Abstract
Background:
Several disinfection techniques have been recently introduced with the main objective of improving root canal disinfection in the inaccessible areas of the root canal system. This in vitro study was done to evaluate the antimicrobial effect and viability of Enterococcus faecalis biofilms using conventional irrigation, EndoActivator (Dentsply, Tulsa Dental, USA), diode laser irradiation and photon-initiated photoacoustic streaming (PIPS).
Materials and Methods:
Root canals of 130 single rooted mandibular premolars, standardized to a uniform length of 20 mm were instrumented until finishing file, F1 (Universal Protaper Rotary System, Dentsply, Tulsa Dental Specialties, USA). After smear layer removal and sterilization, five teeth were randomly selected to assure sterility before bacterial inoculation. The remaining 125 samples were contaminated with E. faecalis suspension, incubated for 21 days and divided into five groups (n = 25). In Group 1; untreated group (positive control), the root canals were not subjected to any disinfection procedure. Sampling was performed within the canals and the colony-forming unit count was evaluated for 20 samples. Five samples were selected to visualize the pattern of colonization at Level 1 (4 mm from the apex) and Level 2 (1 mm from the apex) by confocal laser scanning microscopy. Samples in Groups 2-5 namely conventional needle irrigation, EndoActivator, diode laser and PIPS were subjected to their respective disinfection procedures. Postdisinfection sample evaluation criteria was followed for all groups as same as that for Group 1.
Results:
Diode laser displayed the highest antibacterial efficacy and least viable bacteria than the other three disinfection techniques.
Conclusion:
Diode laser group showed better antibacterial efficacy and least viable bacteria when compared to conventional needle irrigation, PIPS and EndoActivator groups in minimally instrumented, experimentally infected root canals.
Keywords: Confocal laser scanning microscopy, Enterococcus faecalis, disinfection, diode laser, root canal, minimal instrumentation
INTRODUCTION
Pulp and periapical diseases occur as a result of microorganisms invading into dental hard tissues and their further progression into the root canal system of the affected teeth. Therefore, the goal of root canal treatment is to eliminate the microorganisms from the root canal system and radicular dentin. After mechanical instrumentation, enormous areas of the root canal system remains untouched, regardless of the manual or rotary system used for cleaning and shaping.[1,2] Therefore, additional methods to the traditional treatment have been proposed in order to overcome the limitations of incomplete elimination of biofilm from the root canal system.[3]
Sodium hypochlorite (NaOCl) is widely used as an irrigant at concentrations ranging from 0.5% to 6%. It is a potent antimicrobial agent and effectively dissolves organic debris.[4] Irrigants have been traditionally delivered using a syringe and needle. The shortcoming with this technique is inadequate replacement of the irrigant throughout the root canal system.
Numerous irrigation regimens have been proposed to enhance the effectiveness of NaOCl. Such active irrigation has been showed to facilitate the disruption of biofilms and make the cell membrane of bacteria more permeable to NaOCl. EndoActivator (Dentsply, Tulsa Dental, USA) is a sonic device that uses a noncutting polymer tips to vigorously agitate the irrigant in the root canal.[5]
Various laser systems have been examined as adjuncts to currently used disinfection protocols as they can reach areas that are inaccessible with traditional techniques (e.g., bacteria located deeply in fins, isthmuses, lateral canals and in the dentinal tubules). Among the new tendencies in the field of lasers, diode laser is interesting because of its small dimensions of the delivery tips, low cost, optional power output ranges and easy operating modes such as continuous wave, pulsed power, and gated mode.[6,7]
Laser activated irrigation permits fluid interchange and removal of organic tissue and microbes from intricate canal anatomy, resulting in tubular disinfection.[8] Photon-induced photoacoustic streaming (PIPS) uses extremely low energy settings to create power spikes that generate a profound shock wave which travels three-dimensionally throughout the root canal system.[8]
The enlargement of the apical third of the root canal has been advocated to ensure an adequate depth of penetration of the irrigant for better cleansing. The drawbacks of larger apical preparations include undesirable deviation from the original shape of the canal, weakening of the root and procedural complications such as ledge formation, transportations, and perforations. The conservation of tooth structure and the preventing extrusion of obturating materials have been cited as primary advantages of minimal apical enlargements.[9]
This study was undertaken to determine and compare the antibacterial efficacy and viability of Enterococcus faecalis biofilms with needle irrigation, EndoActivator, diode laser and PIPS with erbium, chromium:yttrium, scandium, gallium, garnet (Er, Cr:YSGG) laser in minimally instrumented root canals using the colony-forming unit (CFU) count and confocal laser scanning microscopy (CLSM).
