Abstract
Aim:
To evaluate the cytotoxicity of three root repair materials, mineral trioxide aggregate (MTA), Endosequence Root Repair Material and Biodentine in human periodontal ligament fibroblasts.
Materials and Methods:
Periodontal ligament fibroblasts were cultured from healthy premolar extracted for orthodontic purpose. Cells in the third passage were used in the study. The cultured fibroblast cells were placed in contact with root repair materials: (a) Biodentine, (b) MTA, (c) Endosequence, (d) control. The effects of these three materials on the viability of Periodontal ligament (PDL) fibroblasts were determined by trypan blue dye assay after 24 hours and 48-hour time period. Cell viability was determined using inverted phase contrast microscope.
Statistical Analysis:
Cell viability was compared for all the experimental groups with Wilcoxons matched pair test.
Results:
At the 24-hour examination period, all the materials showed increased cell viability. At 48-hour time period, there is slight decrease in cell viability. Mineral trioxide aggregate showed statistically significant increase in the cell viability when compared to other root repair materials.
Conclusion:
Mineral trioxide aggregate was shown to be less toxic to periodontal ligament fibroblasts than Endosequence Root Repair Material and Biodentine.
Keywords: Biocompatibility, periodontal ligament fibroblasts, root repair material
INTRODUCTION
An annual estimation of endodontic procedures suggests that approximately 5.5% of all treatments performed involve apical root-end surgery and root perforation repair.[1] The aim of the surgical procedures is to correct problems and successfully eliminate inflammatory processes that would not otherwise be successfully treated with non surgical root canal treatment. Iatrogenic complications that can occur during the non-surgical root canal treatment might also be treated by surgical means. Whether the aim is to place a retrofill material or repair an iatrogenic procedural accident, the material used should demonstrate the ability to form a seal with the dentin and maintain biocompatibility with the periapical tissues.[2] An ideal root repair material should have the ability to adhere to dentin, maintain a sufficient seal, be insoluble in tissue fluids, be dimensionally stable, non-resorbable over time, radiopaque, easily manipulated, adequate compressibility, adequate working time, quick setting time, and be biocompatible with human tissues.[3] Numerous materials have been advocated as root repair materials including amalgam, zinc oxide-eugenol-based cements such as Super ethoxy benzoic acid (EBA) and intermediate restorative material (IRM), Cavit, composite resins, glass ionomer cements, and Portland-based cements.[4]
During pulp capping, perforation repair, and retrograde filling, the cytotoxicity of the material used may influence the viability of periradicular cells and cause cell death by apoptosis or necrosis. To promote healing and restoration of the function of the tooth, dental materials should either stimulate repair or be biologically neutral. Therefore, it is important to avoid dental materials that are toxic to the pulpal and periapical tissues that might compromise the clinical outcome.[5]
Although mineral trioxide aggregate (MTA) as root-end filling material and root repair material has gained widespread use since its introduction into the endodontic market, it is not an easy material to handle. A major drawback of MTA is the inability to obtain consistent results when mixed according to the manufacturer's directions. This results in difficulty while placing the product during treatment. The second most disadvantage of MTA (ProRoot MTA) is the long setting time. Efforts have been made to overcome these shortcomings. However, introducing new compositions of MTA or using various additives can affect MTA's ideal characteristics and should await comprehensive investigations.[6]
Brasseler USA (Savannah, GA) has recently introduced the Endosequence Root Repair Material (RRM) which uses bioceramic technology. Bioceramic material refers to the combination of calcium silicate and calcium phosphate that is applicable for dental use. This material is available as a premixed product to provide the clinician with a homogeneous and consistent material.[6,7]
Biodentine (Septodont) is also a bioceramic material with the addition of calcium chloride as a setting accelerant, available in premixed capsules and designed to set within 10-12 minutes. The powder is tricalcium silicate, with the addition to the powder of calcium carbonate (CaCO3) and Zirconium oxide (ZrO2 ). The liquid is a solution of calcium chloride (CaCl2 ) with a water-reducing agent. Advantages of this material are chemico-mechanical bonding with tooth and composite, high compressive and flexure strength. This material has demonstrated superior dentin calcium uptake when compared with MTA.[8]
The purpose of this study was to evaluate the cytotoxic effect of three commercially available root repair materials, MTA, EndoSequence Root Repair Material and Biodentine using human periodontal ligament fibroblasts.
