Abstract
Endoneurial fibroblast-like cells (EFLCs) are one of the cell populations present in the peripheral nervous system. The role and immunophenotypic characteristics of EFLCs are not well known and led us to perform a histological and cytological study of EFLCs in normal human and rat peripheral nerves. We found that all EFLCs express CD34, neural/glial antigen 2 (NG2), and prolyl-4-hydrolase-beta. In addition, half of the EFLCs in normal peripheral nerves express platelet-derived growth factor receptor-β (PDGFR-β) and some also express the intermediate filament nestin in vivo (at a lower level than Schwann cells, which express high levels of nestin). Using cell cultures of purified EFLCs, we characterized subpopulations of EFLCs expressing PDGFR-β alone or PDGFR-β and nestin. Experimental nerve lesions in rat resulted in an increase in nestin-positive EFLCs, which returned to normal levels after 8 days. This suggests that some EFLCs could have a different proliferative and/or regenerative potential than others, and these EFLCs may play a role in the initial phase of nerve repair. These “activated” EFLCs share some immunophenotypic similarities with pericytes and Interstitial cells of Cajal, which have progenitor cell potentials. This raises the questions as to whether a proportion of EFLCs have a possible role as endoneurial progenitor cells.
Keywords: cell markers, colocalization, endoneurial fibroblast-like cells, peripheral nerve, nestin, PDGFR-β, CD34, NG2
Introduction
The peripheral nervous system is composed of three different compartments: the endoneurium, the perineurium, and the epineurium. There is a variety of cell types present within peripheral nerves in addition to axons and Schwann cells, including perineurial cells, mast cells, pericytes, endothelial cells and endoneurial fibroblast-like cells (EFLCs). As early as in 1932, Nageotte stated that the endoneurium is a connective tissue structure with a collagenous framework molded around the nerve fiber and that “all of these collagenous formations which constitute the endoneurium are associated with fibroblasts” (Nageotte 1932). In various tissues, fibroblasts were shown to constitute a heterogeneous population (Ali-Bahar et al. 2004; Azzarone and Macieira-Coelho 1982; Fritsch et al. 1999; Harper and Grove 1979; Nolte et al. 2008; Parker 1932; Schafer et al. 1985; Sempowski et al. 1995; Sorrell and Caplan 2004). Fibroblasts have often been considered as a relatively inert population of cells but this view may radically change, as some authors now consider them as a central and vibrant component of tissue biology (Sorrell and Caplan 2009). EFLCs have not been extensively studied, probably because most of the time they were regarded as supportive cells in the peripheral nerve. In opposition to “common” fibroblasts, which have a mesenchymal origin, EFLCs appear to derive from the neural crest (Joseph et al. 2004). It should be noted, however, that a proportion of EFLCs may originate from the bone marrow during pathological conditions, as noted by Maurer et al. (2003) in an animal model of inherited neuropathy. A few reports showed that EFLCs express cell markers not typical of common fibroblasts, such as CD34 (Weiss and Nickoloff 1993; Maurer et al. 2003) and neural/glial antigen 2 (NG2) (Martin et al. 2001; Morgenstern et al. 2003; Schneider et al. 2001). In pathological conditions, EFLCs also express particular cell markers, such as tenascin-C (Hill 2009; Yamamoto et al. 2011), and have been shown to produce several cytokines (Be’eri et al. 1998; Groh et al. 2012; Rotshenker 2011; Shamash et al. 2002).
We tried to extensively characterize EFLCs by various means (immunohistochemistry, electron microscopy, immune-electron microscopy, cell culture), in order to better understand their functions and potential role in the peripheral nerve.
Materials & Methods
Normal Human Nerve Samples
Human sensory nerves were harvested during diagnostic nerve biopsy using a protocol from our laboratory, according to previously described techniques for histological, immunohistological and ultrastructural studies (Weis et al. 2012).
Patients were recruited in the Neurology Department of the Limoges University Hospital. This study was approved by the Institutional Review Board of the University Hospital. Written informed consent was obtained from all patients included in this study. Biopsy was indicated because an inflammatory disorder was suspected; however, in all cases, this diagnosis was refuted and the nerves were considered as normal.
Rat Normal Sciatic Nerves and Crush Injury
All animal procedures were approved by the animal ethics committee of the University of Limoges. Twelve adult male Sprague Dawley (SD) rats weighing approximately 250 g were used. The rats were deeply anesthetized by inhalation of 3% isoflurane in combination with a mixture of nitrous oxide and oxygen (1:2, V/V), then shaved and washed with antiseptic solution before positioning for surgery. Crush injury was carried out with the rats n=6 placed prone under sterile conditions and the nerves were used for culture and cytochemistry.
In animals undergoing a sciatic nerve crush (lesioned nerves were only used for cell culture), the right sciatic nerve was exposed unilaterally through a skin incision followed by a muscle incision. After nerve mobilization, a non-serrated clamp exerting a constant force was used for a period of 30 s to create a 3 mm-long crush injury. Complete crush was confirmed by the presence of a translucent band across the nerve. The left unoperated sciatic nerve was used as a control. The muscle and skin were then closed with sutures and staples. All rats were observed for general well-being and had ad libitum access to food and water throughout the study. Three days after crush injury, both sciatic nerves were dissected and the rats were euthanized by intra-cardiac injection of a lethal dose of ketamine.
Nerve Tissue Preparation
Each nerve sample was divided into three parts. One part was fixed in 10% formalin for 24 hr and embedded in paraffin, whereas another part was frozen in liquid nitrogen-cooled isopentane and stored in a -80C freezer until use; paraffin and frozen sections were used for conventional immunohistochemistry. The last nerve part was fixed in 2.5% glutaraldehyde and embedded in two different resins: epoxy resin for conventional electron microscopy and London Resin White (LRW) for immuno-electron microscopy. Semi-thin sections (1-µm thick) were examined using a light microscope and areas appropriate for further ultrathin sectioning were then selected and examined using an transmission electron microscope (JEM-1011; JEOL Ltd., Tokyo, Japan).
