Abstract
Background
Roughly one in ten persons in the industrialized world suffers from hip osteoarthritis, a disease for which there is no cure. The goal of conservative therapy is to relieve symptoms, preferably with methods that let patients assume responsibility for their own treatment, e.g., physical training.
Methods
In a randomized controlled trial, we studied the effectiveness of twelve weeks of exercise therapy in patients with hip osteoarthritis (THüKo), compared to no treatment (control group) and placebo ultrasound treatment of the hip (placebo ultrasound group). The primary endpoint was a comparison of the pain scores of the intervention versus control groups on the generic SF-36 health questionnaire. Secondary endpoints included comparisons across all three study groups of scores on the 7 other scales of the SF-36 and on the pain, physical function, and stiffness scales of the osteoarthritis-specific WOMAC Index. The statistical analysis was performed with ANCOVA, with baseline values as a covariate. Between-group effects were subsequently tested pairwise (two-tailed t-tests, alpha = 0.05).
Results
As for the primary endpoint, pain reduction was significantly greater in the intervention than in the control group (mean difference 5.7 points, 95% confidence interval [CI] 0.4–11.1 points, p = 0.034). The comparisons across all three study groups (i.e., secondary endpoints, with 71 subjects in the intervention group, 68 in the control group, and 70 in the placebo group) revealed no significant between-group effects with respect to the SF-36. On the WOMAC Index, however, statistically significant differences were found for pain reduction between the intervention and control group (mean difference 7.4 points, 95% CI 3.0–11.8, p = 0.001) and between the intervention and placebo group (mean difference 5.1 points, 95% CI 0.7–9.4, p = 0.024). Comparable mean differences were also found for functional improvement.
Conclusion
Twelve weeks of exercise therapy in hip osteoarthritis patients of normal vitality reduced pain and improved physical function. No significant improvement was found in these patients‘ general health-related quality of life.
Osteoarthritis of the hip is a progressive, degenerative disorder that affects the musculoskeletal system in adults; its estimated prevalence is 11% (1). The early stages are characterized by pain after intense joint loading; the later stages feature “start-up” pain in the morning and, later still, symptoms during periods of rest and at nighttime. Relieving postures result in a decrease in joint mobility, pathological load distributions, and evasive movements (2). The resulting restrictions on everyday activities and participation in social life are accompanied by a reduction in health-related quality of life (1, 3).
To date, hip osteoarthritis remains incurable (4). At an advanced stage of the disease, surgical intervention is often required in view of the increasingly high degree of suffering experienced by patients (5, 6). Up to that point, however, the conservative therapeutic measures for symptomatic treatment of hip osteoarthritis should be exhaustively utilized. Therapy furthermore aims to restrict the progress of the disease and the degeneration of the joint, as well as to educate patients about hip osteoarthritis and how to deal with it (6, 7).
Conservative therapy entails a combination of pharmacological and non-pharmacological forms of treatment. Measures that can be conducted by the patients themselves, for which they take responsibility, should be given priority (6). These measures include physical exercise, whose possible mechanisms of action are thought to be an improvement to the mechanical environment of the hip joint and an accompanying reduction in the stress put on the joint (8). Further to strengthening the muscles and improving joint mobility, physical exercise also improves proprioception (9). In patients with knee osteoarthritis this has been proven to lead to pain reduction and functional improvements (10).
However, the assumption has to be that the therapeutic effects do not affect every arthritically changed joint in the same way. A separate proof of efficacy of exercise-based interventions in patients with hip joint disorders by means of studies with adequate sample sizes has been explicitly called for (11, 12). This proof of efficacy was provided only this year (2014) in an updated meta-analysis (8), which also found that exercise therapy in hip osteoarthritis was effective in terms of pain relief and functional improvement. However, the study did not identify any positive effect on general health-related quality of life.
A more differentiated look at the studies included in the meta-analysis, with 549 subjects, shows that only five of the total of 10 studies recruited explicitly patients with hip osteoarthritis and that only two studies had minimum case numbers of 50 subjects or more per study arm. The studies investigated different intervention programs that differed substantially in terms of application frequency, content, dosage, duration, time period, and treatment frequency. The pooled standardized mean differences (SMD) for pain reduction and functional improvement were SMD = 0.38 (8). This value is comparable to the placebo effect of 0.36 that has been described for hip osteoarthritis (13). In addition to the proof of efficacy of hip-specific exercise therapy in hip osteoarthritis in general, the evaluation of different programs and dose–response relations in studies with sufficiently large sample sizes that include a placebo intervention is crucially needed.