MATERIALS AND METHODS
One hundred and thirty single rooted human mandibular premolars were standardized to a uniform length of 20 mm. After determining the working length, the samples were instrumented to working length using Universal Protaper Rotary System (Dentsply, Tulsa Dental Specialties, USA) until finishing file, F1. After root canal instrumentation, the teeth were irrigated with 17% EDTA (Prime Dental Products Pvt. Ltd., India) for 1 min for smear layer removal, followed by a final rinse with 5% NaOCl (Nice Chemicals Pvt. Ltd., India) for 1 min and saline (Baxter, India). The samples were dried using paper points (Dentsply, Tulsa Dental, USA) and were sterilized in an autoclave (Confident Dental Equipments Pvt. Ltd., India) at 134°C for 17 min, stored in a sterile pouch until use. Five samples were randomly segregated to assure the sterility of the specimens before bacterial inoculation. The remaining 125 samples were inoculated with American Type Culture Collection 29212 E. faecalis suspension and incubated at 37°C for 21 days.[10]
The samples were randomly allocated into five groups (n = 25). The samples in Group 1: Untreated group (positive control) was not subjected to any disinfection procedure.
In Group 2: Conventional needle irrigation, the samples were irrigated with a 30-gauge side vented needle (Max-i-Probe, Dentsply, Tulsa Dental, USA) introduced 2 mm short of the working length and irrigated with 5% NaOCl for 1 min, rinsed with sterile saline. Following which the samples were irrigated with 17% EDTA for 1 min and rinsed with sterile saline. In Group 3: EndoActivator group, the irrigation protocol was the same as in Group 2 except that the ISO size 20 tip of the EndoActivator was used to activate the irrigant during the disinfection procedure. The tip was introduced 1 mm short of the working length during the activation procedure at 10,000 cycles/min. In Group 4: Diode laser group, after the irrigation protocol as in Group 2, the samples were irradiated with 940 nm diode laser (Ezlase 940, Biolase, California, USA) using a 200 μm tip introduced 1 mm short of the working length for 1 min at peak power of 3.5 W. In Group 5: PIPS group, the irrigation protocol was as in Group 2, except that the irrigant was activated by a 2940 nm Er, Cr:YSGG laser (Biolase, California, USA) at 20 Hz and 75 mJ using a 200 μm tip during the irrigation procedure. The tip was placed in the coronal reservoir only during activation.[8]
After the disinfection procedures, 20 samples from each group were subjected to evaluation of CFU count and 5 samples were subjected to CLSM for viability assessment. For CFU evaluation, 100 μl of sterile saline was deposited within the canal, and agitated with an ISO size 10 K-file (Dentsply Maillefer, Ballaigues, USA). Sterile paper point was introduced to working length, allowed to saturate and placed in sterile vials containing 2 ml of brain-heart infusion (BHI). After vortexing for 20 s, ten-fold serial dilution were prepared in saline, 100 μl were inoculated onto BHI plates (Biomerieux, Firenze, Italy), incubated at 37°C for 48 h and CFU count was evaluated using colony counter (Hi-Media, India) followed by transformed into actual counts based on the known dilution factors.
