Abstract
Introduction:
The aim of this study was to compare the shear bond strength of resin modified glass ionomer cement to conditioned and unconditioned mineral trioxide aggregate surface.
Materials and Method:
White Mineral Trioxide Aggregate (WMTA) and Resin Modified Glass Ionomer Cement (RMGIC) were used for the study. 60 WMTA specimens were prepared and stored in an incubator at 37° C and 100% humidity for 72 hrs. The specimens were then divided into two groups- half of the specimens were conditioned and remaining half were left unconditioned, subsequent to which RMGIC was placed over MTA. The specimens were then stored in an incubator for 24 hrs at 37° C and 100% humidity. The shear bond strength value of RMGIC to conditioned and unconditioned WMTA was measured and compared using unpaired 't ’ test.
Results:
The mean shear bond strength of value of RMGIC to conditioned and unconditioned WMTA was 6.59 MPa and 7.587 MPa respectively. Statistical analysis using unpaired t-test revealed that the difference between values of two groups was not statistically significant (P > 0.05).
Conclusions:
During clinical procedures like pulp capping and furcal repair, if RMGIC is placed as a base over MTA, then conditioning should be done to increase the bond strength between RMGIC and dentin and any inadvertent contact of conditioner with MTA will not significantly affect the shear bond strength value of RMGIC to MTA.
Keywords: Conditioner, mineral trioxide aggregate, resin modified glass ionomer cement, shear bond strength
INTRODUCTION
An ideal endodontic repair material should seal the pathways of communication between the root canal system and its surrounding tissues.[1] It is desirable for the selected material to induce or conduct bone deposition, provide a good seal against bacteria and fluids, have antibacterial property, set in wet environment, be unaffected by blood contamination, and have reasonable compressive strength and hardness.[2] Mineral trioxide aggregate (MTA) was introduced in the field of dentistry by Torabinejad et al.3, which was used for pulp capping, perforation repair, apexification, root end filling material and pulpotomy. When compared with calcium hydroxide [(Ca(OH)2] as pulp capping agent, MTA forms faster, thicker and uniform dentinal bridge, possess less pulpal inflammation, lower solubility, better marginal adaptation and better sealing ability.-[4,5,6]
Mineral trioxide aggregate is a mineral powder that consists of hydrophilic particles, tricalcium silicate, dicalcium silicate, bismuth oxide, calcium dialuminate, and calcium sulfate dehydrate along with some trace elements such as iron, nickel, copper, and strontium.[7] Although MTA does not include Ca(OH)2 in its composition, after it hardens, it contains calcium oxide that could react with tissue fluids to form Ca(OH)2. Calcium hydroxide dissociates into calcium and hydroxyl ions, which increase pH value to approximately 12.5 creating an antibacterial environment. Released calcium ions lead to cell attachment and proliferation, encourage differentiation and migration of hard tissue producing cells, form hydroxyapatite on surface, and provide biologic seal.[4,6]
In the event of using composite resin to restore a tooth after pulp capping or furcal repair with MTA, a sandwich technique could be employed using glass ionomer cement (GIC). Currently resin-modified glass ionomer cement (RMGIC) has overcome the disadvantages of conventional GIC and hence is recommended.[8] Polyacrylic acid conditioner[9] is used to improve the bond strength of RMGIC to dentin, whereby the MTA that has been placed would also get exposed to the polyacrylic acid. There are few studies available in the current literature evaluating bond strength of RMGIC to conditioned and unconditioned MTA. Thus, the research hypothesis tested was that conditioning MTA with 10% polyacrylic acid would adversely affect the shear bond strength of RMGIC to MTA.
