Abstract
The marginal zone (MZ) region of the spleen plays an important role in leukocyte traffic and the removal of blood-borne pathogens by resident macrophages. Macrophage receptor with a collagenous structure (MARCO), expressed by MZ macrophages, recognizes several microbial ligands and is also involved in the retention of MZ B cells. Here, we report that MARCO is also associated with follicular dendritic cells (FDCs) in the spleen. In its FDC-associated form MARCO is arranged in 0.3–0.5-μm diameter granular-fibrillar structures with an appearance similar to the white pulp conduit system formed by fibroblastic reticular cells (FRCs), but with different compartment preference. The follicular display of MARCO resists irradiation and requires the presence of both MZ macrophages and differentiated FDCs. The follicular delivery of MARCO is independent from the shuffling of marginal zone B cells, and it persists after clodronate liposome-mediated depletion of MZ macrophages. Our findings thus indicate that MARCO is distributed to both MZ and follicles within the spleen into conduit-like structures, where FDC-bound MARCO may mediate communication between the stromal microenvironments of MZ and follicles.
Keywords: MARCO, marginal zone macrophages, follicular dendritic cells, conduit
Introduction
The splenic marginal zone (MZ) is a complex anatomic compartment of the spleen in both humans and mice, where both innate and adaptive immune responses are generated against blood-borne pathogens. The innate immune responses are mediated by resident macrophages, which display a range of pattern-recognition receptors, whereas adaptive immune reactions involve specialized MZ B cells (Mebius and Kraal 2005; Kraal and Mebius 2006).
MZ macrophages are closely associated with MZ B cells that react preferentially with a restricted set of antigens associated with encapsulated bacteria in a T cell-independent manner. In addition, MZ B cells are also involved in the follicular delivery of soluble antigens, thus contributing to the initiation of germinal center reactions within the follicles of white pulp (Kraal and Mebius 2006). Here, follicular dendritic cells (FDCs) provide directional cues via the production of the CXCL13 chemokine and also the adhesion molecules for migration of B and TFH cells as well as survival factors and antigenic fragments retained on their surface; this creates a suitable environment for the clonal expansion of activated B lymphocytes (Fu et al. 1998; Ansel et al. 2000; Cinamon et al. 2008). Both the differentiation of FDCs and the formation and preservation of proper architecture of the splenic MZ depend on the presence of B cells following the early postnatal period which, in turn, significantly influence the organism’s capacity to establish effective humoral immune responses (Kapasi et al. 1993; Fu et al. 1998; Mackay et al. 1998; Balogh et al. 2001; Nolte et al. 2004).
Within the murine MZ, two main macrophage subsets can be distinguished according to their tissue location and phenotypic characteristics, and are arranged around the marginal sinus lining cells, which express MAdCAM-1 (mucosal vascular addressin cell-adhesion molecule 1) (Mebius and Kraal 2005; Kraal and Mebius 2006). The follicular aspect of the MAdCAM-1+ sinus-lining cell region contains metallophilic macrophages, which express sialoadhesin (Sn)/CD169 and other ligands for the cysteine-rich domain of mannose receptor (CR-MR/CD206). The red pulp aspect of the MAdCAM-1+ marginal sinus MZ contains macrophages (MZMs) expressing macrophage receptor with collagenous structure (MARCO) and SIGN-R1/CD209 (Mebius et al. 2004; Martinez-Pomares and Gordon 2012). Although SIGN-R1 and MARCO are co-expressed largely by the same MZM subpopulation, the development of MARCO+ macrophages is independent from the activity of macrophage colony stimulating factor (M-CSF), as op/op mice with defective M-CSF production still present MARCO+ macrophages but both SIGN-R1+ MZMs and Sn/CD169+ metallophilic macrophages are absent (Ito et al. 1999). MARCO belongs to the Class A scavenger receptor family, which can also be induced by red pulp macrophages by exposure to TLR4 ligands (Elomaa et al. 1995; Kraal et al. 2000).
Several cellular and molecular connections between the MZ and follicles contribute to the initiation of immune responses. MZ B cells regularly enter the follicles and deliver complement-coated antigens onto FDCs (Cinamon et al. 2008), during which SIGN-R1, expressed by MZ macrophages, may bind complement C1q and lead to the subsequent local activation of the complement system (Kang et al. 2006). In addition, soluble ligands for CR-MR released from metallophilic macrophages may also be transported into follicles in immune responses, thus possibly representing a mechanism for the delivery of mannosylated antigens followed by FDC-mediated capture (Taylor et al. 2005). Therefore, both SIGN-R1 and Sn/CD169 produced by MZ macrophage subsets have been implicated in antigen transport processes from the marginal zone towards the follicles. MARCO, however, although prominently displayed by MZ macrophages, has not been investigated as a potential contributor in MZ-follicular communication. In this paper, we studied whether MARCO is involved in a transport route connecting MZ to follicles. We found that, in a tissue-specific fashion, MARCO may be distributed to FDCs in a process requiring differentiated FDCs and proper MZ architecture, and its retention by FDCs differs from that of activated complement C4.
Materials & Methods
Mice and Tissue Samples
Wild-type BALB/c mice at various ages were obtained from the SPF Unit of the Faculty of Medicine. Nkx2-3-/- mice (Pabst et al. 1999) were backcrossed onto BALB/c background through 14 generations and genotyped as described (Czömpöly et al. 2011), and maintained atthe Department of Immunology and Biotechnology. Lymphotoxin beta-receptor (LTβR)-deficient mice (Fütterer et al. 1998) were kindly provided by Dr. Falk Weih (Jena, Germany). RAG1-deficient mice were purchased from The Jackson Laboratory (West Grove, PA). Clodronate liposome treatment (from ClodronateLiposome.org; Amsterdam, The Netherlands) was performed as previously described (van Rooijen and van Nieuwmegen 1984). All procedures involving live animals were conducted with the approval of the Ethics Committee on Animal Experimentation of the University of Pécs. Spleen tissue samples from mice deficient for CXCL13 and CXCR5 (Förster et al. 1996; Ansel et al. 2000) and expressing mutated S1PR1TSS (Arnon et al. 2011) were kindly provided by Dr. Jason Cyster (San Francisco, CA).