Five samples were subjected to CLSM for evaluation of viability of bacteria at 4 mm (Level 1) and 1 mm (Level 2) from the apex of the root. For CLSM, the samples were stained with fluorescent stains, SYTO 9 and Propidium Iodide (BaclightTM, Carlsbad, CA, USA) and the coronal ends were mounted onto methacrylate blocks. The preparation was cut into two evenly distributed transverse sections 1 mm from the apical most portions as well as from the 4 mm level using a hard tissue microtome (Leica SP 1600, Hard Tissue Microtome, Germany). Sections were examined by CLSM (LSM 510 META NLO, Axiovert 200; Carl Zeiss Ltd., Jena, Germany) and the fluorescent images were analyzed with Amira 5.0 and the image stacks were viewed with LSM browser. The initial stacks, comprising both green and red fluorescence were split into individual component color channels and saved as grayscale image and calibrated to define voxel size. The total fluorescence in each optical slice was obtained by adding the number of voxels calculated from the grayscale images of the respective red and green channels. Bacterial survival was then expressed as the proportion (%) of green voxels from the total fluorescence[11,12] [Figure 1].
Figure 1.
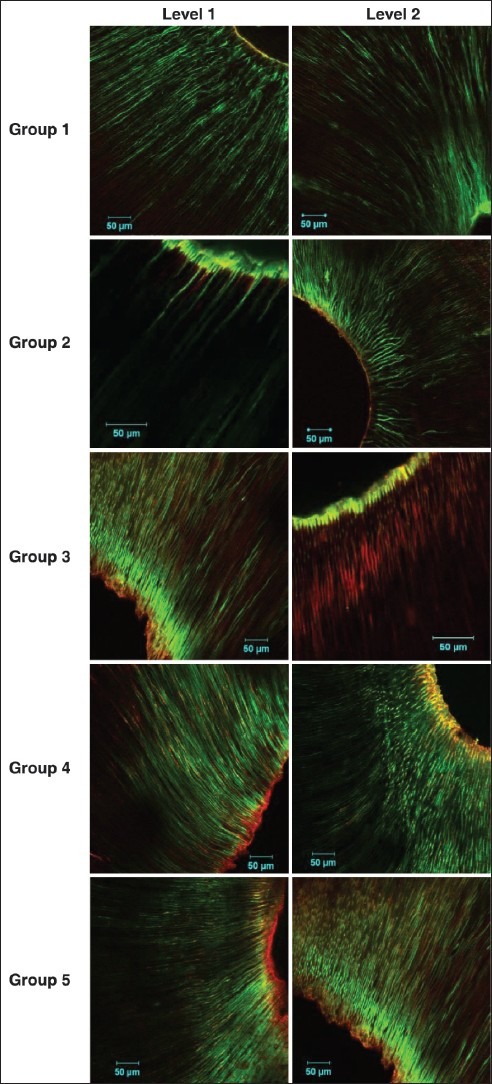
Confocal laser scanning microscopy images for Groups 1-5 at Levels 1 and 2
Statistical analysis
Data were analyzed statistically using Statistical Package for Social Sciences, (SPSS) version-10 software for windows. Data were expressed in its mean and standard deviation and were analyzed using Kruskal–Wallis ANOVA and post-hoc Mann–Whitney U-test.
RESULTS
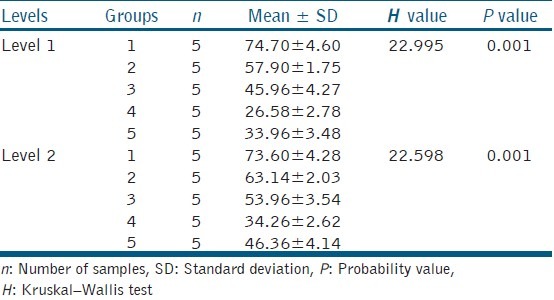
There was a significant reduction in the CFU count for all the techniques tested when compared with the positive group (3.35 ± 0.42 × 106 CFU/ml) (P < 0.005, Bonferroni correction). Diode laser displayed the highest antibacterial efficacy (0.73 ± 0.22 × 106 CFU/ml) and conventional needle irrigation (2.45 ± 0.22 × 106 CFU/ml) least [Table 1]. There was statistically significant difference in the viability of bacteria at Levels 1 and 2 between the groups (P < 0.005). Pair-wise comparison revealed statistically significant difference in viability between diode laser and positive control. Viability of bacteria at Level 2 was found greater than of Level 1 for all the four disinfection techniques used [Table 2].
Table 1.
