Abstract
Prenatal nutrient restriction (NR) culminating in intra-uterine growth restriction (IUGR) with postnatal catch up growth leads to diabesity. In contrast, postnatal NR with growth restriction (PNGR) superimposed on IUGR (IPGR) protects young and aging adults from this phenotype. We hypothesized that PNGR/IPGR will compromise the blood-brain metabolic profile impairing neurobehavior and predisposing to Alzheimer’s disease (AD). NR (50%) in late gestation followed by cross-fostering of rat pups to either ad lib fed (CON) or NR (50%) lactating mothers generated CON, IUGR, PNGR and IPGR male (M) and female (F) offspring that were examined through the life span. In PNGR/IPGR plasma/CSF glucose and lactate decreased while ketones increased in (M) and (F) (PN21, PN50). In addition increased brain glucose transporters, Glut1 & Glut3, greater brain derived neurotrophic factor (BDNF), reduced Glut4, with unchanged serotonin transporter concentrations were noted in (F) (PN50-60). While (F) displayed more hyperactivity, both (F) and (M) exhibited anxiety although socially and cognitively unimpaired (PN25-28&50). Aging (15-17m) (F) not (M), expressed low plasma insulin, reduced brain IRS-2, pAkt, and pGSK-3pSer9, unchanged pPDK1, pTau or lipoprotein receptor related protein 1 (LRP1), higher glial fibrillary acidic protein (GFAP) and spinophilin but a 10-fold increased amyloid-p42. We conclude that therapeutically superimposing PNGR on IUGR (IPGR) should be carefully weighed in light of unintended consequences related to perturbed neurobehavior and potential predilection for AD.
Keywords: nutrient restriction, Alzheimer’s disease, insulin, amyloid beta, anxiety
1. Introduction
Prenatal nutrient restriction with postnatal ad lib access to food results in obesity, type 2 diabetes mellitus, hypertension, cardiovascular disease and dyslipidemia (Barker et al., 2005; Burdge et al., 2009; Evensen et al., 2009; Godfrey et al.; Horton, 2005; Morrison et al.; Zigman and Elmquist, 2003). More recently, introduction of postnatal caloric restriction imposed on prenatal caloric restriction with intra-uterine growth restriction (IUGR), protected the offspring from obesity and type 2 diabetes in males (Garg et al., 2012a; Garg et al., 2012b) and gestational diabetes in females (Garg et al., 2010). While a positive effect of postnatal caloric restriction is encountered on the long term metabolic outcome, a negative impact of postnatal caloric restriction on neuropsychiatric outcomes could prove to be a limiting factor in considering this intervention for overcoming the expected metabolic phenotype. Certain rodent (Medina-Aguirre et al., 2008) and human (Costello et al., 2007; Dellava et al.) studies have associated neuropsychiatric disorders with early nutrient restriction.
Epidemiological investigations have supported the association between early nutrient restriction and the subsequent acquisition of depression and schizophrenia (Guimaraes et al., 2008; Loh et al., 2006; Strassnig et al., 2005). In addition, specific aberrations in the metabolic profile such as hypoglycemia have recently been linked with autism (synaptic disconnectivity syndrome) and attention deficit disorders (neurotransmitter-linked) in children (Ito, 2009; Quackenbush et al., 1999; Udani et al., 2009). Aberrant neurological development as encountered in children with Down syndrome has led to the acquisition of Alzheimer’s disease subsequently with aging (Cenini et al., 2012; Coppus et al., 2011; Moncaster et al., 2010). Further, in adults the association of type 2 diabetes with Alzheimer’s disease has uncovered a relationship between features of insulin resistance encountered in insulin signaling with hyperphosphorylation of microtubule associated Tau protein intra-cellularly necessary for forming neurofibrillary tangles and extracellular amyloid- β plaque development, key characteristics of Alzheimer’s disease (Carro and Torres-Aleman, 2004; Devaskar and Thamotharan, 2007; Finder et al., 2010; Gouras et al., 2000; Holscher, 2011; Irie et al., 2005; Keeney et al.; Li et al., 2007; Liu et al., 2008; Masters et al., 2006). In addition, more recently conditions in humans and mice with insulin deficiency as encountered in type 1 diabetes mellitus (Devi et al., 2012; Liu et al., 2011; Menon and Farina, 2011; Wang et al., 2010) or serum insulin-like growth factor deficiency (Poirier et al., 2012) have also been associated with Alzheimer’s disease. However insulin signaling in brain within the context of insulin deficiency and characteristics of Alzheimer’s disease has not been fully delineated.
We hypothesized that postnatal nutrient restriction by itself or when superimposed on IUGR would 1) disturb the early neuropsychiatric equipoise reliant on circulating and brain metabolic homeostasis in male and female offspring, 2) alter brain glucose transporters and insulin signaling mediating hyperphosphorylation of the microtubule-associated tau protein and/or accumulation of amyloid-β protein, characteristics of Alzheimer’s disease, upon aging. Our testing of these hypotheses led to the observations that postnatal nutrient restriction by itself (PNGR) but more so when imposed on IUGR (IPGR): 1) disturbed the blood-CSF (cerebrospinal fluid) metabolic profile and caused anxiety in both sexes with hyperactivity in females alone; these changes were associated with enhanced brain glucose transporters (Glut1, Glut3) and brain-derived neurotrophic factor (BDNF) 2) reduced circulating insulin and brain insulin signaling (IRS2, pAkt, Glut4) while activating pGSK-3βSer9 and enhancing spinophilin and glial fibrillary acidic protein expression in aging females promoted amyloid β42 accumulation consistent with features of Alzheimer’s disease.
2. Results
The study design generating four experimental groups consisting of CON, IUGR, PNGR and IPGR is shown schematically in Figure 1 (Garg et al., 2012b; Shin et al., 2012).
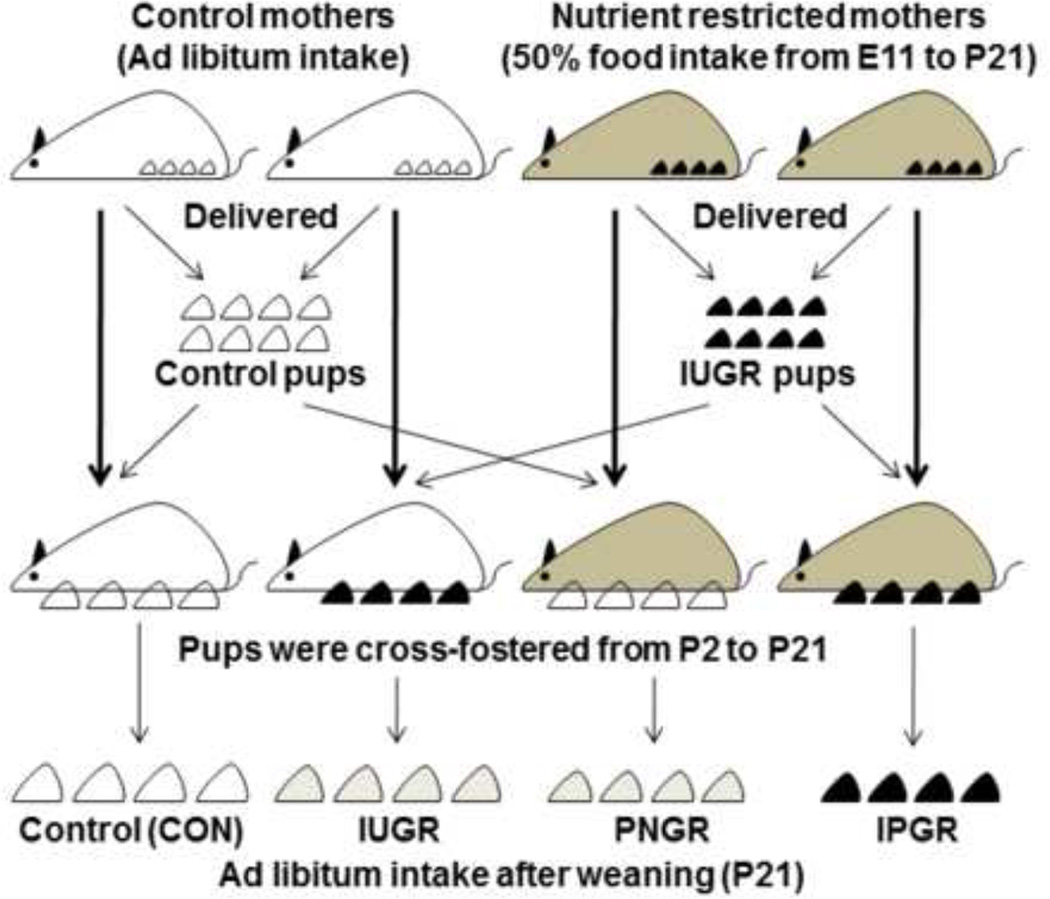
Figure 1. Scheme of the Study Design.
Study design demonstrating the four experimental groups obtained by cross-fostering postnatal rat pups. Nutrient restricted mothers received 50% of daily food intake from mid- to late pregnancy [embryonic day (E) 11 to E21] through lactation [postnatal day (P) 1 to P21].
I. Post-weaned and Young Adult investigations
Body weights
PNGR and IPGR are lighter than CON while female IUGR but not male IUGR is heavier than CON at PN21 (table 1) and PN50 (table 2). This reduction in weight was more prominent at PN50 than PN21 in both males and females of PNGR and IPGR groups (tables 1 and 2).
Table 1.
Phenotype changes in PN21
| A male | CON | IUGR | PNGR | IPGR |
|---|---|---|---|---|
| Body weight (g) | 65.7 ± 1.7 | 64.3 ± 1.0 | 22.8 ± 0.7*** | 23.5 ± 0.7*** |
| Plasma glucose (mM) | 12.3 ± 1.3 | 9.60 ± 0.61* | 7.61 ± 0.70** | 7.82 ± 0.69** |
| CSF glucose (mM) | 3.55 ± 0.28 | 3.25 ± 0.23 | 2.30 ± 0.15** | 3.11 ± 0.25 |
| Plasma lactate (mM) | 2.47 ± 0.06 | 2.24 ± 0.33 | 2.73 ± 0.29 | 2.23 ± 0.27 |
| CSF lactate (mM) | 1.22 ± 0.05 | 1.10 ± 0.10 | 1.65 ± 0.23 | 1.34 ± 0.12 |
| Plasma ketones (µM) | 526 ± 46 | 658 ± 101 | 899 ± 64** | 934 ± 60** |
| CSF ketones (µM) | 182 ± 24 | 261 ± 57 | 615 ± 37* | 506 ± 12* |
| B female | CON | IUGR | PNGR | IPGR |
| Body weight (g) | 56.0 ± 0.9 | 59.8 ± 1.7* | 22.7 ± 0.6*** | 22.3 ± 0.6*** |
| Plasma glucose (mM) | 11.4 ± 0.31 | 8.65 ± 0.19*** | 6.65 ± 0.21*** | 5.36 ± 0.19*** |
| CSF glucose (mM) | 3.84 ± 0.14 | 3.50 ± 0.13 | 2.81 ± 0.21*** | 2.84 ± 0.12*** |
| Plasma lactate (mM) | 0.762 ± 0.077 | 0.843 ± 0.051 | 0.783 ± 0.111 | 0.723 ± 0.080 |
| CSF lactate (mM) | 0.618 ± 0.067 | 0.698 ± 0.038 | 0.614 ± 0.126 | 0.624 ± 0.086 |
| Plasma ketones (µM) | 457 ± 80 | 544 ± 86 | 669 ± 56 | 560 ± 50 |
| CSF ketones (µM) | 191 ± 27 | 227 ± 36 | 514 ± 60*** | 485 ± 20*** |
Data are shown as means ± SE (n=5–6).