The present study aimed to evaluate the effectiveness of a standardized, hip-specific exercise intervention in patients with hip osteoarthritis compared with an untreated control group and a placebo intervention.
Methods
Study design and study arms
In order to answer the research question we conducted a prospective randomized controlled trial (evidence level 1B). A 12-week exercise therapy intervention (Tübinger Hüftkonzept THüKo, the Tübingen exercise therapy approach) was compared with one control group receiving no intervention (control group) and one group that received once-weekly, 15-minute placebo ultrasound treatment of the hips (placebo ultrasound group). An additional intervention group received active ultrasound treatment. The placebo and active ultrasound groups were single-blinded for participants. Ultrasound was given to only a partial cohort, with a randomization ratio of 1:10 compared with the respective other groups.
The THüKo exercise therapy approach entails a once-weekly group intervention (60–90 minutes) in addition to a twice-weekly home exercise program (30–40 minutes each) (14). The therapeutic program entailed education and social interaction, as well as exercises to strengthen the muscles and to improve proprioception, balance and flexibility. Training and pain were documented. The study protocol includes a detailed description of the therapeutic methods, as does the book on the THüKo exercise therapy approach (14, 15).
Patients were recruited via sports orthopedics outpatient clinics and via the press. Recruitment and randomization were done in four sequential blocks. On the basis of a telephone interview assessing eligibility, suitable patients were invited for an orthopedic examination. Their final inclusion in the study was decided on the basis of unilateral or bilateral hip osteoarthritis, according to the criteria of the American College of Rheumatology (16). Subjects were also included in the study if they had unilateral hip osteoarthritis and a hip endoprosthesis in the contralateral joint. The eBox lists further inclusion and exclusion criteria.
eBox. Inclusion and exclusion criteria for the study.
-
Inclusion criteria
Age between 18 and 85 years
Osteoarthritis (OA) of one or both hip joint(s) (clinical criteria of the American College of Rheumatology)
The subject gives voluntary consent to study participation after receiving oral and written information about study content and objectives
The subject has the time available to undertake the exercises and attend the measurings
The subject is physically fit for the intervention measure (as ascertained during the examination conducted by the principal investigator). “Fitness” in this setting relates to the physical as well as the psychological condition of the subject. (Subjects will not be excluded if they have one hip endoprosthesis, as long as the contralateral hip is affected by osteoarthritis according to the listed criteria.)
The subject has capacity to consent
-
Exclusion criteria
Unstable anchoring of endoprosthetic hip joint
Hip dislocation after endoprosthetic joint replacement
Further disorders affecting the lower extremities or lower back that require treatment by a physician/therapist and which are not connected to the OA and are currently being treated.
The presence of osteoarthritis in several joints (for example, hip and knee) is NOT an exclusion criterion.
