Abstract
Background
The German Standing Committee on Vaccination (STIKO) recommends vaccination against human papillomaviruses (HPV) of the high-risk types 16 and 18. The duration of protection afforded by HPV vaccines has been reported in multiple studies to date but has not been systematically evaluated.
Method
Systematic literature review and meta-analysis on the efficacy of vaccination, with assessment of evidence by the GRADE criteria (Grading of Recommendations Assessment, Development and Evaluation).
Results
15 studies were identified: 10 randomized controlled trials (RCTs) and 5 observational studies. The RCTs included a total of 46 436 participants. The duration of follow-up was short (median, 3 years) in 8 RCTs and long (median, 6 years) in 2 RCTs. During the period of short-term follow up, the pooled efficacy of vaccination for the study endpoint of incident HPV infection (percentage of infections prevented) was 83% (95% confidence interval [CI]: 70–90%), while the pooled efficacy against persistent HPV infection was 90% (95% CI: 79–95%). In this period, CIN 2+ lesions were prevented with 84% efficacy (95% CI: 50–95%), and CIN 3+ lesions with 94% efficacy (95% CI: 83–98%). During the period of long-term follow-up, incident infections were prevented with 94% efficacy (95% CI: 80–98%) and persistent infections with 95% efficacy (95% CI: 84–99%). The long-term efficacy against CIN 2+ lesions was 86% (95% CI: –166–99%). No data are available on the long-term efficacy of vaccination against CIN 3+ lesions.
Conclusion
Long-term observation does not indicate any loss of antiviral protection after vaccination against HPV 16 and 18, although the evidence for long-term protection is of lesser quality than that for short-term protection.
Every year, 4600 women in Germany develop cervical cancer (1). The raw incidence rate for 2014 has been estimated at 11.2 cases per 100 000 individuals (1). Persistent infection with a high-risk human papillomavirus (HPV) type is a necessary prerequisite for the development of dysplasia and neoplasia of the cervix (2); incident infections, in contrast, are not a risk factor. HPV types 16 and 18 are among the most common high-risk types in Germany (3).
Dysplasia, or cervical intraepithelial neoplasia (CIN), is classified by severity (grades 1 to 3). The cervical cancer risk increases with the severity of CIN (2). For CIN 2 lesions the risk of developing cervical cancer within 5 to 10 years is 20 to 30%, while CIN 3 lesions that persist for more than two years are associated with a 50% risk (2, 4).
A vaccine for HPV types 6, 11, 16, and 18 has been available since 2006; since 2007 a further vaccine for types 16 and 18 has been available. The addition of HPV vaccination to the vaccination recommendations of the German Standing Committee on Vaccination (Ständige Impfkommission, STIKO) in 2007 led to heated discussions of the benefits of vaccination (5). Since then, further data has been generated that can be seen as confirming the benefit of HPV vaccination identified in its authorization trials, which found that it could prevent persistent HPV infection in HPV-naive girls and young women (6, 7). As of 2012, HPV vaccination recommendations for girls were part of national vaccination plans in 21 of 29 EU countries (8).
The duration of vaccine protection was a central element in the discussion from the very beginning, in both national and international public and specialist debate (5). When this study was conducted, systematic reviews on the efficacy of vaccination were already available (9– 14), but none of these addressed the duration of vaccine protection. In addition, these systematic reviews have limitations in terms of the included study types, and some of them analyzed vaccines (sometimes monovalent) not authorized in Germany and/or have methodological shortcomings in their search for studies and data analysis.
In this context, we performed a systematic search of the literature and a meta-analysis of the existing studies that addressed the duration of protection following HPV vaccination. In particular, we aimed to clarify whether, in individuals vaccinated in childhood, there is still sufficient protection against HPV several years later, when sexual activity and therefore the risk of infection begin. A rapid drop in vaccine protection would raise the question of the need for booster vaccination, or affect the preferred age of vaccination.
Methods
A systematic review was performed to address the following primary questions:
In long-term follow-up (≥5 years following initial immunization) after HPV vaccination, is vaccination less effective than in short-term follow-up (<5 years following initial immunization) in terms of preventing high-risk HPV infection or the development of CIN 2 or CIN 3 lesions?
What is the quality (according to the GRADE guidelines) of the evidence on the efficacy of vaccination in long-term follow-up in comparison to evidence obtained during short-term follow-up?
In order to draw conclusions for the target group of STIKO‘s vaccination recommendation (15), these primary questions must be answered independently of which vaccine is used in girls and young women not previously infected with HPV.
Search of the literature
This systematic review was carried out in line with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement (16). The study protocol was registered in the PROSPERO Register (Prospective International Register of Systematic Reviews; www.crd.york.ac.uk/prospero) at the beginning of the study (reg. no. CRD42013006085).