P<0.05;
P<0.01;
P<0.001 vs. CON by one-way ANOVA.
Table 2.
Phenotype changes in PN50
| A male | CON | IUGR | PNGR | IPGR |
|---|---|---|---|---|
| Body weight (g) | 276 ± 5 | 254 ± 5** | 184 ± 1*** | 198 ± 4*** |
| Plasma glucose (mM) | 8.72 ± 0.32 | 7.74 ± 0.25 | 5.85 ± 0.23*** | 5.32 ± 0.64*** |
| CSF glucose (mM) | 3.29 ± 0.20 | 2.85 ± 0.13 | 2.15 ± 0.11*** | 2.04 ± 0.24*** |
| Plasma lactate (mM) | 1.16 ± 0.08 | 1.08 ± 0.07 | 0.906 ± 0.054* | 0.947 ± 0.056 |
| CSF lactate (mM) | 1.23 ± 0.04 | 1.17 ± 0.06 | 1.05 ± 0.03** | 1.15 ± 0.03 |
| Plasma ketones (µM) | 460 ± 69 | 381 ± 66 | 396 ± 41 | 302 ± 65 |
| CSF ketones (µM) | 271 ± 10 | 271 ± 6 | 433 ± 29* | 413 ± 59 |
| B female | CON | IUGR | PNGR | IPGR |
| Body weight (g) | 168 ± 6 | 189 ± 5* | 153 ± 3** | 148 ± 4** |
| Plasma glucose (mM) | 9.27 ± 0.59 | 8.90 ± 0.53 | 7.31 ± 0.94 | 9.74 ± 1.23 |
| CSF glucose (mM) | 2.75 ± 0.37 | 2.33 ± 0.11 | 2.92 ± 0.41 | 3.55 ± 0.47 |
| Plasma lactate (mM) | 1.97 ± 0.22 | 1.81 ± 0.17 | 1.26 ± 0.14** | 1.39 ± 0.17 |
| CSF lactate (mM) | 1.54 ± 0.06 | 1.52 ± 0.07 | 1.35 ± 0.07 | 1.23 ± 0.03* |
| Plasma ketones (µM) | 688 ± 38 | 766 ± 45 | 647 ± 75 | 456 ± 134 |
| CSF ketones (µM) | 149 ± 28 | 180 ± 17 | 124 ± 18 | 91.1 ± 32.9 |
Data are shown as means ± SE (n=5–6).
P<0.05;
P<0.01;
P<0.001 vs. CON by one-way ANOVA.
Plasma and CSF metabolites
Only PNGR and IPGR displayed low plasma and CSF glucose concentrations at PN21 (table 1) and PN50 (table 2) in males and females. Reduced plasma and CSF lactate concentrations were seen in male and female offspring at PN50 (table 2) with no such difference at PN21 (table 1). In contrast, CSF ketones were increased in both male and female offspring at PN21 (table 1). Plasma ketones were only increased in PN21 male PNGR/IPGR versus CON, with no similar change in female (table 1). At PN50, no inter-group difference existed in plasma ketone concentrations in both males and females. CSF ketones were differentially elevated only in PN50 male PNGR with a similar trend in IPGR. However no change in PN50 female CSF ketones was observed (table 2).
Brain serotonin transporter, BDNF and Glucose transporter concentrations
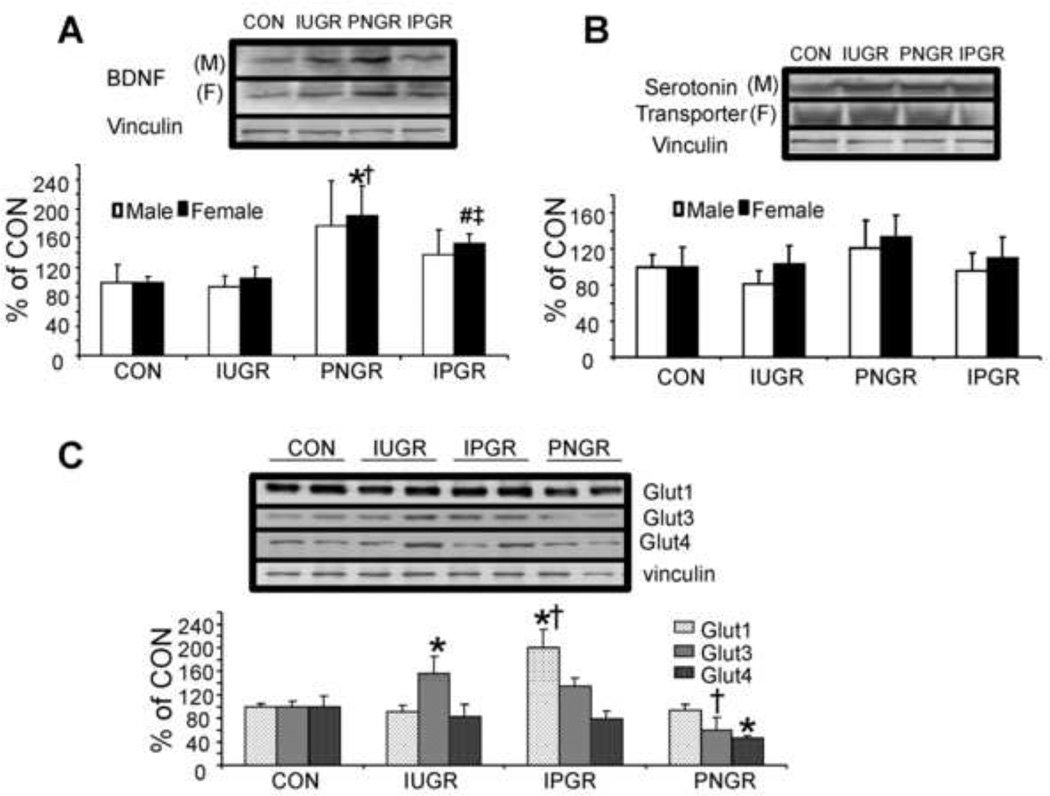
At PN60 particularly in females brain BDNF concentrations (regulates synaptic function, structure and plasticity) were elevated in both PNGR and IPGR compared to CON (p<0.02, p<0.01 Fig. 2A), while brain serotonin transporter protein concentrations were no different between the four groups in both males and females (Fig. 2B). At PN60, as seen in the female offspring, brain Glut1 concentrations increased in IPGR versus CON (p<0.05) while brain Glut4 concentrations in PNGR were reduced versus CON (p<0.05) (Fig. 2C). In contrast, brain Glut3 concentrations were noted to increase only in the IUGR group versus that of CON (p<0.05) declining to control concentrations in both the IPGR and PNGR groups (Fig. 2C).
Figure 2. Brain BDNF, serotonin and glucose transporters.
Effect of early nutrient restriction on brain derived neurotrophic factor (BDNF) (A), serotonin transporter (B), glucose transporters (C, female only) protein concentrations derived from the male and female postnatal day 60 cerebral cortical region. The order of lanes is CON, IUGR, PNGR and IPGR in (A) and (B) with *p<0.02 versus CON, †p<0.02 versus IUGR by one-way ANOVA and Fisher’s PLSD and #p<0.01 versus CON, ‡p<0.05 versus IUGR by unpaired t-test. The order of lanes is CON, IUGR, IPGR and PNGR in (C) alone with *p<0.05 versus CON and †p<0.003 versus IUGR by one-way ANOVA and Fisher’s PLSD. N=6 in each group per protein examined.
Neurobehavioral testing
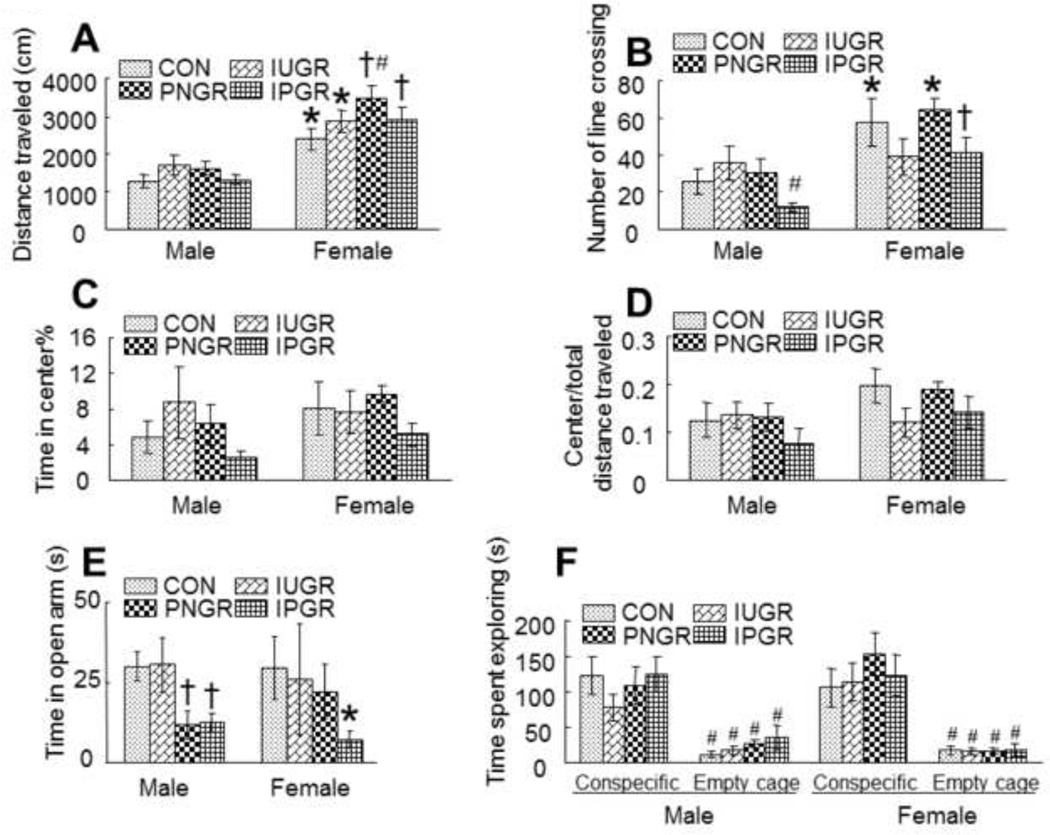
Open Field: On comparing the locomotor activity of the male with female offspring, females in all four groups showed significantly increased locomotor activity and line crossings than their corresponding male counterparts. Particularly while the female PNGR displayed more activity than the sex-matched CON with respect to distance travelled, the male IPGR exhibited less line crossing activity versus the sex-matched CON group (Fig. 3A & B). However, no differences were evident in the open field between the four experimental groups with respect to the time spent in the center versus total distance travelled. The females in the IPGR trended towards spending less time in the center suggesting more time spent in the covered or dark areas related to anxiety, although this difference was not statistically significant (Fig. 3C & D).