Antibodies and Reagents
Rat mAb against mouse fibroblastic reticular cell markers (clone ER-TR7) was kindly provided by Dr Willem van Ewijk (Rotterdam, The Netherlands). Anti-mouse Thy-1 (clone IBL-1) and anti-MARCO (clone IBL-12) mAbs were developed in our laboratory (Balogh et al. 1992; Kvell et al. 2006). Rat mAb against mouse C4 (clone FDC-M2) was provided by Dr. Andras K. Szakal (Taylor et al. 2002). FITC-conjugated anti-mouse CD21/35 mAb (clone 7G6) was purchased from BD Biosciences (Soft Flow Kft; Pécs, Hungary). For multicolor immunofluorescence IBL-12 mAb was conjugated with FITC or Cy3 according to standard procedures. Unlabeled rat mAbs were detected with phycoerythrin-conjugated or FITC-labeled goat anti-rat IgG purchased from BD Biosciences.
Visualization of Splenic Conduit
Ovalbumin (OVA) (Sigma-Aldrich; Budapest, Hungary) was used as a low-molecular weight tracer, labeled with FITC according to standard procedures. For conduit marking, mice were first intravenously injected with unconjugated OVA, followed by FITC-OVA, as described (Nolte et al. 2003). After 10 min, the spleens were removed, snap-frozen and were processed for dual fluorescence labeling of the conduit system and MARCO using a IBL-12 Cy3 conjugate.
Immunofluorescence Staining
Frozen and acetone-fixed, 7-μm-thick sections from spleen and lymph nodes embedded in Killik cryomedium were blocked with 5% BSA in PBS for 20 min. The single- and dual-label immunofluorescence procedures on frozen sections from lymphoid tissues have been described previously (Kvell et al. 2006). For control rat IgG at 10 μg/ml was used. After mounting, the sections were viewed under an Olympus BX61 fluorescence microscope (Olympus Optical Co.; Tokyo, Japan). The acquisition of digital pictures with a CCD camera was performed using the analySIS software; the pictures were processed using Adobe Photoshop 6.0 (Adobe Systems Inc., San Jose, CA) with adjustments for brightness, contrast and black level applied equally to all images.
Confocal Microscopy Analysis and Morphometric Analysis
Confocal fluorescence images were taken using an Olympus Fluoview FV-1000 laser scanning confocal imaging system. Three dimensional reconstructions of Z-stacks were assembled in the Bitplane Imaris 4.2 software (Bitplane AG; Zurich, Switzerland) at the optical section thickness of 0.45 µm. Colocalization was analyzed with the ImageJ software (NIH, Bethesda, MD) using the colocalization plug-in as described elsewhere (Talabér et al. 2009). Briefly, specific staining was distinguished from background based on pixel fluorescence intensities, and the colocalizing pixels were then counted on overlaid images. The rate of colocalized MARCO was determined as colocalized pixels/all MARCO+ pixels.
Bone marrow chimeras
Fetal liver cells from E14.5 embryos or the bone marrow from 2-week-old Wistar rats were isolated by collagenase digestion or mechanical disruption (using a 25-gauge needle attached to a 2 ml syringe) and were used as donor cells for hematopoietic reconstitution (Balázs et al. 1998). Recipient BALB/c mice were irradiated at 10 Gy in split dosage administered 6 hr apart calculated at the midline level of the animals using Co60 source at the Department of Oncotherapy. The mice were then given an intravenous injection of 5×106 rat cells in the volume of 200 μl DMEM via the tail vein. Following reconstitution, the mice were provided with ciprofloxacin (Ciprobay®; Bayer Healthcare; Berlin, Germany) in their drinking water.
Flow Cytometry
Lymphocytes, isolated from the spleen or peripheral lymph nodes (pLN), were incubated with mAbs against complement factor C4 (clone FDC-M2) or MARCO (clone IBL-12) as an undiluted hybridoma supernatant, followed by PE-conjugated goat anti-rat IgG (BD Pharmingen; San Diego, CA). After washing, the residual binding sites of secondary reagent were saturated with 50× diluted normal rat serum, and were further incubated with a cocktail of FITC-labeled anti-CR1/2 (clone 7G6 from BD Pharmingen), Alexa Fluor 647-labeled anti-B220 mAb and biotinylated rat anti-mouse IgM (clone B7.6 from ATCC) in conjunction with streptavidin-PE-Cy5.5 (BD Pharmingen). In hematopoietic chimeras, the degree of chimerism was determined six weeks after reconstitution by flow cytometry of peripheral blood leukocytes using FITC-conjugated rat mAb against mouse CD45 (clone IBL-5/25, produced in our laboratory) and anti-rat CD45 (clone OX-1), as described (Balázs et al. 1998). After incubation and washing, the sample was hemolyzed using BD Biosciences lysing solution and fixed in 1% buffered paraformaldehyde. At least 10,000 lymphocytes were collected by a Becton-Dickinson FACSCalibur cytometer (BD Biosciences; Franklin Lakes, NJ) and analyzed using the CellQuest software gated on FSC/SSC to distinguish between lymphoid and myeloid cells.
Results
Tissue-specific Display of MARCO in Lymphoid Follicles
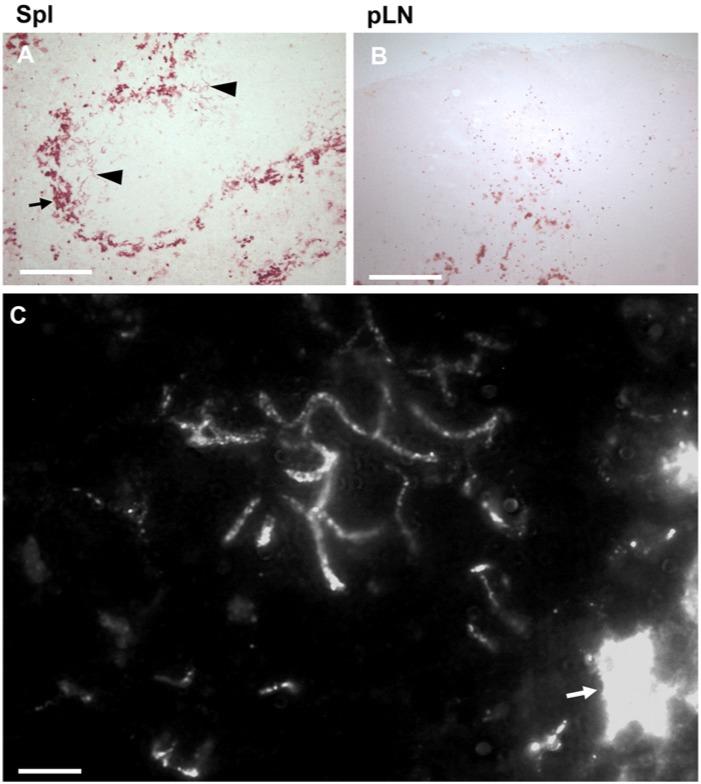
Previous observations indicated that various anti-MARCO antibodies, in addition to robust MZ macrophage labeling, show a specific reticular reactivity within the follicles (Kvell et al. 2006; Birjandi et al. 2011). To study this pattern in more detail, we first investigated the expression of MARCO in other peripheral lymphoid tissues. We confirmed that, in the spleen, MARCO could also be observed in follicles, in addition to its presence in the MZ (Fig. 1A). In contrast, in pLN, MARCO was absent from both the follicles and subcapsular planar floor (Fig. 1B). In Peyer’s patches, we found no discernible staining for MARCO (not shown).