Optic Microscopy
Paraffin Sections
Five micron-thick sections were cut along longitudinal and frontal planes using a microtome. The slides were dewaxed and immersed in water. The samples were boiled for 25 min in a water bath in antigen retrieval solution (Dako-cytomation; Dako, Glostrup, Denmark) and subsequently cooled in the same solution at room temperature. Endogenous peroxidase activity was quenched by treating the sections in 0.3% H2O2 for 30 min. To prevent non-specific binding, the sections were incubated in normal serum diluted in PBS (10%). Samples were incubated with primary antibodies overnight at 4C. The bound antibodies were visualized using appropriate avidin-biotin peroxidase kit (Vectastain; Vector Laboratories, Burlingame, CA) with 3.3’-diaminobenzidine tetrahydrochloride (DAB) as a chromogen (DAB peroxidase substrate kit; Vector Laboratories). Sections were counterstained with Mayer’s hematoxylin, mounted and observed with a Nikon optic microscope (Nikon, Tokyo, Japan).
Frozen Sections
Seven micron-thick sections were cut at along longitudinal or frontal planes using a cryostat. To prevent non-specific binding, the sections were incubated in a phosphate-buffered saline (PBS) solution containing 10% bovine serum albumin (BSA) for 30 min. Samples were incubated with primary antibodies overnight at 4C. After washing in PBS, samples were incubated with secondary antibodies in single- or double-labeled fluorescence studies for 2 hr at room temperature: Alexa Fluor 488-conjugated goat anti-rabbit or goat anti-mouse IgG antibodies or rabbit anti-goat Alexa Fluor 594-conjugated donkey anti-mouse or donkey anti-rabbit or donkey anti-goat (Invitrogen; Carlsbad, CA). After washing in PBS, Hoechst 33258 dye (Sigma-Aldrich; St. Louis, MO) was applied to visualize nuclei and then sections were observed with a Nikon microscope equipped with fluorescence camera. The experiments performed in rats were performed using the nerves of three different animals and gave similar results.
Electron Microscopy
The LR-White technique was adapted from Keita et al. (2002). For labeling of LRW-embedded samples, the following technique was used: to prevent non-specific binding, grids were blocked with PBS containing 10% BSA, 10% normal goat serum (NGS) and 0.1% Tween-20 for 10 min (blocking PBS). Grids were then floated face down on 25 µl drops of primary antibodies diluted in blocking PBS overnight at 4C. After 3×5 min washes in PBS, the grids were incubated on 25 µl drops of goat anti-mouse IgG antibodies or goat anti-rabbit IgG antibodies conjugated to 18-nm colloidal gold for 2 hr at room temperature. Unbound second antibodies were removed with 3×5 min washes in PBS followed by 4×2 min washes in distilled water. The grids were then counterstained with uranyl acetate and analyzed using a JEOL electron microscope at 80 KeV.
Rat Cell Culture
The most commonly used method to establish primary fibroblast cultures from peripheral nerves is the explants culture method (Leal 2004). Both nerves from an anesthetized SD rat were dissected and removed. The epineurium, connective tissue and blood vessels were stripped off with fine forceps under a dissecting microscope. Sciatic nerves were cut into multiple segments of approximately 1 mm and placed as explants into tissue cultures dishes containing Dulbecco’s modified Eagle’s medium (DMEM), 20% fetal bovine serum (FBS), and 1% penicillin/streptomycin (Pen/Strep). Nerve fragments were maintained in contact with the plastic surface to allow cells to migrate out from the tissue pieces over the dish. Cell culture dishes were incubated at 37C with 5% CO2 and 95% humidity. The emergence of migratory cells began after about two days in culture. The medium was replaced every other day. Endoneurial fibroblasts were the first cells to grow out from primary nerve explants. They showed a flattened polymorphic shape with large, round nuclei; Schwann cells (SCs), by comparison, have long bi- or tri-polar processes and cigar-shaped nuclei. Explants were lifted off the surface of the culture dish with fine forceps after four days for control sciatic nerves but not more than 2 or 3 days for crush injuries nerves because the cells grew out from the nerves more rapidly. Cells were fixed for 15 min in 4% paraformaldehyde diluted in PBS and permeabilized with 0.05% Triton X-100 in PBS. After blocking with 3% BSA, the cells were incubated with primary antibodies (Table 1) overnight at 4C. The following day, cells were incubated with Alexa Fluor 488-conjugated goat anti-rabbit or goat anti-mouse IgG antibodies for 2 hr at room temperature. Hoechst dye was applied to the visualize nuclei. Double staining was performed with a mixture of Alexa Fluor 488 (green) and Alexa Fluor 594 (red) (Jackson ImmunoResearch Laboratories; West Grove, PA). The cells were observed using a Leica microscope equipped with phase contrast and Fluorescence optics (Leica Microsystems; Wetzlar, Germany).
Table 1.
Primary Antibodies.
| Antibodies | Dilution | Source | Code | Human or Rat |
|---|---|---|---|---|
| Mouse anti-prolyl-4-hydrolase-beta | 1:50 Cult 1:20 P |
Acris Antibodies; Herford, Germany | AF5110 | Rat |
| Mouse anti-CD34(ICO115) | 1:10 ME 1:50 P+F |
Santa Cruz Biotechnology; Dallas, TX | Sc-7324 | Human |
| Goat anti-CD34 | 1:25 Cult 1:100 F-1:400P |
R&D Systems; Minneapolis, MN | AF4117 | Rat |
| Rabbit anti-NG2 | 1:100 P+Cult+F 1:50 ME |
Millipore | AB5320 | Human-Rat |
| Rabbit anti-S100 | 1:500 P+Cult+F 1:1000 ME |
Dako Ltd; Glostrup, Denmark | Z311 | Human-Rat |
| Mouse anti-Nestin Rat 401 | 1:50 Cult 1:100 F |
Santa Cruz Biotechnology |
Sc-33677 | Rat |
| Mouse anti-Nestin 25/Nestin | 1:10 ME | BD Biosciences, Franklin Lakes, NJ | 611658 | Human |
| Mouse anti-EMA (epithelial membrane antigen) | 1:100 P | Dako Ltd | M 0613 | Human |
| Rabbit Anti-Glut1 | 1:5000 P 1:50 ME |
EMD Millipore, Billerica, MA | 07-1401 | Human-Rat |
| Rabbit anti-c-kit (CD117) | 1:50 P | Dako Ltd | A 4502 | Human |
| Mouse anti-CD68 (KP1) | 1:100 P+F+ME | Dako Ltd | M0814 | Human |
| Mouse anti-CD68 (ED1) | 1:100 P+F+ME | AbD Serotec, Oxford, UK | MCA 341 | Rat |
| Rabbit anti-PDGFR-beta | 1:100 F+P | Abcam, Cambridge, MA | Ab32570 | Human-Rat |
| Rabbit anti-PDGFR-alpha | 1:100 F+P | Santa Cruz Biotechnology |
Sc-338 | Human-Rat |
| Mouse anti-CD90 (Thy 1) | 1:20 Cult | AbD Serotec | MCA47R | Human-Rat |
| Rabbit anti-CD45 | 1:100 F+P | Santa Cruz Biotechnology |
Sc-25590 | Human-Rat |
P: paraffin; Cult: culture; ME: electron microscopy; F: frozen.