MATERIALS AND METHODS
Cell culture Preparation
In this study, PDL fibroblasts were obtained from the healthy premolar extracted for orthodontic purpose. Atraumatically extracted tooth was washed in phosphate buffer solution. The method of preparation of cell cultures was previously described by Melahat, Goduysus et al.,[9] which was followed in this study. Tissue was detached using 5ml of 0.125% trypsin and 0.1% collagenase for 10 minutes and then incubated at 37°C for 1 hour. The specimens were incubated with culture medium. The culture medium was alpha minimal essential medium (α MEM) supplemented with 1 ml of 10% fetal calf serum, 50 μg/ml streptomycin, and 3 μg/ml of Amphotericin. Cell pellet was collected by centrifuging the supernatant obtained. Cell cultures were grown in 60-mm culture dish containing culture medium. The tissue samples were grown at 37°C in a humidified atmosphere of 5% carbon dioxide in air. Experiments were conducted by using third passage cells. Exponentially growing cells were seeded in 24-well plates at a density of total of 1.5 × 104 cells per well.
Preparation of test materials
Endosequence Root Repair Material was available as packaged premixed by the manufacturer and do not require preparation before use. MTA and Biodentine were mixed according to the manufacturer's instructions.
Group-I: Biodentine
Group-II: MTA
Group-III: Endosequence
Group-IV: Control, (α MEM culture medium)
Cytotoxicity evaluation with trypan blue
All the materials taken were standard amounts of 1 milligram (mg) of test material per 5 milliliters (ml) of culture medium and placed in 24-well polycarbonate transwells insert. So that in each well plate 1 ml is placed. After incubation period of 24 hours (hrs) and 48 hrs, cell viability was evaluated by trypan blue dye exclusion assay; 20 microliter (μl) of trypan blue was mixed to 20 μl of treated cellular suspension, spread onto a hemocytometer. The cell number was determined by counting the cells in a hemocytometer under inverted phase contrast microscope. Cytotoxicity of the various root repair materials to PDL fibroblasts was measured. Nonviable cells appear blue-stained. The percentage of viable cells is the number of viable cells divided by the number of total cells multiplied with 100.[10]
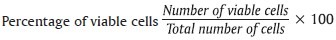
Statistical analysis
All the assays were repeated three times to ensure reproducibility. Mean and standard deviation were calculated for all groups. Cell viability was compared for all the experimental groups with Wilcoxons matched pair test.
RESULTS
Human periodontal ligament fibroblasts were treated with test materials in culture medium for 24 hrs and 48 hrs. The results were summarized in Table 1. After 24-hour time period, MTA, Endosequence, Biodentine, and control group resulted in cell viabilities of 94.26%, 86.19%, 85.97%, and 96.17%. After 48-hour time period, Endosequence, MTA, Biodentine, and control group resulted in cell viabilities of 82.94%, 82.31%, 81.81%, and 93.34%. Table 2 showed that all the test materials showed no statistical significant difference between them (P > 0.05). There was statistically significant difference between the control group and test materials (P < 0.05). Control group showed increased cell viability than all the experimental groups at both 24 hrs and 48 hrs. There is increased cell viability for all the groups at 24 hrs than at 48 hrs time period. Statistical analysis of the data showed no significant difference between the three test materials at all experimental periods (24 hrs and 48 hrs) [Figure 1] (P > 0.05).
Table 1.
Comparison of cell viability at 24-hr and 48-hr exposure to three root repair materials by Wilcoxon matched pairs test
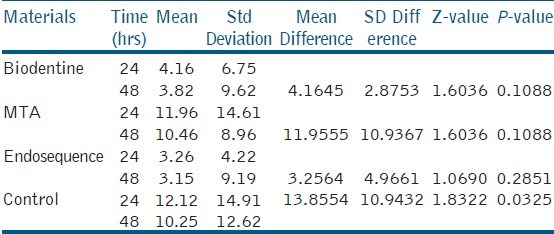
Table 2.
Mean comparison between different groups at 24 hrs and 48 hrs by unpaired t-test
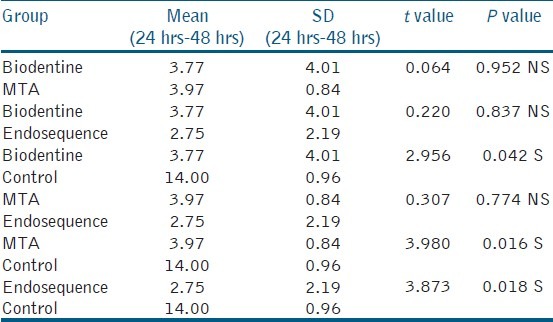
Figure 1.
