Abstract
Numerous root-associated bacteria (rhizobacteria) are known to elicit induced systemic resistance (ISR) in plants. Bacterial cell-density-dependent quorum sensing (QS) is thought to be important for ISR. Here, we investigated the role of QS in the ISR elicited by the rhizobacterium, Serratia marcescens strain 90–166, in tobacco. Since S. marcescens 90–166 produces at least three QS signals, QS-mediated ISR in strain 90–166 has been difficult to understand. Therefore, we investigated the ISR capacity of two transgenic tobacco (Nicotiana tabacum) plants that contained either bacterial acylhomoserine lactone-producing (AHL) or -degrading (AiiA) genes in conjunction with S. marcescens 90–166 to induce resistance against bacterial and viral pathogens. Root application of S. marcescens 90–166 increased ISR to the bacterial pathogens, Pectobacterium carotovorum subsp. carotovorum and Pseudomonas syringae pv. tabaci, in AHL plants and decreased ISR in AiiA plants. In contrast, ISR to Cucumber mosaic virus was reduced in AHL plants treated with S. marcescens 90–166 but enhanced in AiiA plants. Taken together, these data indicate that QS-dependent ISR is elicited by S. marcescens 90–166 in a pathogen-dependent manner. This study provides insight into QS-dependent ISR in tobacco elicited by S. marcescens 90–166.
Keywords: AiiA, N-Acyl homoserine lactone, plant-growth promoting rhizobacteria, quorum sensing
Induced systemic resistance (ISR) is the resistance of plants to disease induced by root-associated bacteria (rhizobacteria), also referred to as plant-growth promoting rhizobacteria (PGPR) (Kloepper et al., 1992). Diverse bacterial determinants have been proposed to play a role in the elicitation of ISR (Kloepper et al., 2004). These include bacterial surface components, such as flagellin, lipopolysaccharides, and exopolysaccharides, and bacterial metabolites, such as siderophores and antibiotics (De Vleesschauwer and Hofte, 2009). In addition to these bacterial determinants, it has also been reported that volatile organic compounds (VOCs), including acetoin and 2,3-butanediol, emitted by Bacillus subtilis GB03 activate an ISR pathway in Arabidopsis seedlings inoculated with Pectobacterium carotovorum (syn. Erwinia carotovora subsp. carotovora) (Ryu et al., 2004a).
ISR can be triggered by acyl-homoserine lactones (AHLs), which are quorum sensing (QS) molecules that control the expression of various genes to regulate a variety of physiological functions in various Gram-negative bacteria in a cell density-dependent manner (Miller and Bassler 2001; Schuhegger et al., 2006). The application of Serratia liquefaciens MG1 (a PGPR strain that produces two different AHLs) to the roots of tomato (Solanum lycopersicum L.) plants resulted in ISR to the leaf fungal pathogen, Alternaria alternata, but an AHL-null mutant of S. liquefaciens MG1 treatment reduced elicitation of ISR (Schuhegger et al., 2006). In the same pathosystem, Pseudomonas putida F117 (an AHL non-producing mutant) was less effective than its wild type, P. putida IsoF (a PGPR strain that produces four different AHLs), at eliciting an ISR response. These indicate that bacterial QS molecules contribute to the capacity of P. putida IsoF to elicit ISR responses.
The findings of these previous studies led us to ask the following questions about the role of AHLs in eliciting ISR responses:
Are single AHLs or combinations of AHLs responsible for eliciting ISR responses when a bacterium produces more than one AHL?
Can ISR be indirectly elicited by production of secondary metabolite(s) regulated by QS rather than elicited via. AHL production by PGPR?
What is the plant response to AHLs?
In answer to the first question, many studies report that direct application of several AHLs comprising different acyl lengths resulted in different plant responses. The addition of 10 μM N-hexanoyl (C6) homoserine lactone (HHL) and N-butanoyl (C4) homoserine lactone to the roots of tomato plants was enough to increase the induction of defense gene expression (Schuhegger et al., 2006). A recent study evaluated the defense responses of Arabidopsis and barley (Hordeum vulgare L.) to a variety of AHLs ranging from C4 to C14 in length (Schikora et al., 2011).
