Abstract
Background:
A number of different surgical techniques are effective for treatment of drug-resistant medial temporal lobe epilepsy. Of these, transsylvian selective amygdalohippocampectomy (SA), which was originally developed to maximize temporal lobe preservation, is arguably the most technically demanding to perform. Recent studies have suggested that SA may result in better neuropsychological outcomes with similar postoperative seizure control as standard anterior temporal lobectomy, which involves removal of the lateral temporal neocortex.
Methods:
In this article, the authors describe technical nuances to improve the safety of SA.
Results:
Wide sylvian fissure opening and use of neuronavigation allows an adequate exposure of the amygdala and hippocampus through a corticotomy within the inferior insular sulcus. Avoidance of rigid retractors and careful manipulation and mobilization of middle cerebral vessels will minimize ischemic complications. Identification of important landmarks during amygdalohippocampectomy, such as the medial edge of the tentorium and the third nerve within the intact arachnoid membranes covering the brainstem, further avoids operator disorientation.
Conclusion:
SA is a safe technique for resection of medial temporal lobe epileptogenic foci leading to drug-resistant medial temporal lobe epilepsy.
Keywords: Epilepsy surgery, medial temporal lobe epilepsy, neurosurgical procedure, selective amygdalohippocampectomy, transsylvian
INTRODUCTION
Drug-resistant epilepsy is defined as recurrent unprovoked seizures refractory to two appropriately selected antiepileptic medication trials.[4,14,23] Medial temporal lobe epilepsy (MTLE) is known to be the most common type of drug-resistant epilepsy.[14,38] A number of studies have demonstrated the efficacy of surgery in treating MTLE and improving the patient's quality of life.[10,15,27,44] Although the optimal procedure is a matter of debate, the goal of each operation is to remove the epileptogenic zone while preserving the surrounding normal anatomy, thereby limiting the morbidity associated with the procedure.
Two common procedures are often described in the literature as effective treatments for MTLE. Anterior temporal lobectomy (ATL) has been shown in a randomized, controlled trial to be superior to medical management for relief of MTLE[44] and selective amygdalohippocampectomy (SA) was developed to preserve the lateral temporal neocortex, especially on the dominant hemisphere.[46] Even though two studies have suggested that better seizure control occurs after ATL,[3,8] other studies have demonstrated comparable outcomes after SA versus ATL.[2,9,30,35,40,43] An improved neuropsychological outcome has been associated with SA compared with ATL.[9,18,28,30,43]
Seizure outcomes after SA were previously studied in a retrospective analysis that demonstrated a Class I Engel outcome (patient free of disabling seizures) in 27 of 30 (90%) patients when magnetic resonance imaging (MRI), electroencephalography (EEG), and histopathologic results were concordant.[46] In another series involving 369 patients who underwent SA, an Engel Class I outcome was achieved in 67%.[45] Both of these studies demonstrated the efficacy of SA.
Because of the technical complexity of SA, presumed risks, and required familiarity with microsurgical techniques on the part of the surgeon, the use of SA has been limited compared with ATL. There is minimal information available regarding the necessary technical nuances needed to minimize SA complications. The narrow operative corridor through the transsylvian route requires that the surgeon be thoroughly familiar with the surgical anatomy of the region and handling of important adjacent cerebrovascular structures. In this article, we describe the technical aspects of SA, focusing on preoperative evaluation and, more importantly, on surgical anatomy and technique to avoid complications. We describe operative nuances based on the senior author's (ACG) experience with this procedure.