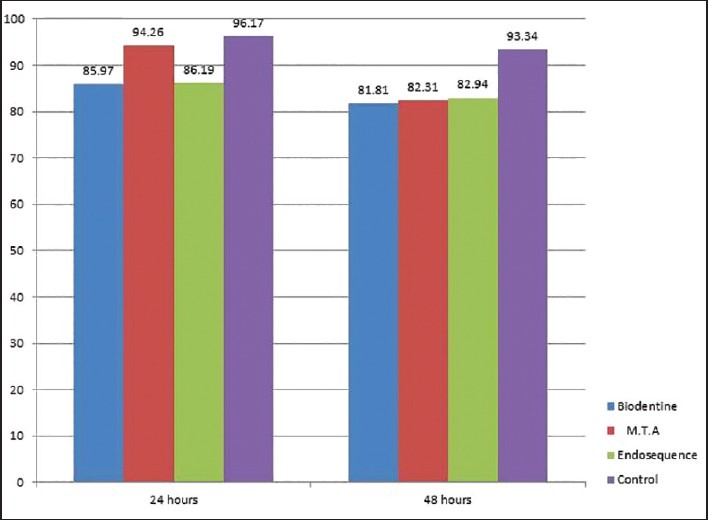
Comparison of percentage of cell viability at 24 hrs and 48 hrs exposure to root repair materials
DISCUSSION
Root canal perforation is an undesirable incident that can occur at any stage of root canal therapy. Although caries or resorptive processes may cause perforations, most root perforations are induced iatrogenically.[11] A perforation, irrespective of location or etiology, hampers the prognosis of endodontic therapy.[12] According to the Washington study, root perforations result in endodontic failures accounting for approximately 10% of all failed cases. It has been speculated that the important factors in determining the success of a perforation repair procedure are the location of the perforation, time lapse between the occurrence of the perforation and repair, the ability of the material to seal the perforation site, and the biocompatibility of the repair material.[11] A root repair material is placed in intimate contact with the periodontium and thus must be nont-oxic and biocompatible with host tissues.[8] To date, a variety of methods, including in vivo and in vitro systems, have been used to evaluate the biocompatibility of filling materials.
Biodentine has been promoted as a dentin substitute, which can also be used as an endodontic repair material.[13] This can also stimulate cells growth and induce hydroxyapatites (Has) formation on the surface of the material when exposed to the simulated body fluid.[14]
Endosequence Root Repair Material Paste (ERRM Paste; Brasseler USA) has been developed as ready-to-use, premixed bioceramic materials recommended for perforation repair, apical surgery, apical plug, and pulp capping. They are excellent physical and biological properties and are easy to work with; they are hydrophilic, insoluble, radiopaque, aluminum-free, and of high pH.[6]
The cytotoxicity of MTA has been investigated in many studies. Several in vitro studies using MTA showed a similarly good biocompatibility. MTA was biocompatible when tested with human periodontal ligament fibroblasts for cell viability, apoptosis, and mitochondrial dehydrogenase activity.[15,16] As there is limited literature for regarding biocompatibility of Biodentine and Endosequence, in this study, the biocompatibility of Biodentine and Endosequence was evaluated in comparison with MTA. Several methods and strategies are available for cytotoxicity testing of materials, each with its merits and limitations.
Primary cell strains derived from living tissues are necessary for specific sensitivity testing, we chose human periodontal ligament fibroblasts to simulate the clinical environment. Assays to measure proliferation, viability, and cytotoxicity are commonly used to monitor the response and health of cells in cultures. Cell counting using viability dyes such as trypan blue or calcein-AM can provide the percentage of viable cells. Cell cultures provide a convenient, controllable, and reproducible instrument for the preliminary evaluation of biological response. The results obtained from in vitro assays might be indicative of the effects observed in vivo. In vivo testing of dental materials may be influenced by skill of the dentist, technical properties of the material, and uncontrollable patient factors. Furthermore, in vitro tests are simple to perform, repeatable, cost-effective, relevant, and suitable as an alternative to in vivo experiments. The trypan blue exclusion test can be used to indicate cytotoxicity, where dead cells take up the blue stain of trypan blue.
In this study, in vitro cytotoxic effects of three root repair materials on PDL fibroblasts were analyzed after 24- and 48-hour incubation periods. The results showed that MTA showed increased cell viability than Endosequence and Biodentine at 24-hour exposure. At 48-hour exposure Endosequence root repair material showed increased cell viability than MTA and Biodentine. However, there were not any statistically significant differences among the MTA, Biodentine and Endosequence and at different time intervals. The results were in accordance with the study conducted by Amer Z Al, Anezi et al. with mouse fibroblasts has shown that the cell viability of Endosequence root repair material is comparable to both gray and white ProRoot MTA.[17] Endosequence root repair material has shown less cell viability than MTA at 24 hrs, which was in accordance to the study conducted by Damas et al.[2] Hui min Zhou et al. evaluated the cytotoxicity of MTA and Biodentine and stated that there was no significant difference between the cell viabilities of fibroblasts exposed to the materials, the results of this study correlated with this regarding MTA and Biodentine.[18]
CONCLUSION
Within the limitations of this study, it was observed that MTA showed good cell viability when compared with other cell viable materials like Endosequence and Biodentine. This study supports the view that MTA is a better biocompatible root repair material. Further investigations for Endosequence and Biodentine should be carried out by using genotoxicity, sensitization, and tissue implantation tests to establish a more general outlook on the clinical application of these materials. Along with the periodontal ligament fibroblasts, other cell lines such as mesenchymal cells, osteoblasts, and osteoclasts should also be evaluated to assess the effect of irrigants.