Elevated Maze test: This trend in female IPGR towards a difference in anxiety level observed by open field testing was further confirmed by the elevated plus maze test which assesses anxiety responses in rodents. Anxiety was determined by comparing time spent in open versus closed arms of the elevated maze. The IPGR male and female and the PNGR male alone spent significantly less time in the open arms versus their sex-matched and age-matched CON counterparts (Fig. 3E), consistent with enhanced anxiety affecting the amygdala-limbic system.
Sociability test: While both males and females in all four groups spent more time with the conspecific mouse rather than in the empty cage, no inter-group differences in sociability that tested the pre-frontal cortex were evident (Fig. 3F).
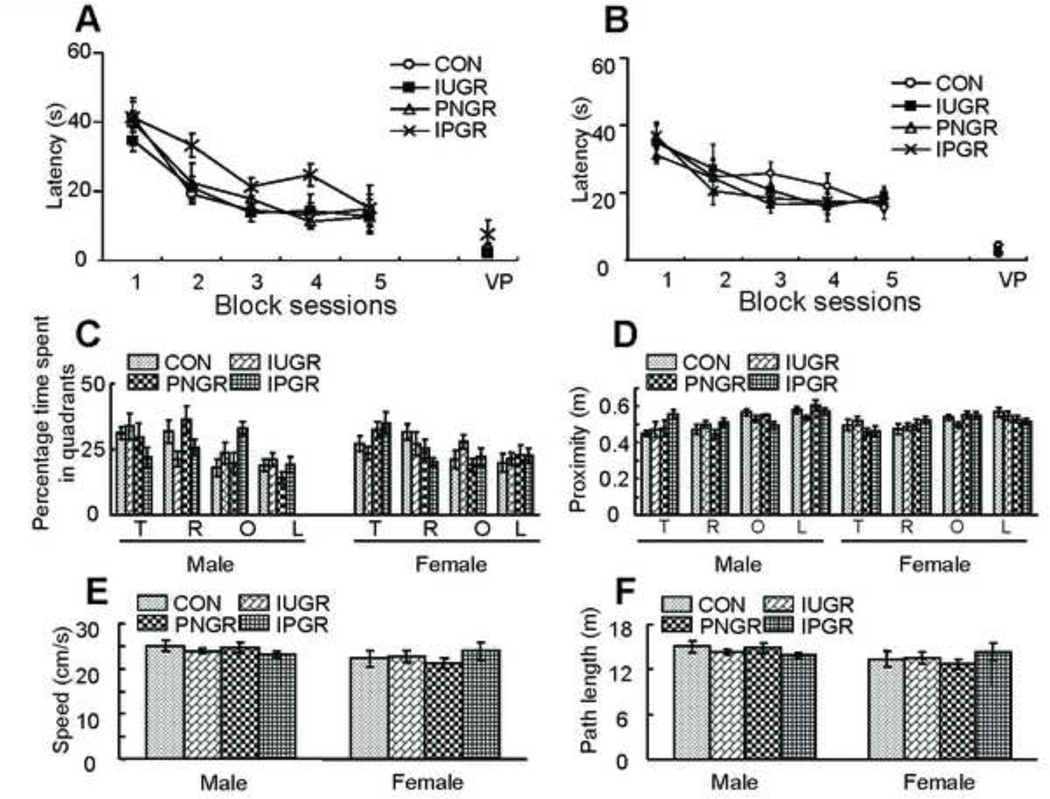
Water Maze test: The Morris Water Maze is designed to test spatial learning during the training block sessions and memory during the probe trial. This test of spatial cognition involves a hippocampus-dependent task. All animals were trained to learn to escape from the pool during the hidden version of the Morris Water Maze. Analysis of escape latencies revealed a trend towards longer escape latency in the IPGR males although not statistically significant (Fig. 4A), suggestive of impaired spatial cognition. No significant difference was observed in terms of escape latency in males or females in all four groups (Fig. 4A & B). Spatial learning memory was assessed by a probe trial conducted after completion of the training block trial sessions. Analysis of quadrant occupancy and proximity displayed the IPGR male to trend towards lower target quadrant occupancy scores suggestive of altered spatial memory, however, no significant difference was observed among the four groups whether male or female (Fig. 4C & D). Total path length and swim speed were no different between the sexes or groups (Fig. 4E & F). Thus, no differences were observed in perceptive, motivational and motor skills between the groups, male or female.
Figure 3. Neurobehavioral testing of Activity and Anxiety.
Effect of early nutrient restriction on activity and anxiety-like behavior in an open field test. A: Two-way ANOVA revealed significant effects of early nutrient restriction (F=2.873, P=0.043) and gender difference (F=66.166, P<0.001). Tukey’s post hoc analysis demonstrated that female rats had a significantly increased activity level than their corresponding male counterparts (*P<0.01, male vs. female in control or IUGR; †P<0.001, male vs. female in PNGR or IPGR). While no statistical difference was noted between the male groups, the PNGR female traveled more distance than the sex-matched CON (*P<0.05). B: Two-way ANOVA demonstrated no significant effect of nutrient restriction (F=2.207, P=0.096) but a significant gender effect (F=16.723, P<0.001). Tukey’s post hoc analysis revealed that female rats overall had a significantly increased number of line crossings between center and periphery than their corresponding male counterparts (*P<0.01, female vs. male in CON or PNGR; †P<0.05, female vs. male in IPGR). In male, IPGR displayed a reduced number of line crossings between center and periphery when compared to IUGR (#P<0.05). C: Time spent in the center zone did not show any significant difference between the various nutrient treatment groups (Two-way ANOVA, F=1.066, P=0.37). D: Female rats displayed increased exploration of the center in the open field test, expressed as the ratio of center to total distance traveled, compared to male rats (Two-way ANOVA, F=5.395, P=0.023). However, no significant difference was noted between different nutrient treatment groups (Two-way ANOVA, F=1.738, P=0.168). E: Effect of early nutrient restriction on anxiety-like behavior in elevated plus maze test. Two-way ANOVA showed a significant effect of early nutrient restriction (F=3.069, P=0.034). Tukey’s post hoc analysis demonstrated that in male, PNGR and IPGR tended to spend less time in the open arm when compared with control (†p<0.03), in female, IPGR had a tendency to spend less time in the open arm (*p<0.05 vs male). F: Effect of early nutrient restriction on social behavior in sociability test. All animals spent more time actively exploring the cage containing the conspecific rat than the empty cage (Three-way ANOVA, F=98.018, P<0.001; Tukey’s post hoc analysis, #P<0.001, conspecific vs. empty cage in CON, IUGR, PNGR and IPGR). However, neither early nutrient restriction group (Three-way ANOVA, F=.0.947, P=0.422) nor sex-specific effects (Three-way ANOVA, F=0.233, P=0.631) were noted in sociability testing.
Figure 4. Neurobehavioral testing of Spatial learning and memory.
Effects of early nutrient restriction on escape latency in the Morris Water Maze test. A: Two-way repeated-measures ANOVA analysis demonstrated that a nonsignificant trend toward higher escape latencies was observed in IPGR male (A) group (F=2.74, P=0.07 between different nutrient restriction treatments). No early nutrient restriction effect was observed in females (B) (Two-way repeated-measures ANOVA, F=0.446, P=0.723 between different nutrient restriction treatments). Data from four training sessions were averaged and are shown as one block session. C: T=target, O=opposite, R=right and L=left quadrants. Three-way ANOVA revealed a significant effect of early nutrient restriction (nutrient restriction × quadrant interaction: F=2.367, P=0.015) but no gender effect (sex × quadrant interaction: F=1.162, P=0.326) in quadrant occupancy scores. D: T=target, O=opposite, R=right and L=left quadrants. Three-way ANOVA demonstrated no significant effect of early nutrient restriction (nutrient restriction × quadrant interaction: F=1.672, P=0.1) and no gender effect (sex × quadrant interaction: F=1.448, P=0.231) in proximity to the target location. One-way ANOVA analysis demonstrated that early nutrient restriction had no effect on either speed (E) (Male, n=6, P=0.522; Female, n=6, P=0.687) or path length (F) (Male, n=6, P=0.517; Female, n=6, P=0.678).
II. Aging Investigations
Plasma insulin and IGF-1 (Insulin-like growth factor 1) concentrations
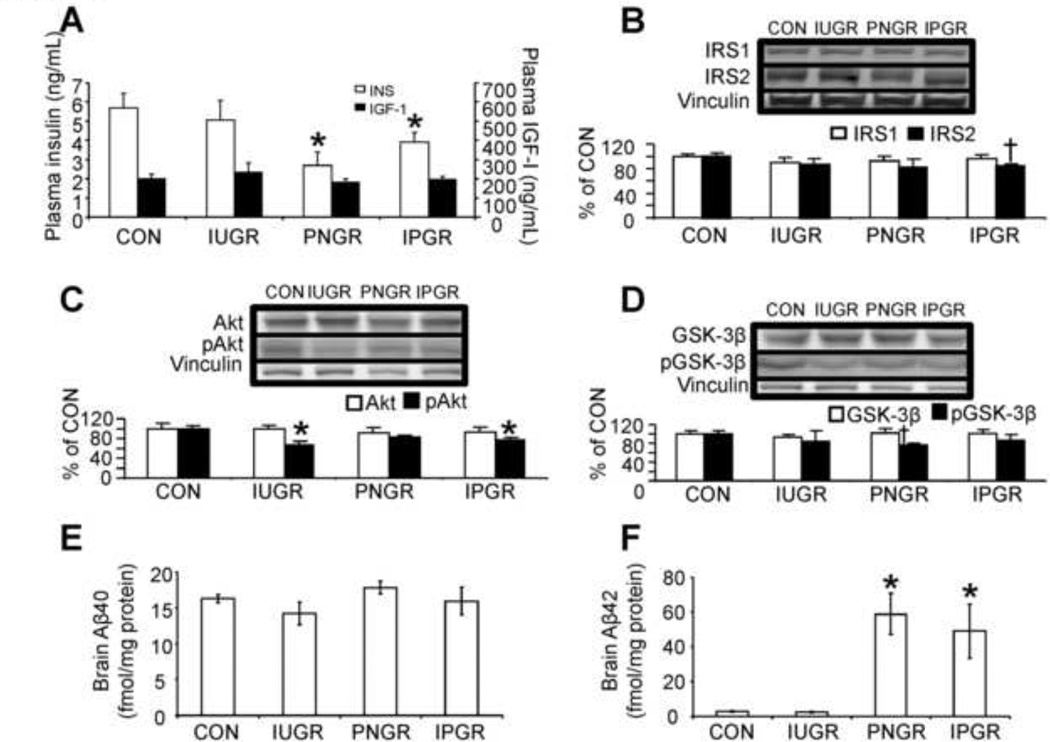
At 15–17m of age, circulating insulin concentrations in the female offspring were lower in PNGR and IPGR when compared to CON and IUGR (Fig. 5A) as previously reported (Garg et al., 2006). No changes in IGF-1 concentrations were seen in any of the four groups.