In answer to the second question, many studies show that bacterial secondary metabolites regulated by QS act to increase ISR. The PGPR strain, S. plymuthica HRO-C48, secretes a strong antibiotic, pyrrolnitrin, via QS regulation, and protects cucumber seedlings against infection by the soil-borne pathogen, Pythium aphanidermatu, and tomato plants against the foliar pathogen, Botrytis cinerea (Pang et al., 2009). Indirect evidence for the role of secondary metabolites in eliciting ISR also exists for P. aeruginosa PAO1 and S. plymuthica RVH1 (Moons et al., 2011; Reimmann et al., 2002; Whiteley et al., 1999). Also, pyocyanin from P. aeruginosa 7NSK2 has been defined as a bacterial determinant of ISR in tomato (Audenaert et al., 2002). During fermentation of glucose, S. plymuthica, like many other bacterial species, is able to switch from producing mixed acids to producing natural chemicals such as acetoin and 2,3-butanediol to avoid the lethal acidification of its environment. N-(3-oxo-hexanoyl)-L-homoserine lactone (OHHL) plays an important role in acetoin and 2,3-butanediol production (Moons et al., 2011).
In answer to the third question, the first demonstration of plant responses to bacterial QS signals was the description of extensive proteome changes in a plant in response to physiological concentrations of AHL (Mathesius et al., 2003). The levels of over 150 proteins in the model legume, Medicago truncatula, were altered in response to 3-oxo-C12-HSL or 3-oxo-C16:1-HSL, with the response depending on the concentration and identity of the AHLs, suggesting that plants can differentiate between QS signals from different bacteria. In addition, AHL treatment affected diverse metabolites such as phenol containing chemicals secreted by the roots, including the AHL mimics (Mathesius et al., 2003; Teplitski et al., 2000). Recently, treatment of Arabidopsis roots with oxo-C14-HSL was shown to promote stronger activation of the mitogen-activated protein kinases AtMPK3 and AtMPK6 and the defense-related transcription factors WRKY22 and WRKY29 (Schikora et al., 2011). These results suggest that long chain AHLs, such as C12, C14 and C16, can efficiently activate plant resistance.
S. marcescens 90–166 is a well-known PGPR strain. When applied to seeds or roots, it results in ISR to a broad spectrum of foliar pathogens, including bacterial, fungal, and viral pathogens (Kloepper and Ryu, 2006). The salicylic acid (SA) non-producing mutant of S. marcescens 90–166 retained ISR to anthracnose in cucumber and to Cucumber mosaic virus (CMV) in Arabidopsis, while the iron-chelation enzyme null mutant abolished ISR capacity against anthracnose in cucumber but had no effect against CMV in Arabidopsis (Press et al., 2001; Ryu et al., 2004b). These data indicate that a specific bacterial determinant can be involved in inducing pathogen-specific resistance, or that more than one bacterial determinant can be involved in ISR induction for each pathosystem. More interestingly, an ISR-negative mini-Tn5phoA mutant of S. marcescens 90–166 reduced AHL production (Wilson et al., 1997). This result led us to investigate whether QS-dependent bacterial traits of S. marcescens 90–166 contribute to the elicitation of ISR against diverse pathogens. Studies of the role of QS-regulated ISR-related traits are limited due to a lack of genetic tools to manipulate S. marcescens 90–166 to generate AHL-negative mutants, or to obtain genome information. To obtain clear evidence about the role of QS-regulated traits that influence ISR, null mutants of all three AHL-producing genes in S. marcescens 90–166 would be required.