A search of MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and the Database of Abstracts of Reviews of Effects was performed (search date: November 19, 2013). The full search strategy is shown in eBox 1. In addition, the ClinicalTrials.gov database was searched for unpublished studies. In addition to these online databases, a manual search of the references listed in the publications included and a search of all identified review articles were also performed. Studies were included regardless of their publication status and the language in which they were written. Further details on the search of the literature can be found in eBox 1.
eBox 1. Search strategy.
Search of Medline, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Database of Abstracts of Reviews of Effects (filter: publication year: 2000 to 2013; species: human; search date: November 19, 2013):
#1 papillomaviridae
#2 tumor virus infections
#3 papillomavirus
#4 HPV
#5 #1 OR #2 OR #3 OR #4
#6 uterine cervical neoplasm
#7 cervical intraepithelial neoplasia
#8 uterine cervical disease
#9 uterine cervical dysplasia
#10 #6 OR #7 OR #8 OR #9
#11 vaccin*
#12 cervarix
#13 gardasil
#14 #11 OR #12 OR 13
#15 #5 AND #10 AND #14
Data extraction
For each original study that met the inclusion criteria, two independent investigators (YD and TH) extracted study characteristics and data and transferred them to standardized data extraction sheets. Discrepancies between the investigators were discussed until consensus was reached (see eBox 2: Details of data extraction).
Assessment of risk of bias
The Cochrane Risk of Bias Tool was used to investigate the risk of bias of the studies included (17).
Assessment of evidence quality
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group guidelines were used to assess evidence quality (18). According to the GRADE guidelines, a body of evidence is assigned one of four possible levels of evidence quality: + very low, ++ low, +++ moderate, or ++++ high. The GRADE guidelines are detailed in eBox 3.
Data synthesis
Relative risks (RRs), absolute risk differences (RDs), and 95% confidence intervals were estimated on the basis of the extracted data or taken directly from the publications. Both vaccines were analyzed jointly. Efficacy of vaccination was calculated as (1 – RR) × 100. Meta-analysis was performed where data on one outcome was available from more than one study (see eBox 4).
Study selection
The study inclusion criteria were set using the PICO (population, intervention, control, outcome) question established before the beginning of the study and stated in the study protocol (Table). There were no limitations on study design. The inclusion criteria themselves determined that data should be analyzed for two predefined subgroups in order to draw conclusions on the duration of protection: follow-up lasting less than five years was defined as short-term, while follow-up lasting for five years or more was defined as long-term. Where multiple publications examining the same outcomes for the same study population at different times during short- or long-term follow-up were available, analysis for short-term follow-up involved the data obtained closest to the median follow-up duration (2.5 years). For long-term follow-up, the publication corresponding to the longest possible follow-up duration was analyzed in such cases (see eBoxes 2 to 4 for details).
Table. Systematic review inclusion criteria (PICO question).
| Population |
|
| Intervention |
|
| Control |
|
| >Outcome |
|
*At least two positive samples, 6 months apart
Results
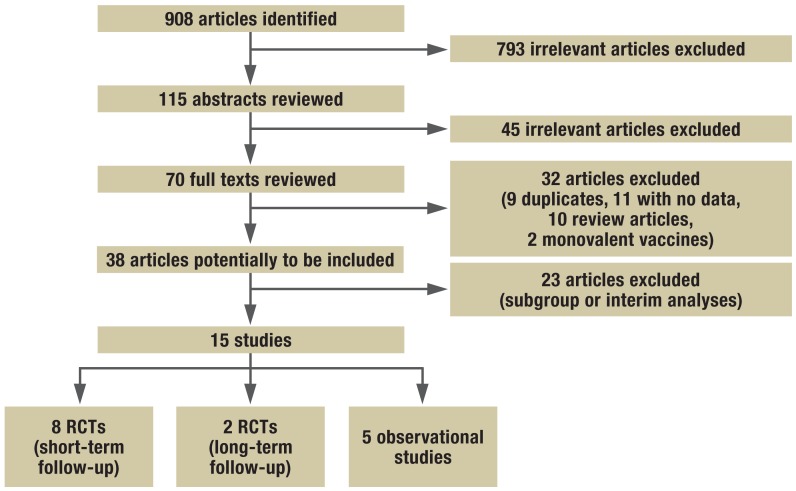
The search strategy shown in eBox 1 identified 908 potentially relevant publications. After their titles, abstract, and full text were examined, 38 publications remained in the study pool, of which 23 had to be excluded because they were interim or subgroup analyses of identical study populations. The final analysis therefore included 15 primary studies (19– 33). Of these, 10 were randomized controlled trials (RCTs) (19– 28), and the remaining five were observational studies (29– 33) (see Figure 1 and eTable for details of the study selection process; further details available from the authors).
Figure 1.