Medication or alcohol misuse
Participation in a clinical study in the preceding 4 weeks
Lack of compliance
Acute illness
Use of walking aids
Previous trauma in the hip and pelvis area with accompanying development of secondary osteoarthritis
Known endocrinological causes of hip osteoarthritis
Confirmed metabolic causes of hip osteoarthritis
State after aseptic bone necrosis (Perthes‘ disease)
Cardiocirculatory disorders or other comorbidities that result in severely restricted everyday physical capacity and that are contraindications to physical exertion (for example, heart failure NYHA III–IV, terminal renal failure stage IV)
Medical exercise therapy, physiotherapy on resistance machines in the preceding 3 months, with a total treatment frequency of more than 6 units
Systematic group or individual therapy to treat the osteoarthritis (systematic in the sense of a minimum of 1x/week for 30 minutes or more) in the preceding 3 months
Physical therapy to treat the osteoarthritis (systematic in the sense of regular, prescribed application at least 1x/week) in the preceding 3 months
Newly initiated exercise/movement therapy in the preceding 3 months (sports and movement therapy defined as taking place a minimum of 1x/week, getting out of breath, minimum duration 30 minutes)
Corticosteroid injection into the hip joint in the preceding 12 months
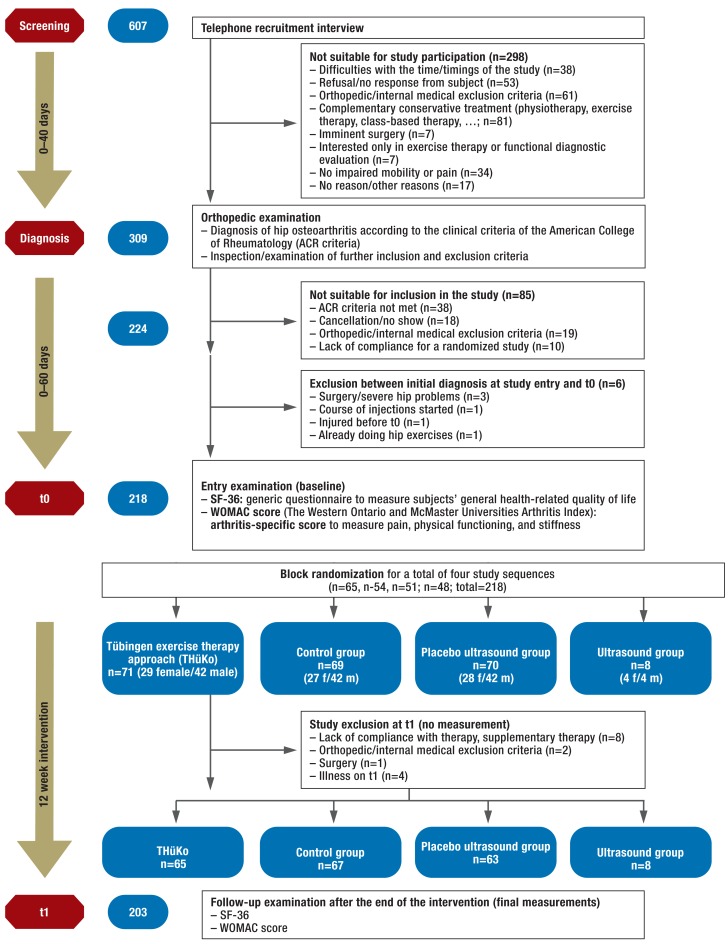
Included subjects were invited to attend an introductory examination (t0), during which the baseline measurements of the target variables were documented for the first time and participants were randomized to one of the four study arms. Randomization was done stratified by sex. Allocation concealment was guaranteed by using a sealed opaque envelope that neither the investigators nor the participants were able to view. The subsequent intervention period was 12 weeks, the target variables were documented again on the second measurement day (t1), shortly after the end of the intervention period (Figure).
Figure.
Included and excluded patients during the course of the study
A detailed description of the study protocol has been published (15). The study was registered with the German Clinical Trials Register (DRKS, Deutsches Register für Klinische Studien), No DRKS00000651. University Hospital Tubingen‘s ethics committee approved the study.
Target variables and sample size calculation
The primary endpoint of the study is the comparison of the Bodily Pain subscale of the SF-36 between the control group and the group that received the THüKo intervention. According to the study protocol, n = 70 subjects were needed for each study arm, taking into account a dropout rate of n = 10 per study arm, in order to prove the superiority of the THüKo concept over no therapy, for the time period from t0 to t1 (power = 0.8, alpha = 0.05). The comparison of the placebo ultrasound group with the THüKo and control group was not part of our primary research question; instead they were the subject of secondary analyses (15). For the ultrasound group, which had an exploratory design, the target case number was n = 7. The complementary secondary target variables of the SF-36 are the comparison of the subscales of all study arms with regard to
Physical Functioning;
Role-Physical;
Role-Emotional;
Social Functioning;
Vitality;
General Health;
Mental Health.
The SF-36 is a generic instrument for the purposes of measuring health-related quality of life, which allows comparisons with intervention studies and with other populations as well as comparisons with a German normative population sample.
The WOMAC index was another secondary end point under study. This index considers three subscales that relate to the symptoms of patients with osteoarthritis of the knee and/or hip. Five items relating to pain are measured, and, additionally, 17 items relating to physical functioning and two items about joint stiffness.
