Abstract
Acetaminophen (APAP) overdose is a major cause of acute liver failure (ALF). Numerous studies have shown that APAP hepatotoxicity in mice involves mitochondrial dysfunction, and recent data suggest this is also the case in humans. We have previously shown that glutamate dehydrogenase (GDH), mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) fragments can be measured in circulation of overdose patients as mechanistic biomarkers of mitochondrial damage and damage-associated molecular patterns. In the present study, our goal was to determine if these biomarkers are higher in serum from non-survivors of APAP-induced ALF (AALF) compared with survivors. GDH, mtDNA and nDNA fragments were measured in serum from AALF patients who did (n = 34) or did not (n = 35) recover. Importantly, all three were significantly increased in patients who died compared with those who survived (GDH: 450±73 vs. 930±145 U/L; mtDNA: 21±6 vs. 48±13 and 33±10 vs. 43±7 ng/mL for two different genes; nDNA fragments: 148±13 vs. 210±13 % of control). Receiver operating characteristic (ROC) curve analyses revealed that nDNA fragments, GDH and mtDNA were predictive of outcome (AUC, study admission: 0.73, 0.70 and 0.71 or 0.76, respectively, p < 0.05; AUC, time of peak ALT: 0.78, 0.71 and 0.71 or 0.76, respectively, p < 0.05) and the results were similar to those from the model for end-stage liver disease (MELD) (AUC, peak MELD: 0.77, p < 0.05).
Conclusions
Our data suggest that patients with more mitochondrial damage are less likely to survive, demonstrating that mitochondria are central in the mechanisms of APAP hepatotoxicity in humans. Clinically, serum nDNA fragments, GDH and mtDNA could be useful as part of a panel of biomarkers to predict patient outcome.
Keywords: Acute liver failure, hepatotoxicity, mechanistic biomarkers, prognosis, translational research
INTRODUCTION
Acetaminophen (APAP) hepatotoxicity is a major clinical problem. In the US alone, approximately 78,000 emergency department visits each year and approximately 500 deaths can be attributed to APAP overdose.1 The pathophysiology of APAP hepatotoxicity has been thoroughly studied in rodents. We know that APAP is converted to a reactive metabolite which depletes liver glutathione (GSH) and binds to proteins.2,3,4 Protein binding is believed to cause mitochondrial dysfunction and oxidative stress.5,6 The increased reactive oxygen species (ROS) produced can activate the mitogen-activated protein kinases apoptosis signal-regulating kinase 1 (ASK1)7 and mixed lineage kinase 3 (MLK3),8 which then activate the c-Jun N-terminal kinases (JNK) 1/2.9 Recent work has shown that both RIP1 and RIP3 are also involved, and likely also work upstream of JNK.8,10 Active JNK can then translocate to mitochondria, where it increases the oxidative stress.11,12 The mitochondrial membrane permeability transition pore (MPTP) complex then forms and the damaged mitochondria lose membrane potential.13 The resulting swelling and lysis of mitochondria, as well as translocation of Bax into the outer mitochondrial membrane, result in release of mitochondrial contents, including endonucleases which move into the nucleus and fragment nuclear DNA.14,15 The final result of this cascade of mechanistic events is oncotic necrosis.
Although it has been shown that GSH depletion and protein binding occur in patients and volunteers exposed to APAP,16,17,18 little else has been done in humans to translate the mechanisms of toxicity. Clearly, additional work is needed. Because liver biopsy is contraindicated for the diagnosis of APAP overdose due to coagulopathy, we are limited to blood samples in our approach. In our initial efforts, we were able to show that the mitochondrial matrix enzyme glutamate dehydrogenase (GDH), mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) fragments can be measured as mechanistic biomarkers of mitochondrial damage in the circulation of patients with APAP-induced liver injury.19 This was based in part on our finding that these three biomarkers are increased in the serum of mice treated with APAP, but not in mice treated with furosemide which causes a similar pattern of centrilobular necrosis without affecting mitochondria.19 In the present study, we hypothesized that this mitochondrial damage is critical in the mechanisms of APAP hepatotoxicity in humans, as it is in mice. Based on this, we predicted that nDNA fragments, GDH and mtDNA would be higher in serum from acetaminophen-induced acute liver failure (AALF) patients who died compared with those who survived. We also performed receiver operating characteristic (ROC) curve analysis and logistic regression to determine whether or not these biomarkers can predict death. Our results provide important new insight into the mechanisms of APAP toxicity in humans. Moreover, it has recently been suggested that nuclear and mitochondrial DNA and mitochondrial proteins can act as damage-associated molecular patterns (DAMPs) to initiate sterile inflammation in drug hepatotoxicity,20 and our data have implications for this area of research.