MATERIALS AND METHODS
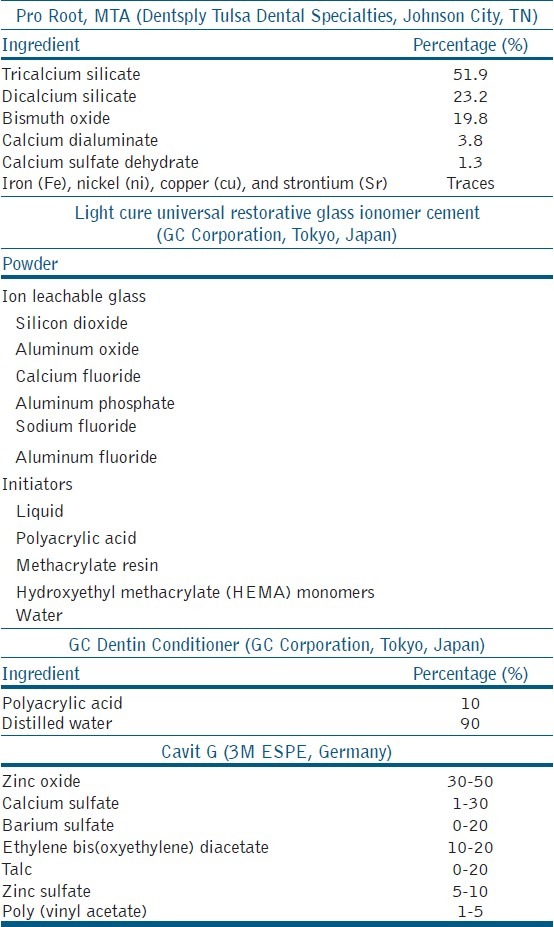
The materials used in the study were Pro Root MTA (DENTSPLY Tulsa Dental Specialties, Johnson City, TN), Light Cure Universal Restorative Glass Ionomer cement (GC Corporation, Tokyo, Japan), GC Dentin conditioner — 10% polyacrylic acid (GC Corporation, Tokyo, Japan), Cavit G (3M ESPE, Germany), cold cure acrylic (Acralyn R, India) composition as shown in Table 1.
Table 1.
Composition of materials used in the study
Preparation of MTA specimens
In all, 60 white mineral trioxide aggregate (WMTA) specimens were prepared by using cylindrical acrylic blocks. Each block had a central hole measuring 4 mm diameter and 2 mm depth. The WMTA powder was mixed with sterile water in a 3:1 powder to liquid ratio as per manufacturer's instructions, placed into the holes in the acrylic blocks, and covered with moist cotton pellet and a temporary filling material Cavit G (3M ESPE). These MTA-filled acrylic blocks were stored in an incubator for 72 hours at 37°C and 100% humidity. After removal of specimens from incubator, the temporary restoration and moist cotton pellet were removed, and then RMGIC was placed immediately.
Placement of the RMGIC
Specimens were divided in two groups (30 specimens each). In Group I before placement of RMGIC, the surface of WMTA was conditioned using 10% polyacrylic acid dentin conditioner (GC) for 20 seconds using a micro brush. The surface was then rinsed with water and dried by gentle blast of air. In Group II, the surface of MTA was left untreated.
Then, RMGIC was placed in the center of MTA surface by packing the material in plastic tubes which had an internal diameter of 3 mm and height 4 mm. The specimens were allowed to set for 10 minutes within the plastic tubes to ensure completion of the initial setting reaction of the RMGIC. The plastic tubes were then removed carefully and RMGIC was again cured for 10 seconds on each side. These specimens were stored at 37°C and 100% humidity for 24 hours to encourage setting in an incubator. All samples were prepared by the same operator.
Shear bond strength measurement
The specimens were mounted in an Instron Universal Testing Machine. A crosshead speed of 0.5 mm per minute was applied to each specimen by using a knife-edged blade, until the bond between the MTA and RMGIC failed.
Statistical analysis
Unpaired t-test was used to determine, if there were statistically significant differences between the shear bond strength values of conditioned and unconditioned groups.
RESULTS
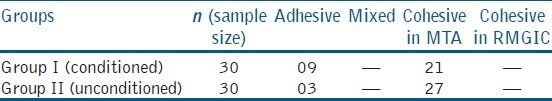
The highest shear bond strength values for conditioned and unconditioned groups were found to be 13.47 MPa and 15.89 MPa, respectively; the lowest value for conditioned was 2.84 MPa and for the unconditioned group was 2.85 MPa. The mean shear bond strength was calculated and found to be 6.59 MPa with standard deviation of 2.854 for the conditioned group (Group I) and 7.587 MPa with standard deviation 3.586 for the unconditioned group (Group II) as shown in Table 2. Figure 1 shows the individual shear bond strength values in MPa of the conditioned and unconditioned groups. Unpaired “t”-test revealed that the difference between the values of two groups at 95% confidence interval (CI) was not statistically significant (P > 0.05). The fracture modes of all samples are as represented in Table 3. Most of the observed modes of failures were cohesive in nature.