The questionnaires were completed by the study participants themselves. All data were entered twice into a database and subsequently checked for consistency. Missing values, re-coding, and transformation of the values were done in accordance with the questionnaire manual‘s provisions (17, 18). WOMAC and SF-36 were transformed to a 0–100 scale. In WOMAC, higher values represent a higher degree of impairment, whereas in the SF-36, higher values are associated with a better general health-related quality of life.
Statistical analysis
We assessed the distributions on the basis of the histograms and their skewness and kurtosis (19). At the suggestion of a reviewer, and taking into account the recommendations for the analysis of randomized controlled trials (20), we did not follow the study protocol‘s requirement for a simple analysis of variance (ANOVA) (15) but conducted an analysis of covariance in order to review the target criteria. To this end, the final measurements (t1) of the target variables were adjusted to their respective baseline measurements (t0). Interaction terms from baseline values and study arms did not reach significance and were not included in the model. Because of the definition of the primary analysis (comparison of THüKo with control group), the confirmatory test, in the strict sense of the word, was restricted to this particular comparison.
The secondary analysis focused on the between-group effects (analysis of covariance) and pair-wise comparisons of the remaining study groups (two-sided, alpha = 0.05). For the subscales of the SF-36 a non parametric test (Kruskal Wallis test) was used in addition to ANCOVA to account for violations of the normal distribution assumption. The data evaluation was done by intention-to-treat analysis with the last observation carried forward (21). For reasons of clarity and comprehensibility we did not explain nor analyze the results from the ultrasound group (n = 8).
Results
Out of 607 interested parties, 225 patients with hip osteoarthritis were included in our study. All patients gave their written consent to participating. 43 subjects had bilateral symptoms. At study entry, 16 persons already had a hip endoprosthesis in the contralateral side, and one person had a knee endoprosthesis. 218 patients took part in the baseline examinations. The figure shows the allocation of the subjects to the study arms and the numbers of participants across the different time points of the study. The first study participant was recruited in September 2010. The last follow-up examination after the 12-week intervention period was done in April 2012.
One control group subject out of 218 could not be included in the analysis owing to missing measurements for t0. Without the 8 patients of the exploratory study arm ultrasound, the study population whose data were available for analysis therefore came to n = 209. The baseline measurements regarding age, sex, body mass index (BMI), and the collected target variables are shown in Table 1. No statistically significant differences to time point t0 were seen. Table 1 also shows for the SF-36 the comparison measurements of the German normative population sample in the age groups 51–60 and 61–70 (18).
Table 1. Baseline values at t0; mean values (standard deviations) reported.
| General | SF-36 (0–100)*1 | WOMAC (0–100)*2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BMI | Age | Bodily Pain | Physical Functioning | Role-Physical | General Health | Vitality | Social Functioning | Role-Emotional | Mental Health | Physical Function | Pain | Stiffness | |
| Control group | 68 | 27.5 (3.2) |
60 (9) |
57 (18) |
65 (20) |
74 (33) |
68 (14) |
63 (17) |
87 (18) |
93 (20) |
80 (13) |
27 (15) |
28 (17) |
38 (22) |
| Placebo ultrasound group | 70 | 27.2 (4.3) |
58 (10) |
53 (18) |
62 (20) |
72 (35) |
63 (17) |
62 (16) |
87 (20) |
87 (29) |
78 (13) |
29 (16) |
29 (17) |
38 (22) |
| Exercise therapy (THüKo) | 71 | 26.9 (3.7) |
58 (10) |
57 (19) |
66 (20) |
79 (34) |
65 (16) |
64 (17) |
91 (14) |
91 (24) |
80 (13) |
27 (18) |
28 (17) |
32 (20) |
| Total | 218 | 27.1 (3.8) |
59 (10) |
56 (18) |
64 (20) |
76 (34) |
65 (16) |
63 (17) |
88 (18) |
90 (24) |
79 (13) |
28 (16) |
28 (17) |
36 (21) |
| P value*3 | 0.63 | 0.34 | 0.36 | 0.41 | 0.46 | 0.16 | 0.65 | 0.35 | 0.38 | 0.72 | 0.55 | 0.90 | 0.24 | |
| German normative population sample(51–60 years) | 73 (27) |
84 (20) |
81 (32) |
61 (19) |
61 (17) |
87 (18) |
89 (27) |
72 (16) |
||||||
| German normative population sample (61–70 years) | 71 (27) |
76 (23) |
73 (35) |
59 (18) |
61 (18) |
87 (18) |
89 (26) |
76 (17) |
||||||
*1100 points in SF-36 = best possible quality of life
*2100 points in WOMAC = maximum impairment
*3P value refers to the between-group effects of the baseline values per column BMI, body mass index
Sixty-five of the 71 ThüKo participants completed the exercise program successfully (92%). The compliance of these subjects was 93% for group therapy; self-reported compliance with the exercise-at-home activities reached 95%. No subject had to leave the intervention because of therapy-related adverse effects. 64 of the 70 subjects in the placebo group participated in the placebo ultrasound treatment up to the end (92%). Of these 64 persons, one was not able to participate in t1 for reasons of illness.