Figure 5.
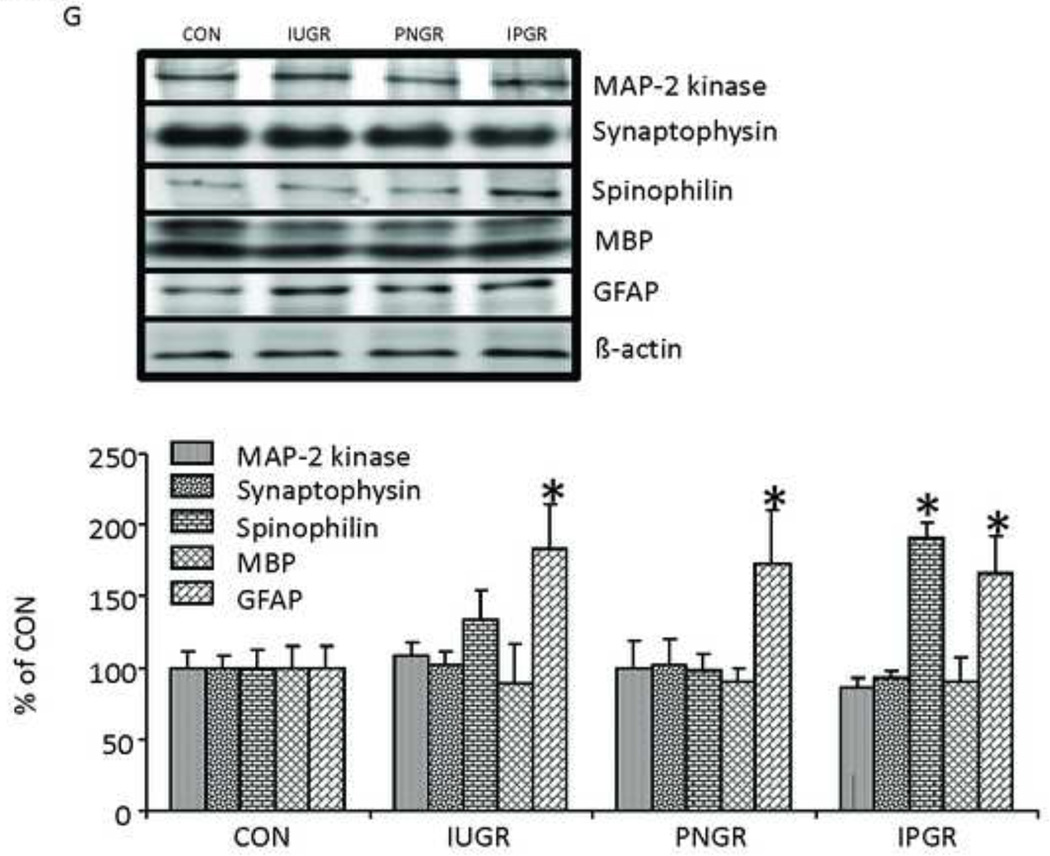
Aging Brain Insulin Signaling, Amyloid-β and Cell-specific Marker Proteins. In 15–17 month old female rats, plasma concentrations of insulin (INS) and insulin-like growth factor 1 (IGF-I) (A), brain insulin signaling proteins, IRS1 & IRS2 proteins (B), total Akt and pAkt proteins (C), total GSK-3β and pGSK-3β proteins (D), amyloid-β40 (E), amyloid-β42 (F) and Cell-specific markers (MAP-2 kinase, synaptophysin, spinophilin, myelin basic protein [MBP] and GFAP) (G) are shown. n=5–6 per group per protein. Vinculin or beta-actin served as the internal control. Representative Western blots are shown on top (B–D,G), and the corresponding densitometric quantification analyses of protein bands are shown as % of control (CON) at the bottom. *P<0.05 vs. CON by one-way ANOVA and Fisher’s PLSD. †P<0.05 vs. CON by Student’s unpaired t-test.
Brain Insulin Signaling pathway
While no inter-experimental group differences in insulin receptor substrate 1 (IRS-1) was observed (Fig. 5B), a decline in IRS-2 was evident in IUGR, PNGR and IPGR versus CON with IPGR alone achieving statistical significance (Fig. 5B). p(phosphorylated form) PDK (phosphoinositide-dependent protein kinase) 1 and pmTOR (mammalian target of rapamycin) concentrations were no different in IUGR, PNGR and IPGR versus CON (Supplemental Fig. A, B). In contrast, a decline in pAkt was seen in the IPGR and an activation seen as a decline in serine 9 phosphorylation of GSK-3β (pGSK-3β; glycogen synthetase kinase) was seen in PNGR versus CON (Fig. 5C, D).
Brain tau and amyloid-β proteins
While no statistical differences were noted, a slight trend towards increasing hyperphosphorylation of tau (~20%) was noted particularly in the female PNGR and IPGR groups versus that of CON (Supplemental Fig. C). Lipoprotein receptor related protein (LRP) 1 concentrations were no different in female IUGR, PNGR and IPGR versus CON although a trend towards a decrease (~20%) was evident in IUGR and PNGR (Supplemental Fig. D). Additionally, no difference in female brain amyloid β40 isoform was observed between the four experimental groups (Fig. 5E). In contrast a striking increase in female brain amyloid β42 protein concentration was observed in the PNGR and IPGR groups when compared to both the CON and IUGR groups (Fig. 5F). Examination of the male aging counterpart revealed no inter-group differences in any of these measurements including amyloid β42 (negative data not shown).
Brain cell specific marker proteins
We observed no inter-group differences in MAP2 kinase (marker of neuronal dendrites), synaptophysin (marker of pre-synaptic neuronal axons) and myelin basic protein (MBP, marker of oligodendrocytes) between IUGR, PNGR, IPGR and CON. In contrast, while an increase in spinophilin (marker of post-synaptic dendritic spines) was observed in IPGR, a uniform increase in glial fibrillary acidic protein (GFAP; marker of astrocytes) was seen in IUGR, PNGR and IPGR when compared to CON (Fig. 5G).
3. Discussion
This is the first investigation that has systematically examined the blood-brain metabolic profile along with brain transport mechanisms and neurobehavior in the post-weaned and young adult male and female offspring exposed to early nutrient restriction. While the IUGR with postnatal catch up growth demonstrated mild if any metabolic derangements, the IPGR and PNGR, revealed reduced circulating and CSF glucose concentrations. These changes were more pronounced at PN21 versus PN50 and seen in males and females. Plasma and CSF lactate concentrations declined in PNGR/IPGR PN50 males and females when consuming a high carbohydrate containing rat chow diet. Both the PN21 PNGR and IPGR demonstrated enhanced circulating and CSF ketone concentrations when consuming a high fat milk diet. Thus, in the presence of a limited supply of glucose and lactate, ketones must serve as the fuel supplying brain energy metabolism during this early phase of life. However at PN50 while the PNGR/IPGR males continued to demonstrate protection by elevated CSF ketones, this protection was absent in age-matched females. While we did not measure insulin or IGF-I at these early stages in this study, we have previously assessed them and noted insulin and IGF-I to be lower in PNGR and IPGR groups versus CON (Abbasi et al., 2011; Shin et al., 2012; Thamotharan et al., 2005). These hormonal changes are congenial for the use of alternate substrates to fuel brain energy metabolism. In addition to these presently observed changes, we and others have previously demonstrated deficiencies in macronutrients and micronutrients with early nutrient restriction, all of which can influence brain development of the offspring (Beard et al., 2005; Bhasin et al., 2009; Eden, 2005; Rivera et al., 2003; Thamotharan et al., 2005; Torres et al., 2010).
We also noted that the glucose transporter isoform (Glut1) that predominantly resides in the blood-brain barrier was increased significantly in the IPGR group, perhaps to compensate for the diminution in circulating glucose concentrations. A similar observation was made earlier in life at fetal day 22, PN1 to PN21 in the offspring born to rat mothers with uteroplacental insufficiency and fetal growth restriction (Sadiq et al., 1999). While the neuronal Glut3 concentrations were unperturbed in IPGR and PNGR, increased concentrations were noted in the IUGR alone, again perhaps reflecting in-utero compensation to reduced availability of substrate (glucose). In contrast, a reduction in the insulin responsive Glut4 was seen in only the PNGR group signifying reduced insulin signaling at this early young adult stage. While no previous studies defining brain Glut3 and Glut4 in the adult offspring exposed to early nutrient restriction exist, chronic corticosterone exposure in the adult rat was observed to reduce hippocampal Glut4 concentrations (Piroli et al., 2007). No change in brain serotonin transporter (present on post-synaptic neurons) concentrations was observed. However, the increase in BDNF concentrations seen only in PNGR and IPGR females is reflective of a compensatory mechanism, perhaps targeting preservation of synaptic function and plasticity, when subjected to an early life metabolic injury. Previous investigations in transgenic female mice overexpressing BDNF displayed higher anxiety scores suggesting that greater concentrations seen in female PNGR and IPGR in our present study may have a similar behavioral effect (Papaleo et al., 2011). Hence we explored this association with neurobehavioral tests.
While early life nutrient restriction perturbed the blood/CSF metabolic profile in both sexes and resulted in compensatory changes of key brain proteins favoring the female, neurobehavioral changes appeared to be sex-specific. The young IPGR females were more hyperactive while the male IPGR were less active, in keeping with the elevated brain BDNF concentrations in the females. This observed hyperactivity was not related to perceptual, motivational or motor skill abnormalities, similar to the previously described transgenic BDNF overexpressing female mice (Papaleo et al., 2011). However both females and males IPGR/PNGR exhibited signs of heightened anxiety. In contrast both males and females retained normal sociability and spatial learning and memory, despite the enhanced anxiety. These encountered neurobehavioral features were reminiscent of an anxiety disorder and/or attention deficit disorder that constitute neuropsychiatric conditions (Aiello and Whitaker-Azmitia, 2011; Pfaff et al., 2011). Retention of normal sociability and spatial learning and memory perhaps rules out conditions akin to autism spectrum disorders or other cognitive dysfunctional disorders (Mair et al., 1991; Silverman et al., 2011; Tanda et al., 2009). While brain serotonin transporter concentrations were preserved in both sexes, since we did not measure serotonin or dopamine concentrations, it is difficult to speculate on the neurotransmitter related mechanisms for the display of these aberrant neurobehaviors in the young PNGR/IPGR adults. However, our investigation supports a role for early, particularly postnatal nutrient restriction in perturbing neurobehavior during development stages reflective of childhood or young adult.