Preoperative preparation
Currently, the American Academy of Neurology recommends that patients with drug-resistant MTLE resulting in disabling complex partial seizures be referred to an epilepsy surgery center.[15] In addition, the results from the Early Randomized Surgical Epilepsy Trial (ERSET) suggest that patients with MTLE would likely benefit from early surgical referral, rather than continued trials of antiepileptic medications.[14]
All patients should undergo a thorough neurologic examination and have a seizure history documented before surgical consideration. Complex partial seizures are the most common type of seizure resulting from MTLE, and secondary generalization is common.[16] In a study by French et al.,[16] 64 of 67 (96%) patients with known MTLE described an aura, most commonly an abdominal visceral sensation (39 of 64, 61%), fear (5 of 64, 8%), or olfactory sensation (5 of 64, 8%).[16] In this same study, 64 of 67 (96%) patients had completely normal neurologic exams.[16]
Before consideration for surgery, a number of tests may be completed to help define the patient's epileptogenic focus. A high-resolution MRI is necessary, not only for identification of abnormal anatomy, including neoplastic lesions and subtle cortical dysplasia, but also for surgical planning and neuronavigation intraoperatively. In a study of 175 patients who underwent ATL, unilateral hippocampal atrophy on MRI was predictive of postoperative seizure control.[32] This was especially true when the location of lesions identified on MRI were concordant with interictal findings on EEG.[32] Coronal T2-weighted and fluid attenuated inversion recovery (FLAIR) images are the most sensitive sequences for identification of medial temporal lobe sclerosis.[34]
Concordance of interictal epileptiform discharges and the location of ictal onset on EEG are independent predictors of postoperative seizure control.[32] Therefore, patients should undergo preoperative continuous video EEG monitoring. When scalp EEG fails to identify or lateralize the epileptogenic focus, invasive EEG monitoring may be useful.[25] This is especially true when discordant results occur from different testing methods or if bilateral hippocampal sclerosis is evident.[25] In these instances, subdural or hippocampal depth electrodes may be implanted to help lateralize the epileptogenic focus.[25]
If EEG and/or MRI are unsuccessful in localizing an epileptogenic focus, a nuclear imaging study may be helpful. Single-photon emission computed tomography (SPECT) allows indirect measurement of regional cerebral blood flow.[11,20] In temporal lobe epilepsy, SPECT studies will frequently demonstrate hypoperfusion during the interictal period and hyperperfusion during the ictal period.[5] The sensitivity of SPECT in localizing a temporal lobe epileptogenic focus is highest during the ictal and postictal phases (97% and 75%, respectively) and lowest during the interictal phase (44%).[11] For this reason, scanning should be performed during the ictal phase and the results compared with the findings during the interictal phases.[48] Positron emission tomography (PET) allows measurement of cerebral metabolism and can be helpful in identifying hypometabolic foci during the interictal phase.[20] However, when directly comparing the ability of ictal SPECT and interictal PET to lateralize a temporal lobe epileptogenic focus in 35 patients, Ho et al.[20] found ictal SPECT to be more sensitive (89% vs. 63%, respectively).
Finally, neuropsychological testing is an essential part of the preoperative workup in epilepsy surgery. It provides a baseline regarding the patient's preoperative memory, language, intelligence, attention, frontal lobe function, and visuospatial abilities.[22] With regard to MTLE, patients with dominant hemisphere foci frequently present with verbal memory deficits.[19,22,33] Moreover, baseline memory performance can be used to predict a patient's risk of postoperative functional decline.[17,22] Specifically, patients who are higher functioning preoperatively are at a greater risk of postoperative functional decline after a dominant hemisphere temporal lobe resection.[6,33] Conversely, patients with nondominant temporal lobe epilepsy may demonstrate deficits in spatial memory and learning after temporal lobe resection.[12]
The intracarotid amobarbital procedure (the WADA test) is a technique used to localize hemispheric dominance. The WADA test also provides insight into the contralateral hemisphere's capability of supporting memory after resection.[25] In this procedure, amobarbital is injected directly into the internal carotid artery to cause temporary hemianesthesia.[22] Neuropsychological testing can then be performed and compared with the contralateral side, allowing for determination of dominance and assessment of memory function.
Surgical technique for transsylvian selective amygdalohippocampectomy [video 1]
There are three commonly described approaches for SA. The subtemporal approach was developed in an attempt to avoid unnecessary damage to the lateral temporal lobe.[21,31] Takaya et al.[39] demonstrated an increase in glucose metabolism in the remaining temporal lobe remote from the resection site after subtemporal SA for MTLE. This study also demonstrated postoperative improvement in verbal memory, attention, and delayed recall scores following the subtemporal approach. The disadvantages to this approach include an increased need for temporal lobe retraction and risk of injury to the vein of Labbé.[21,31]
The transcortical approach is technically simpler, with the drawback of damage to the lateral temporal neocortex.[31] Finally, the transsylvian approach is the third surgical option and the preferred approach of the senior author. Although technically more difficult, this approach spares the lateral temporal lobe structures damaged in the transcortical approach and requires minimal retraction, unlike the subtemporal route.
Patient positioning and skull clamp placement
The transsylvian SA (referred to as SA) as described by Yaşargil[46] is accomplished through a standard pterional craniotomy. The patient is placed on the operating room table in a supine position. The single pin of the skull clamp is placed behind the ipsilateral ear above the superior nuchal line, while the double pin is placed on the contralateral superior temporal line. The patient's head should be rotated approximately 30° toward the contralateral side and extended until the zygoma reaches the highest point on the face. This arrangement allows for gravity retraction of the frontal lobe and prevents the temporal cortex from obscuring the transsylvian operative corridor.
Incision and craniotomy
After completion of the standard pterional incision, the temporalis muscle is reflected in one layer along with the scalp flap. After the bone flap is elevated, the orbital roof is flattened and both the greater and lesser wings of the sphenoid wing are drilled away to the level of the superior orbital fissure. These latter maneuvers minimize retraction on the frontotemporal opercula and enhance more flexible anterior operative working angles through the transsylvian route. Please see video 1 for details of the technique (http://www.youtube.com/watch?v=eZ1jFtFXQYgand feature=em-upload_owner)
Dural opening and sylvian fissure dissection
The dura is incised in a circular manner and reflected anteriorly. The dural opening should be flush with the newly flattened sphenoid and orbital floor.