Flow diagram showing procedure used to search literature
eTable. Randomized controlled trials included in meta-analysis.
| Study | Country | Enrolment year | Inclusion criteria | Age at enrolment (years; mean) |
Duration of follow-up (months) | Vaccine/ comparator | Study sponsorship |
n randomized (vaccine/ comparator group) |
n evaluated (vaccine/ comparator group) |
Definition of evaluated population | Outcomes | Risk of bias*1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Harper et al. (19) |
Brazil, Canada, USA |
2000 | Healthy; age 15 to 25; no more than 6 previous sexual partners; no history of abnormal PAP findings or cervical treatment; negative smear test; seronegative for HPV 16. 18. and 14 other HPV types (up to 90 days before enrolment) |
20.5 | 27 | Bivalent/ placebo |
GSK | 560/553 | 560/553 | Intention to treat: meets inclusion criteria |
Incident infection; persistent infection |
Low |
| Villa et al. (27) |
Brazil, Sweden, Finland, Norway, USA |
N/S | Healthy; age 16 to 23; not pregnant; no more than 4 previous male sexual partners; no history of abnormal PAP findings; women not yet sexually active: age ≥18 |
20.2 (vaccine group)/ 20 (placebo group) |
36 | Quadrivalent/ placebo |
Merck | 276/275 | 199 to 224/ 198 to 224*2 |
Per protocol: naive to relevant HPV types at enrolment |
Persistent infection |
Low |
| Villa et al. (28) (continued from Villa et al. [27]) |
Brazil, Sweden, Finland, Norway |
N/S | Healthy; age 16 to 23; not pregnant; no more than 4 previous male sexual partners; no HPV abnormal PAP findings; women not yet sexually active: age ≥18; Brazil and Nordic countries only |
20.5 (vaccine group)/ 20.3 (placebo group) |
60 | Quadrivalent/ placebo |
Merck | 277/275 | 114/127 | Per protocol: naive to relevant HPV type at enrolment |
Persistent infection |
Low |
| Garland et al. (21) (FUTURE I) |
16 countries*3 | 2002 | Healthy; not pregnant; no more than 4 previous sexual partners; no history of abnormal cervical test results |
Vaccine group: 20.2; control group: 20.3 |
36 | Quadrivalent/ placebo |
Merck | 2723/2732 | 2241/2258 | Per-protocol susceptible: seronegative and DNA- negative for HPV types 6, 11, 16, and 18 at enrolment |
CIN 2, CIN 3 (HPV 16- or HPV 18- positive) |
Low |
| Future II Study Group (22) |
13 countries*4 | 2002 | Healthy; not pregnant; no more than 4 previous sexual partners; no history of abnormal cervical test results |
Vaccine group: 20; control group: 19.9 |
36 | Quadrivalent/ placebo |
Merck | 6087/6080 | 5305/5260 | Per-protocol susceptible: seronegative and DNA- negative for HPV types 6, 11, 16, and 18 at enrolment |
CIN 2, CIN 3 (HPV 16- or HPV 18- positive) |
Low |
| Paavonen et al. (25) (PATRICIA) |
14 countries*5 | 2004 | No more than 6 previous sexual partners; contraception; intact cervix |
20 | 14.8 | Bivalent/ hepatitis A vaccine |
GSK | 9319/9325 | 6344/6402 | Total vacci- nated cohort (TVC) naive: seronegative and DNA- negative for HPV 16/18 at enrolment |
Persistent infection (≥6 months) |
Low |
| Paavonen et al. (26) (PATRICIA) |
14 countries*5 | 2004 | No more than 6 previous sexual partners; contraception; intact cervix |
20 | 34.9 | Bivalent/ hepatitis A vaccine |
GSK | 9319/9325 | 5449/5436 | Total vacci- nated cohort (TVC) naive: seronegative and DNA- negative for HPV 16/18 at enrolment |
CIN 2+, CIN 3+ (regardless of HPV type in lesion) |
Low |
| De Carvalho et al. (20) (partly con- tinued from Harper et al. [19]) |
Brazil | 2000 | Healthy; age 15 to 25; no more than 6 previous sexual partners; no history of abnormal PAP findings or cervical treatment; negative smear test; seronegative for HPV 16, 18, and 14 other HPV types (up to 90 days before enrolment) |
20.5 | 74 | Bivalent/ placebo |
GSK | 258/248 | 222/211 | Meets inclusion criteria |
Incident infection; persistent infection; CIN 2+ |
High*6 |
| Konno et al. (24) |
Japan | 2006 | Healthy; age 20 to 25; negative pregnancy test; contraception throughout vaccination; intact cervix |
Vaccine group: 22.4; control group: 22.5 |
24 | Bivalent/ hepatitis A vaccine |
GSK | 519/521 | 501/501 | According to protocol cohort for efficacy: DNA-negative for at least 1 HPV type at enrolment |
Incident infec- tion; persistent infection; CIN 2+ |
Low |
| Herrero et al. (23) |
Costa Rica | 2004 | Healthy; not pregnant; not breastfeeding; contraception throughout vaccination |
18 to 25 | 50.4 | Bivalent/ hepatitis A vaccine |
National Institutes of Health |
3727/3739 | 2635/2677 | According to protocol cohort: DNA-negative for correspon- ding HPV type at enrolment |
Persistent infection |
Low |
*1Risk of bias recorded using Cochrane Risk of Bias Tool.