In the current study, we established an alternative plant-based system that included two transgenic tobacco plants harboring genes for either AHL degradation (AiiA) or AHL production (AHL). The tobacco plants allowed us to investigate the role of bacterial AHL-based QS molecules on ISR to bacterial and viral pathogens.
Materials and Methods
Plant and PGPR preparation
The AHL-producing transgenic tobacco (AHL tobacco), which was constructed by transforming bacterial YenI that targeted to the chloroplasts, was provided by Dr. Rupert G. Fray, Nottingham University, UK (Fray et al., 1999). The AiiA transgenic tobacco, which was created by transformation of the acylhomoserine lactonase (AiiA) gene from Bacillus sp., was provided by Dr. Lian-Hui Zhang, National University of Singapore (Dong et al., 2001). Seeds of tobacco (Nicotiana tabacum L.) cvs. wild types Samsun NN and GX3 and their transgenic plants, AHL and AiiA plants, respectively, were surface-disinfested by soaking in 70% ethanol for 2 min followed by 1% sodium hypochlorite for 30 min. The seeds were rinsed thoroughly in sterile distilled water (SDW) and transferred to a commercial potting mix, Nongwoo (Nongwoo, Daejeon, S. Korea), without sterilization. Plants were grown in a controlled-environment growth room at 25 ± 2ºC under fluorescent light (a 12 h/12 h day/night cycle, with a light intensity of approximately 7,000 lux). S. marcescens 90–166 was grown in tryptic soy agar (TSA; Difco, St. Louis, MO, USA). For long-term maintenance, S. marcescens 90–166 was preserved in tryptic soy broth (TSB; Difco, St. Louis, MO, USA) containing 15% glycerol (v/v) at −70°C. Three weeks after transfer, plants were inoculated by drenching with 20 mL of a suspension (108–109 colony-forming units (cfu)/mL) of S. marcescens 90–166. These bacterial cells had been grown for 36 h to stationary phase in TSB, pelleted by centrifugation at 10,000 × g, and washed once with SDW. Other treatments applied to the roots of the three-week-old plants included water as a negative control or 1 mM benzothiadiazole (BTH) (Syngenta, NC, USA), which elicits systemic acquired resistance to bacterial and viral pathogens, as a positive control (Hahm et al., 2012; Niu et al., 2011, Ryu et al., 2004b).
AHL detection by thin layer chromatography (TLC) and acetoin detection
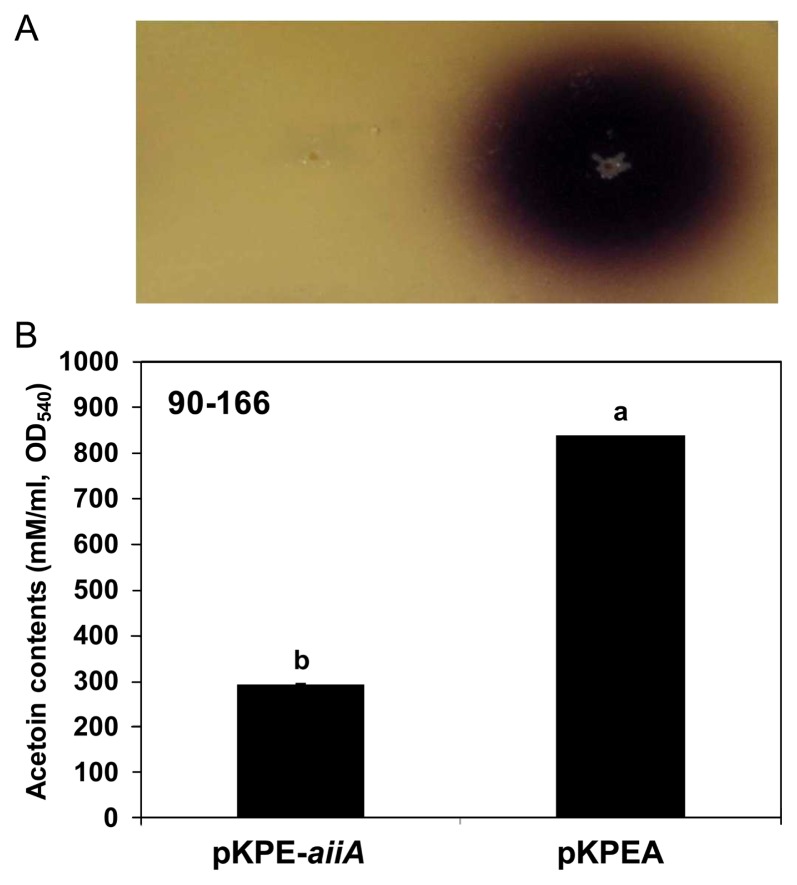
AHLs were extracted and detected as described previously (Schaefer et al., 2000; Shaw et al., 1997). Briefly, AHLs were extracted from spent supernatants of late exponential phase cultures, and partial characterization was carried out by TLC on C18 reversed phase plates. The TLC plates were loaded with the sample extracts and different standards and, after chromatography, overlaid with a soft-agar suspension of the AHL biosensor strain, Agrobacterium tumefaciens NTL4 (pZLR4). Each AHL migrates with a characteristic mobility, and the position and shape of the sample AHL spots can be compared with the spots of different standards in order to identify them. The acetoin production of S. marcescens 90–166 containing pKPE-aiiA and its vector control (pKPEA) that was reported previously (Cho et al., 2007) was determined by the Voges-Proskauer and methyl red assays (van Houdt et al., 2006).