Statistical Analysis
To determine possible contamination of cultured endoneurial fibroblasts by Schwann cells, we performed an S100 immunostaining and counterstained with Hoechst 33258 in the wells of a 12-well culture plate after 3 days of culture. The percentage of S100-positive cells was then determined by visual inspection and counting at 100× magnification.
Differences in the frequency of nestin-positive cells between controls and crush injury samples (at days 3, 5 or 8) were tested using a Student t-test and Bartlett’s test for equal variances using GraphPad Prism (GraphPad Software Inc.; San Diego, CA). Values of p<0.05 were considered significant.
Results
All antibodies used in the different techniques are summarized in Table 1. Immunostaining results obtained for the various antibodies and techniques are summarized in Table 2.
Table 2.
Primary Antibodies and Peripheral Nerve Cells.
| Antibody | Fibroblasts | Schwann Cells | Macrophages* | Perineurial cells* |
|---|---|---|---|---|
| prolyl-4-hydrolase-beta CD90 (Thy 1) |
+++
**
+++ ** |
− | − | − |
| CD34 | +++ | − | − | |
| NG2 | ++ | − | − | ++ |
| Nestin | + *** | +++ | − | − |
| S100 | − | +++ | − | |
| CD117 | − | |||
| EMA (epithelial membrane antigen) | − | − | − | +++ |
| Glut 1 | − | − | − | +++ |
| CD68 | − | − | +++ | |
| PDGFR-beta |
++ | − | ||
| PDGFR-alpha | − | ++ |
: many positive cells, ++: some positive cells, +: a few positive cells, -: negative.
: immunofluorescence and immuno-electron microscopy studies; **: in culture only; ***: in cultures and immuno-electron microscopy only (not visible in immunofluorescence studies).
Light Microscopy: Immunofluorescence and Immunohistochemistry
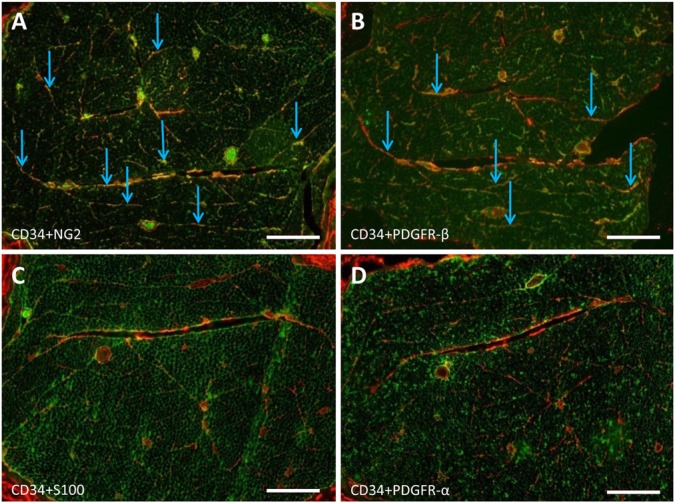
In the endoneurium, EFLCs were diffusely scattered between nerve fibers. Immunofluorescence studies showed that NG2 and CD34 immunoreactivity form an irregular network around Schwann cells. To investigate the co-expression of CD34 and NG2 in normal peripheral nerves, frozen sections were co-incubated with antibodies against CD34 and NG2. EFLCs showed a co-expression of both markers (Fig. 1 and Supplementary Fig. 1 for higher magnification images). In contrast, sections co-stained with antibodies against NG2 and S100 or CD34 and S100 showed no overlap (Fig. 1). NG2- and CD34-positive cells were never immunoreactive for S100, a marker of Schwann cells in normal peripheral nervous tissue. In addition, NG2 antibodies also stained vasa nervorum pericytes and perineurial cells, whereas CD34 antibodies stained endothelial cells but not perineurial cells (Supplementary Fig. 2). Some mesenchymal cells outside the nerves also exhibited CD34 immunoreactivity (data not shown). Because glial cell progenitors in the central nervous system (CNS) co-expressed NG2 and platelet-derived growth factor receptor (PDGFR)-α, whereas mesenchymal fibroblasts expressed PDGFR-β, we tested the expression of both isoforms of PDGFR in peripheral nerves. Frozen sections of rat nerve were co-incubated with antibodies against CD34 and PDGFR-α, or CD34 and PDGFR-β. We observed a co-expression of CD34 and PDGFRβ in approximately half of the CD34-positive EFLC-type structures in normal sciatic nerves (Fig. 1 and Supplementary Fig. 1 for higher magnification images), whereas CD34 and PDGFR-α were not co-expressed (Fig. 1). Some Schwann cells appeared to express PDGFR-α, but none of them expressed PDGFR-β (data not shown).
Figure 1.
Comparison of the localizations of CD34, neural/glial antigen 2 (NG2), platelet-derived growth factor receptor (PDGFR)-β, S100 and PDGFR-α in the endoneurium. Transverse frozen section of a normal rat sciatic nerve (double immunofluorescence, as described in the Materials & Methods). (A) Co-expression of CD34 (red) and NG2 (green) in endoneurial fibroblast-like cells (EFLCs), which are scattered between nerve fibers (arrows). (B) Nearly half of EFLCs co-express CD34 (red) and PDGFR-β (green) (arrows). (C) No co-expression of CD34 (red) and S100 (green). (D) No co-expression of CD34 (red) and PDGFR-α (green). Scale bars, 100 µm.
On cross sections of normal peripheral nerves, a strong nestin immunoreactivity was observed in Schwann cell cytoplasms (similar pattern as that observed for S100). Co-immunostaining for nestin and S100 showed a perfect overlap (data not shown). No EFLCs appeared to be nestin-positive using immunofluorescence techniques, but the high level of nestin expression by all Schwann cells did not allow us to assess this point precisely.