Intergroup comparison of CFU count between five groups
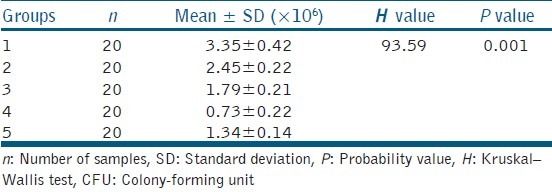
Table 2.
Comparison of viability of bacteria at Levels 1 and 2 between five groups
DISCUSSION
The four different disinfection techniques evaluated in this study were conventional needle irrigation, EndoActivator, diode laser, and PIPS. A minimal instrumentation of ISO size 20 was chosen as the apical preparation limit in this study. Although the taper of 7° in the apical 4 mm of Protaper F1 file may suffix adequate mechanical preparation and enhance the cleaning action of irrigants, it may not correspond to the apical width of mandibular premolars. This discrepancy has no role in this study as the aim of this study was to evaluate the bacterial reduction in minimally instrumented canals using recent disinfection techniques.
In conventional needle irrigation, smaller gauged cannulas are chosen to achieve deeper and more effective placement. In this study, ISO size 20 cannula were used, and the irrigation protocol was a combination of 5% NaOCl and 17% EDTA. Conventional needle irrigation has limitations because a static reservoir of irrigant restricts the potential for any irrigant to penetrate, circulate, and clean into all aspects of the root canal system.[13,14,15] Kouchi et al. have shown that bacteria colonize the periluminal dentine up to a depth of 1100 μ. Chemical disinfectants penetrate only by 100 μ into the dentine, as indicated by Berutti et al.[16,17] This could be the reason for the lower antibacterial efficiency in this study.
The EndoActivator device is a form of active irrigation. Its primary function is to produce vigorous intracanal fluid agitation through acoustic streaming and cavitation. When cavitation bubbles are produced by acoustic waves, they eventually collapse and the energy released is transferred to the root canal, detaching debris found within,[3,4,5] which could be the reason for the reduction of bacteria within the root canals in the present study. However, the performance of subsonic agitation appears to be less effective than diode laser and PIPS group. This may be attributed to the different acoustic streaming velocity and frequency, which positively influence debris removal from the qualitative standpoint. EndoActivator only produces sonic waves.[13] Sonic energy only has the power to produce one node along the length of the instrument, so any constraint of the instrument will eliminate or significantly decrease the acoustic streaming necessary to dislodge and carry away necrotic debris.
The superior bactericidal effect of diode laser irradiation in the present study could be attributed to its greater depth of penetration (up to 1000 μm into the dentinal tubules) when compared to the penetration power of chemical disinfectants, which is limited to 100 μm. It has been found that with progressive decrease in diameter of the deep dentinal tubules, the penetration of irrigants is restricted. However, laser irradiation with its inherent properties of light scattering, local intensity enhancement and attenuation allows light penetration deeper in the dentinal tubules contributing to a superior antimicrobial efficacy. The diode laser causes a thermal photodisruptive action in the unreachable parts of dentin, resulting in enhanced bactericidal effect in root canal dentin.[3,7,18,19]
The reduction of bacterial load by PIPS in this study can be due to the strong modulation in the reaction rate of NaOCl, significantly increasing the production and consumption of available chlorine and oxygen ions.[20,21] They cause photomechanical streaming of fluids with the formation of a cavity containing bubbles inside a fluid (cavitation). This process can allow irrigants to access otherwise inaccessible areas more easily. In addition, the cavitation bubbles expand, become unstable and then collapse (implosion). This implosion will have an impact on the surfaces of the root canal, causing shear forces, surface deformation and the removal of surface material. Each impulse, absorbed by the water molecules, creates a strong shock wave that leads to the formation of effective streaming of fluids inside the canal. The bactericidal effect of laser radiation with a wavelength of 2.78 μm is based on the high absorption constant for OH− groups and H2 O, which is in the order of 5000/cm, destroying E. faecalis when energy is absorbed into the volume of the bacterium.[22,23,24]
Lasers with shorter emission wavelengths like diode can be designed with flexible glass fiber emission tips. The Er, Cr:YSGG exhibits challenges because their large wavelength do not fit into the crystalline molecule of conducting glass.[25] Difference in the physical parameters (wavelength, peak power, and mode) and emission tips between the two laser radiations could have attributed to the greater disinfection efficacy of diode laser.