In addition, our observed neurobehavioral perturbations support involvement of specific brain regions. The noted heightened anxiety in response to early life nutrient restriction implicates the amygdala within the temporal cortex and its wide network of efferent projections to hippocampus and prefrontal cortex. Particularly the basolateral nuclear complex of the amygdala regulates emotional-arousal effects on memory consolidation (Roozendaal et al., 2009), by increasing dorsal hippocampal concentrations of activity regulated cytoskeletal-associated protein (ARC), which is the downstream signaling molecule of BDNF (Moonat et al., 2010). Synaptic plasticity within the amygdala and corticolimbic system influenced by early life nutrient restriction as described previously with stress can cause adaptive or maladaptive responses during adult life (Muhammad and Kolb, 2011a; Muhammad and Kolb, 2011b). Similarly, the dorsal hippocampal region mediates memory and learning processes while the ventral region is related to emotional and anxiety processes (Barkus et al., 2010; Fanselow and Dong, 2010; Snyder et al., 2009). The prefrontal cortex (mainly layers V and VI) and sub-cortical nuclei including the striatum and nucleus accumbens have also been implicated in mediating anxiety behavior (Kambe et al., 2011). Based on these previous studies our present neurobehavioral findings in male and female PNGR and IPGR young adult groups supports involvement of the central and medial amygdala and ventral hippocampus that mediate anxiety. In contrast, the normal water maze and sociability test results in our present study suggest sparing of the baso-lateral amydala, dorsal hippocampus and prefrontal cortex that collectively mediate consolidation of memory and cognition.
Long term follow-up of the aging offspring subjected to postnatal nutrient restriction revealed a reduction in circulating insulin concentrations in the PNGR and IPGR females in our present study. Instead hyperinsulinemia is a hallmark of insulin resistance and type 2 diabetes mellitus (Haan, 2006; Takeda et al., 2011). However, this reduction in the insulin ligand of the insulin signaling pathway led to a reduction in brain IRS-2 and diminished activation of Akt (pAkt) with increased activation of pGSK-3β Ser9. While no similar reduction in either IRS-1 or pmTOR was evident, the changes observed are consistent with reduced IRS-2-Akt with activated pGSK-3βSer9 mediated brain insulin signaling in female aging IPGR/PNGR offspring. Previously transgenic overexpression of pGSK-3β led to neurodegeneration of the dorsal hippocampal region resulting in altered cognition and memory, while preserving the ventral hippocampus that mediates emotional anxiety states (Fuster-Matanzo et al., 2011), findings different from our present neurobehavioral changes observed earlier in the young adult. Exposure to postnatal nutrient restriction also perturbs the developing pancreatic β-cells (Matveyenko et al., 2010) with what appears to be permanent effects seen as reduced circulating insulin concentrations lasting into aging. The consequent reduction in brain insulin signaling due to early nutrient restriction mimics that seen with hypoinsulinemia of type 1 diabetes mellitus (Brodsky and Devlin, 1996; Hamadeh and Hoffer, 2003) as well as that observed with brain insulin resistance present in adult acquired type 2 diabetes mellitus (Kahn et al., 2006; Taguchi et al., 2007). The two conditions of early nutrient restriction and type 1 diabetes mellitus are a result of chronic and persistent hypoinsulinemia while type 2 diabetes mellitus is in association with hyperinsulinemia.
Previous investigations in adult onset type 2 diabetes mellitus have demonstrated that a reduction in insulin signaling (IRS2-pAkt) (Schubert et al., 2003) along with activation of GSK-3β (seen as reduced serine 9 pGSK-3β) lend themselves to hyperphosphorylating the intrinsic microtubule-associated Tau protein. Such hyperphosphorylation is required for the formation of neurofibrillary tangles which begin initiating the extracellular accumulation of amyloid protein which ultimately forms plaques that destroy neurons, thereby leading to the clinical features of Alzheimer’s disease (Finder et al., 2010; Keeney et al., 2011; Kirkitadze and Kowalska, 2005; Yin et al., 2007). In separate adult rat investigations, exogenous delivery of amyloid-β42 into the hippocampus impaired Akt-dependent insulin signaling and membrane-associated Glut4 (Pearson-Leary and McNay, 2012). In our present investigation, IPGR and PNGR displayed no change only a trend towards hyperphosphorylation of tau while both PNGR and IPGR groups demonstrated a ten-fold increase in the amyloid-β42 which represents the main fibrillar species most likely responsible for the neuropathology of Alzheimer’s disease (Deng et al., 2006; Irie et al., 2005; Kim et al., 2007). Aβ42 is first elevated in the brain of patients with AD (Gouras et al., 2000; Iwatsubo et al., 1994), implying that abnormal accumulation of Ap42 is a critical early stage in AD neuropathology. Aβ42 is known to accumulate in response to axonal autophagy rather than overt damage (Nixon, 2007), which is a possibility in our present investigation. Therefore, 15–17 month old female rats in PNGR and IPGR groups are likely to be in the early stages of AD. Similar accumulation of amyloid-β42 was not seen in aging PNGR or IPGR males. This observation in rodents is supported by human evidence of aging women having a higher incidence of Alzheimer’s disease compared to men (Musicco, 2009; Nedoschill et al., 1999; Schmidt et al., 2008; Thies and Bleiler, 2012; Vina and Lloret, 2010).
In addition, the female aging brains revealed increased signs of astrogliosis in IUGR, PNGR and IPGR groups. However, no major changes in oligodendrocytes or pre-synaptic (synaptophysin) or post-synaptic neurons (MAP2-kinase) were otherwise evident in any of these groups, supporting early stages of amyloid-β42 accumulation prior to overt neuronal destruction and perhaps loss. Enhanced astrogliosis is also observed with dystrophic neurites, hippocampal atrophy and amyloid accumulation in vessels as seen in transgenic Alzheimer disease mouse models (Wiedlocha et al., 2012). Hence it is possible that astrogliosis with accumulating amyloid β I-42 particularly in the aging female PNGR and IPGR unlike the younger adult counterpart may affect cognition and memory (Chen et al., 2000) and anxiety (Espana et al., 2010; Wiedlocha et al., 2012) depending on the brain regions and cell types involved.
However the significance of increased spinophilin (marker of post-synaptic neuronal dendritic spines) in IPGR was not known. Spinophilin mutated mice expressed increased dendritic spine density with reduced long term depression during the early developmental stages (Feng et al., 2000). Thus increased spinophilin in the aging IPGR female rat may signify the opposite, namely decreased dendritic spine density that supports reduced cognitive abilities. Alternatively, increased spinophilin as observed in the IPGR group may signify compensatory enhanced dendritic spines and synaptic activity in keeping with the anxiety phenotype seen earlier in life. Previous studies have demonstrated increased dendritic spine density observed in the amygdala to be associated with anxiety states (Moonat et al., 2010). Anxiety in turn manifests when intraneuronal amyloid β peptide accumulates in the baso-lateral amygdala as seen in Alzheimer’s disease (Espana et al., 2010).
Amyloid-β production is promoted by GSK-3α and reduced by GSK-3 inhibitors (Phiel et al., 2003). Although the mechanism whereby GSK-3 promotes Aβ production remains to be clarified, GSK-3-mediated phosphorylation of presenilin-1 (Uemura et al., 2007) which is part of the y-secretase complex, may be a mechanism that is required for Aβ production (Brunkan and Goate, 2005). Furthermore, the elevated production of Aβ42, with no significant change in Aβ40, has been reported in transgenic mice bearing familial AD mutations in presenilin-1 (Chui et al., 1999). Accordingly, it is possible that the decreased plasma insulin influences brain insulin signaling including the activation of GSK-3β which in turn promotes the Aβ42 production in PNGR and IPGR groups either via presenilin-1 or enhanced phosphorylation of Tau protein (only a trend noted here). However, since we did not measure brain presenilin 1 in this study, and there was GSK-3β activation seen by us in PNGR alone, we can only speculate on the role of presenilin 1 in producing amyloid-β42 in aging IPGR/PNGR females. In the absence of a diminution in LRP1 (only a trend seen here) which in turn serves as the clearance pathway preventing accumulation of amyloid β peptide (Sagare et al., 2007), we can surmise that in our present study amyloid-β42 accumulates not due solely to a defect in the clearance but rather secondary to enhanced production perhaps reliant on reduced insulin signaling related activation of GSK-3β.
Postnatal nutrient deficiency seen in PNGR and IPGR perturbed brain insulin signaling and increased Aβ42 accumulation in aging females. Linkages between early life nutrient deficiency and glucose-insulin related metabolic perturbations (Devaskar and Thamotharan, 2007; Garg et al., 2006; Oak et al., 2006; Ozanne et al., 2003; Thamotharan et al., 2005) have been established as has been the link between insulin deficiency and AD-like changes in the brain (Devi et al., 2012; Li et al., 2007; Liu et al., 2011; Menon and Farina, 2011; Wang et al., 2010). However, this is the first direct evidence demonstrating a link between postnatal nutrient restriction and sex-specific AD-like brain changes with aging. Although animal studies inclusive of c. elegans, mice and monkeys demonstrate that nutrient restriction imposed on adults promotes successful brain aging including the prevention of Aβ neuropathology (Anson et al., 2003; Gillette-Guyonnet and Vellas, 2008; Kastman et al., 2012; Mair et al., 2003; Qin et al., 2006; Schulz et al., 2007; Wang et al., 2005; Willette et al., 2012), instead postnatal nutrient restriction which takes place during a critical window of brain development increases the risk of AD with aging.
In conclusion, we have shown that postnatal nutrient restriction either by itself or when imposed on IUGR, have neurological implications in the post-weaning, young adult and aging stages of life. Early on between post-weaning and young adulthood, sex-specific changes in neurobehavior consistent with hyperactivity in females but heightened anxiety in both males and females are encountered. These neuropsychiatric effects are consequent to perturbed blood-brain metabolic profile and greater BDNF concentrations despite the compensatory increase in ketones and specific brain glucose transporters. In contrast to these symptoms in the young adult, the aging female adult offspring demonstrates signs of astrogliosis and perturbed dendritic spines along with features consistent with early Alzheimer’s disease. These findings are of considerable significance as interventions related to curbing postnatal nutrient intake to overcome the metabolic maladaptive consequences of IUGR are being considered. One must balance the metabolic benefits achieved against the subsequent neuropsychiatric and perhaps aging associated cognitive impairments that are unintended consequences of postnatal nutrient restriction. Future studies fine tuning such postnatal nutritional interventions towards optimization must maximize the metabolic benefit while minimizing the long lasting neurobehavioral impairments that are detrimental throughout the life span. Additionally alternate postnatal neuroprotection should be sought such as enhancing endogenous ketone supply.
4. Experimental Procedure
Animals
Sprague-Dawley rats (Charles River Laboratories, Hollister, CA) were housed in individual cages, exposed to 12h light/dark cycles at 21–23°C, and allowed ad lib access to standard rat chow (composition: carbohydrate 63.9%, fat 4.5% and protein 14.5%). Animal care and use were approved by the Animal Research Committee of the University of California, Los Angeles.