The superficial sylvian veins and the divergent frontal and temporal cortical arteries mark the sylvian fissure.[46] Wide opening of this fissure is required. The sylvian fissure dissection begins superficially, anterior to the sylvian venous confluence, where the sylvian cistern is often most generous. At this level, microdissection of the frontotemporal opercula is carried to the floor of the sylvian fossa to allow the surgeon to find the distal M2 branches [Figure 1].[1,46] Care should be taken to minimize the manipulation of these vessels at all points during the operation and thus decrease the risk of postoperative vasospasm. Papaverine-soaked gelfoam pledgets may be placed on these vessels to relieve vasospasm if necessary.
Figure 1.
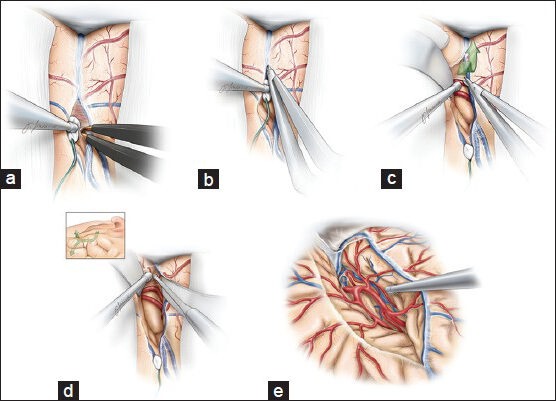
The dissection of the Sylvian fissure is carried out in a deep to superficial fashion (a-d). A wide opening of the fissure is necessary to obviate the need to fixed rigid retractors. The M1 segment is identified as it curves medially (d-e). Care is taken to minimize retraction on the frontotemporal opercula and manipulation of all the vessels coursing through the fissure. This figure is from The Neurosurgical Atlas, ©Aaron A. Cohen-Gadol, MD, MSc, used with permission.
We do employ the classification schemes designed for estimating the difficulty of fissure dissection before surgery.[29,47] We have found these classification methods unreliable.[29,47] Retraction should also be kept to a minimum throughout the procedure, and rigid retractors should be avoided to minimize the risk of retraction injury and venous infarction. The remaining dissection of the proximal sylvian fissure is carried out in a deep to superficial manner [Figure 1]. The fissure is opened to the level of the M1 segment as it curves medially. Such a dissection is extended toward the temporal lobe and limen insulae as the inferior parainsular sulcus and vein are exposed along the temporal stem.
Relevant microsurgical anatomy
The medial temporal lobe surgical anatomy is complicated and must be understood in its entirety before undertaking this procedure to avoid injury to the important adjacent cerebrovascular structures; most at risk are the middle cerebral artery (MCA) branches, the diencephalon/brainstem, the anterior choroidal artery, and the third nerve. The angle of exposure of the ventricle can disorient the surgeon, and therefore neuronavigation is helpful. All relevant boundaries of the structures at risk should be kept in mind. As described by Tubbs et al.,[41] an imaginary line drawn from the MCA bifurcation to the inferior choroidal point (defined as the most anterior aspect of the choroid plexus where the anterior choroidal artery enters the ventricle) is a reliable border between the amygdala inferiorly and the globus pallidus superiorly. Medially, the amygdala extends to the level of the basal cisterns as the uncus, abutting the third nerve. The inferior portion of the amygdala makes up the anterior roof and wall of the temporal horn.
Both the head and body of the hippocampus can be identified within the anterior aspect of the temporal horn. The pes of the hippocampus forms the medial border of the anterior temporal horn, whereas the superior border contacts the posteroinferior border of the amygdala. The body of the hippocampus makes up the medial aspect of the temporal horn floor and is bordered laterally by the collateral eminence, which forms the lateral floor of the temporal horn.[42]
The choroidal fissure, which lies between the choroid plexus (attached to the thalamus) and the fimbria, makes up the medial wall of the posterior two-thirds of the temporal horn.[42] Opening the choroidal fissure provides access to the ambient cistern—this maneuver is avoided during the operation.[42] Choroidal fissure is an important landmark in SA as all structures lying medial to the choroidal fissure belong to thalamus, whereas all structures lateral to it can be removed.[42] Exposure of MCA bifurcation, temporal horn, and choroid plexus orients the surgeon to the relevant anatomy.