Table 2.
Representation of mean, standard deviation and range of group I and group II
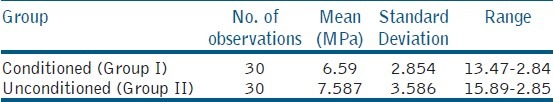
Figure 1.
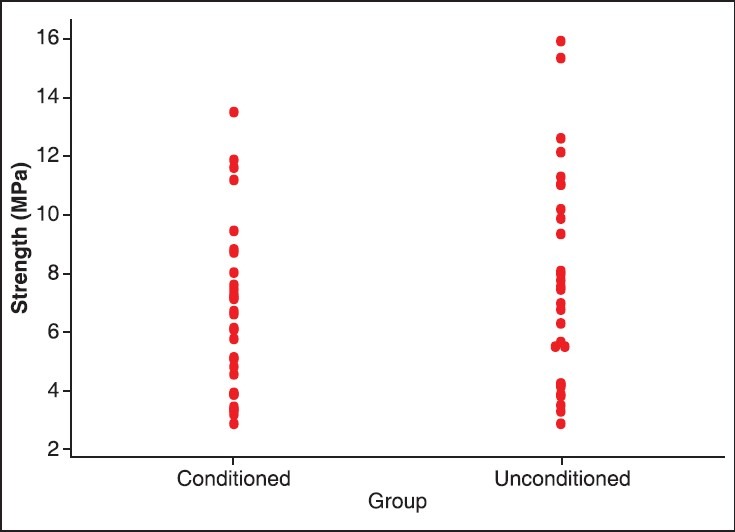
Individual value plot displaying all data values of conditioned and unconditioned group
Table 3.
Mode of failure of all specimens
DISCUSSION
Mineral Trioxide Aggregate has shown excellent potential in a variety of surgical and non-surgical endodontic applications. For the success of the restoration, the bond strength between two restorative materials is of significance. Shear bond strength is the measure of strength between two restorative materials, estimates the local stress that the bonding layer can withstand, and determines the integrity of the materials. High shear bond strength implies better bonding between two interfaces, provides favorable adhesion, and increases retention.[10] Another important clinical significance of high shear bond strength is its relation to microleakage. Higher the shear bond strength, lesser the microleakage.[11]
Pretreatment with 10% polyacrylic acid removes smear layer, leaving the smear plugs and a slightly demineralized dentin and hydroxyapatite around exposed collagen fibrils accessible for interaction. This results in chemical interaction of the carboxylic groups from RMGIC and calcium of hydroxyapatite crystals from dentin and mechanical bonding via hybrid layer formation between dentin and RMGICs.[12] In this study, half of the samples of MTA were conditioned to simulate clinical conditions where MTA surface comes in contact with conditioner while conditioning dentin before placement of RMGIC. Remaining half of the samples were not conditioned in order to compare the effect of conditioning on MTA. Conditioner is used during the placement of GIC. In vitro studies have demonstrated the effect of various acids on Portland cement. Lota et al demonstrated considerable changes in the microstructure of hydrated cement occurring in the presence of polyacrylic acid.[13] Rai et al reported that hydration of Portland cement was considerably retarded when malic acid was added.[14] In the presence of tartaric acid, the silicate hydration phase of Portland cement was retarded strongly.[15] Acid etching using 37% phosphoric acid affects the morphology of intact and the etched MTA surface. Under scanning electron microscopy (SEM) analysis, following etching, a selective loss of matrix from around the crystalline structures was observed. No needle-like crystals were seen over the etched surface. It was further stated that due to loss of matrix, the sealing ability and physical properties of MTA decreased.[2] Therefore, in situations like pulp capping or furcal repair, before the placement of RMGIC, the tooth surface needs to be conditioned; on the other hand any contact of polyacrylic acid with MTA may deteriorate the properties of MTA. It was observed that the hypothesis was rejected as conditioning did not alter the shear bond strength between the two groups tested.