Animal Model of Early Life Nutrient Restriction
Pregnant rats received 50% of their daily food intake (11g/day) beginning from day 11 through day 21 of gestation, which constitutes mid- to late gestation, compared to their control counterparts that fed ad libitum and consumed 20g/day of rat chow. Both groups had free access to drinking water. At birth, the litter size was culled to six to ensure no inter-litter postnatal nutritional variability. Cross-fostering of pups generated four experimental groups as previously described by us (Garg et al., 2006; Shin et al., 2012). Pups born to ad lib feeding control mothers were reared by either mothers on nutrient restriction from PN1 to PN21 (PNGR) or by control mothers (CON). During the suckling phase, the progeny born to nutrient restricted mothers was reared by either control mothers (IUGR) or by nutrient restricted mothers (IPGR). After weaning from mothers, all groups received ad lib access to rat chow and water and were raised until 15 to 17 months of life. Examinations were undertaken at various ages ranging from postnatal (PN) 21, PN25, PN26, PN28, PN35–40, PN50 and PN60 for studies in the young and at 15–17 months for studies during aging.
Cerebrospinal fluid (CSF), plasma and brain collections
Rats were anesthetized at PN21 and PN50 with intraperitoneal injection of sodium pentobarbital (50 mg/kg) (Shin et al., 2012). CSF was collected from the cisterna magna compartment as previously described (Pegg et al., 2010). Briefly, the tissue above the cisterna magna was retracted to expose the translucent meninges overlying the cisterna magna. The surrounding area was gently cleaned with cotton swabs to remove any residual blood. A small 25g needle was used to penetrate the meningeal membranes covering the cisterna magna and 5–10 µL of CSF collected. Blood was subsequently collected from the common carotid artery and the plasma was separated by centrifugation and stored at −80°C. Brains were collected at PN21, PN50–60 and 15–17 months of age and immediately snap-frozen in liquid nitrogen and powdered on dry ice. All samples were stored at −80°C until further analysis.
Glucose, Lactate, and total ketone measurement
Glucose, lactate, and total ketone concentrations were respectively measured by Liquid Glucose Measurement Set (Pointe Scientific, Lincoln Park, MI), Lactate Assay Kit (BioVision, Mountain View, CA), and Autokit Total Ketone Bodies (Wako, Osaka, Japan) following instructions according to manufacturers’ protocols.
Plasma Insulin and IGF-I Assays
Plasma Insulin and IGF-I were quantified by enzyme-linked immunoabsorbent assays using anti-rat insulin antibody (sensitivity: insulin = 0.2 ng/ml; Linco Research, St. Charles, MO) or using anti-IGF-I antibody (sensitivity: mouse/rat IGF-I=3.5 pg/ml; R&D Systems, Minneapolis, MN), respectively.
Brain Amyloid-β Assay
Powdered brain was homogenized in 10 volumes of Tris/sucrose buffer (250 mM sucrose, 20 mM Tris, 1 mM EDTA, and 1 mM EGTA, pH 7.4) containing protease inhibitors. Subsequently, protein extracts were prepared by 1:1 dilution of the initial homogenate with ice-cold 0.4% diethylamine (DEA) in 100 mM NaCl, centrifuged at 100,000g at 4°C for 1 hour, and neutralized by adding 0.5 M Tris-HCl, pH 6.8. Concentrations of Aβ40 and Aβ42 in protein extracts were quantified by ELISA using Human/Rat β Amyloid (40) ELISA kit Wako II (sensitivity; 0.049 pmol/L, Wako, Osaka, Japan) and Human/Rat β Amyloid (42) ELISA kit Wako High-Sensitive (sensitivity; 0.024 pmol/L, Wako, Osaka, Japan), respectively.
Antibodies
Rabbit anti-serotonin transporter and rabbit anti-brain derived neurotrophic factor (BDNF) were purchased from Millipore (Temecula, CA). Rabbit anti-IRS1, anti-IRS2, anti-Akt, anti-phosphorylated (p) Akt (Ser473), anti-PDK1, anti-pPDK1 (Ser241), anti-GSK-3β, anti-pGSK-3β (Ser9), anti-mTOR, and anti-pmTOR (Ser2448) were from Cell Signaling Technology (Danvers, MA). Mouse anti-Tau (Tau-5) and rabbit anti-pTau (Ser396) were purchased from Invitrogen (Camarillo, CA). Synaptophysin and spinophilin antibodies were obtained from Millipore (Temecula, CA). MAP2-kinase, myelin basic protein and glial fibrillary acidic protein (GFAP) antibodies were purchased from Neuromics (Edina, MN). Glut1, Glut3, and Glut4 antibodies were from sources as previously described (Shin et al., 2004), Mouse anti-vinculin and anti-beta actin antibodies were from Sigma Chemical Co. (St. Louis, MO).
Western blot analysis
Powdered brain was homogenized and sonicated either in cell lysis buffer (Cell Signaling Technology) or in PBS containing protease inhibitors (20 µg/ml pepstatin A, 20 µg/ml leupeptin, 30 µg/ml aprotinin, and 2 mM PMSF), 1% Nonidet P-40, and 5 mM EDTA as previously described (Shin et al., 2012). The resulting suspension was centrifuged at 10,000g at 4°C for 10 min, and the supernatant was subjected to Western blot analysis as previously described (Oak et al., 2006). Briefly, samples were solubulized in lysis buffer, separated on SDS-PAGE and transferred to a nitrocellulose membrane by electroblotting. The membrane was then blocked in 5% nonfat dry milk in phosphate-buffered saline containing 0.1% Tween-20 (PBST) for 1 hour and incubated with specific primary antibody [1:1000 dilution for serotonin transporter, BDNF, IRS1, IRS2, Glut1, Glut3, Glut4, Akt, pAkt (Ser473), PDK1, pPDK1 (Ser241), GSK-3β, pGSK-3β (Ser9), mTOR, and pmTOR (Ser2448); 1:2000 dilution for Tau and pTau (Ser396); 1:10,000 for vinculin] overnight at 4°C. In the case of antibodies against cell-specific markers, 1:15,000 for synaptophysin, 1:1000 for spinophilin, 1:15,000 for MAP2-kinase, 1:20,000 for myelin basic protein and 1:60,000 for GFAP with either vinculin at 1:10,000 or beta-actin at 1:2,500 dilution as internal controls were employed over 1hr at room temperature. The membrane was washed with PBST and subsequently incubated with the appropriate secondary horseradish peroxidase-conjugated antibody at an optimal dilution for 1hr at room temperature. After washing the membrane with PBST, protein bands were visualized using ECL plus kit (GE Healthcare, Buckinghamshire, UK) and Typhoon Scanner (GE Healthcare). The quantification of protein bands was performed by densitometry using Image Quant software (GE Healthcare). The optical density was corrected for inter-lane loading variability using an internal control, vinculin (beta-actin in the case of cell-specific markers), and expressed as a percent of CON.
Behavior studies
Animals were housed in groups of three in plastic cages and kept in a room on a 12-h light/dark schedule at 23°C, 60% humidity, with food and tap water available ad libitum. On the day of behavior testing, the rats were brought to the behavior room at least an hour before testing time and left undisturbed in a quiet setting. After each behavior test, the apparatus was cleaned with 70% ethanol, wiped with hand towels and allowed to air dry in between animal testing. At first open-field test was conducted following a rest period of 1 h, the same animals were tested in light-dark exploration test the same day.
Open field test: Open field, locomotor activity and exploratory behavior were measured in the open field test on day two (PN25) after acclimation. The rats were placed in the center of the square Plexiglas enclosure and were left free to explore the arena for 20 min. Activity was quantified using a computer-operated Topscan system (Cleversys, Inc, Reston, Virginia). The program tabulated total distance traveled, ratio of center distance traveled to total distance traveled and percentage of time spent in center.
Elevated plus maze, anxiety behavior was tested on the elevated plus maze on PN26. The elevated plus maze, described in detail elsewhere (Langley-Evans, 2007), consisted of two open arms (50 cm × 10 cm) crossed at right angles with two opposed arms of the same size. Two of the opposed arms were enclosed by wooden walls 40 cm high, except for the central part where the arms crossed. The whole apparatus was elevated 50 cm above the floor. Rats were placed in the central area facing an open arm on the maze, and their behavior was video recorded for 5 min as they explored the maze. Video recordings were scored for time spent by each rat in each segment of the maze (open arms, closed arms and center). An arm entry was defined as a rat’s head and both forepaws occupying the arm.
Sociability test: The sociability test was adapted from Crawley et al. An apparatus (length, width and height) was split into three chambers; the two side chambers each contained one empty perforated plastic container (7 × 7 × 16 cm). First, test animals (PN27 to PN28) were placed into the center chamber and allowed to acclimate to all three chambers for 10 min. After habituation, a stimulus animal (an age- and sex- matched novel rat) was placed into one of the perforated plastic containers (social chamber), whereas the other container remained empty (non-social chamber). The test animal was returned to the apparatus for 10 min. Active exploration (approach, sniffing, rearing) of the conspecific and the empty container by the test rat was recorded and scored offline.
Morris water maze: Morris water maze (PN30–35) was performed to assess spatial learning. The water maze consists of a white circular tank with a diameter of 1 meter. The water was made opaque with white tempura paint and maintained at 24°C. The pool was arbitrarily divided into four quadrants. A circular transparent escape platform of 12 cm diameter was submerged 1.5 cm below the surface of the water in the pool quadrant (the target quadrant). The position of the platform was kept constant throughout acquisition period. During training trials, the rat was placed into the pool at a randomly chosen starting location, in one of the three quadrants that did not contain the hidden platform. Each training trial lasted 1 min, or until the rat remained on the platform for 1 s. Rats that did not find the platform during the trial were placed on the platform for 5 s post-trial. A single probe trial was given after completion of the training, in which the platform was removed. Visible platform training was given after the hidden version of the Morris water maze had been completed. Tracking information was processed by the HVS Water maze software package (HVS Image, Buckingham, UK). Primary measures of interest were escape latency in training trials and quadrant occupancy and proximity to target in probe trials.
Data Analysis
Data are expressed as mean±standard error of the mean. The analysis of variance models were used to compare various treatment groups and the F-values obtained. Inter-group differences were determined by the Fisher’s PLSD (one way) or Turkey’s (2-way or 3-way) tests when ANOVA revealed significance. When only two experimental groups were compared, the Student’s unpaired t-test was used. Significance was assigned when the p value was < 0.05.
Supplementary Material
Highlights for Review.
Early nutrient restriction reduces CSF glucose and lactate but increases ketones.
These metabolic changes increase brain glucose transporters and BDNF during the young adult stage.
Females are hyperactive and both males and females demonstrate anxiety.
During aging, the females demonstrate features characteristic of Alzheimer’s disease.
