Abstract
The present study evaluated survival effects of N-acetylcysteine (NAC) on cultured corneal endothelial cells exposed to oxidative and endoplasmic reticulum (ER) stress and in a mouse model of early-onset Fuchs endothelial corneal dystrophy (FECD). Cultured bovine corneal endothelial cell viability against oxidative and ER stress was determined by CellTiter-Glo® luminescent reagent. Two-month-old homozygous knock-in Col8a2L450W/L450W mutant (L450W) and C57/Bl6 wild-type (WT) animals were divided into two groups of 15 mice. Group I received 7 mg/mL NAC in drinking water and Group II received control water for 7 months. Endothelial cell density and morphology were evaluated with confocal microscopy. Antioxidant gene (iNos) and ER stress/unfolded protein response gene (Grp78 and Chop) mRNA levels and protein expression were measured in corneal endothelium by real time PCR and Western blotting. Cell viability of H2O2 and thapsigargin exposed cells pre-treated with NAC was significantly increased compared to untreated controls (pitalic>0.01). Corneal endothelial cell density (CD) was higher (p=0.001) and percent polymegathism was lower (p=0.04) in NAC treated L450W mice than in untreated L450W mice. NAC treated L450W endothelium showed significant upregulation of iNos, whereas Grp78 and Chop were downregulated compared to untreated L450W endothelium by real time PCR and Western blotting. NAC increases survival in cultured corneal endothelial cells exposed against ER and oxidative stress. Systemic NAC ingestion increases corneal endothelial cell survival which is associated with increased antioxidant and decreased ER stress markers in a mouse model of early-onset FECD. Our study presents in vivo evidence of a novel potential medical treatment for FECD.
Keywords: N-acetylcysteine (NAC), Fuchs endothelial corneal dystrophy (FECD), oxidative stress, ER stress, corneal endothelium
Fuchs endothelial corneal dystrophy (FECD) is a degenerative corneal endothelial disease which is characterized by progressive corneal endothelial cell (CEC) loss with concomitant drop-like excrescences (guttae) and thickening of the endothelial basement membrane (Azizi et al., 2011). FECD affects an estimated 5% of the US population and is one of the most common causes for corneal transplantation (Baratz et al., 2010). Corneal endothelial cell apoptosis due to oxidative stress and endoplasmic reticulum (ER) stress may play a central pathogenic role in FECD (Jurkunas et al., 2010; Engler et al., 2010)
N-acetylcysteine (NAC), a thiol-containing antioxidant and radical scavenger, has been used clinically for many years as a reduced glutathione precursor (Arakawa and Ito, 2007). NAC also blocks bovine serum albumin (BSA)-induced ER stress effects such as GRP78 activation, eIF2α phosphorylation, SGLT expression, and α-MG uptake (Lee et al., 2009). Previously, we described two alpha 2 collagen VIII (Col8a2) transgenic knock-in mouse models of early-onset Fuchs endothelial corneal dystrophy which show early endothelial cell ER stress/unfolded protein response (UPR) and apoptosis (Jun et al., 2012; Meng et al., 2013). These knock-in mutations specify a glutamine to lysine change at amino acid 455 (Q455K) and a leucine to tryptophan change at amino acid 450 (L450W) (Biswas et al., 2001; Gottsch et al., 2005).
The purpose of this study is to evaluate the effect of NAC as an inhibitor of oxidative and ER stress on corneal endothelial cell survival in cell culture and in the L450W alpha 2 collagen VIII transgenic knock-in mouse model of Fuchs endothelial corneal dystrophy.
Bovine corneal endothelial cells (BCECs) were scraped from the excised corneas of freshly enucleated globes with a surgical blade according to an established protocol (Chifflet et al., 2003), and primary cultures were established by resuspending cells in Dulbecco’s modification of Eagle’s minimum essential medium (DMEM) (Cellgro, Manassas, VA) supplemented with 10% fetal calf serum, antibiotic antimycotic solution (10,000 units of penicillin (base), 10,000 μg of streptomycin (base), and 25 μg of amphotericin B/ml) (Invitrogen, Grand Island, NY) and 50 μg/ml of gentamicin (Invitrogen) at 37 °C and 5% CO2 in air on a 6 well plate (Cytoone, Orlando, FL). After confluency was reached (usually 7–10 days), the primary cultures were subcultured to 96 wells (Cytoone) in the same medium.