Amygdalohippocampectomy
Following identification of the M1 and proximal M2 branches and generous exposure of the inferior insula and temporal stem, we make the initial cortical incision in the inferior insular sulcus (10-20 mm), starting just posterior to the temporopolar artery and lateral to the inferior parasylvian vein, which should be coagulated [Figure 2a].[46] Incising the temporal stem allows access to the temporal horn of the lateral ventricle [Figure 2b] and will ultimately become the corridor through which both the amygdala and hippocampus can be viewed [Figure 2c]. The temporal horn may not be immediately apparent due to the collapse of the temporal horn caused by release of cerebrospinal fluid (CSF) at the beginning of the procedure.[46] We use neuronavigation to guide white matter dissection and removal of the lateral aspect of the amygdala to expose the anterior tip of the temporal horn.[46] Still, it is imperative to have a clear understanding of relevant surgical landmarks in the area. Medially, the amygdala extends to the basal cisterns (chiasmatic, crural, and ambient) as the uncus. The uncus is divided into several segments. The semilunar gyrus (or the anterosuperior aspect of the uncus) represents the cortical projection of the amygdala, whereas the intralimbic gyrus (or posterior aspect of the uncus) represents the cortical projection of the hippocampus. The endorhinal sulcus and the optic tract separate the semilunar gyrus from the anterior perforated substance, the lateral margin of which is formed by the limen insulae. Before resection of the amygdala, a portion of this structure is biopsied and sent for histologic examination. The entire amygdala is carefully removed using ultrasonic aspirator, bipolar cautery, and suction [Figure 3].[46] This brings the medial tentorium and cranial nerve III into view through the remaining arachnoid membrane [Figure 4].[46]
Figure 2.
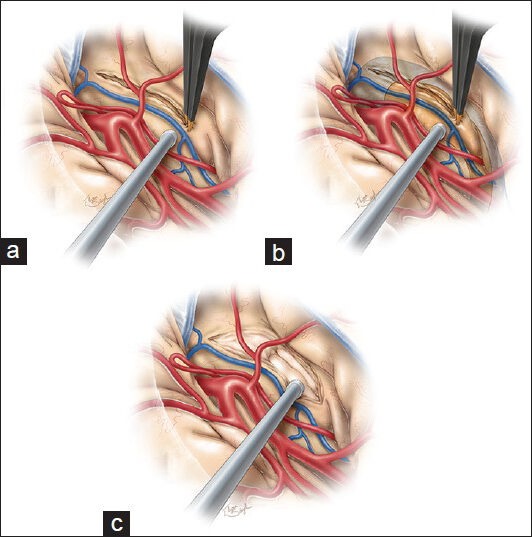
Once the temporal stem has been exposed, a 10–20 mm cortical incision is made along the inferior insular sulcus. This incision begins just posterior to the temperopolar artery and lateral to the inferior parasylvian vein (a). This maneuver will allow access to the anterior temporal horn and hippocampus (b) and will create the corridor through which the amygdala and hippocampus are viewed (c). The tip of the temporal horn should be exposed to orient the surgeon regarding the adjacent anatomy. This figure is from The Neurosurgical Atlas, ©Aaron A. Cohen-Gadol, MD, MSc, used with permission.
Figure 3.
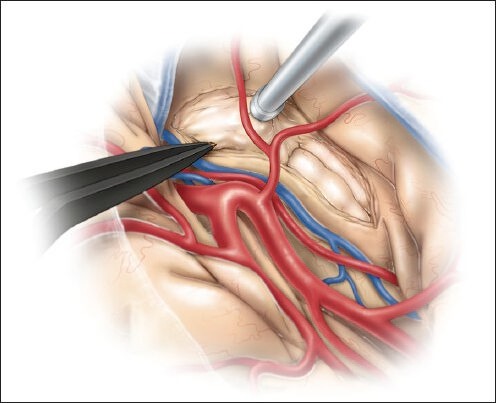
The amygdala is removed using an ultrasonic aspirator, bipolar cautery, and suction. This figure is from The Neurosurgical Atlas, ©Aaron A. Cohen-Gadol, MD, MSc, used with permission.
Figure 4.
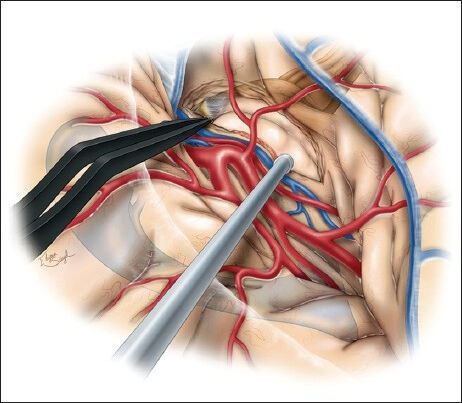
After removal of the amygdala, cranial nerve III and the medial tentorium become visible through the corresponding arachnoid membranes. Careful identification of these structures is necessary to ensure their preservation. This sketch also contains an overlay of the brainstem and the ventricular system to illustrate the relationship of the surrounding important structures. This figure is from The Neurosurgical Atlas, ©Aaron A. Cohen-Gadol, MD, MSc, used with permission.