Figure 1.
Differential expression of MARCO in follicles in lymph nodes and spleen. In spleen (Spl) (A), MARCO is strongly expressed in the marginal zone (MZ) macrophages (arrow), and in fine reticular form in the white pulp (arrowheads). In peripheral lymph nodes (pLN) (B), only medullary macrophage cords show intense cellular MARCO reactivity. High-powered fluorescent labeling of splenic sections (C) shows a continuous MARCO-positive fibrillary structure with granular enhancement of reactivity, in addition to the strong MZ labeling, as indicated by the arrow (n=4). Bar size in A, B=100 µm, in C=10 µm.
In the spleen, the follicle-associated MARCO had a strikingly different fine, thin, reticular morphology compared with the strong cell membrane-associated labeling in the MZ. High-powered inspection by immunofluorescence revealed that MARCO in follicles is arranged in 0.3–0.5-μm diameter branching fibrillar structures, with frequent granular enhancement of reactivity along the fibrils (Fig. 1C).
Follicular MARCO in the Spleen Is Associated with an FDC-based Conduit
We hypothesized FDCs as the probable candidate follicular stromal cell type that might be involved in the reticular display of MARCO within the splenic follicles. Using dual immunofluorescence, we found a close association between FDCs expressing a high level of CR1/2 (CD21/35) and reticular MARCO separate from the T-cell zone (Fig. 2A). The follicular MARCO appeared as a long, thin, linear reactivity, which, at higher power inspection, was revealed as a granular deposition arranged along impressions on the FDC cell membrane with occasional branching (Fig. 2B-2D). Three-dimensional reconstitution also indicated that follicular MARCO is retained in granular-fibrillar arrangement on the FDC surface (Fig. 2E). We also observed a similar co-localization using the FDC-M2 mAb against activated complement factor C4 as a passively acquired ligand for FDCs (Fig. 2F-2G). Morphometric quantitation revealed that approximately 60% of MARCO co-localizes with FDCs either as CR1/2high or FDC-M2-positive cells (Fig. 2H), whereas approximately 10–20% of FDCs associate with MARCO.
Figure 2.
Follicular expression of MARCO is associated with follicular dendritic cells (FDCs). (A) A representative sample of young adult spleen labeled for T cells (blue, Thy-1/CD90), FDCs (green, CR1/2) and MARCO (red) show the orientation (bar size, 50 µm). High-powered magnification of CR1/2 reactivity reveals long linear impressions on the FDC membrane (in B indicated by arrows, bar size=10 µm) filled with MARCO-containing granules (C). The merged picture (D) shows the area outlined in (C). (E) 3D reconstitution of Z-stacks of a 15-µm-thick section reveals close spatial relationship between FDCs (green) and follicular MARCO (red). (F, G) Complement factor C4 (FDC-M2) bound to FDCs also co-localizes with MARCO-positive fibrils, with the area outlined in (F) represented in (G) (bar size in F, 50 µm). (H) Bar diagram showing the ratio (mean ± SEM) of intrafollicular MARCO co-localized with CR1/2 or FDC-M2 (open bars) and CR1/2 or FDC-M2+ FDCs associated with MARCO (filled bars) in young adult mouse spleen, quantified as described in Materials and Methods (n=5–7). T-zone-associated conduit material stained with the fibroblastic reticular cell marker ER-TR7 (I) or in vivo tracing with FITC-conjugated ovalbumin (FITC-OVA) (J) indicates only sparse co-labeling (n=5; bar size, 10 µm).
This reticular staining raised the possibility that some MARCO might be associated with the splenic conduit system, a network established by fibroblastic reticular cells (FRCs) (Nolte et al. 2003; Lokmic et al. 2008). The conduit system can be identified by its reactivity with ER-TR7 mAb; therefore, we double-stained splenic sections with anti-MARCO and ER-TR7 mAbs. We found only occasional association between ER-TR7 reactivity and follicular MARCO expression (11% of follicular MARCO colocalizes with ER-TR7; data not shown), indicating that the physical platform that retains MARCO in the follicles is mostly distinct from the conduit system created by the FRC network (Fig. 2I). In addition, in vivo tracing of the conduit system by FITC-OVA also revealed that the follicular MARCO-positive compartment contained very little fluorescent OVA tracer, while FITC-OVA readily dissipated into the conduit network within the periarteriolar region, with only occasional co-labeling for MARCO (Fig. 2J). The combined in vivo OVA accumulation and MARCO immunofluorescence thus indicates that there is a clear segregation between the B-zone-associated MARCO-positive fibrils and T-zone-associated conduit, although some communication may exist between the two structures.
The Follicular MARCO Deposition Requires Both Mature FDCs and MZ Macrophages
The parallel presence of follicular MARCO in spleen and its robust MZ-associated expression prompted us to determine which cells are required for this coupled display.