METHODS AND PATIENTS
Acetaminophen-induced acute liver failure patients
To compare patients who did and did not survive, serum samples from AALF patients were obtained through the Acute Liver Failure Study Group (ALFSG) network. In each case, the diagnosis of APAP overdose was made by site investigators using standard criteria: history of acetaminophen overdose ingestion, detectable acetaminophen level (documenting ingestion), and/or aminotransferase level of ≥ 1,500 IU/L. Sera were chosen from consecutive patients, but were balanced by the number of those who died and those surviving without a transplant. In other respects, standard ALF criteria were used: abnormal coagulation (INR ≥ 1.5), hepatic encephalopathy, and liver failure within 26 weeks of illness onset in the absence of chronic liver disease. Due to hepatic encephalopathy in this AALF cohort, consent was obtained from next of kin. Samples were centrifuged on-site to obtain serum and stored at −80°C. Only samples from patients with evidence of extensive tissue necrosis (peak in-study ALT ≥ 1,000 U/L) were used for our measurements. Upon receipt after shipping, the samples were re-centrifuged at 20,000 g for 10 min to pellet any intact mitochondria in the serum, as previously discussed,21 and supernatants were transferred to fresh tubes before processing for experiments. The investigator measuring the biomarkers was blinded to outcome before and during the experiments. Time course samples were also obtained from healthy volunteers and from two representative APAP overdose patients at the University of Kansas Medical Center. Informed consent in writing was obtained from each patient and the study protocol was approved by the internal review boards (IRB) of all participating institutions and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Clinical data
Prothrombin time (PT), serum ALT, serum bilirubin, and serum creatinine were measured in clinical laboratories at the investigation sites using standard clinical assays and procedures. The data from these measurements were provided by the ALFSG.
Nuclear DNA fragments
Nuclear DNA fragments were measured as previously described19 using an anti-histone ELISA with an anti-DNA secondary antibody (Roche, Indianapolis, IN).
Glutamate dehydrogenase
GDH was measured as described.19 Briefly, sample aliquots were mixed with 200 mM imidazole buffer containing ammonium acetate (25 mM), NADH (200 μM), ADP (100 μM), and 0.05% bovine serum albumin (final pH = 8.0). The loss of NADH absorbance at 340 nm was monitored to take a baseline reading (or was monitored until flat), then an aliquot of 2 mM α-ketoglutarate was added to begin the GDH-catalyzed reaction. Baseline activity was subtracted to obtain the true activity of GDH in the sample.
Mitochondrial DNA
mtDNA was measured by absolute quantification real-time PCR as previously described,19 with one minor difference. The DNA samples were not diluted to the same concentration of total DNA, as a comparison of diluted and undiluted aliquots of the same set of serum samples did not show an effect on the final concentrations. Briefly, DNA was isolated from serum using the QIAamp Blood Mini Kit (Qiagen). Standards for mtDNA were prepared from mitochondria isolated from the human liver cell line HepaRG. Two mtDNA-encoded human genes were measured in the patient samples: NADH dehydrogenase (NADH Deh) and cytochrome c oxidase subunit III (Cyt C Ox).19
Mitochondrial biomarker damage index
A mitochondrial biomarker damage index (MDBI) was calculated by adding the standardized value for nDNA fragments with the standardized natural logarithms of GDH and mtDNA (NADH Deh). Standardization was achieved by taking the value for each patient and subtracting the overall mean value of the parameter for all injury groups, then dividing by the overall standard deviation of the parameter for all injury groups. The equation is:
where D is the value for nDNA fragments, G is the value for GDH, and M is the value for mtDNA (NADH Deh).
Clinical scores
The King’s College Criteria for APAP overdose patients were used. These criteria are pH < 7.3 or concurrent serum creatinine > 3.4 mg/dL, INR > 6.5, and encephalopathy grade ≥ 3.22 MELD score was calculated as 3.78 × ln(serum billirubin) + 11.2 × ln(INR) + 9.57 × ln(serum creatinine) + 6.43.23 The highest MELD score achieved after study enrollment was reported.
Statistics
All data were non-normal, as determined using the Shapiro-Wilk test. The Mann-Whitney U-test was used to compare data from survivors and non-survivors. Logistic regression was performed to test for associations between biomarkers and outcome. Receiver operating characteristic (ROC) curve analysis was used to compare the predictive strength of biomarkers with chance. Both logistic regression and ROC analysis were performed using SAS (SAS Institute Inc., Cary, NC). Other statistical tests were run in SigmaPlot (Systat Software, San Jose, CA). In all cases, p < 0.05 was considered significant.