The anterior hippocampus is now safely removed in an anterior-to-posterior fashion to the level of the P2 bifurcation (where its tail curves medially) and laterally to the level of collateral eminence [Figure 5].[1,46] The techniques for removal of the hippocampus are similar to those described above for amygdala. Resection is conducted along the subpial planes lateral to the choroid plexus and superior to the arachnoid membranes along the basal cisterns; these membranes are protected while the parahippocampus is excised in subpial manner. A rim of brain tissue may be left along the lateral aspect of the choroid plexus and on the arachnoid of the basal cisterns to avoid injury to the brainstem and diencephalic structures [Figure 6]. The anterior choroidal artery is not exposed. The posterior cerebral artery (PCA) perforators entering the posterior hippocampus are coagulated and cut. Their avulsion is avoided since this maneuver may injure the PCA, evident through the arachnoid of the basal cisterns. The operative working distance for the SA is long, and any injury to the MCA branches during aggressive retraction or manipulation should be avoided. The shaft of instruments may be used as dynamic retractors. Generous opening of the sylvian fissure during the earlier stages of the operation avoids the need for aggressive retraction of the frontotemporal opercula. The medial edge of tentorium remains a good landmark to protect the structures within the basal cisterns. Significant tension on the arachnoid layers along the edge of the tentorium may lead to temporary postoperative diplopia related to neuropraxia of the trochlear nerve.
Figure 5.
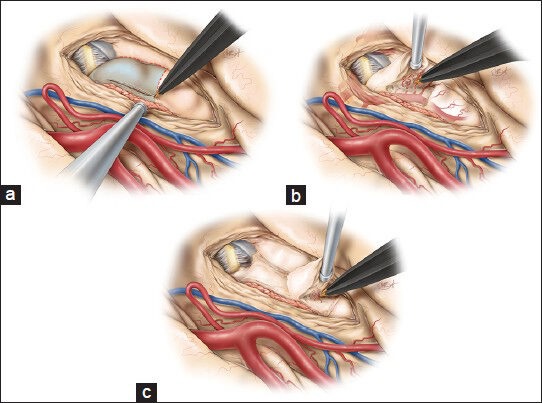
Once all relevant structures have been identified, the hippocampus can be safely removed in an anterior-to-posterior fashion in two steps. The first step involves removal of the anterior or Pes hippocampus (a) while making an incision just lateral to the choroid plexus. Care should be taken to carefully coagulate the PCA branches to the hippocampus and prevent their avulsion, which could damage the PCA (b). The posterior hippocampus is removed in a second step. Preservation of a small layer of brain tissue along the arachnoid membrane of the basal cisterns ensures the safety of the diencephalic structures (c). This figure is from The Neurosurgical Atlas, ©Aaron A. Cohen-Gadol, MD, MSc, used with permission.
Figure 6.
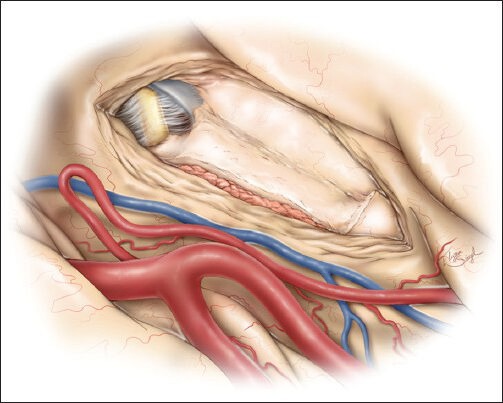
The final result demonstrates removal of the amygdala and hippocampus without a need to expose or dissect the cerebrovascular structures housed within the basal cisterns. This figure is from The Neurosurgical Atlas, ©Aaron A. Cohen-Gadol, MD, MSc, used with permission.
Potential complications
Meticulous handling of the superficial Sylvian veins decreases the risk of venous injury and resultant infarction. There is often a need for extensive microdissection through the sylvian fissure and medial mobilization of the MCA's temporal trunk to create additional operative space for the cortical incision along the inferior parainsular sulcus to enter the ventricle. These maneuvers may be risky if the principles of microsurgery are not carefully followed. Undue retraction on the frontotemporal opercula may lead to language dysfunction in the dominant hemisphere. Perforators from the temporal trunk of the MCA may be injured if these vessels are placed under tension. As discussed above, generous sylvian fissure opening is a key step in SA.