Detection of root-associated bacteria on the roots of AHL and AiiA plants
To compare the concentrations of root-colonizing microorganisms in the rhizosphere of tobacco plants, a whole root system for each treatment was taken 4 weeks after transplanting, cleaned to remove soil particles and then placed in a 250 mL Erlenmeyer flask containing 100 mL of 0.1 M MgSO4, which was vigorously shaken in an incubator at 30°C for 30 min. The root colonization capacity of each root was determined by plating a dilution series onto 1/10 strength TSA and incubating plates for 2–3 days at 30°C. The population density of Gram-negative bacteria was determined by counting colonies on 1/10 TSA plates supplemented with 10 mg/mL vancomycin to inhibit growth of Gram-positive bacteria. The population density of Gram-positive bacteria was determined by counting colonies on plates that were heat treated at 80°C for 30 min to kill Gram-negative bacteria and fungal spores (Yang et al., 2011).
Bacterial pathogen challenges
To investigate the strength of ISR responses to foliar pathogens in the presence of S. marcescens 90–166, tobacco plants were challenged with the following: Pseudomonas syringae pv. tabaci, a causal pathogen of wild fire on tobacco (Zang et al., 2004); and P. carotovorum subsp. carotovorum, a soft rot pathogen (Cho et al., 2007; Ryu et al., 2004b). P. syringae pv. tabaci and P. carotovorum subsp. carotovorum were grown on Pseudomonas Agar F (Difco, St. Louis, MO, USA) and TSA, respectively. Prior to use in the experiments, fully-grown bacteria were scraped off the plates and resuspended in SDW.
To inoculate tobacco with P. syringae pv. tabaci, cells were diluted to 106–107 cfu/mL in SDW and then sprayed onto the leaves. Seven days after pathogen challenge, disease severity was measured. Inoculated plants were placed in a dew chamber (100% humidity) in the dark for 2 days and then transferred to a greenhouse at 25°C under average 60% humidity condition. To assess the wild fire symptoms caused by P. syringae pv. tabaci, the “percentage disease” was measured by recording the percentage of total plant leaf surface that showed symptoms. To inoculate tobacco with P. carotovorum subsp. carotovorum, cells were diluted to 106 cfu/mL (based on optical density), and five leaves per plant were inoculated with two spots per leaf. To assess soft rot caused by P. carotovorum subsp. carotovorum, the number of symptomatic leaves (0–5) per plant was counted at 24 h after inoculation. The > 20% maceration area on the leaf was counted as a symptomatic leaf. This experiment was designed as a randomized complete block (RCB) with 12 replications (one plant per replication). The experiment was repeated three times.