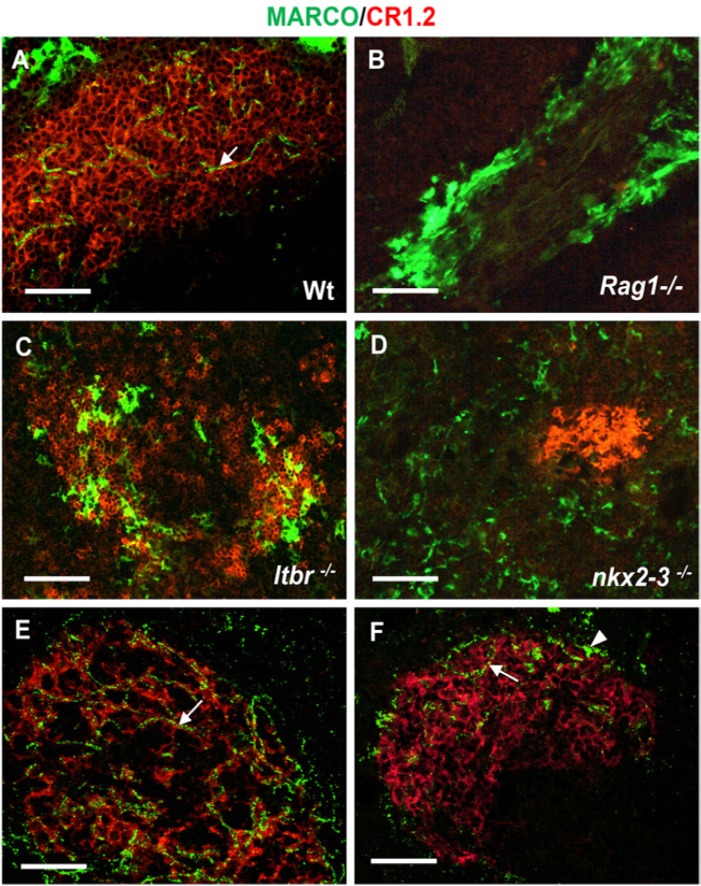
Using immunofluorescence, we first compared the distribution of MARCO in adult wild-type animals (Fig. 3A) to mice with various mutations (hematopoietic or stromal) that affect the formation of white pulp or the MZ. Hematopoietic alterations in Rag1-/- mice include the absence of mature T and B cells, which leads to atrophic white pulp formation and a blockade of FDC maturation (Fu et al. 1998). Stromal defects in mice lacking the Nkx2-3 homeodomain transcription factor (Nkx2.3-/-) show defective MZ but with preserved differentiation of FDCs (Pabst et al. 1999; Czömpöly et al. 2011). Finally, in mutants lacking lymphotoxin beta-receptor (ltbr-/- mice), the MZ is severely disturbed and FDCs are absent (Fütterer et al. 1998).
Figure 3.
Follicular display of MARCO requires mature follicular dendritic cells (FDCs) and marginal zone (MZ). Splenic sections from wild-type spleen (A) or from mice with various mutations (indicated in each micrograph, B–D) that affect the splenic architecture are shown. The association between CR1/2 (red) and MARCO (green) in WT spleen (A) is indicated (arrow). Mice with blocked lymphoid development (RAG1-deficient mice, B) lack mature FDCs expressing CR1/2 and follicular MARCO+ conduits, but contain MZ macrophages. Mice deficient for LTβR (C) have disorganized white pulp and MZ structure, and lack white pulp-associated MARCO. Nkx2-3-deficient mice (D) are unable to establish follicular MARCO-positive conduits in the absence of MARCO-positive MZ macrophages (n=5 from each genotype). (E) Clodronate liposome-mediated depletion of MZ macrophages efficiently depleted the producer cells within 48 hr, whereas the follicle-associated MARCO persisted in a close association with FDCs. (F) Ten days later, both follicle-associated MARCO and partial MZ repopulation by MARCO-positive MZ macrophages can be observed (n=6). Arrows in A, E and F point to follicular MARCO; arrowhead in F indicates a MARCO-expressing MZ macrophage. Bar size, 50 µm.
In Rag1-/- mice, we found that, despite the presence of complete MARCO-positive MZ macrophage ring, there was no detectable MARCO-deposition within the small white pulp area (Fig. 3B). As expected, CD21/35-positive FDCs and B cells were absent.
Although ltbr-/- mice have a more discernible white pulp area, they lack CD21high FDCs, and no reticular MARCO-reactivity could be observed, despite the presence of scattered CD21dim B cells. Although some clusters of MARCO-positive cells were present at the boundary between the white pulp and red pulp, these cells did not form a continuous rim (Fig. 3C). In an inverse fashion, in Nkx2-3-/- spleen samples demonstrated the presence of compacted FDCs with intense CD21 expression, yet no FDC-associated MARCO could be detected. In these mice, only scattered MARCO-expressing cells were present in the spleen, without any characteristic appearance of MZ macrophages (Fig. 3D). Furthermore, the intravenous administration of clodronate liposomes (van Rooijen and van Nieuwmegen 1984) resulted in the depletion of MARCO-positive MZ macrophages within 48 hr, yet follicular MARCO persisted, and remained detectable until 10 days, when the MZ MARCO-positive macrophages started to repopulate the MZ (Fig. 3E-3F).
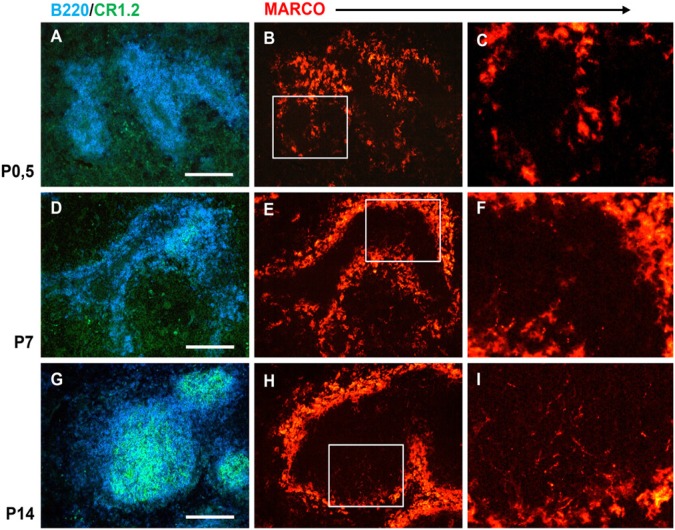
As the splenic stromal architecture in mice is established after birth with characteristic maturation sequence of both FDCs and MZ macrophages (Balogh et al. 2001; Nolte et al. 2003), next we studied the time course of the follicular MARCO emergence during the postnatal period in wild-type mice (Fig. 4). Using triple immunofluorescence for MARCO, FDC-associated CR1/2 (CD21/35) and B220 as B-cell marker in neonatal (P0.5), day 7 (P7) and day 14 (P14) spleens, respectively, we found that, in neonates, despite the presence of the MARCO-positive rim separating the red pulp and white pulp regions, there were no conduit-like MARCO components in the white pulp (Fig. 4A-4C). The immature stage of the follicles was revealed by the absence of FDCs and the perivascular accumulation of B cells. By P7, focal FDC maturation was evident by the appearance of small CR1/2-positive foci, and the presence of small, discontinuous, MARCO-positive granules in the white pulp area where discrete B cell clusters had formed (Fig. 4D-4F). By the second week, fully developed follicles with extensive CR1/2-positive reticula were present, containing deeply penetrating MARCO-positive fibrils (Fig. 4G-4I). Taken together, these findings indicate that both organized MZ macrophages, which produce large quantities of MARCO, and mature FDCs are required together for the follicular deposition of MARCO. For the establishment of follicular MARCO-positive conduits, the emergence of mature FDCs appears as the rate-limiting event, which coincides with the establishment of B cell follicles.