Cerebral Vasospasm: One of the most frequently discussed postoperative complications of SA is cerebral vasospasm resulting from subarachnoid bleeding within the operative field and aggressive manipulation of the corresponding vessels. Intracranial blood flow velocities increase after SA.[36,37] Schaller et al.[37] documented this increased blood flow velocity, but did not find any associated neurological deficit clinically. Lackner et al.[24] found that patients who were females and those with larger amounts of postoperative blood identified on CT scan had increased risk for vasospasm, but these authors identified no difference in vasospasm rates between transsylvian SA and ATL.
The skill of the surgeon in navigating the complex anatomy of the temporal lobe while minimizing blood loss and vessel manipulation/retraction is paramount.
Visual field deficit: Similarly to ATL, visual field defects are common after SA.[13,26] The defect manifests as a contralateral superior hemiquadrantanopia resulting from damage to the Meyer's loop bending around the temporal horn of the lateral ventricle. Two studies have sought to compare the rates of visual field deficits after SA versus ATL. In these studies, both transcortical SA and ATL resulted in frequent visual field deficits.[13,26] Interestingly, a cadaveric study by Choi et al.[7] suggested that the transsylvian approach using an incision made in the inferior insular sulcus at the level of the limen insulae or 5 mm just posterior would protect the optic radiations traversing the area.
This potential morbidity should be discussed with the patient before the operation. In addition, care should be taken intraoperatively to minimize retraction on the temporal lobe in an attempt to decrease both the edema and ischemia created within the region. If the surgeon is not intimately familiar with temporal lobe anatomy and does not readily have access to neuronavigation, anteromedial neocortical removal followed by resection of medial structures is a reasonable alternative option.
CONCLUSIONS
SA is an effective treatment for drug-resistant MTLE. Through careful dissection with minimal retraction, the transsylvian approach provides an adequate operative field to remove the epileptogenic focus while minimizing damage to the surrounding vascular and cortical structures.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/133/140651
Contributor Information
Timothy J. Kovanda, Email: tkovanda@iupui.edu.
R. Shane Tubbs, Email: shane.tubbs@chsys.org.
Aaron A. Cohen-Gadol, Email: acohenmd@gmail.com.
REFERENCES
- 1.Adada B. Selective amygdalohippocampectomy via the transsylvian approach. Neurosurg Focus. 2008;25:E5. doi: 10.3171/FOC/2008/25/9/E5. [DOI] [PubMed] [Google Scholar]
- 2.Arruda F, Cendes F, Andermann F, Dubeau F, Villemure JG, Jones-Gotman M, et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40:446–50. doi: 10.1002/ana.410400314. [DOI] [PubMed] [Google Scholar]
- 3.Bate H, Eldridge P, Varma T, Wieshmann UC. The seizure outcome after amygdalohippocampectomy and temporal lobectomy. Eur J Neurol. 2007;14:90–4. doi: 10.1111/j.1468-1331.2006.01565.x. [DOI] [PubMed] [Google Scholar]
- 4.Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, et al. How long does it take for partial epilepsy to become intractable? Neurology. 2003;60:186–90. doi: 10.1212/01.wnl.0000031792.89992.ec. [DOI] [PubMed] [Google Scholar]
- 5.Bonte FJ, Devous MD, Sr, Stokely EM, Homan RW. Single-photon tomographic determination of regional cerebral blood flow in epilepsy. AJNR Am J Neuroradiol. 1983;4:544–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Chelune GJ, Naugle RI, Luders H, Awad IA. Prediction of cognitive change as a function of preoperative ability status among temporal lobectomy patients seen at 6-month follow-up. Neurology. 1991;41:399–404. doi: 10.1212/wnl.41.3.399. [DOI] [PubMed] [Google Scholar]
- 7.Choi C, Rubino PA, Fernandez-Miranda JC, Abe H, Rhoton AL., Jr Meyer's loop and the optic radiations in the transsylvian approach to the mediobasal temporal lobe. Neurosurgery. 2006;59(4 Suppl 2):ONS228–35. doi: 10.1227/01.NEU.0000223374.69144.81. [DOI] [PubMed] [Google Scholar]
- 8.Clusmann H, Kral T, Gleissner U, Sassen R, Urbach H, Blumcke I, et al. Analysis of different types of resection for pediatric patients with temporal lobe epilepsy. Neurosurgery. 2004;54:847–59. doi: 10.1227/01.neu.0000114141.37640.37. [DOI] [PubMed] [Google Scholar]