To examine the effects of NAC on BCECs exposed to oxidative and ER stress, corneal endothelial cells were incubated in quadruplicate concentrations (0 to 10 mM) of N-acetylcysteine (Sigma, St. Louis, MO) in cell culture medium for 48 hours. NAC was freshly dissolved in phosphate-buffered saline (PBS; Gibco-BRL, Carlsbad, CA) at room temperature before use. After pretreatment with NAC for 48 hours, cell culture medium containing NAC was then replaced with 100μL cell culture medium without NAC and containing thapsigargin (28 μM) for 24 hours or H2O2 (0.6 mM) for 4 hours. Negative control (no NAC treatment for 48 hours and no thapsigargin for 24 hours or H2O2 for 4 hours) was also included to determine baseline cell viability. At the end of incubation in H202, thapsigargin, or control conditions as described above, cell viability was determined by adding 100μL of CellTiter-Glo® luminescent reagent (Promega, Madison, WI), and the luminescence, produced by the luciferase-catalyzed reaction of luciferin and ATP, was measured using a FLUOstar OPTIMA spectrophotometer (Optima, Tokyo, Japan). The cell viability (%) relative to control was calculated as 100 x luminescence in H2O2 or thapsigargin conditioned cells/luminescence in negative control cells.
Weaned 2 month old animals were divided into two groups of 30 mice for each treatment. Group I (NAC group) received standard rodent chow orally and 7 mg/mL NAC drinking water (Whitehead et al., 2008) for 7 months. Group II (control group) received standard rodent chow orally and pure drinking water for 7 months. Each group was divided into two subgroups of 15 mice each. These subgroups included mice with the Col8a2 L450W mutation (Meng et al., 2013) or wild-type mice. Animal care and use conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All animal protocols were approved by the Institutional Animal Care and Use Committee of Johns Hopkins University.
All mice were euthanized with isoflurane (Vedco Inc., St. Joseph, MO) verified by checking for the absence of respirations and followed by cervical dislocation. Immediately after euthanasia, corneal endothelial cells were examined by confocal microscope (Nidek Confoscan 3, Fremont, CA). Mice were placed on a customized platform and the head was fixed with the right eye pointing towards the objective. Lubricant gel (Genteal; Novartis, East Hanover, NJ) was used as an immersion fluid, and images of the central corneal endothelium were acquired as previously described (Jun et al., 2012). Corneal endothelial cell imaging and quantitative analysis including cell density and percent polymegathism were performed using the Confoscan 3 software. Cells were manually identified for analysis by a single observer (ECK).
After confocal microscopy examination, eyes were extracted and Descemet membranes (DM) were stripped with a forceps. Total RNA was extracted from murine corneal endothelium on stripped DMs (four eyes per sample, i.e. a single sample (n) included two animals of the same genotype, with 5 samples from 10 animals (n=5) per subgroup analyzed) using TRIzol reagent (Invitrogen) followed by RNeasy column (Qiagen, Hilden, Germany) purification. Thus, 2 mice were included in each sample with n=5 (10 mice) samples for each of four subgroups analyzed, including mutant mice with and without NAC, and wild-type mice with and without NAC. Complementary DNA was generated using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The resulting complementary DNA (3μL cDNA) was preamplified with TaqMan® PreAmp MasterMix and mouse specific primers (Applied Biosystems, real time PCR primers for the examined genes are Nos2(iNos); Mm00440502_m1 (assay number), Nfe2l2(Nrf2); Mm00477784_m1, Ptgs2(Cox2); Mm00478374_m1, DDIT3(Chop); Mm01135937_g1, Hspa5(Grp78); Mm00517690_g1, and β-actin; Mm00607939_s1). Real time-polymerase chain reaction (real time PCR) was performed in triplicate using the TaqMan® Gene Expression Master Mix (2×) (Applied Biosystems), preamplified cDNA products (diluted 1:20; 5μl), nuclease free water, and mouse specific primers in a 20μL reaction volume. The relative gene expression in Col8a2L450W/L450W and WT endothelium was normalized to the housekeeping gene beta-actin (β-actin). A no-template control was included in each quantitative real time PCR experiment to confirm the absence of DNA contamination in the reagents used for amplification. All assays used similar amplification efficiency, and a ΔCT experimental design was used for relative quantification. Data analysis was performed using StepOne™ software (Version 2.2, Applied Biosystems). Statistical analysis of quantitative real time PCR data between the groups was performed using the ANOVA and DataAssist™ Software v3.0 (Applied Biosystems). A p-value bold> 0.05 was considered statistically significant.
Descemet membrane and endothelial cells were stripped from freshly dissected, 10 month-old mouse corneas and homogenized in Tissue Protein Extract Reagent (Thermo Fisher Scientific, Rockford, IL) with 1% protease inhibitor cocktail (Sigma) and 1% ethylenediaminetetraacetic acid (Sigma). Each sample contained both corneas of the same animal from a total of four subgroups including mutant mice with and without NAC and WT mice with and without NAC. Each subgroup included n=5 mice. The mixture was then microcentrifuged at 48°C for 10 min at 12 000 rpm. The lysate was removed and the protein concentration was quantified by BCA Protein Assay Kit (Thermo Fisher Scientific). Eight micrograms of protein was mixed with 10 μl of 4× loading dye (Invitrogen) with 2-mercaptoethanol (Sigma) and heated at 65°C for 5 min.