*2Per protocol, for specific outcome. For technical reasons, randomized participant numbers were used as the denominator for this analysis.
*3Includes: Austria, Brazil, Canada, Colombia, Czech Republic, Germany, Hong Kong, Italy, Mexico, New Zealand, Peru, Puerto Rico, Russia, Thailand, UK, USA.
*4Includes (the following 14 countries are listed in study appendices): Brazil, Colombia, Denmark, Finland, Iceland, Mexico, Norway, Peru, Poland, Puerto Rico, Singapore, Sweden, UK, USA.
*5Includes: Australia, Belgium, Brazil, Canada, Finland, Germany, Italy, Mexico, the Philippines, Spain, Taiwan, Thailand, UK, USA.
*6Losses during follow-up are high and differ between groups; selective outcome reporting (participant numbers differ between outcomes); only one of the study populations initially included (Brazil) was followed up. N/S: Not stated
Study characteristics
The RCTs involved a total of 46 436 participants (23 211 individuals vaccinated against HPV, 23 225 control participants). Eight RCTs (19, 21– 27) reported data from short-term follow-up, and two (20, 28) from long-term follow-up. The RCTs had been conducted in a total of 30 countries on four continents. For most of the RCTs the mean age of participants at the beginning of the studies was 20 years. The average duration of follow-up was three years for short-term follow-up and six years for long-term follow-up. Six studies involved a bivalent vaccine and the other four a quadrivalent vaccine. Only one study (23) was not initiated and funded by a vaccine manufacturer.
The studies yielded the following short-term follow-up data:
Five studies: data on persistent HPV infections (19, 23– 25, 27)
Four studies: data on the outcome CIN 2+ lesions (21, 22, 24, 26)
In long-term follow-up, one study provided data from a study on incident infections and CIN 2+ lesions (20), and two on persistent infections (20, 28). No studies reported CIN 3+ lesions in long-term follow-up.
Risk of bias
The eTable shows the risk of bias for each RCT, in addition to their characteristics. While the risk of bias was assessed as low in all studies that reported short-term follow-up data, it was high in one of the two studies on long-term follow-up (details available from the authors).
Study findings
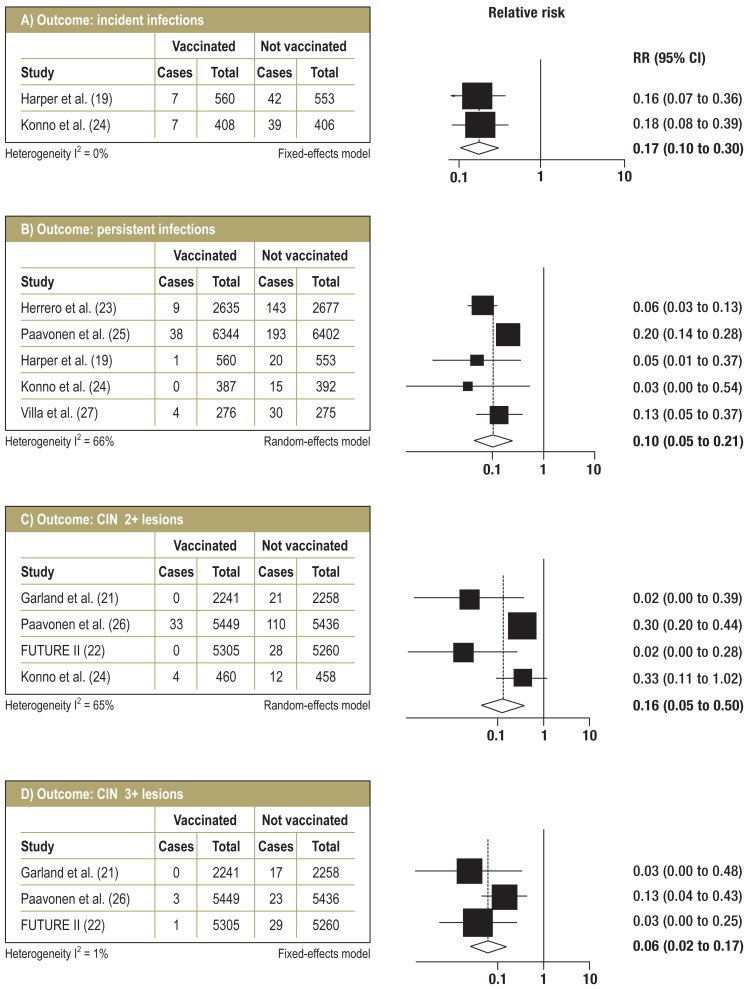
RCTs—short-term follow-up: Figure 2 shows the relative risks and pooled estimates with 95% confidence intervals (95% CIs) from meta-analysis of the RCTs for short-term follow-up. With a median follow-up duration of 25.5 months, incident HPV 16 and HPV 18 infections were prevented with 83% efficacy (95% CI: 70 to 90%). For persistent infections (lasting six months or longer), pooled efficacy was estimated at 90% (95% CI: 79 to 95%) with a median follow-up duration of 27 months, although there was moderate, statistically significant heterogeneity. CIN 2+ cervical lesions were prevented with 84% efficacy (95% CI: 50 to 95%) after a median of 36 months‘ follow-up, also with moderate, statistically significant heterogeneity. Subgroup analysis showed that this heterogeneity was caused by outcome definitions: in the PATRICIA trial (26) and the trial by Konno et al. (24), CIN 2+ lesions were analyzed as an outcome irrespective of the type of HPV found in the lesion; in contrast, the FUTURE I (21) and FUTURE II (22) studies reported CIN 2+ lesions as HPV 16- or HPV 18-positive lesions for participants with no previous infection, which are the participants relevant to this review article (per-protocol susceptible population). None of these two latter studies reported any lesions independent of HPV type for this group of participants. Subgroup analysis for the first two studies mentioned (24, 26) showed pooled vaccination efficacy against CIN 2+ lesions, regardless of HPV type, of 70% (95% CI: 56 to 79%), while efficacy against HPV 16- or HPV 18-positive CIN 2+ lesions specifically was 98% (95% CI: 86 to 100%) according to the FUTURE studies (21, 22). CIN 3+ lesions were prevented with 94% efficacy (95% CI: 83 to 98%) with a median follow-up duration of 36 months.