On paraffin sections of rat sciatic nerves, prolyl-4-hydrolase-beta, a classical marker of mesenchymal fibroblasts, faintly stained the cytoplasms of EFLCs (data not shown). No positive staining could be obtained in human nerves.
In normal human and rat nerves, EFLCs were not stained by antibodies against EMA, Glut1, CD68 (macrophage marker), c-Kit (CD117), or CD45 (leukocyte common antigen) (data not shown). EMA was only expressed in perineurial cells. Glut1 expression was observed in the perineurium and in pericytes of endoneurial blood vessels, which exhibited an intense immunoreactivity (Supplementary Fig. 2).
Electron and Immuno-electron Microscopy
Using conventional electron microscopy, EFLCs appeared as cells with no lamina basalis, having long lamellar processes projecting between nerve fibers. Their cytoplasms contained abundant rough endoplasmic reticulum, lysosomes, and Golgi apparatus. The cytoplasmic membranes of EFLCs showed pinocytotic vesicles (Richard et al. 2012). A significant number of EFLCs were located beneath the perineurium where they usually lie in parallel under the perineurial cells. EFLCs were also diffusely scattered between nerve fibers and were more frequently located near capillaries. They appeared to form a reticular network within the endoneurium, but we could not identify direct evidence of intercellular communications, such as gap junctions, between cells (data not shown).
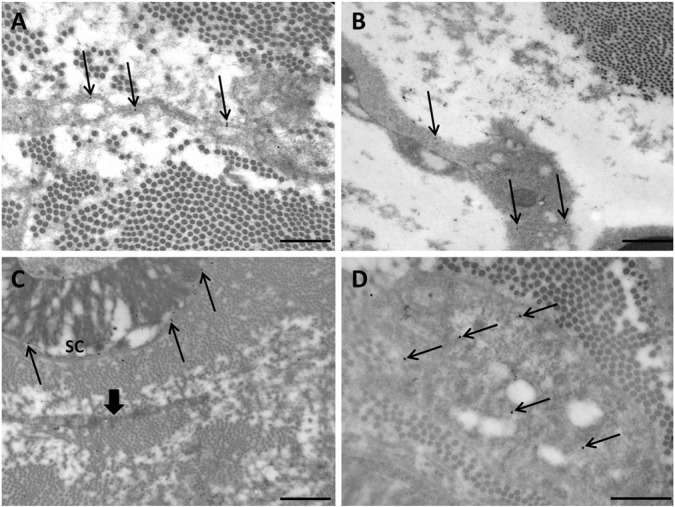
Immuno-electron microscopy studies showed positive staining for CD34 at the level of the EFLC plasma membrane in normal human nerves (Fig. 2). In addition to EFLCs, mastocytes and endothelial cells were CD34-positive. NG2 immunolabeling was positive in EFLCs (Fig. 2) and perineurial cells. Nestin immunolabeling appeared strong in Schwann cell cytoplasms. Unexpectedly, some EFLCs also showed a less intense but significant nestin immunolabeling (Fig. 2). We confirmed these findings by studying a large number of ultrathin sections, and always observed that a subset of EFLCs displayed a cytoplasmic nestin staining (Supplementary Fig. 3).
Figure 2.
Immuno-electron microscopy studies of normal human nerves (transverse section, London resin white staining). (A) Only endoneurial fibroblast-like cells (EFLCs) show positive staining for CD34 (electron-dense gold particles) at the level of plasma membrane (arrows); scale bar, 500 nm. (B) EFLCs are also stained by the anti-NG2 antibody (arrows); scale bar, 1 µm. (C) Only Schwann cells are stained by the anti-S100 antibody (arrows indicate gold particles at the level of the abaxonal cytoplasm of a myelinating Schwann cell: SC); a nearby EFLC is not stained (large arrow); scale bar, 1 µm. (D) An EFLC shows positive staining for nestin (cytoplasmic staining, arrows); scale bar, 500 nm.
Only the layered perineurium showed immunoreactivity for Glut1; both luminal and contraluminal basement membranes in the processes of perineurial cells were labeled (data not shown). EFLCs were not labeled by anti-CD68 and anti-S100 antibodies (Fig. 2)
Cell Culture
After three to four days, two main different cell types were observed in cultures obtained from nerve explants: most of the cells were flattened cells with a polygonal shape, blunt cytoplasmic processes and big round nuclei, which were identified as fibroblasts; very few of the cells were spindle-shaped with an oval cell body and a strong tendency to line up end-to-end, typical of Schwann cells. Immunofluorescence labeling of cultured cells with anti-S100 (marker of Schwann cells) and anti-prolyl-4-hydrolase-beta (P4Hb, a marker of fibroblasts) antibodies established that 98% of cultured cells were EFLCs and 2% were Schwann cells. We, as others (Niapour et al. 2010), observed that fibroblasts were the first cells to exit from nerve explants, 2 or 3 days after the beginning of cell culture; it is therefore advisable to remove the nerve pieces as soon as possible in order to avoid Schwann cell contamination and obtain EFLC cultures that are as pure as possible.
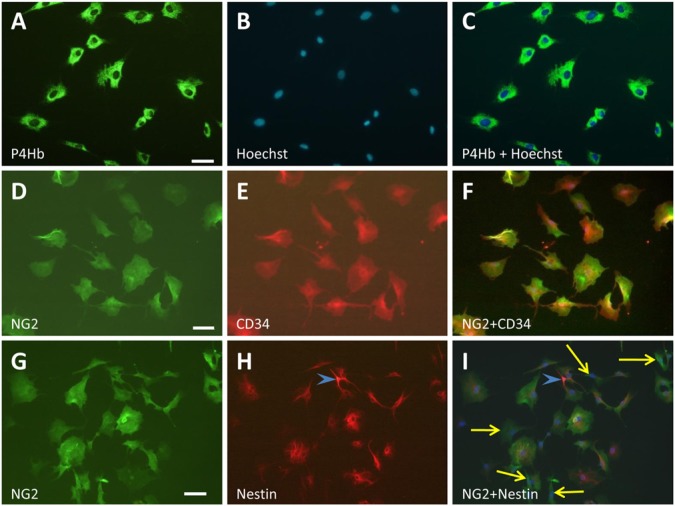
These cultures were labeled with antibodies against NG2, CD34, P4Hb, nestin, Thy1 and PDGFR-β. Both NG2 and CD34 antibodies labeled a population of flat, irregular cells with large nuclei, which were also immunoreactive for P4Hb and Thy-1. When performing double immunostaining, all EFLCs appeared to be positive for CD34 and NG2 (Fig. 3), NG2 and P4Hb, and CD34 and P4Hb (data not shown). A subpopulation of EFLCs showed immunoreactivity for PDGFR-β (40% of EFLCs), and half of the PDGFR-β-positive cells showed nestin immunoreactivity (Fig. 4). In these cells, nestin immunostaining was preferentially distributed around the nucleus (Figs. 3 and 4). In nestin-expressing EFLCs, the fluorescence intensity was markedly weaker than that observed in Schwann cells (Fig. 3). Double staining with anti-S100 and anti-nestin antibodies showed an overlap in Schwann cells only. EFLCs in culture were not stained by the anti-S100 antibody (data not shown).When performing a double immunostaining against nestin and NG2, all EFLCs displayed NG2 immunoreactivity and a minority of them (~20%) were also positive for nestin (Fig. 3). No immunostaining for CD68, c-Kit and CD45 were found in the EFLCs.