Figure 4.
Postnatal kinetics of the establishment of follicular MARCO-positive conduits. Multiple immunofluorescence on splenic sections from neonatal (P0.5, A-C), 1-week-old (P7, D-F) and 2-week-old (G-I) mice are shown. B cells (blue) and follicular dendritic cells (FDCs; green) illustrate the gradual organization of follicles (A, D and G; bar size, 100µm). MARCO staining (red) identifies MZ macrophages and follicular MARCO-positive conduits (high-power magnification in C, F and I corresponds the white rectangles in B, E and H; n=5).
Long-term Follicular Retention of MARCO by FDC Reticulum
As FDCs in mice have been demonstrated to be resistant to irradiation (Humphrey et al. 1984), next we tested whether the replacement of mouse hematopoietic cells with rat-derived leukocytes could affect the distribution of MARCO. Using either rat fetal liver (FL) or young adult bone marrow as a donor tissue, we found a high degree of rat chimerism six weeks after reconstitution in the peripheral blood in both lymphoid and myeloid lineages (Fig. 5A). In the immunofluorescent evaluation of the chimeric spleens, we observed deeply penetrating follicular MARCO associated with intensely CR1/2-positive cells. However, this CR1/2 staining between the chimeric and control samples was clearly different, as in chimeric spleens the CR1/2 labeling was restricted to the radiation-resistant FDCs (Fig. 5B), whereas in control samples, B cell-associated staining for CR1/2 was also detectable (Fig. 5C). Subsequent immunohistochemical analysis of the anti-MARCO rat mAb on wild-type rat spleen sections excluded its reactivity with the rat counterpart (not shown), indicating that the follicular MARCO in chimeric mice was of mouse origin. Therefore, we conclude that, despite the overwhelming replacement of circulating myeloid and lymphoid lineage cells in their blood, mouse MARCO was retained by radiation-resistant FDCs in irradiated chimeras.
Figure 5.
Radiation resistance of marginal zone (MZ)-follicular MARCO distribution pathway. BALB/c mice were irradiated and repopulated using rat fetal liver hematopoietic or rat bone marrow cells. The peripheral blood was analyzed by flow cytometry and compared with that of control mice. (A, top pair) Lymphoid (Ly) and myeloid (My) cells were defined according to their size (forward scatter/FSC) and granularity (side scatter/SSC). (A, bottom four) The degree of chimerism is shown on the basis of rat (r) CD45 or mouse (m) CD45 expression in peripheral blood leukocytes (middle/lymphoid gate and lower/myeloid gate density plots, respectively), where the quadrant numbers correspond to the percentage values of gated cells (n=6). (B, C) Splenic sections from 6-weeks post-transplant (B) chimeric and (C) control BALB/c mice stained for MARCO (red) and CR1/2 (green) show the follicular retention of MARCO (arrows) (n=6). Bar size, 50µm.
MZ B cells Do Not Transport MARCO into Follicles
MZ B cells are known to transport complement-coated soluble antigens for FDC-mediated retention within the follicles (Ferguson et al. 2004; Cinamon et al. 2008). This is thought to occur under the guidance of BLC/CXCL13 chemokine binding to its receptor, CXCR5, in a process also requiring the desensitization of MZ B cells’ S1PR1 receptor through its C-terminal region (Arnon et al. 2011). To investigate whether MZ B cells also deliver MARCO for follicular display, first we tested if MZ B cells co-express MARCO. Using multicolor flow cytometry, we found that MZ B cells (identified as B220+, IgMhigh and CD21high subset absent in pLNs) do not display MARCO, while they clearly express membrane-associated complement component C4 (Fig. 6A), as reported earlier (Cinamon et al, 2008). [3].
Figure 6.
Transport of marginal zone (MZ) MARCO into follicles is independent of MZ B-cell shuffling. Splenic lymphocytes from BALB/c mice were labeled with anti-CR1/2 and anti-IgM to distinguish MZ B cells (in R1 gate) from follicular B cells (in R2 gate) and immature B cells (in R3 gate), and also lymphocytes from peripheral lymph node cells, used as a staining control (A, left top and lower). Histogram overlays show the lack of MARCO (top right) and the presence of detectable complement C4 (lower right) on MZ B cells corresponding to R1 gate (filled histogram) as compared with the isotype control (empty histogram). Numbers in the representative histogram overlays indicate the ratio of mean fluorescence intensities (MFI) between the expression of MARCO or C4 and isotype control (n=5). (B-E) shows the distribution of MARCO (red) and follicular dendritic cell (FDC)-associated CR1/2 (green) in (B) Blc-/-, (C) Cxcr5-/- and (D) S1pr1TSS transgenic mutants affecting follicular migration of B cells as compared with the (E) B6 wild-type control sample. Note the absence of follicles and CR1/2high FDCs in mice deficient for BLC/CXCL13 and CXCR5. Arrows in (D) and (E) point to FDC-associated MARCO (n=2–4). Bar size in B/C, 100µm; in D/E, 20µm.
Next, we tested how blockade of the CXCL13/CXCR5 chemokine signaling pathway affects the follicular deposition of MARCO. We found that MARCO was restricted to the marginal zone in both CXCL13 and CXCR5 deficiencies (Fig. 6B-6C), in addition to the ring-like distribution of B cells and the absence of FDCs (Förster et al. 1996; Ansel et al. 2000).