Figure 2.
Forest plots for follow-up <5 years (RCTs)
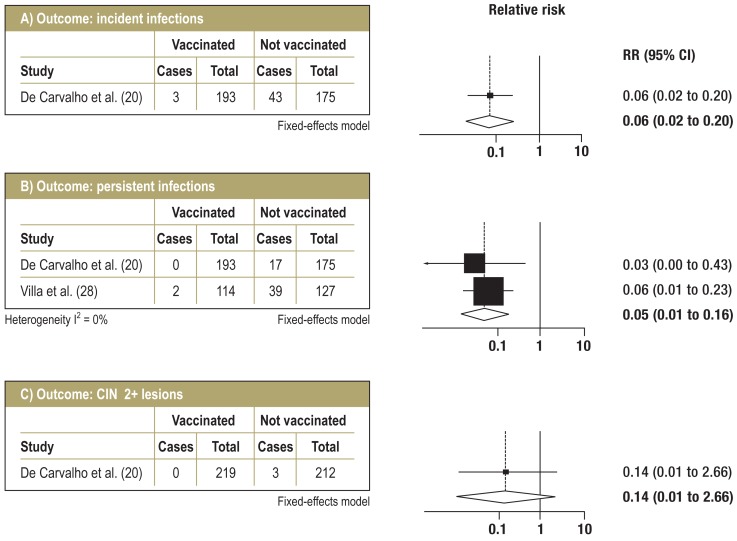
RCTs—long-term follow-up: Figure 3 shows the data from meta-analysis of the RCTs on long-term follow-up. The efficacy of prevention of incident infections was 94% (95% CI: 80 to 98%); this data was obtained from a single trial with a follow-up duration of seven years (20). Persistent infections were prevented with a pooled efficacy of 95% (95% CI: 84 to 99%) over a period of six years (median) (20, 28). For CIN 2+ lesions as well, data was obtained from only one study (20). It showed 86% vaccination efficacy (95% CI: –166 to 99%) for HPV 16- or HPV 18-positive lesions after seven years‘ follow-up, which is a non-significant effect with a very wide confidence interval. The same study reported vaccination efficacy of 40.6% (95% CI: –106 to 84.7%) for CIN 2+ lesions regardless of HPV type. No data was available on CIN 3+ lesions.
Figure 3.
Forest plots for follow-up ≥5 years (RCTs)
Observational studies: All five observational studies that were identified reported results from short-term follow-up. Two were cohort studies (30, 33), while the other three were geographical association studies (29, 31, 32). The latter provided indirect evidence for population-based efficacy of HPV vaccination, as they compared data from before and after the introduction of vaccination for specific geographical areas (England, UK [31,], Victoria, Australia [29], and Connecticut, USA [32]) and found a decrease in the incidence of HPV infections (31) or CIN 2+ lesions (29, 32) (population-based before-and-after studies). The cohort studies identified relationships between HPV vaccination status and CIN 2+ lesions (33) or HPV infections (30). No estimates (relative risks) of vaccination efficacy could be obtained from any of the observational studies, so ultimately these studies could not be included in the analysis and evaluation of evidence. Instead, they were taken only as supporting evidence for the efficacy of vaccination in terms of specific outcomes (details available from the authors).
One further observational study was published after the search of the literature had been completed. This analyzed data from the Australian vaccination program for the state of Victoria (34). It was not included in the analysis, as it did not meet all the inclusion criteria (no data on participants‘ infection status).