Virus inoculation and enzyme-linked immunosorbent assay (ELISA)
CMV strain Fny was provided by Peter Palukaitis (Scottish Crop Research Institute, Invergowrie, Scotland) and was used for all virus-based experiments in the current study. Leaf inoculation with CMV was performed as described previously by Ryu et al. (2004b) and Murphy et al. (2003). Briefly, CMV was maintained in N. tabacum cultivar Kentucky 14 by mechanical passage in a temperature-controlled greenhouse. Plants were inoculated with CMV at 4 weeks after planting. The CMV inoculum consisted of systemically-infected Kentucky 14 tissue ground in 50 mM potassium phosphate buffer (pH 7.0) and 10 mM sodium sulfite (1 g tissue in 10 mL buffer). All inoculation materials were chilled to 4°C prior to use and maintained on ice during the inoculations. A fresh preparation of CMV inoculum was used for whole experiments. CMV was mechanically inoculated by rub inoculation onto the oldest three leaves (each of which was lightly dusted with carborundum prior to inoculation) of each tobacco plant 7 days after treatment with PGPR or BTH (positive control treatments). Plants were also mock-inoculated with 50 mM potassium phosphate buffer containing 10 mM sodium sulfite in the same manner.
Inoculated plants were rated for disease severity at 14 days after inoculation with CMV. The disease severity scale for CMV was assigned a score of 0 to 5 as follows: 0 = no symptoms; 1 = mild deformation and mosaic of the youngest two leaves; 2 = pronounced leaf deformation and mosaic of the youngest two leaves with progression of symptoms into sequentially older leaves; 3 = pronounced leaf deformation and mosaic progression beyond the two youngest leaves, with all leaves expressing some form of CMV-induced symptoms; 4 = similar symptoms to those described for a rating of 3, with plants also showing stunted growth (note that this stunting included both reduced internode extension and smaller leaves); 5 = plants were severely stunted with a majority of leaves being small, severely deformed and tightly bunched together. Each treatment included eight replications (one plant per replication). Mock treatment indicated non-virus treated plant.
Detection of CMV in tobacco tissue was determined by indirect ELISA using an anti-CMV coat protein antibody as described by Ryu et al., (2004b). Inoculated leaves were tested for CMV infection at 7 days post inoculation (dpi), and non-inoculated leaves were tested at 7, 14 and 21 dpi. At each period of testing, samples were collected from a set of plants that had not been sampled for previous tests. Leaf samples (50 to 100 mg) were placed in a 1.5-mL Eppendorf tube and ground in 1 mL of 50 mM carbonate buffer, pH 9.6, using a plastic homogenizer. ELISA reactions were measured with a Sunrise microtiter plate reader (Phenix Research Products, Hayward, CA, USA). Samples were considered positive for the presence of CMV when the absorbance value was greater than the mean plus three standard deviations of the healthy control samples. Each ELISA test included a series of known concentrations of purified CMV as an internal standard.
Data analysis
Data were analysed by ANOVA using JMP software (ver. 4.0; SAS Institute Inc., Cary, NC, USA), with significant differences between treatments determined by the magnitude of the F value at P = 0.05. When a significant F value was obtained for a treatment, separation of the means was performed using Fisher’s protected least-significant difference (LSD) at P = 0.05. The results of repeated trials for each of the experiments outlined above were similar. Hence, one representative trial of each experiment is reported.