For the evaluation of the target variable Bodily Pain of the SF-36, the data of one patient (THüKo) were excluded since the patient had severe flu in the weeks before t0 and reported severe bodily pain at t0, which had ceased by the time of t1. The evaluation of the primary target criterion showed statistically significant differences between the control group and the THüKo group. The secondary analysis (ANCOVA considering all groups: THüKo, placebo, control) did not yield results of statistical significance (alpha = 0.05) (Table 2), in spite of significant results in pair-wise comparisons.
Table 2. SF-36 subscale Bodily Pain; analyzed by intention to treat; primary end point in pairwise comparison THüKo versus control group; complementary analysis of variance with THüKo, placebo ultrasound group and control group; ŋ 2= adjusted effect estimate of model.
| P value ANCOVA |
Control group (n=68) |
Placebo ultrasound group (n=70) *1 |
THüKo (n=70) *1*2 |
ŋ*2 | Mean difference ThüKo vs control group (95-%-CI) |
P value | Mean difference THüKo vs placebo ultrasound group (95-%-CI) |
P value | |
|---|---|---|---|---|---|---|---|---|---|
| SF-36 Bodily Pain: | |||||||||
| Baseline scores | 56.6 (17.5) |
53.1 (18.2) |
57.9 (18.4) |
||||||
| Final scores | 56.4 (19.5) |
54.7 (19.0) |
63.1 (20.1) |
||||||
| Change scores t1–t0*3 | –0.1 (17.3) |
1.6 (15.9) |
5.2 (17.6) |
||||||
| ANCOVA*4 | p=0.061 | 56.0 (1.9)*3 |
56.5 (1.9)*3 |
61.8 (1.9)*3 |
0.37 | 5.7 (0.4–11.1) |
p=0.034*5 | 5.3 (-0.1–10.6) |
p=0.049 |
THüKo: exercise therapy, CI: confidence interval
*1Mean (standard deviation), unless described otherwise
*2One subject (THüKo) was excluded because of an extreme score (flu in the week preceding t0: t0 = 10 and t1 = 100).
*3Differences between t0 and t1 without baseline adjustment
*4Analysis of covariance: final scores adjusted to baseline scores. Reported as means and standard errors.
*5P value of the primary analysis
For all other subscales of the SF-36, no between-group effects were found, The mean differences are between 0 and 6 points, the median of the differences for all scales and all groups is 0 (Table 3). The subscales Role-Emotional, Role-Physical, and Social Functioning show ceiling effects, since more than half of the subjects in the named scales had the maximum number of 100 points at t0, and positive improvements at t1 were therefore not possible.
Table 3. SF-36 subscales: changes in scores between t0 and t1; mean (standard deviation) | median, rounded to whole numbers.