RESULTS
Patient information
Serum samples (N = 69) from AALF patients acquired from the ALFSG network were equally balanced between those who survived and recovered (n = 34) and those who died (n = 35) (Table 1 and 2). Samples from transplant recipients were not included in order to avoid ambiguity in our results. Currently, transplant decisions are based on expert opinion rather than on strongly predictive empirical data. Thus, it is likely that including transplant recipients would have resulted in inclusion of some patients in our poor outcome group who could have spontaneously survived. In addition to the ‘rescue’ of transplantation, prediction of death is complicated by other factors such as infection and extent of brain damage due to encephalopathy. The median time from hospital admission to study enrollment was 2 days (Table 2). Approximately 40% of the non-survivors and 32% of the survivors were enrolled within 1 day of hospital admission, while a total of 77% in both groups were enrolled within 0 – 3 days. The remainders were spread out over a week. Most of the patients who were enrolled in the study later than 1 – 2 days after hospital admission were transferred to the study site from an outlying hospital that was not a tertiary care center. For comparison, serum was also obtained from a small cohort of healthy volunteers at the University of Kansas Medical Center.
Table 1.
All Patient Information
| Volunteers | AALF Patients | |
|---|---|---|
|
|
||
| N | 6 | 69 |
| Age (median, range) | 25, 24 – 29 | 34, 18 – 80 |
| Sex(% F) | 67 | 76 |
| Peak ALT (U/L) | 23 ± 2 | 5870± 535 |
| Peak bilirubin (mg/dL) | NA | 7.6 ± 0.6 |
| Peak PT (s)a | NA | 41 ± 3 |
| Peak creatinine (mg/dL) | NA | 3.3 ± 0.3 |
When available. PT = prothrombin time. NA = Not available.
Table 2.
AALF Patient Information
| Survivors | Non-survivors | |
|---|---|---|
|
|
||
| N | 34 | 35 |
| Age (median, range) | 34, 18 – 78 | 35, 21 – 80 |
| Sex(% F) | 79 | 74 |
| Days to Enrollment (median, range)a | 2, 0 – 5 | 2, 0 – 7 |
| Peak ALT (U/L) | 5148 ± 754 | 6483 ± 755 |
| Peak bilirubin (mg/dL) | 7 ± 1 | 8 ± 1 |
| Peak PT (s) | 31 ± 2 | 52 ± 4 |
| Peak creatinine (mg/dL) | 2.9 ± 0.5 | 3.6 ± 0.3 |
Time from hospital admission to study enrollment. PT = prothrombin time. NA = Not available.
Mitochondrial damage biomarkers were elevated in AALF patients