Results
AHL detection by TLC
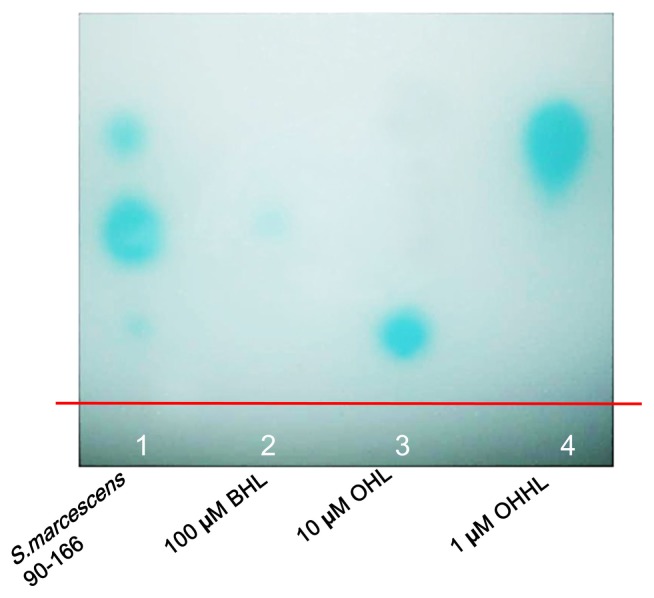
First, we investigated whether S. marcescens 90–166 produced AHL signal molecules using a modified TLC method, with A. tumefaciens NTL4 as the indicator strain (Shaw et al., 1997). A green pigment was noted after applying the AHLs produced by S. marcescens 90–166 to the reporter strain (Fig. 1). The three green spots were similar in position to that produced by BHL, OHL, and OHHL, indicating that S. marcescens 90–166 produces at least three AHLs. This result requires verification by chemical identification and quantification of the molecules by liquid chromatography-mass spectrometry (LC-MS).
Fig. 1.
Detection of AHL molecules from S. marcescens 90–166 by thin layer chromatography with reporter strain A. tumefaciens NTL4 (pZLR4). Control treatments include OHL, OHHL and BHLThe blue dots represent individual AHL molecule indicated by standard AHLs (OHL, OHHL and BHL).
Population density of bacteria on AHL and AiiA plant roots
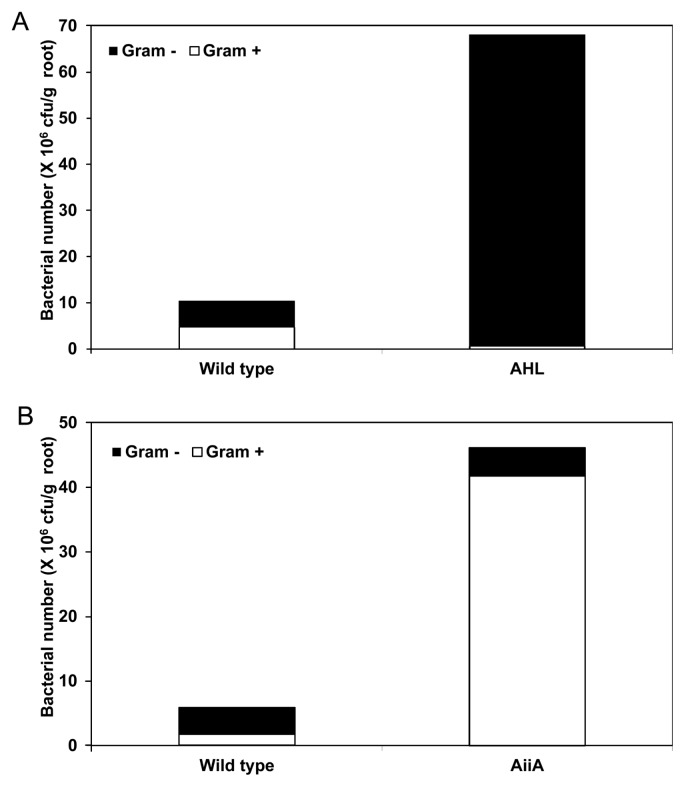
A dilution plating method was employed to investigate whether AHL and AiiA production in tobacco plants affected the population dynamics of rhizosphere bacteria. The total number of bacteria on the wild type AHL and AiiA plants was similar (10.41 × 106 and 5.52 × 106 cfu/mL, respectively; Fig. 2). The average total number of bacteria on the AHL plants was 6.5-fold higher than that on the wild-type plants (Fig. 2A). In the wild type (cv. Samsun NN) for AHL plants, 55.0% of the total bacteria were Gram negative and showed diverse colony morphology on TSA medium. On AHL plants, 99.2% of the total bacteria were Gram negative. As for the AHL plants, the average number of total bacteria on the AiiA plants was 7.8-fold higher than that on the wild-type plants (Fig. 2B). In the wild type (cv. GX3) for AiiA plants, 71.1% of total bacteria were Gram positive, whereas 90.3% were Gram positive in the AiiA plants.