| Physical Functioning | Role- Physical | General Health | Vitality | Social Functioning | Role- Emotional | Mental Health | |
|---|---|---|---|---|---|---|---|
| Control group | 2 (18) | 0 | 3 (33) | 0 | 0 (16) | 0 | 0 (12) | 0 | –2 (15) | 0 | 2 (14) | 0 | –2 (10) | 0 |
| Placebo ultrasound group | 1 (12) | 0 | 6 (27) | 0 | 2 (15) | 0 | 1 (15) | 0 | –1 (16) | 0 | 0 (26) | 0 | 0 (12) | 0 |
| Exercise therapy (THüKo) | 2 (14) | 0 | 2 (35) | 0 | 3 (14) | 0 | –1 (15) | 0 | –2 (13) | 0 | 1 (27) | 0 | –1 (11) | 0 |
| P value NPar*1 P value ANCOVA*2 |
0.83 0.72 |
0.72 0.94 |
0.47 0.66 |
0.50 0.76 |
0.80 0.86 |
0.91 0.45 |
0.29 0.78 |
*1NPar: Analysis of non-adjusted changed scores between t0 and t1 by Kruskal-Wallis test
*2Analysis of covariance: final scores adjusted to baseline scores
For the disease-specific WOMAC, significant between-group effects were seen for the subscales Physical Functioning and Pain. For both scales, the THüKo group showed significantly greater effects than the control group and the placebo ultrasound group. No between-group effects were seen for the Stiffness subscale (Table 4).
Table 4. WOMAC index; analysis by intention to treat; analysis of variance with THüKo, placebo ultrasound group, and control group; ŋ 2= adjusted effect estimate of model used.
| P value ANCOVA | Control group (n=68) *1 | Placebo ultrasound group (n=70)*1 | THüKo (n=71)*1 | ŋ2 | Mean difference control group vs ThüKo*4 (95-%-CI) | P value | Mean difference placebo ultrasound group vsTHüKo*5(95-%-CI) | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Subscale Pain | |||||||||
| Baseline scores | 28.3 (16.9) |
28.8 (17.2) |
27.5 (16.7) |
||||||
| Final scores | 27.0 (18.4) |
25.0 (16.2) |
19.1 (16.5) |
||||||
| Change scores t1–t0*2 | –1.3 (15.3) |
–3.9 (14.3) |
–8.5 (13.9) |
||||||
| ANCOVA*3 | p=0.004 | 26.9 (1.6) |
24.6 (1.6) |
19.5 (1.6) |
0.42 | 7.4 (3.0–11.8) |
p=0.001 | 5.1 (0.7–9.4) |
p=0.024 |
| Subscale physical function | |||||||||
| Baseline scores | 26.7 (15.5) |
29.3 (16.5) |
26.5 (17.5) |
||||||
| Final scores | 24.7 (15.8) |
25.3 (15.8) |
18.1 (16.1) |
||||||
| Change scores t1–t0*2 | –2.1 (12.9) |
–3.9 (12.4) |
–8.4 (13.4) |
||||||
| ANCOVA*3 | p=0.002 | 25.1 (1.4) |
24.2 (1.4) |
18.7 (1.4) |
0.48 | 6.4 (2.5–10.3) |
p=0.001 | 5.5 (1.6–9.3) |
p=0.006 |
| Subscale stiffness | |||||||||
| Baseline scores | 37.8 (21.9) |
37.6 (21.7) |
32.4 (20.2) |
||||||
| Final scores | 30.1 (20.8) |
30.9 (20.0) |
23.4 (19.3) |
||||||
| Change scores t1–t0*2 | –7.7 (17.5) |
–6.6 (17.0) |
–9.0 (20.2) |
||||||
| ANCOVA*3 | p=0.204 | 29.0 (1.9) |
30.0 (1.9) |
25.4 (1.9) |
0.38 | 3.6 (-1.8–9.0) |
p=0.186 | 4.9 (-0.7–9.9) |
p=0.090 |
THüKo: exercise therapy, CI: confidence interval;
*1Mean (standard deviation), unless described otherwise
*2Differences between t0 und t1 without baseline adjustment
*3Analysis of covariance: final scores adjusted to baseline scores; reported as means and standard errors
*4Positive mean differences illustrate greater positive changes for THüKo compared with the control group
*5Positive mean differences illustrate greater positive changes for THüKo compared with the placebo ultrasound group
Discussion
The present study aimed to measure the effectiveness of exercise therapy for general (primary end point) and hip-specific pain symptoms, physical function, joint stiffness, and diverse areas of general health-related quality of life.