To determine whether or not the mitochondrial damage biomarkers GDH, mtDNA, and nDNA fragments were elevated in serum from the AALF patients, these parameters were measured in samples from the healthy volunteers and from all of the AALF patients together (n = 69). For the AALF group, the sample nearest the time of peak ALT was used. The average concentration of nDNA fragments in serum was approximately 2-fold higher in AALF patients compared with healthy volunteers (180±10 % of volunteers, p < 0.05), mtDNA was increased 35-to-40 fold over control (1±0.3 vs. 32±5 ng/mL and 1±0.8 vs. 40±6 ng/mL for NADH Deh and Cyt C Ox, respectively; p < 0.05), and the difference in GDH between healthy controls and AALF patients was even greater (11±1 vs. 684±86 U/L, respectively; p < 0.05).
Mitochondrial biomarkers were higher in AALF patients who died
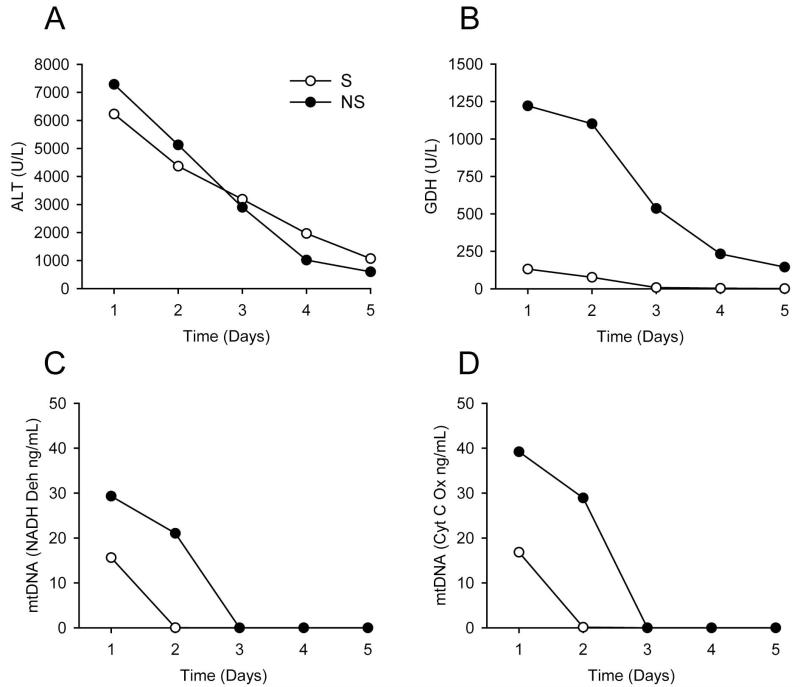
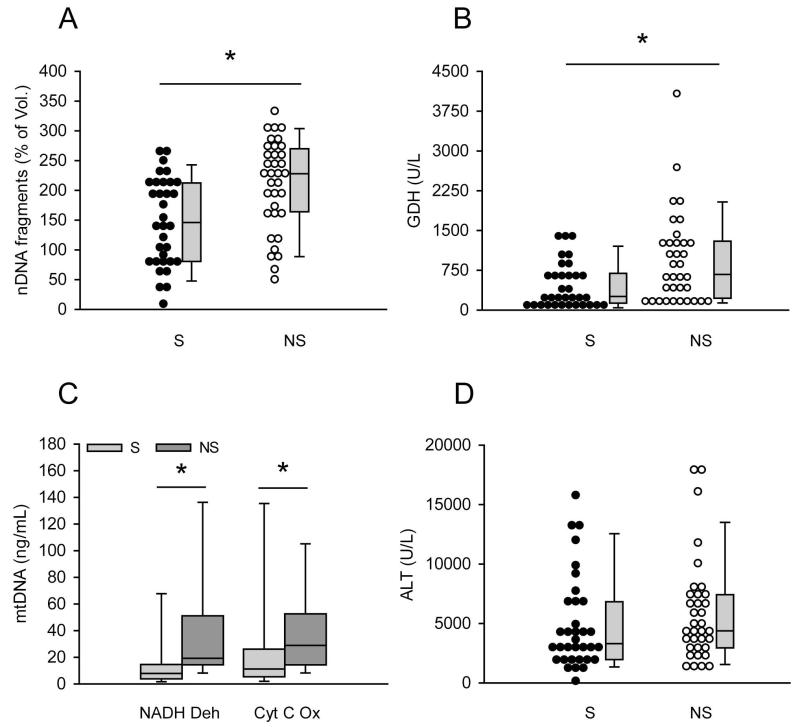
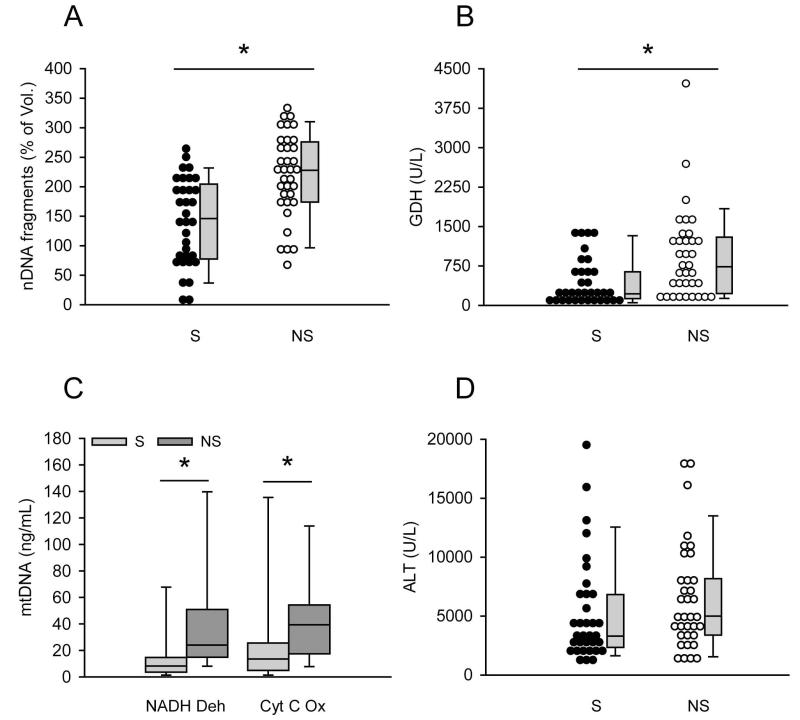
In initial time course studies of mitochondrial damage biomarkers in acetaminophen-induced liver injury, we observed that patients with poor outcome occasionally appeared to have higher levels of some mitochondrial damage biomarkers despite little overall difference in ALT when compared with patients who recovered (Fig. 1). As an example, figure 1 shows time course data from one survivor and one non-survivor of APAP hepatotoxicity. Higher GDH (Fig. 1B) and mtDNA (Fig. 1C,D) were observed in the patient who died, but there was no difference in ALT between the survivor and non-survivor (Fig. 1A). To determine whether or not mitochondrial damage biomarkers are consistently higher in serum from AALF patients who do not survive, the AALF cohort was separated into two groups: those who recovered within 21 days of study admission and those who did not. For all of the patients in the latter group, mortality actually occurred within two weeks. All three parameters were measured in both the first available sample after study admission and in the samples drawn nearest to the time of peak study ALT. Interestingly, all three parameters were significantly higher in the samples from AALF patients who died, both in the first sample after study admission and in the samples drawn nearest to the time of peak ALT. nDNA fragments were approximately 1.6-fold higher (148±13 vs. 210±13 and 140±73 vs. 220±70 % of volunteers for first samples and peak ALT samples, respectively; p < 0.05) (Fig. 2A and 3A), GDH values were more than 2-fold higher (450±73 vs. 930±145 