- 9.Clusmann H, Schramm J, Kral T, Helmstaedter C, Ostertun B, Fimmers R, et al. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg. 2002;97:1131–41. doi: 10.3171/jns.2002.97.5.1131. [DOI] [PubMed] [Google Scholar]
- 10.de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: A cohort study. Lancet. 2011;378:1388–95. doi: 10.1016/S0140-6736(11)60890-8. [DOI] [PubMed] [Google Scholar]
- 11.Devous MD, Sr, Thisted RA, Morgan GF, Leroy RF, Rowe CC. SPECT brain imaging in epilepsy: A meta-analysis. J Nucl Med. 1998;39:285–93. [PubMed] [Google Scholar]
- 12.Dulay MF, Levin HS, York MK, Li X, Mizrahi EM, Goldsmith I, et al. Changes in individual and group spatial and verbal learning characteristics after anterior temporal lobectomy. Epilepsia. 2009;50:1385–95. doi: 10.1111/j.1528-1167.2008.01730.x. [DOI] [PubMed] [Google Scholar]
- 13.Egan RA, Shults WT, So N, Burchiel K, Kellogg JX, Salinsky M. Visual field deficits in conventional anterior temporal lobectomy versus amygdalohippocampectomy. Neurology. 2000;55:1818–22. doi: 10.1212/wnl.55.12.1818. [DOI] [PubMed] [Google Scholar]
- 14.Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: A randomized trial. JAMA. 2012;307:922–30. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel J, Jr, Wiebe S, French J, Sperling M, Williamson P, Spencer D, et al. Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society; American Association of Neurological Surgeons. Practice parameter: Temporal lobe and localized neocortical resections for epilepsy: Report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–47. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 16.French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–80. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 17.Harvey DJ, Naugle RI, Magleby J, Chapin JS, Najm IM, Bingaman W, et al. Relationship between presurgical memory performance on the Wechsler Memory Scale-III and memory change following temporal resection for treatment of intractable epilepsy. Epilepsy Behav. 2008;13:372–5. doi: 10.1016/j.yebeh.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Helmstaedter C, Reuber M, Elger CC. Interaction of cognitive aging and memory deficits related to epilepsy surgery. Ann Neurol. 2002;52:89–94. doi: 10.1002/ana.10260. [DOI] [PubMed] [Google Scholar]
- 19.Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997;54:369–76. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- 20.Ho SS, Berkovic SF, Berlangieri SU, Newton MR, Egan GF, Tochon-Danguy HJ, et al. Comparison of ictal SPECT and interictal PET in the presurgical evaluation of temporal lobe epilepsy. Ann Neurol. 1995;37:738–45. doi: 10.1002/ana.410370607. [DOI] [PubMed] [Google Scholar]
- 21.Hori T, Tabuchi S, Kurosaki M, Kondo S, Takenobu A, Watanabe T. Subtemporal amygdalohippocampectomy for treating medically intractable temporal lobe epilepsy. Neurosurgery. 1993;33:50–6. [PubMed] [Google Scholar]
- 22.Jones-Gotman M, Harnadek MC, Kubu CS. Neuropsychological assessment for temporal lobe epilepsy surgery. Can J Neurol Sci. 2000;27(Suppl 1):S39–43. doi: 10.1017/s0317167100000639. [DOI] [PubMed] [Google Scholar]
- 23.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–77. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 24.Lackner P, Koppelstaetter F, Ploner P, Sojer M, Dobesberger J, Walser G, et al. Cerebral vasospasm following temporal lobe epilepsy surgery. Neurology. 2012;78:1215–20. doi: 10.1212/WNL.0b013e318250d7d6. [DOI] [PubMed] [Google Scholar]
- 25.Mansouri A, Fallah A, Valiante TA. Determining surgical candidacy in temporal lobe epilepsy. Epilepsy Res Treat 2012. 2012:706917. doi: 10.1155/2012/706917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mengesha T, Abu-Ata M, Haas KF, Lavin PJ, Sun DA, Konrad PE, et al. Visual field defects after selective amygdalohippocampectomy and standard temporal lobectomy. J Neuroophthal. 2009;29:208–13. doi: 10.1097/WNO.0b013e3181b41262. [DOI] [PubMed] [Google Scholar]
- 27.Mohammed HS, Kaufman CB, Limbrick DD, Steger-May K, Grubb RL, Jr, Rothman SM, et al. Impact of epilepsy surgery on seizure control and quality of life: A 26-year follow-up study. Epilepsia. 2012;53:712–20. doi: 10.1111/j.1528-1167.2011.03398.x. [DOI] [PubMed] [Google Scholar]