Figure 3.
Immunological characterization of cultured endoneurial fibroblast-like cells (EFLCs). EFLCs isolated from rat normal sciatic nerve show typical fibroblastic shapes when cultured on plastic surfaces. These cells have a flattened polymorphic shape, blunt cytoplasmic processes (A, cytoplasmic staining using anti-P4Hb antibody) and large nuclei (B, Hoechst; C, merged image). Anti-NG2 (D) and anti-CD34 (E) antibodies label all EFLCs, with a perfect overlap in the merged image (F). Some of the NG2-positive EFLCs (G) are also nestin-positive (H, and I: merged image). In this area of the culture dish, most of the NG2-positive EFLCs express nestin. A few EFLCs do not express nestin (yellow arrows) and a single Schwann cell (blue arrowhead) shows strong nestin immunoreactivity but no NG2 staining. Scale bars,100 µm.
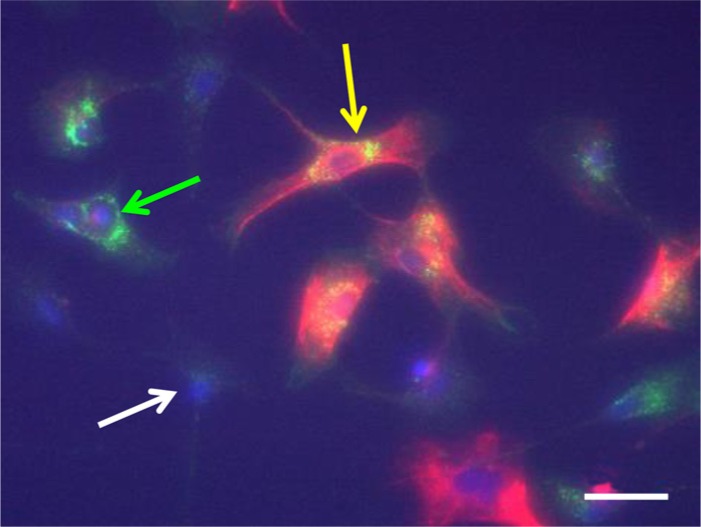
Figure 4.
Characterization of Nestin/PDGFR-β-positive endoneurial fibroblast-like cells (EFLCs). The image shows three types of ELFCs. PDGFR-β staining shows a punctate cytoplasmic pattern mainly in the perinuclear region (green), whereas nestin (intermediate filament) staining forms a dense meshwork in the whole cytoplasm (red). Approximately 60% of all EFLCs in culture are negative for both PDGFR-β and nestin staining (white arrow), ~20% are positive for PDGFR-β and negative for nestin staining (green arrow) and ~20% are positive for both (yellow arrow). Nuclei are labeled with Hoechst. Scale bar, 100 µm.
Finally, we performed in vivo injuries by crushing sciatic nerves in live rats and performing the explant culture method on nerves taken at various time points after the injury (3, 5 and 8 days) (Fig. 5). We compared the expression of nestin and PDGFR-β by EFLCs from crushed nerves and EFLCs from normal nerves (obtained from unoperated animals). We also assessed the expression of nestin and PDGFR-β by EFLCs from the left (uncrushed) nerve of operated animals. No difference was observed between EFLCs from normal unoperated nerves and EFLCs from the uncrushed left nerves of operated animals (40% of PDGFR-β positive cells and 20% of nestin-positive cells at all time points). By contrast, a significant increase in nestin expression was observed in EFLCs from crushed nerves at day 5 (positive in 35% of “crushed” EFLCs vs 20% in controls, p<0.001). The percentage of nestin-positive fibroblasts then decreased, returning to control levels at day 8 (Fig. 5). At day 5, all nestin-positive EFLCs were also positive for PDGFR-β, but a fraction of PDGFR-β-positive EFLCs increased only moderately (51% of PDGFR-β-positive EFLCs at day 5 after crush versus 40% in controls, p=n.s.).
Figure 5.
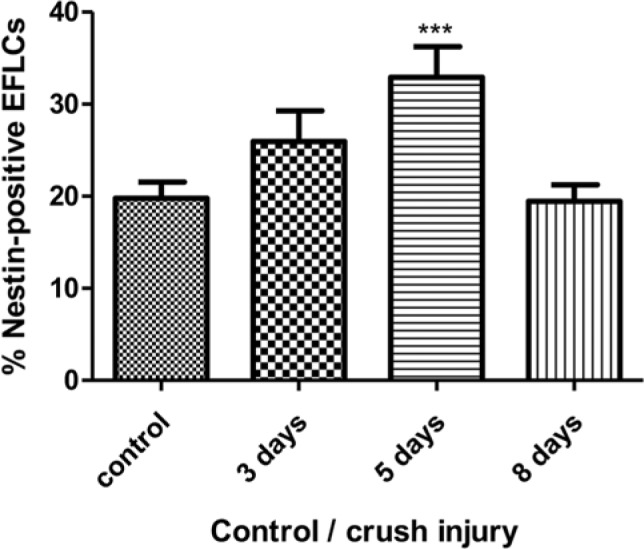
Percentage of EFLCs from the nerves of control and crush injured rats that were Nestin positive at 3, 5 and 8 days after the crushing injury was sustained (***: p≤0.001).