and 443±75 vs. 923±143 U/L for first samples and peak ALT samples, respectively; p < 0.05) (Fig. 2B and 3B) and mtDNA was 1.5 to 2-fold higher (NADH Deh: 21±6 vs. 48±13 and 21±7 vs. 42±8 ng/mL for first samples and peak ALT samples, respectively; p < 0.05) (Cyt C Ox: 33±10 vs. 43±7 and 32±10 vs. 47±7 ng/mL for first samples and peak ALT samples, respectively; p < 0.05) (Fig. 2C and 3C) (Suppl. Fig. 1). Importantly, these increases were not simply due to greater cell contents release in the patients who died, as ALT levels were not different between the two groups either in the first sample drawn after study admission or in the sample drawn nearest to the time of peak ALT (Fig. 2D and 3D) (Table 2). Clinically, ALT is considered the gold standard marker of hepatocyte injury. Together, these data suggest that there may have been more mitochondrial damage in the AALF patients who did not survive.
Figure 1.
Time course of mitochondrial damage biomarkers in representative patients. Alanine aminotransferase (ALT) (A), glutamate dehydrogenase (GDH) (B), and mitochondrial DNA (mtDNA) (C and D) over time in serum from two representative patients with acetaminophen-induced acute liver injury. S = survivor. NS = non-survivor.
Figure 2.
Mitochondrial damage biomarkers were higher in serum from AALF non-survivors on the first study day. Nuclear DNA (nDNA) fragments (A), glutamate dehydrogenase (GDH) (B), mitochondrial DNA (mtDNA) (C) and alanine aminotransferase (ALT) (D) were measured in the first serum sample after study admission from acetaminophen-induced acute liver failure (AALF) patients who did (n = 34) or did not (n = 35) survive. S = survivors. NS = non-survivors. Dot histograms and box plots are shown. Boxes show the median and 25th and the 75th percentiles. Bars show the 10th and the 90th percentiles. *p < 0.05.
Figure 3.
Mitochondrial damage biomarkers were higher in serum from AALF non-survivors at the time of peak ALT. Nuclear DNA (nDNA) fragments (A), glutamate dehydrogenase (GDH) (B), mitochondrial DNA (mtDNA) (C) and alanine aminotransferase (ALT) (D) were measured in the first serum sample after study admission from acetaminophen-induced acute liver failure (AALF) patients who did (n = 34) or did not (n = 35) survive. S = survivors. NS = non-survivors. Dot histograms and box plots are shown. Boxes show the median and 25th and the 75th percentiles. Bars show the 10th and the 90th percentiles. *p < 0.05.