In this study, the conditioner was placed as per the manufacturer's instructions. Washing and rinsing the conditioner did not have any effect on the setting of MTA as RMGIC was placed after 72 hours of placement of the MTA, within which time the MTA had set. Result showed that there was no difference in the shear bond strength of conditioned and unconditioned MTA to RMGIC. It was beyond the scope of the study to check the effect of polyacrylic acid on set MTA, at the microstructure level after 72 hours. In addition the results of the study are comparable with previous studies demonstrating shear bond strength of RMGIC to different substrates, such as 5-17 MPa, when RMGIC was bonded to dentin[12] and 5.286 MPa when RMGIC bonded to composite resin using a self-etch adhesive.[8]
The composition of the conditioner as mentioned by the manufacturer consists of only 10% polyacrylic acid, without any other component in it, which is similar to the ones mentioned in the literature.[9,10,12,16] Hence, it can be concluded that the shear bond strength of RMGIC to MTA has not been affected by any other active component in the conditioner other than 10% polyacrylic acid.
Table 3 shows mode of failure of all specimens. In this study, RMGIC was compacted on the surface of set MTA. Among 60 samples, 48 showed cohesive failure and 12 showed adhesive failures. The separation occurred mostly at the margin of the MTA and RMGIC. However, a section of MTA remained attached to the RMGIC indicating that the forces separating the material did not affect the bond strength between the two. One of the probable reasons could be the porous surface of MTA.[17] Secondly, it could be attributed to the withdrawal of water from the MTA into the glass ionomer. The lack of water in the MTA resulted in the inhibition of complete hydration of the material.[18]
Although we attempted to simulate clinical conditions, there are always limitations associated with any in vitro investigation. In all, 60WMTA specimens were prepared by using cylindrical acrylic blocks. Each block had a central hole measuring 4 mm diameter and 2 mm depth. The measurements were as followed by Yesilyurt et al.[17] and Bayrak et al.[1] A diameter of 4 mm was chosen to provide the surface area for the cylinder of resin-modified glass ionomer to be placed in the center of the MTA, to test for the shear bond strength. The depth of 2 mm was chosen as per standard in vitro studies[1,17] and also to simulate clinical situation, such as a perforation repair wherein a 2-mm-thick MTA needs to be placed, though the thickness of the material may vary. These specimens were then placed in a humidor for 72 hours a 37°C and 100% humidity, to mimic the intraoral conditions. Slyuyk et al. demonstrated that when MTA was used as a furcation perforation repair material, MTA resisted displacement at 72 hours to a significantly greater level than 24 hours.[19] The study kept the parameter of time constant for both groups. Despite these potential limitations and keeping other parameters constant, it was found that conditioning does not significantly affect the shear bond strength value between RMGIC and MTA.
CONCLUSION
Within the limitations of this study it can be concluded that
Shear bond strength value of RMGIC to unconditioned WMTA surface was slightly higher when compared with shear bond strength value of RMGIC to conditioned WMTA surface. However, statistical analysis revealed that the difference between the values of two groups was not statistically significant.
During clinical procedures like pulp capping and furcal repair, if RMGIC is placed as a base over MTA, then conditioning should be done to increase the bond strength between RMGIC and dentin and any inadvertent contact of conditioner with MTA will not significantly affect the shear bond strength value of RMGIC to MTA.
Based on the results of this study, RMGIC can be applied as a base over MTA beneath permanent restorations, when MTA is used for direct pulp capping and furcal repair.