Samples were loaded onto a 10% Tris–HCl Ready Gel (BioRad, Hercules, CA) and subjected to sodium dodecylsulfate–polyacrylamide gel electrophoresis separation for 1 h at 120 V. Proteins were transferred to a polyvinylidene fluoride membrane (BioRad) and incubated in blocking buffer made of 5% non-fat milk in PBS with 0.1% Tween-20. Membranes were then incubated in primary antibodies: iNOS (1:500, Abcam, Boston, MA), CHOP (1:500, Santa Cruz Biotechnology, Santa Cruz, CA), and GRP78 (1:500, Cell Signaling, Danvers, MA) diluted in blocking buffer for 1 hour at room temperature. Subsequently, membranes were washed and incubated in 1:10,000 dilution of anti-rabbit IgG, horseradish peroxidase conjugated antibody (GE Healthcare, Piscataway, NJ) diluted in blocking buffer for 45 min at room temperature. Loading controls were assayed by probing with β-actin (1:1,000, Cell Signaling) as primary antibody after stripping with Restore Stripping Buffer (Thermo Fisher Scientific). Proteins were detected using SuperSignal West Dura (Thermo Fisher Scientific). Densitometry analysis was performed using Image J as previously described (http://www.lukemiller.org/journal/2007/08/quantifying-western-blots-without.html).
Data are presented as mean ± standard deviation (SD). Statistical analysis of cell viability in culture experiments and cell density/polymegathism by confocal microscopy was performed by Mann-Whitney 2-tailed test. Real time PCR and Western blot assay results were also analyzed by Mann-Whitney 2-tailed test. A p-value < 0.05 was considered statistically significant.
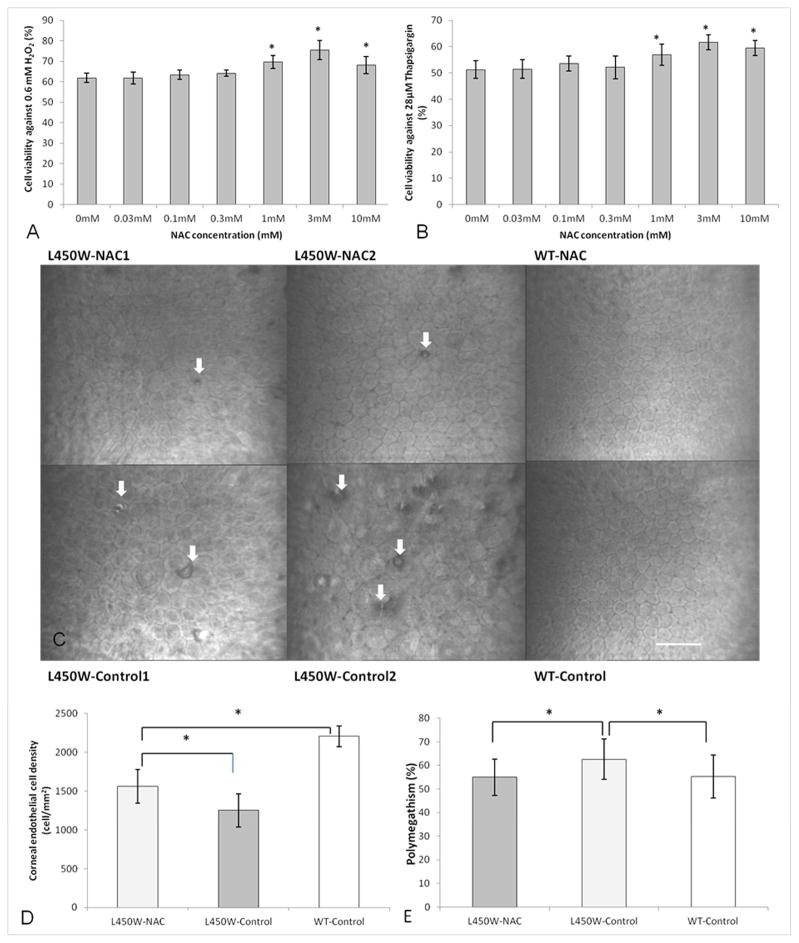
We devised a short term cell culture model to assess whether NAC could improve viability against oxidative and ER stress in corneal endothelial cells. Cell viability of 1 mM (69.6 ± 3.2 %, p=0.004), 3 mM (75.5 ± 4.7 %, 0=0.001), and 10 mM (67.7 ± 4.1 %, p=0.01) NAC pre-treated cells exposed to H202 was significantly increased compared to untreated controls (61.9 ± 2.2 %) (Fig. 1A). Cell viability of 1 mM (56.9 ± 4.0 %, p=0.03), 3 mM (61.7 ± 2.8 %, p=0.001), and 10 mM (59.5 ± 2.8 %, p=0.02) NAC pre-treated cells exposed to thapsigargin was significantly increased compared to untreated controls (51.3 ± 3.2 %) (Fig. 1B).