Mitochondrial biomarkers predict death in AALF
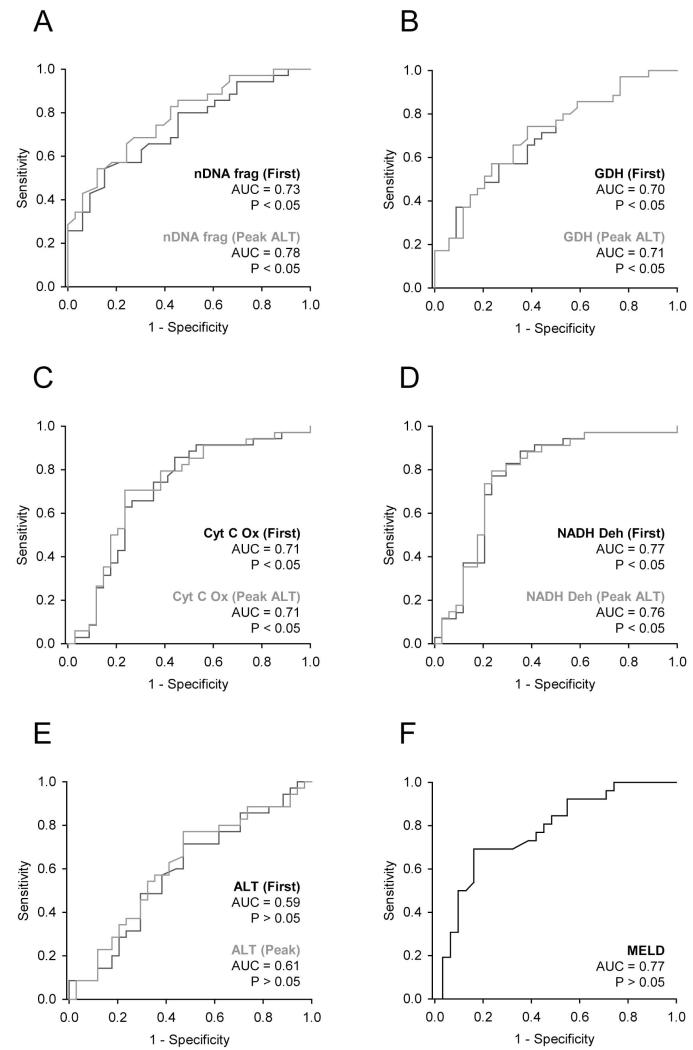
Because mitochondrial damage biomarkers were elevated in serum from AALF patients who died, we next wanted to determine if serum concentrations of these biomarkers can predict patient outcome in AALF patients. Receiver operating characteristic (ROC) curve analysis showed that all three mitochondrial damage biomarkers were predictive of death both at the time of study admission and at the time of peak ALT (Fig. 4A-D). Due to overlap in the parameter values between survivors and non-survivors, sensitivity was relatively low for all three parameters. At the time of admission (sensitivity at 90% specificity, 95% CI): nDNA fragments (0.43, 0.26-0.60), GDH (0.37, 0.21-0.55) and mtDNA (0.14, 0.05-0.3 and 0.09, 0.02-0.23 for Cyt C Ox and NADH Deh, respectively) (Table 3). This suggests that the individual parameters considered separately would have limited clinical utility. Nevertheless, the AUC values were statistically significant. Optimal cutoff values, the values at which the sum of sensitivity and specificity is greatest, were also calculated for each parameter and these results are reported in Table 3. In the future, the best approach to predicting outcome may be a combination of biomarkers: either mitochondrial damage biomarkers with each other, or with other predictive markers, in a biomarker panel. To test this possiblity, we calculated a mitochondrial damage biomarker index (MDBI) as described in the methods section. Indeed, we found that the MDBI performed moderately better in ROC analysis than the individual biomarkers (Suppl. Fig. 2) (Suppl. Table 1). However, it would be ideal to test this index in a unique confirmation cohort before making additional conclusions. For comparison, we also tested for an association between ALT and outcome. ROC curve analysis failed to reveal any relationship between ALT and mortality (Fig. 4E). In contrast, MELD scores overall performed similarly to mtDNA and nDNA fragments (Fig. 4F) (sensitivity at 90% specificity, 95% CI: 0.46, 0.27-0.67). Although KCC results are typically used to predict outcome and to determine the need for liver transplantation in acute hepatotoxicity, only 9 of the 69 patients included in this study did not meet the KCC. This small number prevented us from comparing KCC with outcome. Moreover, it has been shown that MELD scores (due largely to the contribution of INR) predict outcome after the onset of hepatic encephalopathy as well as KCC.24 Thus, MELD scores can be considered a clinical positive control for outcome prediction in this study. The MELD score is calculated from INR and serum levels of creatinine and bilirubin. A correlation coefficient matrix comparing the MELD score and its individual components with peak levels of the mitochondrial damage biomarkers measured in this study revealed a few statistically significant but weak correlations (Suppl. Table 2). The low coefficients demonstrate that these are distinct biomarkers that behave differently from the MELD components.
Figure 4.
Mitochondrial damage biomarkers predict death in AALF patients. Receiver operating characteristic (ROC) curves for nuclear DNA (nDNA) fragments (A), glutamate dehydrogenase (GDH) (B), mitochondrial DNA (mtDNA) (C and D), alanine aminotransferase (ALT) (E) and MELD scores (F). Data are from acetaminophen-induced acute liver failure (AALF) patients who did (n = 34) or did not (n = 35) survive. Either the first sample after study admission (“First”) or the sample drawn nearest to the time of peak ALT (“Peak ALT”) was used. AUC = area under the curve. AUC > 0.5 with p < 0.05 is considered predictive of death.
Table 3.