Discussion
The present study aimed to define the cellular characteristics of EFLCs using histological, ultrastructural, immunohistochemical and cytochemical techniques. This immunological characterization of EFLCs has never been performed until now. Morphologically, EFLCs are defined as fusiform cells with a relatively small amount of cytoplasm, fine filamentous processes and an absence of basal lamina. We have tried to immuno-phenotype EFLCs and found that all EFLCs are uniformly immuno-positive for prolyl-4-hydroxylase-β, which has a central role in the synthesis of collagen, CD34 and NG2. We also showed that the EFLC population is heterogeneous, with ~40% of EFLCs expressing PDGFR-β, and half of these PDGFR-β-positive cells also expressing nestin.
In normal nerves, EFLCs form a reticular network that can be visualized with CD34 immunolabeling. The present immunohistochemical studies on normal human and rat nerves confirmed that CD34 is expressed by all EFLCs, as well as by vasa nervorum endothelial cells, as previously observed in human peripheral nerves (Weiss and Nickoloff 1993). CD34-positive cells have also been reported in some nerve lesions, such as nerve sheath tumors (Weiss and Nickoloff 1993) but the nature of these positive cells was not clarified in this study. Weiss and Nickoloff speculated that CD34-positive cells within the nerve might represent precursor elements or play a supportive role for the Schwann cell. CD34 is a membrane-bound sialomucin that is expressed on primitive hematopoietic stem cells and downregulated as they differentiate into mature cells. Although its precise function remains unknown, the pattern of expression of CD34 suggests that it plays a significant role in early hematopoiesis. CD34 expression is, however, not specific to one type of cell, as it is also present in vascular endothelial cells, including those of capillaries in most tissues and in mast cells (Nielsen et al. 2009). It was also shown to be expressed by a subset of blood-borne “fibrocytes” present in various tissues (Bucala et al. 1994; Keeley et al. 2010). However, EFLCs have been shown to derive (at least in part) from neural crest stem cells (Joseph et al. 2004), and are therefore different from blood-borne fibrocytes, which have a hematopoietic origin. In agreement, EFLCs do not express the leukocyte common antigen CD45, which is a general marker for cells of a hematopoietic origin.
NG2 is a well-characterized integral membrane proteoglycan found principally on the surfaces of oligodendrocyte precursor cells in the CNS (Levine and Nishiyama 1996). Three previous studies found expression of NG2 in the peripheral nerves of mice, rats and humans (Martin et al. 2001; Morgenstern et al. 2003; Schneider et al. 2001). Our study confirms that all EFLCs express NG2 in rats and humans. NG2 expression is not common to all fibroblasts, as it was previously shown that cultured peripheral nerve fibroblasts express large amounts of NG2, whereas skin fibroblasts do not (Morgenstern et al. 2003). This distinction might be related to the fact that most EFLCs are of a neural crest origin (Joseph et al. 2004), whereas common fibroblasts have a mesenchymal origin. The role of NG2 expression in EFLCs remains unclear, although it has been suggested that NG2 could have facilitatory roles with respect to axonal regeneration, by promoting enhanced mobility of non-neuronal cells after injury or confining regenerating axons within the endoneurial compartment (Rezajooi et al. 2004). By analogy with the CNS, NG2-expressing EFLCs might also represent progenitor cells, but their differentiation capabilities remain to be determined. We did not study the expression of tenascin-C (TN-C), which is not expressed by EFLCs in normal conditions. It was recently shown that, after perineurium resection in the rat sciatic nerve, TN-C-positive EFLCs migrate to the outer side of the nerve to regenerate the damaged perineurium (Yamamoto et al. 2011). It would be interesting to determine whether all EFLCs or only a subset of them (for example, those able to differentiate into perineurial cells) express TN-C, in vivo and in culture in follow-up studies that focus on regeneration after a nerve lesion.
By contrast with CD34 and NG2, which appear to be expressed by all EFLCs, PDGFR-β expression is restricted to a subset of EFLCs. PDGF family members include PDGF-A, -B, -C, and -D. PDGFs are major mitogens for many cell types of mesenchymal origin, including fibroblasts and vascular smooth muscle cells (Seppa et al. 1982). These growth factors are assembled as disulfide-linked homo- or heterodimers and activate PDGF receptor (PDGFR)-α and -β. Upon binding to the specific ligand, PDGFRs form homo- or heterodimers and transduce the signal through activation of their intracytoplasmic tyrosine kinase domains (Heldin and Westermark 1999; Tallquist and Kazlauskas 2004). The PDGF-PDGFR system has been implicated in several fibrotic conditions and is assumed to play a role in driving the proliferation of cells of a fibroblast phenotype (Andrae et al. 2008; Bonner 2004; Powell et al. 1999). PDGFR-β-positive EFLCs might therefore represent endoneurial cells with higher proliferative capabilities.
Concerning the peripheral nervous system, PDGF B-chain and PDGFR-α are expressed in Schwann cells in both normal peripheral nerves and in culture (Eccleston et al. 1990; Hardy et al. 1992). In our study, we found that PDGFR-α is not expressed by EFLCs in normal conditions. By contrast, 40–50% of EFLCs expressed PDGFR-β, in normal peripheral nerve as well as in culture. In 2009, Yamazaki et al. detected PDGFR-β expression in a few spindle-shaped cells after nerve lesion (Yamazaki et al. 2009). On western blots, PDGFR-α and -β were found to be activated in peripheral nerves during the early phase after injury and PDGFR-β was also found to be abundantly expressed at the late phase of nerve injury, but the localization and nature of PDGFR-β-positive cells in the endoneurium was not investigated in that study. The spatial and temporal patterns of expression of PDGFR-β in EFLCs after various types of peripheral nerve injuries or in human PNS disorders now have to be determined. This could help us to understand the role of the PDGF-PDGFR system in peripheral nerve fibrosis after injury.