Figure 1.
Cell viability of N-acetylcysteine (NAC) treated bovine corneal endothelial cells compared to untreated control after H2O2 (A) or thapsigargin (B) conditioning (*p<0.05). Confocal microscopy of corneal endothelial cells (C) in two different mutant mice treated with NAC (L450W-NAC1/2) show fewer guttae (white arrows) and relatively normal cell size and shape compared to two different untreated mutant mice (L450W-Control1/2). Wild-type mice with (WT-NAC) and without (WT-Control) NAC treatment show normal corneal endothelium. Scale bar = 60 μm for all images (C). Mutant mice treated with NAC (L450W-NAC) show higher endothelial cell density (D) than mutant mice untreated with NAC (L450W-Control). WT mice untreated with NAC (WT-Control) are shown for comparison (D, *p<0.05). Mutant mice treated with NAC (L450W-NAC) show lower endothelial cell polymegathism (E) than mutant mice untreated with NAC (L450W-Control). WT mice untreated with NAC (WT-Control) are shown for comparison (E, *p<0.05).
Given the above results showing some beneficial effect of NAC in cultured CECs exposed to short term oxidative and ER stress, we sought to assess effects of chronic, systemic NAC in a mouse model of FECD. NAC treated L450W mice showed relatively mild variation of endothelial cell size and hexagonal morphology with few extracellular matrix excrescences (guttae, white arrows) compared to control mice (Fig. 1C). However, untreated L450W mice showed a more severe phenotype with multiple guttae and increased variation in endothelial cell size and shape (Fig. 1C). NAC treated and untreated wild-type mice showed a normal endothelial phenotype with no guttae (Fig. 1C). No differences in behavior, survival, breeding, activity, or morphology were noted between NAC treated and untreated mice.
Corneal endothelial cell density (CD) of NAC treated L450W mice (1559 ± 216 cells/mm2) was significantly higher than for untreated L450W mice (1250 ± 214) (p=0.001, Fig. 1D). CD of untreated WT mice (2207 ± 133 cells/mm2) was significantly higher than both NAC treated (p=0.0001) and untreated (p=0.00001) L450W mice (Fig. 1D). Percent polymegathism (variation in cell size which is a marker of endothelial cell health) was significantly lower in NAC treated L450W mice (54.9 ± 7.6 %) compared with untreated (62.6 ± 8.4) L450W mice (p=0.04, Fig. 1E). No difference in percent polymegathism was noted between NAC treated L450W mice compared with untreated WT control mice (Fig. 1E).
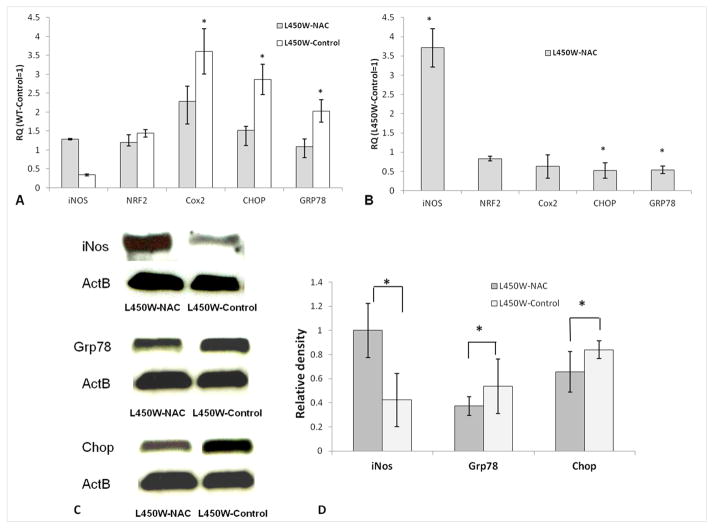
We next sought to confirm increased antioxidant markers (Nrf2 and iNos) (Itoh et al., 1997; Hemmrich et al., 2003; Hillas et al., 2013) and decreased oxidative stress (Cox2) (O’Banion, 1999; Feng et al., 1995) and ER stress/UPR (Grp78 and Chop) (Engler et al., 2010) markers in the endothelium of NAC treated L450W mice compared to untreated L450 mice by real time PCR. There was significant up-regulation of Cox2 (3.60 ± 0.61, p=0.01), Chop (2.86 ± 0.42, p=0.01), and Grp78 (2.03 ± 0.32, p=0.01) mRNA in corneal endothelium of NAC untreated L450W mice compared with NAC untreated WT mice (relative quantity = 1, Fig. 2A) indicating increased oxidative and ER stress in mutant mouse endothelium. The relative expression of iNos (0.41 ± 0.02, p=0.15) and Nrf2 (1.44 ± 0.10, p=0.11) in corneal endothelium of NAC untreated L450W mice was not significantly different compared to NAC untreated WT mice (Figure 2A).