Mitochondrial Damage Biomarker Sensitivity
| Sens (90% Spec) | 95% CI (Sens) | Optimal Ct | Sens, Spec | |
|---|---|---|---|---|
|
|
||||
| nDNA fragments |
0.43 | 0.26, 0.60 | 219% | 0.57, 0.74 |
| GDH | 0.37 | 0.21, 0.55 | 628 U/L | 0.54, 0.85 |
| mtDNA | 0.14 | 0.05, 0.30 | 14 ng/mL | 0.77, 0.76 |
Sens = sensitivity. Spec = specificity. Ct = cutoff.
DISCUSSION
In the last forty years, a great deal has been learned about the mechanisms of APAP hepatotoxicity mainly through studies in mice. Unfortunately, much less is known in humans. We have previously demonstrated that nDNA fragments, GDH and mtDNA can be measured as circulating mitochondrial damage biomarkers in patients with APAP-induced liver injury.19 By comparing APAP hepatotoxicity with furosemide-induced liver injury in mice, we previously provided evidence that serum nDNA fragments, GDH and mtDNA are specific biomarkers of mitochondrial damage.19 Furosemide is known to cause liver injury in rodents that resembles APAP hepatotoxicity but without affecting mitochondria.25 In the present study, these biomarkers were increased 1.5 to 2.1-fold more in serum from AALF patients who died compared with those who survived. Importantly, ALT values, as a clinically accepted marker of general cell contents release, were similar between the two groups. These data support our hypothesis that mitochondrial damage is central in APAP hepatotoxicity in people. Moreover, this work shows that these biomarkers may be useful in prediction of patient outcome if combined with other parameters.
Aside from nDNA fragments, GDH and mtDNA, a number of other biomarkers have recently been shown to correlate with later injury or death in acute liver injury, including APAP hepatotoxicity. Among these are keratin 18 (K18), high mobility group box 1 protein (HMGB1), microRNA-122 (miR-122), and malate dehydrogenase (MDH).26-28 Consistent with data from rodent studies, measurement of circulating full-length and caspase-cleaved K18 revealed that the primary mode of cell death in APAP hepatotoxicity in humans is oncotic necrosis.26 It was also found that high serum concentrations of the full-length form of K18 were predictive of poor outcome after APAP overdose.26 More recently, it was shown that admission levels of K18 can predict the later development of acute liver injury in early-presenting patients.27 Interestingly, it was found in the same study that early GDH activity can also predict later injury, though not as well as other markers. HMGB1 levels have also been shown to be higher in serum from patients who died or received a liver transplant compared to those who spontaneously recovered,26 and early serum levels of both HMGB1 and miR-122 have been shown to predict later injury.27 Similarly, serum MDH levels were found to correlate strongly with the development of liver injury.28 With the exception of caspase-cleaved K18, the mitochondrial damage biomarkers used in this study differ from these other biomarkers because they provide specific mechanistic information.19
Theoretically, mechanistic biomarkers may offer several advantages over other biomarkers. The most obvious benefit is that they can tell us something about the pathophysiology of the injury, and possibly even provide targets for therapeutic intervention. Another possible advantage of mechanistic biomarkers is earlier detection of injury and prediction of outcome. Because the mechanisms of injury precede the injury itself, it is possible that some mechanistic biomarkers could be detected early. This may be especially true for metabolites and other small molecules that are less dependent than large macromolecules upon plasma membrane rupture or exocytosis for release. For example, our group and others have shown that very high concentrations of certain acylcarnitines can be detected during APAP hepatotoxicity in mice,29,30 and our data indicate that this is due to mitochondrial dysfunction.30 However, we were unable to show an increase in serum acylcarnitines in humans, likely because NAC treatment supports mitochondrial function.31 Nevertheless, such a marker could be a tremendous boon for clinical practice. Early and accurate prediction of patient outcome could allow a treatment decision to be made before the injury progresses too far. Finally, it is reasonable to think that mechanistic biomarkers may possess an inherent quantitative association with the extent of injury. For example, if mitochondrial damage is important in the mechanisms of injury, then patients with more mitochondrial damage and therefore higher circulating levels of mitochondrial biomarkers may be less likely to survive. However, although the mitochondrial damage biomarkers in our study were significantly higher in the AALF patients who did not survive, there was considerable overlap in some of the values which may limit clinical utility. In the future, biomarkers such as these may be more useful as part of a clinical panel. One potential weakness of our study is the lack of data regarding the time of NAC treatment with respect to the time of overdose. It is possible that some of the patients in the non-survivor group died because they received NAC later than the patients who survived. However, this seems unlikely as there was no difference in ALT or other markers of injury between the two groups, and patients receiving NAC significantly earlier would be expected to have lower enzymes.19
It has been suggested that APAP-induced liver injury is at least partially mediated by sterile inflammation, and that nDNA fragments, mtDNA, and some mitochondrial and nuclear proteins released from damaged hepatocytes can initiate this response.20 While it is clear from recent data that there is inflammation and neutrophil activation in both mice and humans after APAP overdose,32 their role in the toxicity is controversial.20,33 There are many lines of evidence which contradict the idea that innate immunity is important in the injury phase of APAP toxicity.33 Nevertheless, some evidence suggests that neutrophils and other immune cells play an important role during recovery and regeneration after APAP overdose.32 If true, then the data presented here would suggest that there is a balance between the extent of mitochondrial damage and the sterile inflammation triggered by mitochondrial DNA and proteins, and nuclear DNA. Some release of mitochondrial damage markers could promote inflammation and thus recovery, while too much mitochondrial damage leads to death.