Fig. 2.
Bacterial populations in the root systems of acyl-homoserine lactone-producing (AHL) and -degrading (AiiA) tobacco plants. Number of Gram-negative and -positive bacteria from (A) AHL tobacco and (B) AiiA tobacco roots. The detail methods used to isolate Gram-negative bacteria and Gram-positive bacteria were described in Materials and Method. Bars represent the mean of 10 replications per treatment. The experiment was repeated three times with similar results.
ISR against bacterial pathogens in the AHL and AiiA plants
Although S. marcescens 90–166 is known to be a strong ISR inducer against several phytopathogens, including P. carotovorum subsp. carotovorum and P. syringae pv. tabaci (Press et al., 1997; Zhang et al., 2004), a role for QS molecules in the induction of ISR has not yet been confirmed. BHL was confirmed to be produced from strain 90–166 previously and had elicited induction of resistance by direct application (Schuhegger et al., 2006). Therefore, in this study, we assessed whether QS-regulated metabolic determinant production by S. marcescens 90–166 modulates ISR. It is difficult to identify an ISR determinant regulated by a QS-dependent manner in strain 90–166 because strain 90–166 produces at least three AHLs. We employed two transgenic tobacco plants, one that produced a Gram-negative bacterial QS signal (i.e. carried a gene encoding AHL production) and one that degraded the signal molecule (i.e. carried the AiiA gene from Bacillus sp.) (Dong et al., 2001). These plants, along with their non-transgenic parent strains, were treated with S. marcescens 90–166 and then infected with either P. carotovorum subsp. carotovorum or P. syringae pv. tabaci.
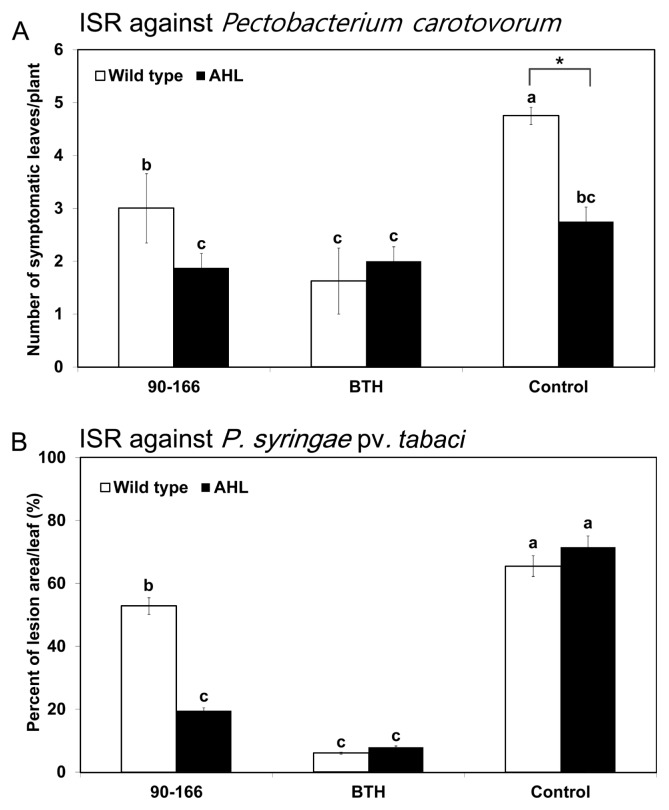
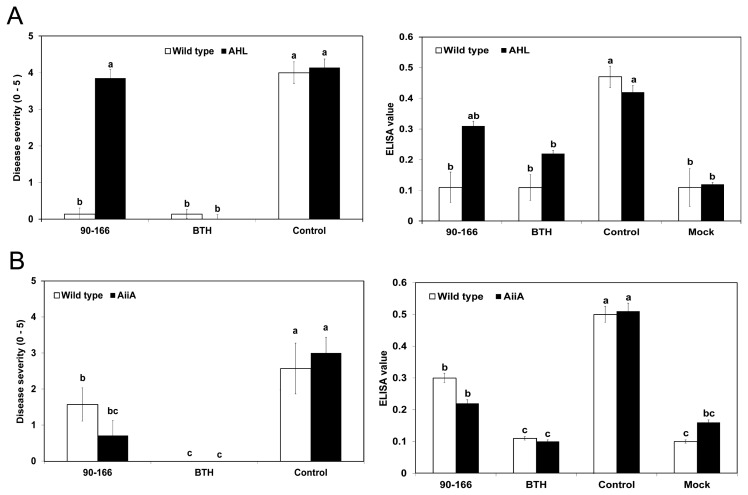
For plants inoculated with S. marcescens 90–166 and infected with P. carotovorum subsp. carotovorum, the average number of symptomatic (soft-rot) leaves on the wild type and AHL plants was 3.0 and 1.8, respectively, compared with 4.7 and 2.7 for negative control (i.e. infected with P. carotovorum subsp. carotovorum but not treated with S. marcescens 90–166) wild-type and AHL plants, respectively (Fig. 