Acknowledgements
This work was supported by grants from the NIH HD 25024, HD 41230 and HD 33997. We thank Shingo Ito for technical input with the brain amyloid beta assays, and Ravikumar Ponnusamy in the UCLA Behavioral Testing Core Laboratory for assistance with neurobehavioral studies.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbasi A, Thamotharan M, Shin BC, Jordan MC, Roos KP, Stahl A, Devaskar SU. Myocardial Macro-Nutrient Transporter Adaptations in the Adult Pre-gestational Female Intra-uterine and Postnatal Growth Restricted Offspring. Am J Physiol Endocrinol Metab. 2011 doi: 10.1152/ajpendo.00539.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello TP, Whitaker-Azmitia PM. Sexual differentiation and the neuroendocrine hypothesis of autism. Anat Rec (Hoboken) 2011;294:1663–1670. doi: 10.1002/ar.21251. [DOI] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 2010;626:49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JL, Hendricks MK, Perez EM, Murray-Kolb LE, Berg A, Vernon-Feagans L, Irlam J, Isaacs W, Sive A, Tomlinson M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr. 2005;135:267–272. doi: 10.1093/jn/135.2.267. [DOI] [PubMed] [Google Scholar]
- Bhasin KK, van Nas A, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes. 2009;58:559–566. doi: 10.2337/db07-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky IG, Devlin JT. Effects of dietary protein restriction on regional amino acid metabolism in insulin-dependent diabetes mellitus. Am J Physiol. 1996;270:E148–E157. doi: 10.1152/ajpendo.1996.270.1.E148. [DOI] [PubMed] [Google Scholar]
- Brunkan AL, Goate AM. Presenilin function and gamma-secretase activity. J Neurochem. 2005;93:769–792. doi: 10.1111/j.1471-4159.2005.03099.x. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Lillycrop KA, Jackson AA. Nutrition in early life, and risk of cancer and metabolic disease: alternative endings in an epigenetic tale? Br J Nutr. 2009;101:619–630. doi: 10.1017/S0007114508145883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Torres-Aleman I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer's disease. Eur J Pharmacol. 2004;490:127–133. doi: 10.1016/j.ejphar.2004.02.050. [DOI] [PubMed] [Google Scholar]
- Cenini G, Dowling AL, Beckett TL, Barone E, Mancuso C, Murphy MP, Levine H, 3rd, Lott IT, Schmitt FA, Butterfield DA, Head E. Association between frontal cortex oxidative damage and beta-amyloid as a function of age in Down syndrome. Biochim Biophys Acta. 2012;1822:130–138. doi: 10.1016/j.bbadis.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QS, Kagan BL, Hirakura Y, Xie CW. Impairment of hippocampal long-term potentiation by Alzheimer amyloid beta-peptides. J Neurosci Res. 2000;60:65–72. doi: 10.1002/(SICI)1097-4547(20000401)60:1<65::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Chui DH, Tanahashi H, Ozawa K, Ikeda S, Checler F, Ueda O, Suzuki H, Araki W, Inoue H, Shirotani K, Takahashi K, Gallyas F, Tabira T. Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nat Med. 1999;5:560–564. doi: 10.1038/8438. [DOI] [PubMed] [Google Scholar]
- Coppus AM, Schuur M, Vergeer J, Janssens AC, Oostra BA, Verbeek MM, van Duijn CM. Plasma beta amyloid and the risk of Alzheimer's disease in Down syndrome. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Worthman C, Erkanli A, Angold A. Prediction from low birth weight to female adolescent depression: a test of competing hypotheses. Arch Gen Psychiatry. 2007;64:338–344. doi: 10.1001/archpsyc.64.3.338. [DOI] [PubMed] [Google Scholar]
- Dellava JE, Thornton LM, Hamer RM, Strober M, Plotnicov K, Klump KL, Brandt H, Crawford S, Fichter MM, Halmi KA, Jones I, Johnson C, Kaplan AS, Lavia M, Mitchell J, Rotondo A, Treasure J, Woodside DB, Berrettini WH, Kaye WH, Bulik CM. Childhood anxiety associated with low BMI in women with anorexia nervosa. Behav Res Ther. 2010;48:60–67. doi: 10.1016/j.brat.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Tarassishin L, Kallhoff V, Peethumnongsin E, Wu L, Li YM, Zheng H. Deletion of presenilin 1 hydrophilic loop sequence leads to impaired gamma-secretase activity and exacerbated amyloid pathology. J Neurosci. 2006;26:3845–3854. doi: 10.1523/JNEUROSCI.5384-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaskar SU, Thamotharan M. Metabolic programming in the pathogenesis of insulin resistance. Rev Endocr Metab Disord. 2007;8:105–113. doi: 10.1007/s11154-007-9050-4. [DOI] [PubMed] [Google Scholar]
- Devi L, Alldred MJ, Ginsberg SD, Ohno M. Mechanisms underlying insulin deficiency-induced acceleration of beta-amyloidosis in a mouse model of Alzheimer's disease. PLoS One. 2012;7:e32792. doi: 10.1371/journal.pone.0032792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden AN. Iron deficiency and impaired cognition in toddlers: an underestimated and undertreated problem. Paediatr Drugs. 2005;7:347–352. doi: 10.2165/00148581-200507060-00003. [DOI] [PubMed] [Google Scholar]
- Espana J, Gimenez-Llort L, Valero J, Minano A, Rabano A, Rodriguez-Alvarez J, LaFerla FM, Saura CA. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer's disease transgenic mice. Biol Psychiatry. 2010;67:513–521. doi: 10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Evensen KA, Steinshamn S, Tjonna AE, Stolen T, Hoydal MA, Wisloff U, Brubakk AM, Vik T. Effects of preterm birth and fetal growth retardation on cardiovascular risk factors in young adulthood. Early Hum Dev. 2009;85:239–245. doi: 10.1016/j.earlhumdev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci U S A. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finder VH, Vodopivec I, Nitsch RM, Glockshuber R. The recombinant amyloid-beta peptide Abeta1-42 aggregates faster and is more neurotoxic than synthetic Abeta1-42. J Mol Biol. 2010;396:9–18. doi: 10.1016/j.jmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Fuster-Matanzo A, Llorens-Martin M, de Barreda EG, Avila J, Hernandez F. Different susceptibility to neurodegeneration of dorsal and ventral hippocampal dentate gyrus: a study with transgenic mice overexpressing GSK3beta. PLoS One. 2011;6:e27262. doi: 10.1371/journal.pone.0027262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M, Thamotharan M, Rogers L, Bassilian S, Lee WN, Devaskar SU. Glucose metabolic adaptations in the intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab. 2006;290:E1218–E1226. doi: 10.1152/ajpendo.00474.2005. [DOI] [PubMed] [Google Scholar]
- Garg M, Thamotharan M, Pan G, Lee PW, Devaskar SU. Early exposure of the pregestational intrauterine and postnatal growth-restricted female offspring to a peroxisome proliferator-activated receptor-{gamma} agonist. Am J Physiol Endocrinol Metab. 2010;298:E489–E498. doi: 10.1152/ajpendo.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M, Thamotharan M, Dai Y, Lee PWN, Devaskar SU. Hepatic de novo lipogenesis: Indicator of adaptations in lipid and glucose metabolism expressed in response to intrauterine and postnatal calorie restriction. Diabetes. 2012a under review. [Google Scholar]
- Garg M, Thamotharan M, Dai Y, Thamotharan S, Shin BC, Stout D, Devaskar SU. Early Postnatal Caloric Restriction Protects Adult Male Intra-Uterine Growth Restricted Offspring from Obesity. Diabetes. 2012b;61:1–8. doi: 10.2337/db11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette-Guyonnet S, Vellas B. Caloric restriction and brain function. Curr Opin Clin Nutr Metab Care. 2008;11:686–692. doi: 10.1097/MCO.0b013e328313968f. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes LR, Jacka FN, Gama CS, Berk M, Leitao-Azevedo CL, Belmonte de Abreu MG, Lobato MI, Andreazza AC, Cereser KM, Kapczinski F, Belmonte-de-Abreu P. Serum levels of brain-derived neurotrophic factor in schizophrenia on a hypocaloric diet. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1595–1598. doi: 10.1016/j.pnpbp.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Haan MN. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat Clin Pract Neurol. 2006;2:159–166. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- Hamadeh MJ, Hoffer LJ. Effect of protein restriction on sulfur amino acid catabolism in insulin-dependent diabetes mellitus. Am J Physiol Endocrinol Metab. 2003;284:E382–E389. doi: 10.1152/ajpendo.00295.2002. [DOI] [PubMed] [Google Scholar]
- Holscher C. Diabetes as a risk factor for Alzheimer's disease: insulin signalling impairment in the brain as an alternative model of Alzheimer's disease. Biochem Soc Trans. 2011;39:891–897. doi: 10.1042/BST0390891. [DOI] [PubMed] [Google Scholar]
- Horton TH. Fetal origins of developmental plasticity: animal models of induced life history variation. Am J Hum Biol. 2005;17:34–43. doi: 10.1002/ajhb.20092. [DOI] [PubMed] [Google Scholar]
- Irie K, Murakami K, Masuda Y, Morimoto A, Ohigashi H, Ohashi R, Takegoshi K, Nagao M, Shimizu T, Shirasawa T. Structure of beta-amyloid fibrils and its relevance to their neurotoxicity: implications for the pathogenesis of Alzheimer's disease. J Biosci Bioeng. 2005;99:437–447. doi: 10.1263/jbb.99.437. [DOI] [PubMed] [Google Scholar]
- Ito T. Children's toxicology from bench to bed--Liver injury (1): Drug-induced metabolic disturbance--toxicity of 5-FU for pyrimidine metabolic disorders and pivalic acid for carnitine metabolism. J Toxicol Sci. 2009;34(Suppl 2):SP217–SP222. doi: 10.2131/jts.34.sp217. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kambe T, Motoi Y, Inoue R, Kojima N, Tada N, Kimura T, Sahara N, Yamashita S, Mizoroki T, Takashima A, Shimada K, Ishiguro K, Mizuma H, Onoe H, Mizuno Y, Hattori N. Differential regional distribution of phosphorylated tau and synapse loss in the nucleus accumbens in tauopathy model mice. Neurobiol Dis. 2011;42:404–414. doi: 10.1016/j.nbd.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Kastman EK, Willette AA, Coe CL, Bendlin BB, Kosmatka KJ, McLaren DG, Xu G, Canu E, Field AS, Alexander AL, Voytko ML, Beasley TM, Colman RJ, Weindruch RH, Johnson SC. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J Neurosci. 2012;32:11897–11904. doi: 10.1523/JNEUROSCI.2553-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney JT, Swomley AM, Harris JL, Fiorini A, Mitov MI, Perluigi M, Sultana R, Butterfield DA. Cell Cycle Proteins in Brain in Mild Cognitive Impairment: Insights into Progression to Alzheimer Disease. Neurotox Res. doi: 10.1007/s12640-011-9287-2. [DOI] [PubMed] [Google Scholar]
- Keeney JT, Swomley AM, Harris JL, Fiorini A, Mitov MI, Perluigi M, Sultana R, Butterfield DA. Cell Cycle Proteins in Brain in Mild Cognitive Impairment: Insights into Progression to Alzheimer Disease. Neurotox Res. 2011 doi: 10.1007/s12640-011-9287-2. [DOI] [PubMed] [Google Scholar]
- Kim J, Onstead L, Randle S, Price R, Smithson L, Zwizinski C, Dickson DW, Golde T, McGowan E. Abeta40 inhibits amyloid deposition in vivo. J Neurosci. 2007;27:627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkitadze MD, Kowalska A. Molecular mechanisms initiating amyloid beta-fibril formation in Alzheimer's disease. Acta Biochim Pol. 2005;52:417–423. [PubMed] [Google Scholar]