Another novel finding of our study is the demonstration (by immuno-electron microscopy in peripheral nerves and immunofluorescence in cultured EFLCs) that a subpopulation of EFLCs expresses the intermediate filament nestin. In culture, nestin-positive EFLCs represent a subset (nearly half) of PDGFR-β-positive EFLCs. This has not been noted before, probably because Schwann cells also express nestin but at a much higher level, which would not allow for the detection of EFLC positivity for this protein in immunofluorescence studies of the peripheral nerve. In light microscopy studies, the resolution is much lower than in immuno-electron microscopy studies, and EFLCs are diffusely scattered between nerve fibers, with very thin cytoplasmic processes. This probably explains the apparent discrepancy between the results of immunofluorescence studies and those of immuno-electron microscopy studies and EFLCs cultures. Nestin is expressed in multipotent stem cells and progenitor cells and is involved in the early stages of lineage commitment, in proliferation and in differentiation. We have observed that the number of nestin-positive EFLCs increases significantly after a sciatic nerve crush injury. It was previously shown that, in the CNS, astrocytes can re-express or upregulate nestin after injury (Lin et al. 1995; Namiki and Tator 1999), which may indicate a reversion to a more immature phenotype. Other studies have documented a reactivation of nestin-expressing cells during tissue regeneration and wound healing (Wiese et al. 2004). The observation that a subset of EFLCs are nestin-positive is not completely surprising, because populations of nestin-positive fibroblast-like cells have recently been found in several “non-neural” tissues (Kishaba et al. 2010; Wu et al. 2013). In light of these findings, we speculate that nestin-positive EFLCs may be endowed with multipotentiality as compared with nestin-negative fibroblasts. This can be related to the fact that all nestin-positive EFLCs are also PDGFR-β-positive, suggesting a higher proliferative potential.
In addition, some immunophenotypic similarities appear between EFLCs, pericytes and interstitial Cajal cells (ICCs), which are considered to be mesenchymal progenitor cells: the three cell types express CD34 and nestin (only a subpopulation of EFLCs for nestin). In addition, pericytes also express NG2 and PDGFR-β. There are, however, marked differences between EFLCs and the latter two cell types. EFLCs do not express c-Kit, whereas ICCs do. Both ICC and pericytes have a basal lamina and display abundant gap junctions (Allt and Lawrenson 2001; Komuro et al. 1999). Ultrastructurally, ICCs are easily distinguished from EFLCs by a combination of features including large gap junctions, abundant intermediate filaments, numerous mitochondria, flattened cisterns of rough endoplasmic reticulum and a characteristic electron density of the cytoplasm (Komuro et al. 1999). Pericytes contain numerous free ribosomes, cisternae of granular endoplasmic reticulum, isolated microtubules, a few mitochondria, and, in some of them, numerous lysosomes (Lafarga and Palacios 1975). However, the immunophenotypic similarities between the three cell types still suggest that EFLCs could constitute a niche of endoneurial progenitor cells.
In conclusion, the combination of various techniques (from cell culture to immuno-electron microscopy) allowed us to precisely characterize and immunophenotype the heterogeneous population of EFLCs. Their role and potential for peripheral nerve maintenance and regeneration remains to be investigated by the combination of these different techniques in pathological conditions. The characterization of EFLC immunophenotype also provides a useful tool for further studies focused on the fate and potential differentiation capability of EFLCs.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the French Ministry of Research and a research grant from the Region Limousin.
References
- Ali-Bahar M, Bauer B, Tredget EE, Ghahary A. (2004). Dermal fibroblasts from different layers of human skin are heterogeneous in expression of collagenase and types I and III procollagen mRNA. Wound Repair Regen 12:175-182 [DOI] [PubMed] [Google Scholar]
- Allt G, Lawrenson JG. (2001). Pericytes: cell biology and pathology. Cells Tissues Organs 169:1-11 [DOI] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. (2008). Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22:1276-1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzarone B, Macieira-Coelho A. (1982). Heterogeneity of the kinetics of proliferation within human skin fibroblastic cell populations. J Cell Sci 57:177-187 [DOI] [PubMed] [Google Scholar]
- Be’eri H, Reichert F, Saada A, Rotshenker S. (1998). The cytokine network of wallerian degeneration: IL-10 and GM-CSF. Eur J Neurosci 10:2707-2713 [PubMed] [Google Scholar]
- Bonner JC. (2004). Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15:255-273 [DOI] [PubMed] [Google Scholar]
- Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. (1994). Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1:71-81 [PMC free article] [PubMed] [Google Scholar]
- Eccleston PA, Collarini EJ, Jessen KR, Mirsky R, Richardson WD. (1990). Schwann Cells Secrete a PDGF-like Factor: Evidence for an Autocrine Growth Mechanism involving PDGF. Eur J Neurosci 2:985-992 [DOI] [PubMed] [Google Scholar]
- Fritsch C, Orian-Rousseaul V, Lefebvre O, Simon-Assmann P, Reimund JM, Duclos B, Kedinger M. (1999). Characterization of human intestinal stromal cell lines: response to cytokines and interactions with epithelial cells. Exp Cell Res 248:391-406 [DOI] [PubMed] [Google Scholar]
- Groh J, Weis J, Zieger H, Stanley ER, Heuer H, Martini R. (2012). Colony-stimulating factor-1 mediates macrophage-related neural damage in a model for Charcot-Marie-Tooth disease type 1X. Brain 135:88-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M, Reddy UR, Pleasure D. (1992). Platelet-derived growth factor and regulation of Schwann cell proliferation in vivo. J Neurosci Res 31:254-262 [DOI] [PubMed] [Google Scholar]
- Harper RA, Grove G. (1979). Human skin fibroblasts derived from papillary and reticular dermis: differences in growth potential in vitro. Science 204:526-527 [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. (1999). Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79:1283-1316 [DOI] [PubMed] [Google Scholar]