In contrast to these two mutants, in S1PR1TSS mice, where a TSS tripeptide replaces the AAA sequence in the C-terminal region of S1PR1 receptor, FDCs develop, and follicles form. Furthermore, the MZ B cells are blocked from entering the follicles and deposit captured immune complexes on the surface of FDCs (Arnon et al. 2011). We found that, despite the follicular exclusion of MZ B cells, S1PR1TSS mice had abundant follicular display of MARCO similar to wild-type mice (Fig. 6D, 6E). Taken together, these findings suggest that MARCO is deposited within the follicles without the involvement of MZ B cell movement. On the other hand, the CXCR5/CXCL13-driven B cell migration into the developing white pulp territory is necessary for the follicular deposition of MARCO through the induction of FDC reticula.
Discussion
In our present work, we describe the follicular distribution of MARCO, a marginal zone macrophage-associated scavenger receptor in mouse spleen, which may point to the existence of a tissue-specific transport system within splenic follicles. MARCO, a Class A scavenger receptor, was first identified as a trimeric protein formed by 518 amino acids monomers, capable of binding bacteria and acetylated low density lipoprotein, with its mRNA expression restricted to the splenic marginal zone (Elomaa et al. 1995; Kraal et al. 2000). Interestingly, although the follicular deposition of MARCO has already been illustrated in other publications (Kvell et al. 2006; Birjandi et al. 2011), its comprehensive characterization is missing. These structures appear to require the simultaneous presence of MARCO-positive marginal zone macrophages and follicular dendritic cells (FDCs), and they differ from the conduit system of the T-zone that has been described for peripheral lymph nodes in two main aspects (Gretz et al. 1996; Nolte et al. 2003; Lokmic et al. 2008): First, follicular MARCO is restricted to the spleen, and is absent in other lymphoid tissues; second, unlike the conduit network created by fibroblastic reticular cell (FRC) ensheathing extracellular matrix components they themselves produce, this MARCO-positive network is probably the result of FDCs passively capturing MARCO generated by MZ macrophages, as evidenced in mutant mice with various abnormalities of distinct regions in the spleen. In this way, a MARCO-transport pathway can be envisaged to exist in the mouse spleen, connecting the MZ macrophages to the follicular stroma.
The splenic T-zone-associated conduit system is characterized by the expression of the ER-TR7 mAb-reactive material, which is produced by FRCs. Double labeling for ER-TR7 and MARCO revealed that the latter network does not expand into the T-zone conduits. Furthermore, in vivo tracing using FITC-OVA also indicated that, although T-zone-associated white pulp conduits expand from the central artery along the ER-TR7-positive meshwork towards the follicles with a decreasing amount of tracer, the transport of MARCO may be directed in the opposite direction from the MZ towards the follicles, without seeping towards the red pulp.
This difference between the dissipation gradient of OVA-tracer and MARCO in the spleen parallels the distinct cellular composition of conduits present in separate compartments of peripheral lymphoid tissues. Ontogenic studies suggest that the formation and maintenance of splenic FDCs involve the peripheral region of white pulp where FDC precursors first appear (Balogh et al. 2001) and their emergence is coupled with the remodeling of the follicular conduit system (Bajénoff et al. 2009). It appears that both mature FDCs and MZ macrophages are required for the proper formation of follicular MARCO-positive conduits, as mice with defective FDC maturation (such as in RAG1 mutants), complex follicular-MZ organization (LTβR), or primarily MZ macrophage deficiency (Nkx2-3 mutation) are unable to establish white pulp/follicular MARCO conduits. In lymph nodes, however, there is no MARCO-source available along the liquid flow upstream to FDCs. Intriguingly, exposure to supralethal irradiation dose—despite the exchange of mouse B cells for rat lymphocytes —did not abolish the follicular retention of MARCO, arguing for the close stromal relatedness of the process. It remains to be investigated that beyond these cellular requirements which extracellular matrix molecules characteristic of lymphoid conduits (Lokmic et al. 2008) are present together with FDC-associated MARCO.
MARCO-deficient mice show delayed formation of MZ architecture, scattered MZ macrophage distribution and a weakened immune response against T-independent Type 2 antigens (Chen et al. 2005), whereas its acute blockade with specific mAb induced the mobilization of MZ B cells, also indicating some role for MARCO in the retention of MZ B cells (Karlsson et al. 2003). However, while anti-MARCO mAbs may interrupt the C4-directed MZ B cell binding to MARCO (thus mobilizing the MZ B cells), the absence of MARCO on MZ B cells excludes the direct involvement of MZ B cells as MARCO carrier cells. Therefore, the role of B cells in the formation of follicular MARCO network probably manifests through their role in the establishment of proper MZ architecture and FDC development (Kapasi et al. 1993; Nolte et al. 2004), but not in the direct transportation, which may occur via other mechanisms.
Complement C4 (Chen et al. 2006)—an important opsonin for subsequent recognition by complement receptors CR1/2, which influences the distribution of immune complexes between MZ and follicles (Zachrau et al. 2004)—was raised as potential endogenous ligand for MARCO. Considering the role of C4 in the antigen handling processes, a B cell-mediated MARCO transport mechanism could be envisaged in which MZ B cells, with a high-level expression of CR1/2, may capture complement-coated immune complexes (Humphrey et al. 1984) and also bind to complement C4 associated with MARCO on MZ macrophages. The absence of detectable MARCO on MZ B cells by flow cytometry, however, excludes this possibility. Because of the size restriction of the white pulp conduit system in accommodating molecules below 70 kDa for follicular transport, it is probable that some reduced variant or monomeric form of MARCO may be processed. As the IBL-12 mAb used in our studies binds to the epitope involved in Ac-LDL binding within the SRCR domain (Kvell et al. 2006), MARCO in a follicular reticular form may potentially preserve this ligand-binding site.
