016/j.ab.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, et al. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009;69:5575–83. doi: 10.1158/0008-5472.CAN-08-4235. [DOI] [PubMed] [Google Scholar]
- 24.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajwa N, Liao C, Nikolovska-Coleska Z. Inhibitors of the anti-apoptotic Bcl-2 proteins: a patent review. Expert Opin Ther Pat. 2012;22:37–55. doi: 10.1517/13543776.2012.644274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang CY, Wang S. Analysis of Flexibility and Hotspots in Bcl-xL and Mcl-1 Proteins for the Design of Selective Small-Molecule Inhibitors. ASC Med Chem Lett. 2012;3:308–12. doi: 10.1021/ml200301w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doi K, Li R, Sung SS, Wu H, Liu Y, Manieri W, et al. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. J Biol Chem. 2012;287:10224–35. doi: 10.1074/jbc.M111.334532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee EF, Czabotar PE, van Delft MF, Michalak EM, Boyle MJ, Willis SN, et al. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol. 2008;180:341–55. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friberg A, Vigil D, Zhao B, Daniels RN, Burke JP, Garcia-Barrantes PM, et al. Discovery of potent myeloid cell leukemia 1 (Mcl-1) inhibitors using fragment-based methods and structure-based design. J Med Chem. 2013;56:15–30. doi: 10.1021/jm301448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27 (Suppl 1):S71–83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–8. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Z, Cao K, Lin C, Li L, Liu HY, Zhao XY, et al. The p53 upregulated modulator of apoptosis (PUMA) chemosensitizes intrinsically resistant ovarian cancer cells to cisplatin by lowering the threshold set by Bcl-x(L) and Mcl-1. Mol Med. 2011;17:1262–74. doi: 10.2119/molmed.2011.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–9. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi Y, Karbowski M, Yamaguchi H, Kazi A, Wu J, Sebti SM, et al. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol Cell Biol. 2005;25:9369–82. doi: 10.1128/MCB.25.21.9369-9382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 37.Hinds MG, Day CL. Regulation of apoptosis: uncovering the binding determinants. Curr Opin Struct Biol. 2005;15:690–9. doi: 10.1016/j.sbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, et al. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–14. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–73. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 41.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.