The CFU evaluation methodology's level of sensitivity was believed to be insufficient for detecting the possible viable cells in the lower concentration. The CLSM analysis used in this study is a convenient, accurate and reproducible method for localizing and quantifying live and dead bacteria within dentinal tubules. They provide information about the severity of dentine infection and vitality of bacteria within the dentinal tubules at the cellular level. They are promising in elucidating the significance of the residual bacteria in uncultivable state.[26,27]
CONCLUSION
Diode laser group showed better antibacterial efficacy and least viable bacteria when compared to conventional needle irrigation, PIPS and EndoActivator groups in minimally instrumented, experimentally instrumented root canals.
PIPS and EndoActivator groups showed promising antibacterial activity and less viable bacteria than conventional needle irrigation; hence, they can be considered as alternatives to diode laser group in eliminating microorganisms from infected root canals.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Meire MA, Coenye T, Nelis HJ, De Moor RJ. Evaluation of Nd:YAG and Er:YAG irradiation, antibacterial photodynamic therapy and sodium hypochlorite treatment on Enterococcus faecalis biofilms. Int Endod J. 2012;45:482–91. doi: 10.1111/j.1365-2591.2011.02000.x. [DOI] [PubMed] [Google Scholar]
- 2.Bago I, Plečko V, Gabrić Pandurić D, Schauperl Z, Baraba A, Anić I. Antimicrobial efficacy of a high-power diode laser, photo-activated disinfection, conventional and sonic activated irrigation during root canal treatment. Int Endod J. 2013;46:339–47. doi: 10.1111/j.1365-2591.2012.02120.x. [DOI] [PubMed] [Google Scholar]
- 3.Anjanyelu K, Nivedita S. Influence of calcium hydroxide on the post treatment pain in endodontics. A systematic review. J Conserv Dent. 2014;17:200–7. doi: 10.4103/0972-0707.131775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasqualini D, Cuffini AM, Scotti N, Mandras N, Scalas D, Pera F, et al. Comparative evaluation of the antimicrobial efficacy of a 5% sodium hypochlorite subsonic-activated solution. J Endod. 2010;36:1358–60. doi: 10.1016/j.joen.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Huffaker SK, Safavi K, Spangberg LS, Kaufman B. Influence of a passive sonic irrigation system on the elimination of bacteria from root canal systems: A clinical study. J Endod. 2010;36:1315–8. doi: 10.1016/j.joen.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Marchesan MA, Brugnera-Junior A, Ozorio JE, Pécora JD, Sousa-Neto MD. Effect of 980-nanometer diode laser on root canal permeability after dentin treatment with different chemical solutions. J Endod. 2008;34:721–4. doi: 10.1016/j.joen.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Kaiwar A, Usha HL, Meena N, Ashwini P, Murthy CS. The efficiency of root canal disinfection using a diode laser: In vitro study. Indian J Dent Res. 2013;24:14–8. doi: 10.4103/0970-9290.114916. [DOI] [PubMed] [Google Scholar]
- 8.Peters OA, Bardsley S, Fong J, Pandher G, Divito E. Disinfection of root canals with photon-initiated photoacoustic streaming. J Endod. 2011;37:1008–12. doi: 10.1016/j.joen.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Saini HR, Tewari S, Sangwan P, Duhan J, Gupta A. Effect of different apical preparation sizes on outcome of primary endodontic treatment: A randomized controlled trial. J Endod. 2012;38:1309–15. doi: 10.1016/j.joen.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Castelo-Baz P, Martín-Biedma B, Ruíz-Piñón M, Rivas-Mundiña B, Bahillo J, Seoane-Prado R, et al. Combined Sodium Hypochlorite and 940 nm Diode Laser Treatment Against Mature E. Faecalis Biofilms in-vitro. J Lasers Med Sci. 2012;3:116–21. [Google Scholar]