Figure 2.
(A) By real time PCR, Cox2, Chop, and Grp78 mRNAs were overexpressed (*p<0.05) in the endothelium of mutant mice untreated with NAC (L450W-Control) compared to wild-type mice untreated with NAC (WT-Control, relative quantity (RQ) = 1), indicating increased oxidative and ER stress in mutant endothelium. (B) By real time PCR, iNos mRNA was overexpressed, but Chop and Grp78 mRNAs were underexpressed (*p<0.05) in mutant mice treated with NAC (L450W-NAC) compared to mutant mice untreated with NAC (L450W-Control, RQ=1), indicating increased antioxidant defense and decreased ER stress in L450W mutant endothelium associated with NAC treatment. Western blotting (C) with densitometry normalized to β-actin (ActB) (D) shows increased levels of iNos, but decreased levels of Grp78 and Chop (*p<0.05), in mutant mice treated with NAC (L450W-NAC) compared to mutant mice untreated with NAC (L450W-Control), indicating increased antioxidant defense and decreased ER stress in L450W mutant endothelium associated with NAC treatment.
There was significant up-regulation of iNos (3.75 ± 0.50, p=0.02), but down-regulation of Chop (0.50 ± 0.24, p=0.01) and Grp78 (0.50 ± 0.16, p=0.01) mRNA in corneal endothelium of NAC treated L450W mice when NAC untreated L450W mice were used as standard (relative quantity = 1, Fig. 2B), indicating increased antioxidant defense and decreased ER stress associated with NAC treatment. The relative expression of Nrf2 (0.83 ± 0.02, p=0.15) and Cox2 (0.63 ± 0.30, p=0.10) mRNAs in corneal endothelium of NAC treated L450W mice was not significantly different when NAC untreated L450W mice were used as standard (Fig. 2B).
Western blotting showed significant up-regulation of the antioxidant enzyme iNos (2.36 ± 0.12-fold, p=0.01), but significant down-regulation of the ER stress proteins Grp78 (0.78 ± 0.15-fold, p=0.03) and Chop (0.70 ± 0.10-fold, p=0.04) in corneal endothelium of NAC treated L450W mice vs. NAC untreated L450W mice (Fig. 2C & 2D). These results indicate that systemic NAC treatment is associated with increased antioxidant defense and decreased ER stress/UPR in L450W mutant endothelium.
Fuchs endothelial corneal dystrophy (FECD) is a slowly progressive eye disease leading to corneal vision loss, mostly affecting people above 40 years old (Czarny et al., 2013). The only definitive treatment of FECD is corneal transplantation (Czarny et al., 2013; Rose et al., 2009). Endoplasmic reticulum (ER) and oxidative stress contribute to the pathogenesis of FECD (Azizi et al., 2011; Engler et al., 2010). N-acetylcysteine (NAC), a prodrug which supplies the cysteine required for reduced glutathione (GSH) synthesis, has been used to combat oxidative stress-induced damage in various tissues (Maziere et al., 1999). NAC also has been shown to prevent ER stress by inhibiting BSA-induced increases in Na-glucose cotransporter (SGLT) protein expression and α-methyl-D-glucopyranoside (α-MG) uptake in renal proximal tubule cells (Lee et al., 2009).
Based on the roles of oxidative and ER stress in the pathogenesis of FECD, the present study was conducted to investigate the potential effect of NAC against ER and oxidative stress in corneal endothelium in vitro and in a mouse model of early onset FECD. Our results demonstrate that NAC pretreatment increases survival in cultured corneal endothelial cells exposed to oxidative and ER stress. Others have shown similar protective effects of NAC on oxidative stress induced by bleomycin in cultured lung vascular endothelial cells (Patel et al., 2012). To our knowledge, however, the present work is the first to report a protective effect of NAC on ER stress induced by thapsigargin.
Given the positive in vitro effects of NAC we observed in cultured corneal endothelial cells under short term stress conditions, we evaluated the effects of chronic, oral, low-dose NAC treatment on the corneal endothelium in vivo using our knock-in mouse model of FECD homozygous for the L450W Col8a2 mutation. L450W mice treated with NAC showed fewer guttae and higher cell density with decreased polymegathism compared with untreated L450W mice. Real time PCR and Western blotting showed evidence for increased antioxidant defense (iNos) and decreased ER stress (Chop and Grp78). Endogenous iNOS expression and activity have key functions in increasing endothelial survival and maintaining function against oxidative damage (Hemmrich et al., 2003). INOS can prevent both inflammation and oxidative stress in clinical trials of chronic obstructive pulmonary disease (Hillas et al., 2013). INOS-derived nitric oxide (NO) may also be involved in the tolerance to ischemia/reperfusion (I/R) injury in a variety of pathophysiological conditions where iNOS expression is increased as a result of oxidative stress (Otani, 2009). INOS-derived NO plays a crucial role in the tolerance to I/R injury in the cardiomyopathic hamster heart (Kyoi et al., 2006). Previously a similar effect of increased iNOS mRNA levels with NAC treatment in a human endothelial cell line was reported (Chen et al., 2000) as we observed in vivo in NAC treated L450W endothelium.