- Langley-Evans SC. Metabolic programming in pregnancy: studies in animal models. Genes Nutr. 2007;2:33–38. doi: 10.1007/s12263-007-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56:1817–1824. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008;582:359–364. doi: 10.1016/j.febslet.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer's disease and diabetes. J Pathol. 2011;225:54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh C, Meyer JM, Leckband SG. A comprehensive review of behavioral interventions for weight management in schizophrenia. Ann Clin Psychiatry. 2006;18:23–31. doi: 10.1080/10401230500464646. [DOI] [PubMed] [Google Scholar]
- Mair RG, Knoth RL, Rabchenuk SA, Langlais PJ. Impairment of olfactory, auditory, and spatial serial reversal learning in rats recovered from pyrithiamine-induced thiamine deficiency. Behav Neurosci. 1991;105:360–374. [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Masters CL, Cappai R, Barnham KJ, Villemagne VL. Molecular mechanisms for Alzheimer’s disease: implications for neuroimaging and therapeutics. J Neurochem. 2006;97:1700–1725. doi: 10.1111/j.1471-4159.2006.03989.x. [DOI] [PubMed] [Google Scholar]
- Matveyenko AV, Singh I, Shin BC, Georgia S, Devaskar SU. Differential effects of prenatal and postnatal nutritional environment on ss-cell mass development and turnover in male and female rats. Endocrinology. 2010;151:5647–5656. doi: 10.1210/en.2010-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Aguirre I, Gutierrez-Ospina G, Hernandez-Rodriguez J, Boyzo A, Manjarrez-Gutierrez G. Development of 5-HT(1B), SERT and thalamocortical afferents in early nutrionally restricted rats: an emerging explanation for delayed barrel formation. Int J Dev Neurosci. 2008;26:225–231. doi: 10.1016/j.ijdevneu.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Menon R, Farina C. Shared molecular and functional frameworks among five complex human disorders: a comparative study on interactomes linked to susceptibility genes. PLoS One. 2011;6:e18660. doi: 10.1371/journal.pone.0018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncaster JA, Pineda R, Moir RD, Lu S, Burton MA, Ghosh JG, Ericsson M, Soscia SJ, Mocofanescu A, Folkerth RD, Robb RM, Kuszak JR, Clark JI, Tanzi RE, Hunter DG, Goldstein LE. Alzheimer's disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS One. 2010;5:e10659. doi: 10.1371/journal.pone.0010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2010;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 2010;25:669–677. doi: 10.1007/s00467-009-1407-3. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Kolb B. Mild prenatal stress-modulated behavior and neuronal spine density without affecting amphetamine sensitization. Dev Neurosci. 2011a;33:85–98. doi: 10.1159/000324744. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Kolb B. Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behav Brain Res. 2011b;223:7–16. doi: 10.1016/j.bbr.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Musicco M. Gender differences in the occurrence of Alzheimer's disease. Funct Neurol. 2009;24:89–92. [PubMed] [Google Scholar]
- Nedoschill JC, Lang CJ, Lanczik M. [Dementia of the Alzheimer type in women] Fortschr Neurol Psychiatr. 1999;67:441–447. doi: 10.1055/s-2007-994994. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120:4081–4091. doi: 10.1242/jcs.019265. [DOI] [PubMed] [Google Scholar]
- Oak SA, Tran C, Pan G, Thamotharan M, Devaskar SU. Perturbed skeletal muscle insulin signaling in the adult female intrauterine growth-restricted rat. Am J Physiol Endocrinol Metab. 2006;290:E1321–E1330. doi: 10.1152/ajpendo.00437.2005. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Olsen GS, Hansen LL, Tingey KJ, Nave BT, Wang CL, Hartil K, Petry CJ, Buckley AJ, Mosthaf-Seedorf L. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. J Endocrinol. 2003;177:235–241. doi: 10.1677/joe.0.1770235. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Silverman JL, Aney J, Tian Q, Barkan CL, Chadman KK, Crawley JN. Working memory deficits, increased anxiety-like traits, and seizure susceptibility in BDNF overexpressing mice. Learn Mem. 2011;18:534–544. doi: 10.1101/lm.2213711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson-Leary J, McNay EC. Intrahippocampal administration of amyloid-beta(1-42) oligomers acutely impairs spatial working memory, insulin signaling, and hippocampal metabolism. J Alzheimers Dis. 2012;30:413–422. doi: 10.3233/JAD-2012-112192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg CC, He C, Stroink AR, Kattner KA, Wang CX. Technique for collection of cerebrospinal fluid from the cisterna magna in rat. J Neurosci Methods. 2010;187:8–12. doi: 10.1016/j.jneumeth.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Rapin I, Goldman S. Male predominance in autism: neuroendocrine influences on arousal and social anxiety. Autism Res. 2011;4:163–176. doi: 10.1002/aur.191. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Piroli GG, Grillo CA, Reznikov LR, Adams S, McEwen BS, Charron MJ, Reagan LP. Corticosterone impairs insulin-stimulated translocation of GLUT4 in the rat hippocampus. Neuroendocrinology. 2007;85:71–80. doi: 10.1159/000101694. [DOI] [PubMed] [Google Scholar]
- Poirier R, Fernandez AM, Torres-Aleman I, Metzger F. Early brain amyloidosis in APP/PS1 mice with serum insulin-like growth factor-I deficiency. Neurosci Lett. 2012;509:101–104. doi: 10.1016/j.neulet.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Qin W, Chachich M, Lane M, Roth G, Bryant M, de Cabo R, Ottinger MA, Mattison J, Ingram D, Gandy S, Pasinetti GM. Calorie restriction attenuates Alzheimer's disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus) J Alzheimers Dis. 2006;10:417–422. doi: 10.3233/jad-2006-10411. [DOI] [PubMed] [Google Scholar]
- Quackenbush EJ, Kraemer KH, Gahl WA, Schirch V, Whiteman DA, Levine K, Levy HL. Hypoglycinaemia and psychomotor delay in a child with xeroderma pigmentosum. J Inherit Metab Dis. 1999;22:915–924. doi: 10.1023/a:1005691424004. [DOI] [PubMed] [Google Scholar]
- Rivera JA, Hotz C, Gonzalez-Cossio T, Neufeld L, Garcia-Guerra A. The effect of micronutrient deficiencies on child growth: a review of results from community-based supplementation trials. J Nutr. 2003;133:4010S–4020S. doi: 10.1093/jn/133.11.4010S. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Sadiq HF, Das UG, Tracy TF, Devaskar SU. Intra-uterine growth restriction differentially regulates perinatal brain and skeletal muscle glucose transporters. Brain Res. 1999;823:96–103. doi: 10.1016/s0006-8993(99)01145-2. [DOI] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Kienbacher E, Benke T, Dal-Bianco P, Delazer M, Ladurner G, Jellinger K, Marksteiner J, Ransmayr G, Schmidt H, Stogmann E, Friedrich J, Wehringer C. [Sex differences in Alzheimer’s disease] Neuropsychiatr. 2008;22:1–15. [PubMed] [Google Scholar]
- Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C, Corfas G, White MF. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 2003;23:7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Shin BC, McKnight RA, Devaskar SU. Glucose transporter GLUT8 translocation in neurons is not insulin responsive. J Neurosci Res. 2004;75:835–844. doi: 10.1002/jnr.20054. [DOI] [PubMed] [Google Scholar]
- Shin BC, Dai Y, Thamotharan M, Gibson LC, Devaskar SU. Pre-and postnatal calorie restriction perturbs early hypothalamic neuropeptide and energy balance. J Neurosci Res. 2012 doi: 10.1002/jnr.23013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Radik R, Wojtowicz JM, Cameron HA. Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus. 2009;19:360–370. doi: 10.1002/hipo.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Self-reported body weight perception and dieting practices in community-dwelling patients with schizophrenia. Schizophr Res. 2005;75:425–432. doi: 10.1016/j.schres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sato N, Rakugi H, Morishita R. Molecular mechanisms linking diabetes mellitus and Alzheimer disease: beta-amyloid peptide, insulin signaling, and neuronal function. Mol Biosyst. 2011;7:1822–1827. doi: 10.1039/c0mb00302f. [DOI] [PubMed] [Google Scholar]
- Tanda K, Nishi A, Matsuo N, Nakanishi K, Yamasaki N, Sugimoto T, Toyama K, Takao K, Miyakawa T. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamotharan M, Shin BC, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. GLUT4 expression and subcellular localization in the intrauterine growth-restricted adult rat female offspring. Am J Physiol Endocrinol Metab. 2005;288:E935–E947. doi: 10.1152/ajpendo.00342.2004. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L. 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Torres N, Bautista CJ, Tovar AR, Ordaz G, Rodriguez-Cruz M, Ortiz V, Granados O, Nathanielsz PW, Larrea F, Zambrano E. Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat. Am J Physiol Endocrinol Metab. 2010;298:E270–E277. doi: 10.1152/ajpendo.00437.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udani V, Munot P, Ursekar M, Gupta S. Neonatal hypoglycemic brain - injury a common cause of infantile onset remote symptomatic epilepsy. Indian Pediatr. 2009;46:127–132. [PubMed] [Google Scholar]
- Uemura K, Kuzuya A, Shimozono Y, Aoyagi N, Ando K, Shimohama S, Kinoshita A. GSK3beta activity modifies the localization and function of presenilin 1. J Biol Chem. 2007;282:15823–15832. doi: 10.1074/jbc.M610708200. [DOI] [PubMed] [Google Scholar]
- Vina J, Lloret A. Why women have more Alzheimer's disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis. 2010;20(Suppl 2):S527–S533. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, Pasinetti GM. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer's disease. Faseb J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- Wang X, Zheng W, Xie JW, Wang T, Wang SL, Teng WP, Wang ZY. Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Mol Neurodegener. 2010;5:46. doi: 10.1186/1750-1326-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedlocha M, Stanczykiewicz B, Jakubik M, Rymaszewska J. Selected mice models based on APP, MAPT and presenilin gene mutations in research on the pathogenesis of Alzheimer's disease. Postepy Hig Med Dosw (Online) 2012;66:415–430. doi: 10.5604/17322693.1001098. [DOI] [PubMed] [Google Scholar]
- Willette AA, Coe CL, Colman RJ, Bendlin BB, Kastman EK, Field AS, Alexander AL, Allison DB, Weindruch RH, Johnson SC. Calorie restriction reduces psychological stress reactivity and its association with brain volume and microstructure in aged rhesus monkeys. Psychoneuroendocrinology. 2012;37:903–916. doi: 10.1016/j.psyneuen.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin YI, Bassit B, Zhu L, Yang X, Wang C, Li YM. {gamma}-Secretase Substrate Concentration Modulates the Abeta42/Abeta40 Ratio: IMPLICATIONS FOR ALZHEIMER DISEASE. J Biol Chem. 2007;282:23639–23644. doi: 10.1074/jbc.M704601200. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Elmquist JK. Minireview: From anorexia to obesity--the yin and yang of body weight control. Endocrinology. 2003;144:3749–3756. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.