- 11.Parmar D, Hauman CH, Leichter JW, McNaughton A, Tompkins GR. Bacterial localization and viability assessment in human ex vivo dentinal tubules by fluorescence confocal laser scanning microscopy. Int Endod J. 2011;44:644–51. doi: 10.1111/j.1365-2591.2011.01867.x. [DOI] [PubMed] [Google Scholar]
- 12.Zapata RO, Bramante CM, de Moraes IG, Bernardineli N, Gasparoto TH, Graeff MS, et al. Confocal laser scanning microscopy is appropriate to detect viability of Enterococcus faecalis in infected dentin. J Endod. 2008;34:1198–201. doi: 10.1016/j.joen.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Ruddle CJ. Endodontic disinfection: Tsunami irrigation. Endod Pract. 2008;11:7–15. [Google Scholar]
- 14.Luddin N, Ahmed HM. The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. J Conserv Dent. 2013;16:9–16. doi: 10.4103/0972-0707.105291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul ML, Mazumdar D, Niyogi A, Baranwal AK. Comparative evaluation of the efficacy of different irrigants including MTAD under SEM. J Conserv Dent. 2013;16:336–41. doi: 10.4103/0972-0707.114367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berutti E, Marini R, Angeretti A. Penetration ability of different irrigants into dentinal tubules. J Endod. 1997;23:725–7. doi: 10.1016/S0099-2399(97)80342-1. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi Z. Sodium hypochlorite in endodontics: An update review. Int Dent J. 2008;58:329–41. doi: 10.1111/j.1875-595x.2008.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 18.Preethee T, Kandaswamy D, Arathi G, Hannah R. Bactericidal effect of the 908 nm diode laser on Enterococcus faecalis in infected root canals. J Conserv Dent. 2012;15:46–50. doi: 10.4103/0972-0707.92606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Souza EB, Cai S, Simionato MR, Lage-Marques JL. High-power diode laser in the disinfection in depth of the root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:e68–72. doi: 10.1016/j.tripleo.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Ordinola-Zapata R, Bramante CM, Aprecio RM, Handysides R, Jaramillo DE. Biofilm removal by 6% sodium hypochlorite activated by different irrigation techniques. Int Endod J. 2013;7:659–66. doi: 10.1111/iej.12202. [DOI] [PubMed] [Google Scholar]
- 21.Olivi G, DiVito E. Photoacoustic endodontics using PIPS TM: experimental background and clinical protocol. J Laser Health Acad. 2012;1:22–5. [Google Scholar]
- 22.Dewsnup N, Pileggi R, Haddix J, Nair U, Walker C, Varella CH. Comparison of bacterial reduction in straight and curved canals using erbium, chromium:yttrium-scandium-gallium-garnet laser treatment versus a traditional irrigation technique with sodium hypochlorite. J Endod. 2010;36:725–8. doi: 10.1016/j.joen.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Franzen R, Esteves-Oliveira M, Meister J, Wallerang A, Vanweersch L, Lampert F, et al. Decontamination of deep dentin by means of erbium, chromium:yttrium-scandium-gallium-garnet laser irradiation. Lasers Med Sci. 2009;24:75–80. doi: 10.1007/s10103-007-0522-2. [DOI] [PubMed] [Google Scholar]
- 24.Licata ME, Albanese A, Campisi G, Geraci DM, Russo R, Gallina G. Effectiveness of a new method of disinfecting the root canal, using Er, Cr:YSGG laser to kill Enterococcus faecalis in an infected tooth model. Lasers Med Sci. 2013 Aug 6; doi: 10.1007/s10103-013-1410-6. [Epub ahead of print] PubMed PMID: 23917414. [DOI] [PubMed] [Google Scholar]
- 25.Meire M, De Moor R. Lasers in endodontics: laser disinfection, an added value.? Endodontic Practice Today. 2007;1:159–72. [Google Scholar]
- 26.Zaura-Arite E, van Marle J, ten Cate JM. Conofocal microscopy study of undisturbed and chlorhexidine-treated dental biofilm. J Dent Res. 2001;80:1436–40. doi: 10.1177/00220345010800051001. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Wang Z, Shen Y, Haapasalo M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod. 2011;37:1380–5. doi: 10.1016/j.joen.2011.06.018. [DOI] [PubMed] [Google Scholar]