In contrast to the presumed antioxidant effect of NAC induced iNos upregulation in the corneal endothelium of NAC treated L450W mice compared with untreated L450W mice, the relative expression of Nrf2 and Cox2 by real time PCR between these groups was not significantly different. However, Cox2 did show a trend for a 37% decreased expression for NAC treated L450W mice compared with untreated L450W mice, and Nrf2 showed a milder trend for a 17% decreased expression for NAC treated L450W mice compared with untreated L450W mice. These findings potentially can be explained by NAC’s direct reactive oxygen species (ROS) scavenging properties as seen in the NAC mediated suppression of Nrf2 transcriptional upregulation in PC12 cells treated with deltamethrin (Li et al., 2007) and NAC’s inhibition of Nrf2 activation in human umbilical vein endothelial cells exposed to laminar shear stress (Hsieh et al., 2009). Thus, we hypothesize that the direct oxidant scavenging effects of NAC, as opposed to increased antioxidant gene expression, could explain the observed downward trend in Cox2 and Nrf2 mRNA levels in NAC treated vs. untreated L450W endothelium.
Accumulation of misfolded proteins results in endoplasmic reticulum stress, a condition that is toxic to cells (Szegezdi et al., 2006). To counteract this stress, cells initiate the unfolded protein response (UPR) which is a comprehensive program to reduce the accumulation of toxic unfolded proteins (Szegezdi et al., 2006). Failure to alleviate endoplasmic reticulum stress by the unfolded protein response can lead to cellular apoptosis (Szegezdi et al., 2006). UPR activation has been shown to play a pathogenic role in the endothelium of FECD patients and the previously reported L450W mouse model of this disease (Engler et al., 2010; Meng et al., 2013). In this study, two markers of ER stress/UPR activation, Chop and Grp78, were significantly downregulated at the mRNA and protein levels in NAC treated vs. untreated L450W mice. A similar effect of NAC on Grp78 expression was previously reported for cadmium-induced ER stress in germ cells (Ji et al., 2013). Thus, our results show that the increased survival effect of NAC on corneal endothelium in the L450W mouse model of FECD is associated with reduced ER stress/UPR activation.
Currently no definitive non-surgical treatments exist for FECD, but several reports suggest the potential benefit of such an approach. Our recent work demonstrated increased corneal endothelial cell survival effects of lithium treatment associated with decreased oxidative and ER stress in a similar mouse model of FECD based on the Q455K Col8a2 mutation (Kim et al., 2013). Furthermore, the inhibition of Rho/Rho kinase (ROCK) signaling by Y-27632, a specific ROCK inhibitor, promoted cell adhesion, inhibited apoptosis, and enhanced cell proliferation in cultured primate CECs (Okumura et al., 2011). Topical instillation of the ROCK inhibitor after transcorneal freezing has been shown to promote corneal endothelial wound healing in a primate model and in human patients with FECD (Okumura et al., 2013). In addition to these reports, our present results suggest the potential efficacy of other agents for treating FECD which target known or yet to be discovered pathologic cellular processes.
The present study is subject to a number of potential limitations. Firstly, the L450W Col8a2 mutation causes the uncommon early onset form of FECD, and the efficacy of NAC in the more common genetic form of the disease associated with the TCF-4 gene (Baratz et al., 2010) is unclear. Secondly, although we feel that mouse models are relevant and useful in the study of corneal endothelial diseases, interspecies differences still exist and could affect the clinical applicability of our results. Thirdly, we did not assess in detail potential ocular or non-ocular side effects arising from systemic NAC use. Such issues could be more problematic in humans with a longer disease course and lifespan and would need to be evaluated carefully in future studies.
In conclusion, NAC increases corneal endothelial survival in a cell culture and mouse model of Fuchs endothelial corneal dystrophy. Thus, our study presents in vivo evidence of a novel potential medical treatment for FECD. We further suggest that chronic NAC treatment acts via its effects on oxidative and ER stress among other candidate cell stress pathways in the corneal endothelium. More detailed evaluation of the pharmacology, efficacy, and potential routes of administration (e.g. topical vs. local) for this drug as a potential treatment of FECD are warranted.
Highlights.
NAC increased cultured endothelial cells survival against oxidative and ER damage.
Col8a2 L450W mutation mice received 7 mg/mL NAC drinking water for 7 months.
Endothelial cell density of NAC-treated L450W mice was higher than untreated-control.