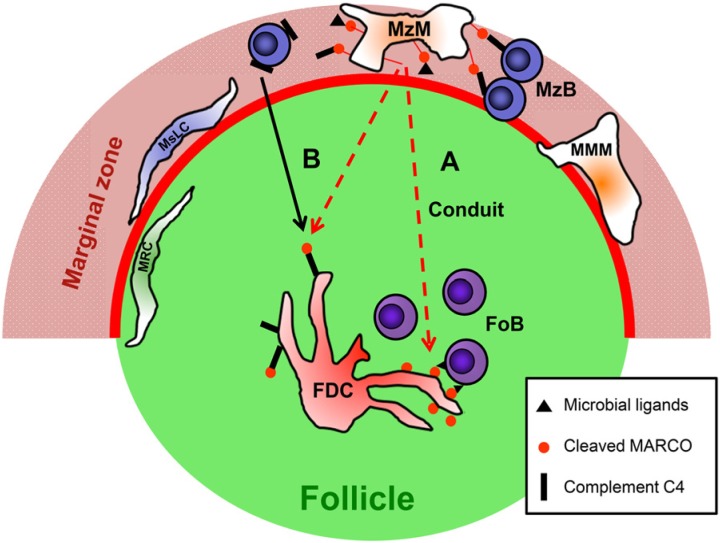
MARCO may bind zymosan, Candida albicans, lipopolysaccharide and lipoteichoic acid and also unopsonized particles, possibly through several ligand-binding sites (Kraal et al. 2000). Once acquired, MARCO on FDCs may support B cell responses via several interactions, including TLR4 expressed by FDCs (El Shikh et al. 2007) or through CR21/35, if MARCO is complexed with C4 degradation products. Only the spleen has been found to contain such a follicular structure, and therefore splenic B cells are likely to be exposed to a different follicular milieu than B cells of other tissues lacking FDC-associated MARCO. Considering the broad microbial ligand-binding potential of MARCO, its association with fungal or bacterial compounds for subsequent recognition by B cells, such as that of LPS by TLR4, may augment the latter cells’ antigen receptor-evoked signaling responses. Alternatively, released MARCO may act as an adsorbent for such microbial components, thus preventing their dissipation into the lymphoid tissue. Moreover, the absence of macrophage-associated MARCO impairs resistance against bacterial infection (Arredouani et al. 2004), but its absence confers protection in influenza viral infection in the lung (Ghosh et al. 2011), indicating that the antimicrobial effects of MARCO probably manifest in an agent-dependent fashion. Figure 7 represents an overview of the proposed formation of follicular MARCO and its possible involvement in connecting the MZ and follicles in the mouse spleen.
Figure 7.
A hypothetical scheme of the follicular transport of MARCO into follicles in the murine spleen. Marginal zone macrophages (MZM) display MARCO (red dot with red rod), which may potentially form a complex with microbial ligands (black triangles) or complement C4 (black rectangles), can bridge MZ B cells via the latter cells’ recognition of CD21 ligands. Following proteolytic cleavage or other processing events, soluble MARCO (with or without complement C4 or microbial compounds) would hypothetically dissipate across the marginal sinus (thick red line) into the follicle via either (A) the pre-formed conduits, which allows it to then pass onto follicular dendritic cells (FDCs), or (B) be passively acquired by C4 bound to CD21 on FDCs, which allows it to be deposited earlier by MZ B cells (continuous black arrow). Thus, FDCs may either immobilize soluble MARCO:C4, or MARCO is deposited into FDC-associated conduits, where microbial ligands bound to MARCO may be recognized by follicular B cells (FoB). Possible roles of marginal metallophilic macrophages (MMM) and other stromal elements (marginal sinus lining cells – MsLC and marginal reticular cells – MRC) remain unknown. The regulatory roles of B cells in the formation of MZ architecture and FDC differentiation are not depicted.
In summary, the splenic MARCO-containing conduit system represents a unique spleen-specific structure, with complex developmental requirements. It may form a physical platform between the marginal zone and follicular stroma, thus promoting the connection between these two distinct lymphoid compartments.
Acknowledgments
The authors gratefully acknowledge the spleen samples of Blc-/-, Cxcr5-/- and S1PR1TSS mice kindly provided by Dr. Jason Cyster (University of California, San Francisco). The ER-TR7 mAb was generously supplied by Dr. Willem van Ewijk.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by SROP-4.2.2/B-10/1-2010-0029 and HEALTH IMPULSE (HUHR/1001/2.1.3/0006) project grant at the University of Pécs. ZK was supported by the by the Apáczai Csere János Fellowship in the framework of TÁMOP 4.2.4. A/1-11-1-2012-0001 ‘National Excellence Program’. P.B. was in part supported by the Research Fund of the Faculty of Medicine of the University of Pécs, and is recipient of the research grant IBD-0341 from the Broad Medical Research Program of The Eli and Edythe Broad Foundation and the Hungarian Scientific Research Fund (OTKA K108429). The authors have no conflicting financial interests.
References
- Ansel KM, Ngo VN, Hyman PL, Luther S.A, Förster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. (2000). A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406:309-314 [DOI] [PubMed] [Google Scholar]
- Arnon TI, Xu Y, Lo C, Pham T, An J, Coughlin S, Dorn GW, Cyster JG. (2011). GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science 333:1898-1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. (2004). The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med 200:267-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajénoff M, Germain RN. (2009). B-cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells. Blood 114:4989-4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balázs M, Horváth G, Balogh P. (1998). Simple determination of donor/host origin and donor leukocyte subsets in rat-mouse chimeras. J Immunol Methods 218:117-121 [DOI] [PubMed] [Google Scholar]
- Balogh P, Bebök Z, Németh P. (1992). Cellular enzyme-linked immunocircle assay. A rapid assay of hybridomas produced against cell surface antigens. J Immunol Methods 153:141-149 [DOI] [PubMed] [Google Scholar]
- Balogh P, Aydar Y, Tew JG, Szakal AK. (2001). Ontogeny of the follicular dendritic cell phenotype and function in the postnatal murine spleen. Cell Immunol 214:45-53 [DOI] [PubMed] [Google Scholar]
- Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. (2011). Alterations in marginal zone macrophages and marginal zone B cells in old mice. J Immunol 186:3441-3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Pikkarainen T, Elomaa O, Soininen R, Kodama T, Kraal G, Tryggvason K. (2005). Defective microarchitecture of the spleen marginal zone and impaired response to a thymus-independent type 2 antigen in mice lacking scavenger receptors MARCO and SR-A. J Immunol 175:8173-8180 [DOI] [PubMed] [Google Scholar]
- Chen Y, Sankala M, Ojala JR, Sun Y, Tuuttila A, Isenman DE, Tryggvason K, Pikkarainen T. (2006). A phage display screen and binding studies with acetylated low density lipoprotein provide evidence for the importance of the scavenger receptor cysteine-rich (SRCR) domain in the ligand-binding function of MARCO. J Biol Chem 281:12767-13775 [DOI] [PubMed] [Google Scholar]