Overall, our data showed that nDNA fragments, GDH and mtDNA were significantly elevated in all AALF patients, and particularly so in those who did not survive. These data suggest that mitochondrial damage is a critical part of the mechanisms of APAP hepatotoxicity in humans. Although the low sensitivity of these biomarkers for prediction of death means that GDH, mtDNA and nDNA fragments individually may not be clinically useful, these might be informative as part of a panel of biomarkers to predict patient outcome. In the future, it would be interesting to measure these parameters in serum from patients with other forms of liver injury that are suspected to involve mitochondrial damage.
Supplementary Material
Acknowledgments
Financial support: The Acute Liver Failure Study Group was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant no. U-01--58369. The data and samples reported here were supplied by the NIDDK Central Repositories. This manuscript does not necessarily reflect the opinions or views of the NIDDK Central Repositories, or the NIDDK. This work was also supported in part by grants from the University of Kansas Medical Center Liver Center (to H.J.), from the National Institutes of Health (R01 DK070195 and R01 AA12916) (to H.J.), and from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 (to M.R.M.) from the National Institute of Environmental Health Sciences.
Abbreviations
- APAP
Acetaminophen
- ALF
Acute liver failure
- AALF
Acetaminophen-induced acute liver failure
- mtDNA
Mitochondrial DNA
- GDH
Glutamate dehydrogenase
- nDNA fragments
Nuclear DNA fragments
- ROC
Receiver operating characteristic
- K18
eratin 18
- HMGB1
High mobility group protein 1
- miR-122
MicroRNA 122
- MDH
Malate dehydrogenase
REFERENCES
- 1.Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40:585–592. doi: 10.1016/j.amepre.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- 3.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- 4.McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- 6.Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012a;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–2108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 14.Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-induced factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- 15.Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- 16.Davis M, Ideo G, Harrison NG, Williams R. Hepatic glutathione depletion and impaired bromosulphthalein clearance early after paracetamol overdose in man and the rat. Clin Sci Mol Med. 1975:495–502. doi: 10.1042/cs0490495. [DOI] [PubMed] [Google Scholar]
- 17.Lauterburg BH, Mitchell JR. Therapeutic doses of acetaminophen stimulate the turnover of cysteine and glutathione in man. J Hepatol. 1987;4:206–211. doi: 10.1016/s0168-8278(87)80081-8. [DOI] [PubMed] [Google Scholar]
- 18.Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM. Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 19.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in mice and humans involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Jaeschke H, McGill MR. Serum glutamate dehydrogenase – biomarkers for liver cell death or mitochondrial dysfunction? Toxicol Sci. 2013;134:221–222. doi: 10.1093/toxsci/kft087. [DOI] [PubMed] [Google Scholar]
- 22.Bernal W, Wendon J. Liver transplantation in adults with acute liver failure. J Hepatol. 2004;40:192–197. doi: 10.1016/j.jhep.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Kamath PS, Kim WR, Acute Liver Disease Study Group The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology. 2007;45:789–796. doi: 10.1002/hep.21503. [DOI] [PubMed] [Google Scholar]
- 25.Wong SG, Card JW, Racz WJ. The role of mitochondrial injury in bromobenzene and furosemide induced hepatotoxicity. Toxicol Lett. 2000;116:171–181. doi: 10.1016/s0378-4274(00)00218-6. [DOI] [PubMed] [Google Scholar]
- 26.Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for model of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, Bateman DN, Goldring CE, Park BK. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schomaker S, Warner R, Bock J, Johnson K, Potter D, Van Winkle J, Aubrecht J. Assessment of emerging biomarkers of liver injury in human subjects. Toxicol Sci. 2013;132:276–283. doi: 10.1093/toxsci/kft009. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Krausz KW, Shah YM, Idle JR, Gonzalez FJ. Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2009;22:699–707. doi: 10.1021/tx800464q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGill MR, Li F, Sharpe MR, Williams CD, Curry SC, Ma X, Jaeschke H. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch Toxicol. 2014;88:391–401. doi: 10.1007/s00204-013-1118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol. 2014;275:122–133. doi: 10.1016/j.taap.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.