For the general pain symptoms (SF-36), positive treatment effects were seen for the THüKo group compared with the control group. The same was true for disease-specific pain and physical function, which were statistically significantly improved by exercise therapy compared with the control group and the placebo group. In terms of the WOMAC index, the extent of pain reduction and functional improvement resulting from the THüKo exercise therapy approach were comparable with the results of a recent meta-analyses on the effectiveness of exercised-based interventions in hip osteoarthritis: patients who underwent exercise therapy reported a pain score of 21 points (scale 0–100), whereas the non-exercising control group reported a pain score of 29 points. For physical function, the end results of the exercise therapy group were 22 points and those of the control group, 29 points. The comparison figures relate to randomized controlled studies with a land-based intervention. Water-based exercise therapies were not included (8, 10, 22). Not only the change scores between exercise group and control group for pain and function are comparable, but also the baseline measurements and end result measurements of both samples. The population of the meta-analysis and the present study are therefore similar in terms of the pain-related and function-related impairment due to hip osteoarthritis.
An important insight gained from the present study is the confirmation that the intervention is effective compared with a placebo intervention, as the effect sizes described, of 0.36, are comparable to effect sizes described in the literature for exercise therapy compared with a control group (8, 13). The available results of the WOMAC index show that exercise therapy is superior to a mere placebo intervention in terms of pain reduction as well as improved physical function.
No significant between-group effects were seen for joint stiffness; with the exception of the SF-36 subscale Bodily Pain; the same is the case for the assessment of health-related quality of life, for which so far no other studies have found a positive mechanism of action for exercise therapy either (8).
What remains unclear is the extent to which our results are generalizable to the treatment of all patients with osteoarthritis of the hip. The sample under study showed moderate disease-related impairments as compared to the average baseline measurements of the WOMAC. In this setting, the SF-36 measurements illustrate that the subjects score higher even than the German normative population sample on the vitality scale and that they have a better perception of their general health. This study is therefore inevitably subject to recruitment bias. The effectiveness of the intervention in worse-affected persons in a generally poorer state of health will need to be investigated in future studies. The same is the case for a differentiated analysis of different forms of intervention in terms of content, dosage, sustainability, and cost effectiveness (1, 12, 23, 24).
The effects of the Tübingen exercise therapy approach (THüKo) as shown in this study relate to the 12-week intervention period and do not allow any conclusions regarding long-term treatment effects. In the present study, follow-up examinations were conducted after three months and nine months. In this time period, subjects were, however, at liberty to seek out further therapeutic options that they had not used previously. This was in order to avoid early dropout by study participants because of non-compliance. This means that the randomized allocation to the treatment arms was effective exclusively for the time period t0 to t1. In addition to the complementary evaluation of the follow-up examinations, therapeutic effects over the long term should be studied in routine clinical practice.
The next important objective will be to integrate the THüKo intervention comprehensively into everyday healthcare provision. Concrete recommendations for exercise therapy in hip osteoarthritis can be given on the basis of our study. In Germany, implementation into everyday healthcare could be done in the context of functional training or rehabilitation exercise in accordance with the social security statutes. Alternatively, the treating physician could prescribe exercise therapy or physiotherapeutic treatment. For the diagnosis of arthritis, the German catalogue of non-physician practitioner services recommends instructing patients in how to undertake their individual independent exercise program and provides information on how best to deal with the injured joint in the sense of a “hip school” (24). The home training program conducted in our study may provide helpful orientation in this setting (14).
Key Messages.
The Tübingen exercise therapy approach (THüKo) is a 12 week exercise intervention for the treatment of patients with hip osteoarthritis, which aims to reduce pain and improve function.
THüKo is based on a combination of a class-based program and instruction for a training program at home. It includes imparting of knowledge, social interaction, and—in particular—exercises aiming to strengthen muscles, improve proprioception, and provide balance training and improve flexibility.
Positive treatment effects for the general health-related quality of life have thus far not been ascertained for the THüKo approach.
The implementation of the THüKo approach into routine healthcare provision can be undertaken, for example, by qualified instructors in the context of rehabilitation exercises or functional training or professional instruction by exercise therapists or physiotherapists.
Future research activities should evaluate the long-term effectiveness of the therapeutic regimen and investigate whether the intervention is also effective for patients with more pronounced impairments in terms of health-related quality of life.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
The authors thank Artzt company for providing the exercise materials.
Footnotes
Conflict of interest statement
The study was supported with training materials by the companies Theraband and Ludwig Artzt.