The result of this study is not in agreement with the hypothesis tested.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bayark S, Tunc ES, Saroglu I, Egilmez T. Shear bond strengths of different adhesive systems to white mineral trioxide aggregate. Dent Mater J. 2009;28:62–7. [PubMed] [Google Scholar]
- 2.Kayahan MB, Nekoofar MH, Kazandag M, Canpolat C, Malkondu O, Kaptan F, et al. Effect of acid-etching procedure on physical properties of mineral trioxide aggregate. Int Endod J. 2009;42:1004–14. doi: 10.1111/j.1365-2591.2009.01610.x. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Pittford TR. The constitution of mineral trioxide aggregate. Dent Mater. 2005;21:297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Zarrabi MH, Javidi M, Jafarian AH, Joushan B. Histologic assessment of human pulp response to capping with mineral trioxide aggregate and novel endodontic cement. J Endod. 2010;36:1778–81. doi: 10.1016/j.joen.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Hakki SS, Bozkurt SB, Hakki EE, Belli S. Effects of mineral trioxide aggregate on cell survival, gene expression associated with mineralized tissues, and biomineralization of cementoblasts. J Endod. 2009;35:513–9. doi: 10.1016/j.joen.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Priv-Doz TD, Stratmann U, Wolff P, Sagheri D, Schafer E. Direct pulp capping with mineral trioxide aggregate: An immunohistologic comparison with calcium hydroxide in rodents. J Endod. 2010;36:814–9. doi: 10.1016/j.joen.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Belio-Reyes IA, Bucio L, Cruz-Chavez E. Phase composition of ProRoot Mineral Trioxide Aggregate by X-Ray powder diffraction. J Endod. 2009;35:875–8. doi: 10.1016/j.joen.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Arora V, Kundabala M, Parolia A, Thomas MS, Pai V. Comparison of the shear bond strength of RMGIC to a resin composite using different adhesive system. An in vitro study. J Conserv Dent. 2010;13:80–3. doi: 10.4103/0972-0707.66716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanumiharja M, Burrow MF, Tyas MJ. Microtensile bond strengths of glass ionomer (polyalkenoate) cements to dentine using four conditioners. J Dent. 2000;28:361–6. doi: 10.1016/s0300-5712(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 10.Suresh KS, Nagarathna J. Evaluation of shear bond strengths of Fuji II and Fuji IX with and without salivary contamination on deciduous molars: An in vitro study. Arch Oral Sci Res. 2011;1:139–45. [Google Scholar]
- 11.Wang L, Sakai VT, Kawai S, Buzalaf MA, Atta MT. Effect of adhesive systems associated with resin modified glass ionomer cements. J Oral Rehabil. 2006;33:110–6. doi: 10.1111/j.1365-2842.2006.01536.x. [DOI] [PubMed] [Google Scholar]
- 12.Mauro SJ, Sundfeld RH, Bedran-Russo AK, Fraga Briso AL. Bond strength of resin — modified glass ionomer to dentin: The effect of dentin surface treatment. J Minim Interv Dent. 2009;2:45–53. [Google Scholar]
- 13.Lota JS, Kendall K, Bensted J. Mechanism for the modification of Portland cement hydration using polyacrylic acid. Adv Cem Res. 2000;12:45–56. [Google Scholar]
- 14.Rai S, Chaturevedi S, Singh NB. Examination of Portland cement paste hydrated in the presence of malic acid. Adv Cem Res. 2004;34:455–62. [Google Scholar]
- 15.Rai S, Singh NB, Singh NP. Intraction of tartaric acid during hydration of Portland cement. Indian J Chem Tech. 2006;13:255–61. [Google Scholar]
- 16.Pereira PN, Yamada T, Tei R, Tagami J. Bond strength and interface micromorphology of an improved resin-modified glass ionomer cement. Am J Dent. 1997;10:128–32. [PubMed] [Google Scholar]
- 17.Yesilyurt C, Yildirim T, Tasdemir T, Kusgoz A. Shear bond strength of conventional glass ionomer cements bound to mineral trioxide aggregate. J Endod. 2009;35:1381–3. doi: 10.1016/j.joen.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri J. Scanning electron microscopic evaluation of the material interface of adjacent layers of dental materials. Dent Mater. 2011;27:870–8. doi: 10.1016/j.dental.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Sluyk SR, Moon PC, Hartwell GR. Evaluation of setting properties and retention characteristics of MTA when used as a furcation perforation repair material. J Endod. 1998;24:768–71. doi: 10.1016/S0099-2399(98)80171-4. [DOI] [PubMed] [Google Scholar]