ACKNOWLEDGEMENTS
We sincerely thank Dr. Ranganathan M.D.S (Professor), Oral and Maxillofacial Pathology, RAGAS Dental College, Chennai, Ms. Jayanthi Sampath, Chennai Research foundation, Chennai and Dr. Kiran M.D.S (Professor), Oral and Maxillofacial Pathology, SIBAR Institute of Dental Sciences, Guntur, for their cooperation, valuable support and encouragement for completing the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Testori T, Capelli M, Milani S, Weinstein RL. Success and failure in periradicular surgery: a longitudinal retrospective analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:493–8. doi: 10.1016/s1079-2104(99)70250-7. [DOI] [PubMed] [Google Scholar]
- 2.Torabinejad M, McDonald NJ. Endodontic surgery. In: Torabinejad M, Walton RE, editors. Endodontics: Principles and Practice. 4th ed. St Louis: Mosby; 2009. pp. 357–75. [Google Scholar]
- 3.Damas BA, Wheater MA, Bringas JS, Hoen MM. Cytotoxicity comparison of mineral trioxide aggregates and endosequence bioceramic root repair materials. J Endod. 2011;37:372–5. doi: 10.1016/j.joen.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Torabinejad M, Chivan N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 5.Witold B, Jadwiga M, Ewa K, Elzbieta A. Cytotoxicity and mutagenicity of N2 cement - Root canal filling material. Adv Clin Exp Med. 2009;18:615–21. [Google Scholar]
- 6.Ma J, Shen Y, Stojicic S, Haapasalo M. Biocompatibility of two novel root repair materials. J Endod. 2011;37:793–8. doi: 10.1016/j.joen.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Lieutenant Commander Jeffrey Hilley, Colonel Kathleen McNally. Bioceramics in Endodontics. Clin Update. 2013:35. [Google Scholar]
- 8.Aggarwal V, Singla M, Miglani S, Kohli S. Comparative evaluation of push-out bond strength of ProRoot MTA, Biodentine, and MTA Plus in furcation perforation repair. J Conserv Dent. 2013;16:462–5. doi: 10.4103/0972-0707.117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorduysus M, Avcu N, Gorduysus O, Pekel A, Baran Y, Avcu F, et al. Cytotoxic effects of four different endodontic materials in human periodontal ligament fibroblasts. J Endod. 2007;33:1450–4. doi: 10.1016/j.joen.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Nagesh B, Sivavenkata MN, Sujana, Kumar KK, Ranganathan, Sarath R. Cytotoxic evaluation of two chlorine releasing irrigating solutions on cutured human periodontal ligament fibroblasts. J NTR Univ Health Sci. 2013;2:42–6. [Google Scholar]
- 11.Main C, Mirzayan N, Shabahang S, Torabinejad M. Repair of root perforations using mineral trioxide aggregate: A long-term study. J Endod. 2004;30:80–3. doi: 10.1097/00004770-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Torabinejad M, Parirokh M. Mineral trioxide aggregate: A comprehensive literature review-part II: Leakage and biocompatibility investigations. J Endod. 2010;36:190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Camps J, Pashley D. Reliability of the dye penetration studies. J Endod. 2003;29:592–4. doi: 10.1097/00004770-200309000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Tulsi S, Nimisha S, Ruchi R. Comparative analysis of sealing ability of biodentin and calcium phosphate cement against mineral trioxide aggregate (MTA) as a furcal perforation repair material. NJIRM. 2013;4:56–9. [Google Scholar]
- 15.Kokate SR, Pawar AM. An in vitro comparative stereomicroscopic evaluation of marginal seal between MTA, glass inomer cement and biodentine as root end filling materials using 1% methylene blue as tracer. Endodontology. 2012;24:36–42. [Google Scholar]
- 16.Lee SJ, Monsef M, Torabinejad M. Sealing ability of mineral trioxide aggregate for repair of lateral root perforation. J Endod. 1993;19:541–4. doi: 10.1016/S0099-2399(06)81282-3. [DOI] [PubMed] [Google Scholar]
- 17.Alanezi AZ, Jiang J, Safavi KE, Spangberg LS, Zhu Q. Cytotoxicity evaluation of endosequence root repair material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e122–5. doi: 10.1016/j.tripleo.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Zhou HM, Shen Y, Wang ZJ, Li L, Zheng YF, Häkkinen L, et al. In vitro cytotoxicity evaluation of a novel root repair material. J Endod. 2013;39:478–83. doi: 10.1016/j.joen.2012.11.026. [DOI] [PubMed] [Google Scholar]