NAC-treated L450W mice increased iNos, but decreased Chop and Grp78 mRNA and protein.
NAC increases corneal endothelial cell survival against oxidative and ER stress.
Acknowledgments
Financial Support: Grants from the National Institutes of Health (EY019874), J. Willard and Alice S. Marriott Foundation, Edward Colburn, Lorraine Collins, Richard Dianich, Mary Finegan, Barbara and Peter Freeman, Stanley Friedler, MD, Herbert Kasoff, Diane Kemker, Jean Mattison, Lee Silverman, Norman Tunkel, PhD (all to ASJ), and Research to Prevent Blindness (to Wilmer Eye Institute).
Footnotes
The authors have no financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arakawa M, Ito Y. N-acetylcysteine and neurodegenerative diseases: Basic and clinical pharmacology. Cerebellum. 2007;6 (4):308–314. doi: 10.1080/14734220601142878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi B, Ziaei A, Fuchsluger T, Schmedt T, Chen Y, Jurkunas UV. p53-regulated increase in oxidative-stress--induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model. Invest Ophthalmol Vis Sci. 2011;52 (13):9291–9297. doi: 10.1167/iovs.11-8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratz KH, Tosakulwong N, Ryu E, Brown WL, Branham K, Chen W, Tran KD, Schmid-Kubista KE, Heckenlively JR, Swaroop A, Abecasis G, Bailey KR, Edwards AO. E2-2 protein and Fuchs’s corneal dystrophy. N Engl J Med. 2010;363 (11):1016–1024. doi: 10.1056/NEJMoa1007064. [DOI] [PubMed] [Google Scholar]
- Biswas S, Munier FL, Yardley J, Hart-Holden N, Perveen R, Cousin P, Sutphin JE, Noble B, Batterbury M, Kielty C, Hackett A, Bonshek R, Ridgway A, McLeod D, Sheffield VC, Stone EM, Schorderet DF, Black GC. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet. 2001;10 (21):2415–2423. doi: 10.1093/hmg/10.21.2415. [DOI] [PubMed] [Google Scholar]
- Chen G, Wang SH, Warner TD. Regulation of iNOS mRNA levels in endothelial cells by glutathione, a double-edged sword. Free Radic Res. 2000;32 (3):223–224. doi: 10.1080/10715760000300231. [DOI] [PubMed] [Google Scholar]
- Chifflet S, Hernández JA, Grasso S, Cirillo A. Nonspecific depolarization of the plasma membrane potential induces cytoskeletal modifications of bovine corneal endothelial cells in culture. Exp Cell Res. 2003;282 (1):1–13. doi: 10.1006/excr.2002.5664. [DOI] [PubMed] [Google Scholar]
- Czarny P, Kasprzak E, Wielgorski M, Udziela M, Markiewicz B, Blasiak J, Szaflik J, Szaflik JP. DNA damage and repair in Fuchs endothelial corneal dystrophy. Mol Biol Rep. 2013;40 (4):2977–2983. doi: 10.1007/s11033-012-2369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Kelliher C, Spitze AR, Speck CL, Eberhart CG, Jun AS. Unfolded protein response in Fuchs endothelial corneal dystrophy: a unifying pathogenic pathway? Am J Ophthalmol. 2010;149 (2):194–202. doi: 10.1016/j.ajo.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Xia Y, Garcia GE, Hwang D, Wilson CB. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-alpha, and lipopolysaccharide. J Clin Invest. 1995;95 (4):1669–1675. doi: 10.1172/JCI117842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch JD, Zhang C, Sundin OH, Bell WR, Stark WJ, Green WR. Fuchs corneal dystrophy: aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Invest Ophthalmol Vis Sci. 2005;46 (12):4504–4511. doi: 10.1167/iovs.05-0497. [DOI] [PubMed] [Google Scholar]
- Hemmrich K, Suschek CV, Lerzynski G, Kolb-Bachofen V. iNOS activity is essential for endothelial stress gene expression protecting against oxidative damage. J Appl Physiol. 2003;95 (5):1937–1946. doi: 10.1152/japplphysiol.00419.2003. [DOI] [PubMed] [Google Scholar]
- Hillas G, Nikolakopoulou S, Hussain S, Vassilakopoulos T. Antioxidants and mucolytics in COPD management: when (if ever) and in whom? Curr Drug Targets. 2013;14 (2):225–234. doi: 10.2174/1389450111314020007. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Hsiao HY, Wu WY, Liu CA, Tsai YC, Chao YJ, Wang DL, Hsieh HJ. Regulation of shear-induced nuclear translocation of the Nrf2 transcription factor in endothelial cells. J Biomed Sci. 2009;16:12. doi: 10.1186/1423-0127-16-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Ji YL, Wang H, Zhang C, Zhang Y, Zhao M, Chen YH, Xu DX. N-acetylcysteine protects against cadmium-induced germ cell apoptosis by inhibiting endoplasmic reticulum stress in testes. Asian J Androl. 