Evaluation of quality of evidence according to GRADE guidelines
For short-term follow-up, evidence quality was rated as “high” for the outcomes incident infections, persistent infections, and CIN 3+ lesions, as there were no shortcomings in terms of risk of bias or the other areas covered by the GRADE guidelines (inconsistency, indirectness, imprecision, publication bias). For the outcome CIN 2+ lesions, evidence quality was assessed as “moderate,” as the wide confidence interval indicated imprecision (details available from the authors).
As the trial by De Carvalho et al. (20) had a very high risk of bias, evidence quality was downgraded for all outcomes of long-term follow-up. Further shortcomings concerned the definition of the outcome persistent infections (indirectness) and the wide confidence interval for the outcome CIN 2+ lesions. Overall, evidence quality for long-term follow-up was therefore low to very low (details available from the authors).
Conclusion
This systematic review shows that there is no evidence from long-term follow-up that vaccine protection following vaccination for HPV types 16 and 18 decreases. While persistent infections (those lasting six months or longer) were prevented with a pooled efficacy of 90% with a median follow-up duration of 27 months, the pooled efficacy for a period of six years (median) was 95%. For the clinical outcome HPV 16- or HPV 18-positive CIN 2+ lesions, 84% vaccination efficacy was calculated after a median of 36 months, and 86% after seven years‘ follow-up. Data on long-term follow-up was taken from only one study (20), which had a considerably smaller number of participants; this explains why the effect was insignificant and the confidence interval very wide. Because only a few RCTs were conducted for five years or longer and these had considerably fewer participants than studies with shorter follow-up, the quality of evidence for long-term protection is lower than that for short-term protection. However, the premise of stable long-term protection is supported by data that shows induction of a robust immune memory following HPV booster vaccination (35).
Our work focused on study participants in whom incident HPV infection with the types of HPV contained in the vaccine was ruled out when they were enrolled in the studies. The highest vaccination efficacy was achieved when girls and young women were vaccinated before their first possible HPV infection. For example, in the FUTURE II study the efficacy of vaccination against HPV 16- and HPV 18-associated medium-grade dysplasia of the cervix (CIN 2+) in HPV-negative women was almost 100%, while in participants in the same study for whom HPV status was not an inclusion criterion it fell to approximately 50% (22). The main route of transmission of HPV infections of the cervix is sexual contact, and the probability of HPV infection rises substantially when an individual becomes sexually active (36). HPV vaccination should therefore be completed before the beginning of sexual activity.
The observational studies we identified provided no information that went beyond the data obtained from the RCTs. This was partly because all the existing observational studies yielded data only on short-term follow-up, for which there is already relatively good evidence from RCTs. It was also partly because the designs of some observational studies were not suitable for generating data on vaccination efficacy, and others did not provide any data on girls or young women without HPV infection, which was the group focused on here. Nevertheless, these studies are an additional source of evidence showing an effect of HPV vaccination on various outcomes following widespread use in the target group.
This systematic review focuses on studies investigating efficacy of vaccination in short- and long-term follow-up; data on adverse drug reactions (ADRs) was not included in the evaluation according to the study protocol. Two recent systematic reviews have analyzed data on ADRs following HPV vaccination; both concluded that the studies included did not show any significant differences between vaccinated and non-vaccinated participants in terms of relevant outcomes, and that the safety profile of vaccination was acceptable (12, 13). A recently published systematic review also demonstrates the efficacy and safety of HPV vaccination when coadministered with other vaccines (37).
The limitations of this article concern the focus on girls and women with no HPV infection. The results are extrapolated to the actual target group for vaccination, girls and young women who are not yet sexually active. It must also be assumed that the data for other target groups such as older women or young men is different in terms of both efficacy data and evidence quality.
The particular strength of this article is that this is the first time a comprehensive systematic review has provided a conclusion on the long-term efficacy of HPV vaccine protection for the most important vaccination target group on the basis of meta-analysis. In addition, the standardized, international GRADE guidelines have been used to provide a conclusion on the quality of the evidence, differentiated by length of follow-up. This supports a critical assessment of this data.
Key Messages.
Every year, approximately 11.2 of every 100 000 individuals in Germany develop cervical cancer. Persistent infection with a high-risk human papillomavirus (HPV) type is a necessary prerequisite for the development of dysplasia and neoplasia of the cervix.
Since 2007, the German Standing Committee on Vaccination (STIKO) has recommended that girls aged between 12 and 17 receive vaccination against the high-risk types 16 and 18 of the human papillomavirus. For the first time, a systematic review has been performed using pooled estimates of the duration of vaccine protection.
This meta-analysis shows that the long-term (five years or longer) follow-up data published to date contains no evidence of a drop in vaccine protection following vaccination against HPV types 16 and 18.
RCTs investigating the duration of protection of HPV vaccination should be continued in order to improve the quality of evidence on long-term protection.
Observational studies estimating the efficacy of vaccination in terms of various outcomes should be initiated to support the evidence.
eBox 2. Details of data extraction.