- Hill R. (2009). Extracellular matrix remodelling in human diabetic neuropathy. J Anat 214:219-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, Lee KF, Meijer D, Anderson DJ, Morrison SJ. (2004). Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 131:5599-5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley EC, Mehrad B, Strieter RM. (2010). Fibrocytes: bringing new insights into mechanisms of inflammation and fibrosis. Int J Biochem Cell Biol 42:535-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita M, Magy L, Richard L, Piaser M, Vallat JM. (2002). LR white post-embedding colloidal gold method to immunostain MBP, P0, NF and S100 in glutaraldehyde fixed peripheral nerve tissue. J Peripher Nerv Syst 7:128-133 [DOI] [PubMed] [Google Scholar]
- Kishaba Y, Matsubara D, Niki T. (2010). Heterogeneous expression of nestin in myofibroblasts of various human tissues. Pathol Int 60:378-385 [DOI] [PubMed] [Google Scholar]
- Komuro T, Seki K, Horiguchi K. (1999). Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol 62:295-316 [DOI] [PubMed] [Google Scholar]
- Lafarga M, Palacios G. (1975). Ultrastructural study of pericytes in the rat supraoptic nucleus. J Anat 120:433-438 [PMC free article] [PubMed] [Google Scholar]
- Leal LPS, Spinel C. (2004). Endoneural fibroblasts isolation and culture. Acta biologica Colombiana 9:57 [Google Scholar]
- Levine JM, Nishiyama A. (1996). The NG2 chondroitin sulfate proteoglycan: a multifunctional proteoglycan associated with immature cells. Perspect Dev Neurobiol 3:245-259 [PubMed] [Google Scholar]
- Lin RC, Matesic DF, Marvin M, McKay RD, Brustle O. (1995). Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol Dis 2:79-85 [DOI] [PubMed] [Google Scholar]
- Martin S, Levine AK, Chen ZJ, Ughrin Y, Levine JM. (2001). Deposition of the NG2 proteoglycan at nodes of Ranvier in the peripheral nervous system. J Neurosci 21:8119-8128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, Muller M, Kobsar I, Leonhard C, Martini R, Kiefer R. (2003). Origin of pathogenic macrophages and endoneurial fibroblast-like cells in an animal model of inherited neuropathy. Mol Cell Neurosci 23:351-359 [DOI] [PubMed] [Google Scholar]
- Morgenstern DA, Asher RA, Naidu M, Carlstedt T, Levine JM, Fawcett JW. (2003). Expression and glycanation of the NG2 proteoglycan in developing, adult, and damaged peripheral nerve. Mol Cell Neurosci 24:787-802 [DOI] [PubMed] [Google Scholar]
- Nageotte J. (1932) In “Cytology and Cellular Pathology of the Nervous System”. In New York, W. Penfield; [Google Scholar]
- Namiki J, Tator CH. (1999). Cell proliferation and nestin expression in the ependyma of the adult rat spinal cord after injury. J Neuropathol Exp Neurol 58:489-498 [DOI] [PubMed] [Google Scholar]
- Niapour A, Karamali F, Karbalaie K, Kiani A, Mardani M, Nasr-Esfahani MH, Baharvand H. (2010). Novel method to obtain highly enriched cultures of adult rat Schwann cells. Biotechnol Lett 32:781-786 [DOI] [PubMed] [Google Scholar]
- Nielsen HH, Ladeby R, Fenger C, Toft-Hansen H, Babcock AA, Owens T, Finsen B. (2009). Enhanced microglial clearance of myelin debris in T cell-infiltrated central nervous system. J Neuropathol Exp Neurol 68:845-856 [DOI] [PubMed] [Google Scholar]
- Nolte SV, Xu W, Rennekampff HO, Rodemann HP. (2008). Diversity of fibroblasts–a review on implications for skin tissue engineering. Cells Tissues Organs 187:165-176 [DOI] [PubMed] [Google Scholar]
- Parker RC. (1932). The functional characteristics of nine races of fibroblasts. Science 76:219-220 [DOI] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. (1999). Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol 277:C183-201 [DOI] [PubMed] [Google Scholar]
- Rezajooi K, Pavlides M, Winterbottom J, Stallcup WB, Hamlyn PJ, Lieberman AR, Anderson PN. (2004). NG2 proteoglycan expression in the peripheral nervous system: upregulation following injury and comparison with CNS lesions. Mol Cell Neurosci 25:572-584 [DOI] [PubMed] [Google Scholar]
- Richard L, Topilko P, Magy L, Decouvelaere AV, Charnay P, Funalot B, Vallat JM. (2012). Endoneurial fibroblast-like cells. J Neuropathol Exp Neurol 71:938-947 [DOI] [PubMed] [Google Scholar]
- Rotshenker S. (2011). Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation 8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer IA, Pandy M, Ferguson R, Davis BR. (1985). Comparative observation of fibroblasts derived from the papillary and reticular dermis of infants and adults: growth kinetics, packing density at confluence and surface morphology. Mech Ageing Dev 31:275-293 [DOI] [PubMed] [Google Scholar]
- Schneider S, Bosse F, D’Urso D, Muller H, Sereda MW, Nave K, Niehaus A, Kempf T, Schnolzer M, Trotter J. (2001). The AN2 protein is a novel marker for the Schwann cell lineage expressed by immature and nonmyelinating Schwann cells. J Neurosci 21:920-933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempowski GD, Borrello MA, Blieden TM, Barth RK, Phipps RP. (1995). Fibroblast heterogeneity in the healing wound. Wound Repair Regen 3:120-131 [DOI] [PubMed] [Google Scholar]
- Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR. (1982). Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol 92:584-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamash S, Reichert F, Rotshenker S. (2002). The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci 22:3052-3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell JM, Caplan AI. (2009). Fibroblasts-a diverse population at the center of it all. Int Rev Cell Mol Biol 276:161-214 [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Caplan AI. (2004). Fibroblast heterogeneity: more than skin deep. J Cell Sci 117:667-675 [DOI] [PubMed] [Google Scholar]
- Tallquist M, Kazlauskas A. (2004). PDGF signaling in cells and mice. Cytokine Growth Factor Rev 15:205-213 [DOI] [PubMed] [Google Scholar]
- Weis J, Brandner S, Lammens M, Sommer C, Vallat JM. (2012). Processing of nerve biopsies: a practical guide for neuropathologists. Clin Neuropathol 31:7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SW, Nickoloff BJ. (1993). CD-34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am J Surg Pathol 17:1039-1045 [DOI] [PubMed] [Google Scholar]
- Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. (2004). Nestin expression–a property of multi-lineage progenitor cells? Cell Mol Life Sci 61:2510-2522 [DOI] [PubMed] [Google Scholar]
- Wu X, Bolger WE, Anders JJ. (2013). Fibroblasts isolated from human middle turbinate mucosa cause neural progenitor cells to differentiate into glial lineage cells. PLoS One 8:e76926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Okui N, Tatebe M, Shinohara T, Hirata H. (2011). Regeneration of the perineurium after microsurgical resection examined with immunolabeling for tenascin-C and alpha smooth muscle actin. J Anat 218:413-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Sabit H, Oya T, Ishii Y, Hamashima T, Tokunaga A, Ishizawa S, Jie S, Kurashige Y, Matsushima T, Furuta I, Noguchi M, Sasahara M. (2009). Activation of MAP kinases, Akt and PDGF receptors in injured peripheral nerves. J Peripher Nerv Syst 14:165-176 [DOI] [PubMed] [Google Scholar]