2013;15 (2):290–296. doi: 10.1038/aja.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun AS, Meng H, Ramanan N, Matthaei M, Chakravarti S, Bonshek R, Black GC, Grebe R, Kimos M. An alpha 2 collagen VIII transgenic knock-in mouse model of Fuchs endothelial corneal dystrophy shows early endothelial cell unfolded protein response and apoptosis. Hum Mol Genet. 2012;21 (2):384–393. doi: 10.1093/hmg/ddr473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkunas UV, Bitar MS, Funaki T, Azizi B. Evidence of oxidative stress in the pathogenesis of Fuchs endothelial corneal dystrophy. Am J Pathol. 2010;177 (5):2278–2289. doi: 10.2353/ajpath.2010.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EC, Meng H, Jun AS. Lithium treatment increases endothelial cell survival and autophagy in a mouse model of Fuchs endothelial corneal dystrophy. Br J Ophthalmol. 2013;97 (8):1068–1073. doi: 10.1136/bjophthalmol-2012-302881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoi S, Otani H, Matsuhisa S, Akita Y, Enoki C, Tatsumi K, Hattori R, Imamura H, Kamihata H, Iwasaka T. Role of oxidative/nitrosative stress in the tolerance to ischemia/reperfusion injury in cardiomyopathic hamster heart. Antioxid Redox Signal. 2006;8 (7–8):1351–1361. doi: 10.1089/ars.2006.8.1351. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Suh HN, Han HJ. Effect of BSA-induced ER stress on SGLT protein expression levels and alpha-MG uptake in renal proximal tubule cells. Am J Physiol Renal Physiol. 2009;296 (6):F1405–F1416. doi: 10.1152/ajprenal.90652.2008. [DOI] [PubMed] [Google Scholar]
- Li HY, Wu SY, Shi N. Transcription factor Nrf2 activation by deltamethrin in PC12 cells: Involvement of ROS. Toxicol Lett. 2007;171 (1–2):87–98. doi: 10.1016/j.toxlet.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Maziere C, Conte M, Degonville J, Ali D, Maziere J. Cellular enrichment with polyunsaturated fatty acids induces an oxidative stress and activates the 456 transcription factors AP1 and NFkappaB. Biochem Biophys Res Commun. 1999;265 (1):116–122. doi: 10.1006/bbrc.1999.1644. [DOI] [PubMed] [Google Scholar]
- Meng H, Matthaei M, Ramanan N, Grebe R, Chakravarti S, Speck CL, Kimos M, Vij N, Eberhart CG, Jun AS. L450W and Q455K Col8a2 Knock-In Mouse Models of Fuchs Endothelial Corneal Dystrophy Show Distinct Phenotypes and Evidence for Altered Autophagy. Invest Ophthalmol Vis Sci. 2013;54 (3):1887–1897. doi: 10.1167/iovs.12-11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Banion MK. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit Rev Neurobiol. 1999;13 (1):45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- Okumura N, Koizumi N, Kay EP, Ueno M, Sakamoto Y, Nakamura S, Hamuro J, Kinoshita S. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2013;54 (4):2493–2502. doi: 10.1167/iovs.12-11320. [DOI] [PubMed] [Google Scholar]
- Okumura N, Koizumi N, Ueno M, Sakamoto Y, Takahashi H, Hamuro J, Kinoshita S. The new therapeutic concept of using a rho kinase inhibitor for the treatment of corneal endothelial dysfunction. Cornea. 2011;30 (Suppl 1):S54–9. doi: 10.1097/ICO.0b013e3182281ee1. [DOI] [PubMed] [Google Scholar]
- Otani H. The role of nitric oxide in myocardial repair and remodeling. Antioxid Redox Signal. 2009;11 (8):1913–1928. doi: 10.1089/ars.2009.2453. [DOI] [PubMed] [Google Scholar]
- Patel RB, Kotha SR, Sauers LA, Malireddy S, Gurney TO, Gupta NN, Elton TS, Magalang UJ, Marsh CB, Haley BE, Parinandi NL. Thiol-redox antioxidants protect against lung vascular endothelial cytoskeletal alterations caused by pulmonary fibrosis inducer, bleomycin: comparison between classical thiol-protectant, N-acetyl-L-cysteine, and novel thiol antioxidant, N,N′-bis-2-mercaptoethyl isophthalamide. Toxicol Mech Methods. 2012;22 (5):383–396. doi: 10.3109/15376516.2012.673089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose L, Kelliher C, Jun AS. Endothelial keratoplasty: historical perspectives, current techniques, future directions. Can J Ophthalmol. 2009;44 (4):401–405. doi: 10.3129/i09-090. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7 (9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead NP, Pham C, Gervasio OL, Allen DG. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586 (7):2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]