- Cinamon G, Zachariah MA, Lam OM, Foss FW Jr, Cyster JG. (2008). Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol 9:54-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czömpöly T, Lábadi A, Kellermayer Z, Olasz K, Arnold HH, Balogh P. (2011). Transcription factor Nkx2-3 controls the vascular identity and lymphocyte homing in the spleen. J Immunol 186: 6981-6989 [DOI] [PubMed] [Google Scholar]
- Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, Thesleff I, Kraal G, Tryggvason K. (1995). Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 80:603-609 [DOI] [PubMed] [Google Scholar]
- El Shikh ME, El Sayed RM, Wu Y, Szakal AK, Tew JG. (2007). TLR4 on follicular dendritic cells: an activation pathway that promotes accessory activity. J Immunol 179:4444-4450 [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Youd ME, Corley RB. (2004). Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int Immunol 16:1411-1422 [DOI] [PubMed] [Google Scholar]
- Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. (1996). A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87:1037-1047 [DOI] [PubMed] [Google Scholar]
- Fu YX, Huang G, Wang Y, Chaplin DD. (1998). B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin α–dependent fashion. J Exp Med 187:1009-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. (1998). The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9:59-70 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Gregory D, Smith A, Kobzik L. (2011). MARCO regulates early inflammatory responses against influenza: a useful macrophage function with adverse outcome. Am J Respir Cell Mol Biol 45:1036-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretz JE, Kaldjian EP, Anderson AO, Shaw S. (1996). Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J Immunol 157:495-499 [PubMed] [Google Scholar]
- Humphrey JH, Grennan D, Sundaram V. (1984). The origin of follicular dendritic cells in the mouse and the mechanism of trapping of immune complexes on them. Eur J Immunol 14:859-864 [DOI] [PubMed] [Google Scholar]
- Ito S, Naito M, Kobayashi Y, Takatsuka H, Jiang S, Usuda H, Umezu H, Hasegawa G, Arakawa M, Shultz LD, Elomaa O, Tryggvason K. (1999). Roles of a macrophage receptor with collagenous structure (MARCO) in host defense and heterogeneity of splenic marginal zone macrophages. Arch Histol Cytol 62:83-95 [DOI] [PubMed] [Google Scholar]
- Kang YS, Do Y, Lee HK, Park SH, Cheong C, Lynch RM, Loeffler JM, Steinman RM, Park CG. (2006). A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell 125:47-58 [DOI] [PubMed] [Google Scholar]
- Kapasi ZF, Burton GF, Shultz LD, Tew JG, Szakal AK. (1993). Induction of functional follicular dendritic cell development in severe combined immunodeficiency mice. Influence of B and T cells. J Immunol 150:2648-2658 [PubMed] [Google Scholar]
- Karlsson MC, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. (2003). Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J Exp Med 198:333-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G, van der Laan LJ, Elomaa O, Tryggvason K. (2000). The macrophage receptor MARCO. Microbes Infect 2:313-316 [DOI] [PubMed] [Google Scholar]
- Kraal G, Mebius RE. (2006). New insights into the cell biology of the marginal zone of the spleen. Int Rev Cytol 250:175-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvell K, Czömpöly T, Pikkarainen T, Balogh P. (2006). Species-specific restriction of cell surface expression of mouse MARCO glycoprotein in murine cell lines. Biochem Biophys Res Commun 341:1193-202 [DOI] [PubMed] [Google Scholar]
- Lokmic Z, Lämmermann T, Sixt M, Cardell S, Hallmann R, Sorokin L. (2008). The extracellular matrix of the spleen as a potential organizer of immune cell compartments. Semin Immunol 20:4-13 [DOI] [PubMed] [Google Scholar]
- Mackay F, Browning JL, Kosco-Vilbois MH, Noelle RJ. (1998). The sequential role of lymphotoxin and B cells in the development of splenic follicles. J Exp Med 187:997-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pomares L, Gordon S. (2012). CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol 33:66-70 [DOI] [PubMed] [Google Scholar]
- Mebius RE, Nolte MA, Kraal G. (2004). Development and function of the splenic marginal zone. Crit Rev Immunol 24:449-464 [DOI] [PubMed] [Google Scholar]
- Mebius RE, Kraal G. (2005). Structure and function of the spleen. Nat Rev Immunol 5:606-616 [DOI] [PubMed] [Google Scholar]
- Nolte MA, Beliën JA, Schadee-Eestermans I, Jansen W, Unger WW, van Rooijen N, Kraal G, Mebius RE. (2003). A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J Exp Med 198:505-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte MA, Arens R, Kraus M, van Oers MH, Kraal G, van Lier RA, Mebius RE. (2004). B cells are crucial for both development and maintenance of the splenic marginal zone. J Immunol 172:3620-3627 [DOI] [PubMed] [Google Scholar]
- Pabst O, Zweigerdt R, Arnold HH. (1999). Targeted disruption of the homeobox transcription factor Nkx2-3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development 126:2215-2225 [DOI] [PubMed] [Google Scholar]
- Talabér G, Boldizsár F, Bartis D, Pálinkás L, Szabó M, Berta G, Sétáló G Jr, Németh P, Berki T. (2009). Mitochondrial translocation of the glucocorticoid receptor in double-positive thymocytes correlates with their sensitivity to glucocorticoid-induced apoptosis. Int Immunol 21:1269-1276 [DOI] [PubMed] [Google Scholar]
- Taylor PR, Pickering MC, Kosco-Vilbois MH, Walport MJ, Botto M, Gordon S, Martinez-Pomares L. (2002). The follicular dendritic cell restricted epitope, FDC-M2, is complement C4; localization of immune complexes in mouse tissues. Eur J Immunol 32:1888-1896 [DOI] [PubMed] [Google Scholar]
- Taylor PR, Gordon S, Martinez-Pomares L. (2005). The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol 26:104-110 [DOI] [PubMed] [Google Scholar]
- van Rooijen N, van Nieuwmegen R. (1984). Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res 238:355-358 [DOI] [PubMed] [Google Scholar]
- Zachrau B, Finke D, Kropf K, Gosink HJ, Kirchner H, Goerg S. (2004). Antigen localization within the splenic marginal zone restores humoral immune response and IgG class switch in complement C4-deficient mice. Int Immunol 16:1685-1690 [DOI] [PubMed] [Google Scholar]