The following data was extracted: study location, study year(s), study type/design, name of vaccine and manufacturer, strains covered by the vaccine, vaccination schedule, sponsorship, inclusion and exclusion criteria for study participants, age at beginning of vaccination, ethnicity, duration of follow-up, number of participants enrolled (for RCTs: randomized), number of participants evaluated, number (or percentage) of vaccinated individuals with a given outcome, number (or percentage) of non-vaccinated individuals with a given outcome, and for observational studies confounding factors taken into account and estimates of effect sizes adjusted for confounding factors. Because the primary question of this systematic review concerned uninfected girls and women, data on study participants who were negative for HPV 16 and/or 18 at the beginning of the study or had not yet become sexually active was used wherever possible.
eBox 3. Details of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group guidelines.
The GRADE guidelines (18) provide a transparent system for assessing evidence and developing recommendations. They were developed by the GRADE Working Group and are used by the World Health Organization (38), the US Advisory Committee on Immunization Practices (ACIP) (39), the German Standing Committee on Vaccination (STIKO) (40), and other bodies.
According to GRADE, evidence quality is a measure of confidence in the correctness of estimates of effect sizes: the higher the quality of evidence (on a four-level scale ranging from “very low” to “high”), the surer the user can be that the effects of an intervention reported in a study correspond to the “true” sizes of the effects.
The units of analysis used by GRADE are outcomes; in other words, evidence quality concerns a single outcome. When the body of evidence, i.e. all available studies (rather than one single study), on an outcome is reviewed as a whole, the results of the systematic review are assigned one of four levels of evidence quality: + very low, ++ low, +++ moderate, or ++++ high.
Bodies of evidence from RCTs are initially rated as ++++ high-quality, while bodies of evidence from observational studies are initially assessed as ++ low-quality. Evidence quality can then be up- or downgraded on the basis of an established set of criteria covering aspects of both internal and external validity. Quality can be downgraded according to five criteria: 1) risk of bias (due to shortcomings in study design or conduct), 2) inconsistency (i.e. dispersion of study findings), 3) indirectness (whether the study findings can be applied to the target group for the recommendation), 4) imprecision (wide confidence interval or large standard deviation), and 5) publication bias (bias in overall results as a result of selective publication of “desirable” study findings).
The GRADE guidelines also allow evidence quality to be upgraded on the basis of three criteria: 1) large effect sizes (e.g. relative risk >2.0); 2) a dose–effect relationship, and 3) if potential confounding factors would have reduced the effect (i.e. all remaining, plausible confounding factors have already reduced the effect, so the effect observed is a conservative estimate). GRADE assessment of evidence is separate from the stage at which the evidence is used as the basis for a recommendation. In other words, high evidence quality does not automatically lead to a strong recommendation, and a strong recommendation for or against an intervention can occasionally be based on moderate- or low-quality evidence as a result of other important factors (such as patients‘ values and preferences, costs, or the balance between positive and adverse effects).
eBox 4. Details of data synthesis.
Where there was heterogeneity (revealed by a statistically significant chi-square test or I2 statistics), a random-effects model was used. In other cases, data was pooled using a fixed-effects model. Due to the limited number of studies per outcome no test for publication bias was performed, in line with the recommendations of the Cochrane Collaboration. All evaluations were performed for a follow-up duration of less than five years (short-term follow-up) and five years or more (long-term follow-up). All calculations were performed using the software program STATA 12 (StataCorp, College Station, TX, USA). The results of the evidence evaluation process and the absolute risk differences were recorded in GRADE evidence profiles using the program GRADEpro (version 3.6; GRADE Working Group) (available from the authors).
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
Footnotes
Conflict of interest statement
Prof. Zepp has received reimbursement of conference fees, travel expenses, and accommodation expenses from GSK. He has received fees for conducting commissioned clinical studies from GSK and Sanofi.
Dr. Harder, PD Wichmann, Dr. Deleré, Dr. van der Sande, and Prof. Klug declare that no conflict of interest exists.
Dr. Terhardt has received fees for continuing education lectures unrelated to any specific product, for which financial recompense was provided by vaccine manufacturers (AstraZeneca, Sanofi).