PD Dr Krauß, Dr Steinhilber, Mr Haupt, and Dr Janßen received honoraria for authorship in the context of the book project “Das Tübinger Hüftkonzept—von der Wissenschaft in die Praxis” (The Tübingen exercise therapy approach: from science into practice) and have received royalties from hellblau publishers.
Ms Miller and Prof Martus declare that no conflict of interest exists.
References
- 1.Fernandes L, Hagen KB, Bijlsma JW, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 2.Debrunner AM. 4 edition. Bern: Verlag Hans Huber, Hogrefe AG; 2005. Orthopädie, Orthopädische Chirurgie. [Google Scholar]
- 3.Reginster JY. The prevalence and burden of arthritis. Rheumatology (Oxford) 2002;41:3–6. [PubMed] [Google Scholar]
- 4.Fransen M, McConnell S, Bell M. Therapeutic exercise for people with osteoarthrosis of the hip or knee. A systematic review. The Journal of Rheumatology. 2002;29:1737–1745. [PubMed] [Google Scholar]
- 5.Rieger H. Köln: Deutscher Ärzte-Verlag GmbH; 2010. Sportverletzt was jetzt? [Google Scholar]
- 6.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Deutsche Gesellschaft für Orthopädie und orthopädische Chirurgie (DGOOC), Berufsverband der Ärzte für Orthopädie (BVO) AMWF-Leitlinien-Register Nr. 033/001. Köln: AWMF Online. AWMF-Leitlinien-Register; 2009. Leitlinien Orthopädie: Koxarthrose. [Google Scholar]
- 8.Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;4 doi: 10.1002/14651858.CD007912.pub2. CD007912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandmann GH, Imhoff AB. Physiologische Wirkmechanismen körperlicher Aktivität. In: Halle M, Schmidt-Trucksäss A, Hambrecht R, Berg A, editors. Sporttherapie in der Medizin. 1 edition. Stuttgart: Schattauer; 2008. pp. 359–360. [Google Scholar]
- 10.Fransen M, McConnell S. Land-based exercise for osteoarthritis of the knee: a metaanalysis of randomized controlled trials. J Rheumatol. 2009;36:1109–1117. doi: 10.3899/jrheum.090058. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Doherty M, Arden N, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2005;64:669–681. doi: 10.1136/ard.2004.028886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2009;(3) doi: 10.1002/14651858.CD007912. CD007912. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67:1716–1723. doi: 10.1136/ard.2008.092015. [DOI] [PubMed] [Google Scholar]
- 14.Haupt G, Janßen P, Krauß I, Steinhilber B. Essen: Verlag hellblau; 2014. Das Tübinger Hüftkonzept. [Google Scholar]
- 15.Krauss I, Steinhilber B, Haupt G, Miller R, Grau S, Janssen P. Efficacy of conservative treatment regimes for hip osteoarthritis - Evaluation of the therapeutic exercise regime „Hip School“: A protocol for a randomised, controlled trial. BMC Musculoskelet Disord. 2011;12 doi: 10.1186/1471-2474-12-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy N. Brisbane: Prof. Nicholas Bellamy; 2009. WOMAC Osteoarthritis Index: User Guide IX. [Google Scholar]
- 18.Bullinger B, Kirchberger I. Göttingen: Verlag Hogrefe; 1998. SF-36 Fragebogen zum Gesundheitszustand, Handanweisung. [Google Scholar]
- 19.Kim HY. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod. 2013;38:52–54. doi: 10.5395/rde.2013.38.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;10(323):1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2009;(4) doi: 10.1002/14651858.CD004376.pub2. CD004376. [DOI] [PubMed] [Google Scholar]
- 22.Sudeck G, Pfeifer K. Woll A, Haag H, Mess F, editors. Evaluation von Bewegungstherapie in der Rehabilitation. Handbuch Evaluation im Sport. Schorndorf: Hofmann. 2012:89–111. [Google Scholar]
- 23.Golightly YM, Allen KD, Caine DJ. A comprehensive review of the effectiveness of different exercise programs for patients with osteoarthritis. Phys Sportsmed. 2012;40:52–65. doi: 10.3810/psm.2012.11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heilmittelkatalog. Ludwigsburg: IntelliMed GmbH. 1th edition auf Basis der geltenden Heilmittelrichtlinie 2011 ed. 2011. Heilmittel der physikalischen Therapie. [Google Scholar]