- 28.Morino M, Uda T, Naito K, Yoshimura M, Ishibashi K, Goto T, et al. Comparison of neuropsychological outcomes after selective amygdalohippocampectomy versus anterior temporal lobectomy. Epilepsy Behav. 2006;9:95–100. doi: 10.1016/j.yebeh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Ngando HM, Maslehaty H, Schreiber L, Blaeser K, Scholz M, Petridis AK. Anatomical configuration of the Sylvian fissure and its influence on outcome after pterional approach for microsurgical aneurysm clipping. Surg Neurol Int. 2013;4:129. doi: 10.4103/2152-7806.119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paglioli E, Palmini A, Portuguez M, Paglioli E, Azambuja N, da Costa JC, et al. Seizure and memory outcome following temporal lobe surgery: Selective compared with nonselective approaches for hippocampal sclerosis. J Neurosurg. 2006;104:70–8. doi: 10.3171/jns.2006.104.1.70. [DOI] [PubMed] [Google Scholar]
- 31.Park TS, Bourgeois BF, Silbergeld DL, Dodson WE. Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy. Technical note. J Neurosurg. 1996;85:1172–6. doi: 10.3171/jns.1996.85.6.1172. [DOI] [PubMed] [Google Scholar]
- 32.Radhakrishnan K, So EL, Silbert PL, Jack CR, Jr, Cascino GD, Sharbrough FW, et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: A multivariate study. Neurology. 1998;51:465–71. doi: 10.1212/wnl.51.2.465. [DOI] [PubMed] [Google Scholar]
- 33.Rausch R, Babb TL. Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Arch Neurol. 1993;50:812–7. doi: 10.1001/archneur.1993.00540080023008. [DOI] [PubMed] [Google Scholar]
- 34.Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- 35.Sagher O, Thawani JP, Etame AB, Gomez-Hassan DM. Seizure outcomes and mesial resection volumes following selective amygdalohippocampectomy and temporal lobectomy. Neurosurg Focus. 2012;32:E8. doi: 10.3171/2011.12.FOCUS11342. [DOI] [PubMed] [Google Scholar]
- 36.Schaller C, Jung A, Clusmann H, Schramm J, Meyer B. Rate of vasospasm following the transsylvian versus transcortical approach for selective amygdalohippocampectomy. Neurol Res. 2004;26:666–70. doi: 10.1179/016164104225015921. [DOI] [PubMed] [Google Scholar]
- 37.Schaller C, Zentner J. Vasospastic reactions in response to the transsylvian approach. Surg Neurol. 1998;49:170–5. doi: 10.1016/s0090-3019(97)00283-8. [DOI] [PubMed] [Google Scholar]
- 38.Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–62. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- 39.Takaya S, Mikuni N, Mitsueda T, Satow T, Taki J, Kinoshita M, et al. Improved cerebral function in mesial temporal lobe epilepsy after subtemporal amygdalohippocampectomy. Brain. 2009;132:185–94. doi: 10.1093/brain/awn218. [DOI] [PubMed] [Google Scholar]
- 40.Tanriverdi T, Olivier A. Cognitive changes after unilateral cortico -amygdalohippocampectomy unilateral selective-amygdalohippocampectomy mesial temporal lobe epilepsy. Turk Neurosurg. 2007;17:91–9. [PubMed] [Google Scholar]
- 41.Tubbs RS, Miller JH, Cohen-Gadol AA, Spencer DD. Intraoperative anatomic landmarks for resection of the amygdala during medial temporal lobe surgery. Neurosurgery. 2010;66:974–7. doi: 10.1227/01.NEU.0000368105.64548.71. [DOI] [PubMed] [Google Scholar]
- 42.Wen HT, Rhoton AL, Jr, de Oliveira E, Cardoso AC, Tedeschi H, Baccanelli M, et al. Microsurgical anatomy of the temporal lobe: Part 1: Mesial temporal lobe anatomy and its vascular relationships as applied to amygdalohippocampectomy. Neurosurgery. 1999;45:549–91. doi: 10.1097/00006123-199909000-00028. [DOI] [PubMed] [Google Scholar]
- 43.Wendling AS, Hirsch E, Wisniewski I, Davanture C, Ofer I, Zentner J, et al. Selective amygdalohippocampectomy versus standard temporal lobectomy in patients with mesial temporal lobe epilepsy and unilateral hippocampal sclerosis. Epilepsy Res. 2013;104:94–104. doi: 10.1016/j.eplepsyres.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 45.Wieser HG, Ortega M, Friedman A, Yonekawa Y. Long-term seizure outcomes following amygdalohippocampectomy. J Neurosurg. 2003;98:751–63. doi: 10.3171/jns.2003.98.4.0751. [DOI] [PubMed] [Google Scholar]
- 46.Yaşargil MG, Krayenbuhl N, Roth P, Hsu SP, Yasargil DC. The selective amygdalohippocampectomy for intractable temporal limbic seizures. J Neurosurg. 2010;112:168–85. doi: 10.3171/2008.12.JNS081112. [DOI] [PubMed] [Google Scholar]
- 47.Yasargil MG. Microneurosurgery. Vol. 36. Stuttgart, Germany: Thieme Publishers; 1984. Operative anatomy; pp. 252–90. [Google Scholar]
- 48.Zubal IG, Spencer SS, Imam K, Seibyl J, Smith EO, Wisniewski G, et al. Difference images calculated from ictal and interictal technetium-99m-HMPAO SPECT scans of epilepsy. J Nucl Med. 1995;36:684–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