References
- 1.Robert Koch-Institut, Gesellschaft der Epidemiologischen Krebsregister in Deutschland e.V. 2013. Krebs in Deutschland 2009/2010. [Google Scholar]
- 2.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 3.Delere Y, Remschmidt C, Leuschner J, Schuster M, Fesenfeld M, Schneider A, et al. Human Papillomavirus prevalence and probable first effects of vaccination in 20 to 25 year-old women in Germany: a population-based cross-sectional study via home-based self-sampling. BMC infectious diseases. 2014;14 doi: 10.1186/1471-2334-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. The lancet oncology. 2008;9:425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 5.Gerhardus A. Wie wirksam ist die HPV-Impfung? Dtsch Arztebl. 2009;106:A 330–A 334. [Google Scholar]
- 6.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30:F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch FX, Broker TR, Forman D, Moscicki AB, Gillison ML, Doorbar J, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31:H1–H31. doi: 10.1016/j.vaccine.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nohynek H, Wichmann O, D Ancona F, Gatekeepers VN. National Advisory Groups and their role in immunization policy-making processes in European countries. Clinical microbiology and infection. the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013;19:1096–1105. doi: 10.1111/1469-0691.12315. [DOI] [PubMed] [Google Scholar]
- 9.Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ: Canadian Medical Association journal = journal de l‘Association medicale canadienne. 2007;177:469–479. doi: 10.1503/cmaj.070948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Torre G, de Waure C, Chiaradia G, Mannocci A, Ricciardi W. HPV vaccine efficacy in preventing persistent cervical HPV infection: a systematic review and meta-analysis. Vaccine. 2007;25:8352–8358. doi: 10.1016/j.vaccine.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros LR, Rosa DD, da Rosa MI, Bozzetti MC, Zanini RR. Efficacy of human papillomavirus vaccines: a systematic quantitative review. International journal of gynecological cancer: official journal of the International. Gynecological Cancer Society. 2009;19:1166–1176. doi: 10.1111/IGC.0b013e3181a3d100. [DOI] [PubMed] [Google Scholar]
- 12.Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC infectious diseases. 2011;11 doi: 10.1186/1471-2334-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rey-Ares L, Ciapponi A, Pichon-Riviere A. Efficacy and safety of human papilloma virus vaccine in cervical cancer prevention: systematic review and meta-analysis. Archivos argentinos de pediatria. 2012;110:483–489. doi: 10.5546/aap.2012.eng.483. [DOI] [PubMed] [Google Scholar]
- 14.Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. The Lancet infectious diseases. 2012;12:781–789. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 15.RobertKoch-Institut. Impfung gegen humane Papillomvieren (HPV) für Mädchen von 12 bis 17 Jahren - Empfehlung und Begründung. 2007. Mitteilung der Ständigen Impfkommission (STIKO) am Robert Koch-Institut. [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration‘s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of clinical epidemiology. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 20.De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine. 2010;28:6247–6255. doi: 10.1016/j.vaccine.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. The New England journal of medicine. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 22.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. The New England journal of medicine. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 23.Herrero R, Wacholder S, Rodriguez AC, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer discovery. 2011;1:408–419. doi: 10.1158/2159-8290.CD-11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konno R, Tamura S, Dobbelaere K, Yoshikawa H. Efficacy of human papillomavirus type 16/18 AS04-adjuvanted vaccine in Japanese women aged 20 to 25 years: final analysis of a phase 2 double-blind, randomized controlled trial. International journal of gynecological cancer: official journal of the International. Gynecological Cancer Society. 2010;20:847–855. doi: 10.1111/IGC.0b013e3181da2128. [DOI] [PubMed] [Google Scholar]
- 25.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 26.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 27.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. The lancet oncology. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 28.Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. British journal of cancer. 2006;95:1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brotherton JM, Fridman M, May CL. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–2092. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 30.Kahn JA, Brown DR, Ding L, et al. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012;130:e249–e256. doi: 10.1542/peds.2011-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesher D, Soldan K, Howell-Jones R, et al. Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine. 2013;32:26–32. doi: 10.1016/j.vaccine.2013.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niccolai LM, Russ C, Julian PJ, et al. Individual and geographic disparities in human papillomavirus types 16/18 in high-grade cervical lesions: Associations with race, ethnicity, and poverty. Cancer. 2013;119:3052–3058. doi: 10.1002/cncr.28038. [DOI] [PubMed] [Google Scholar]
- 33.Powell SE, Hariri S, Steinau M, et al. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine. 2012;31:109–113. doi: 10.1016/j.vaccine.2012.10.092. [DOI] [PubMed] [Google Scholar]
- 34.Crowe E, Pandeya N, Brotherton JM, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. Bmj. 2014;348 doi: 10.1136/bmj.g1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsson SE, Villa LL, Costa RL, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 36.Kjaer SK, Chackerian B, van den Brule AJ, et al. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer epidemiology, biomarkers & prevention. a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:101–106. [PubMed] [Google Scholar]
- 37.Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32:2670–2674. doi: 10.1016/j.vaccine.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 38.Duclos P, Durrheim DN, Reingold AL, Bhutta ZA, Vannice K, Rees H. Developing evidence-based immunization recommendations and GRADE. Vaccine. 2012;31:12–19. doi: 10.1016/j.vaccine.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed F, Temte JL, Campos-Outcalt D, Schunemann HJ Group AEBRW. Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the US. Centers for Disease Control and Prevention (CDC) Vaccine. 2011;29:9171–9176. doi: 10.1016/j.vaccine.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Ständige Impfkommission am Robert Koch-institut (STIKO) Standardvorgehensweise (SOP) www.stiko.de/DE/Content/Kommission/STIKO/Aufgaben_Methoden/methoden_node.html. Last accessed on 23 October 2013.