3A). Statistical analysis revealed that there were significantly fewer symptomatic leaves in AHL plants than in wild-type plants for both the S. marcescens 90–166 and control treatments but no difference in BTH treatment (Fig. 3A). The result is agrees with the previous finding by Fray et al. (1999). The positive control treatment (1 mM BTH) produced wild-type plants with fewer symptomatic leaves than plants treated with S. marcescens 90–166 or the negative controls. No difference in disease severity was noted in AHL plants after any of the treatments.
Fig. 3.
Disease severity of P. carotovorum subsp. carotovorum and P. syringae pv. tabaci infections after treatment of acyl-homoserine lactone-producing (AHL) transgenic tobacco plants with S. marcescens 90–166. (A) Disease severity 24 h after drop inoculation of P. carotovorum subsp. carotovorum at 106 cfu/mL (B) Disease severity 7 days after inoculation of P. syringae pv. tabaci at 108 cfu/mL. Different letters indicate significant differences between treatments (P < 0.05 according to the least significant difference (LSD)). Different letters indicate a significant difference between wild type and AHL/AiiA plants. The experiment was repeated three times with similar results.
In the same experimental system, but using P. syringae pv. tabaci as the pathogen, we found the disease severity in negative control wild-type and AHL plants to be similar (Fig. 3B). For the wild-type plants, the disease severity was similar after treatment with S. marcescens 90–166 and negative control treatment, resulting in lesion areas of 52.8% and 65.5% per leaf, respectively (Fig. 3B). For the AHL plants, disease severity was lower in S. marcescens 90–166 treated plants than in negative control treated plants, with lesion areas of 19.5% and 71.5% per leaf, respectively. AHL plants treated with S. marcescens 90–166 showed 2.7-fold less lesion area than wild-type plants treated with S. marcescens 90–166. BTH treatments resulted in less lesion area in both wild-type and AHL plants than the control treatments, but the lesion areas were similar to those observed after S. marcescens 90–166 treatment in AHL plants (Fig. 3B).
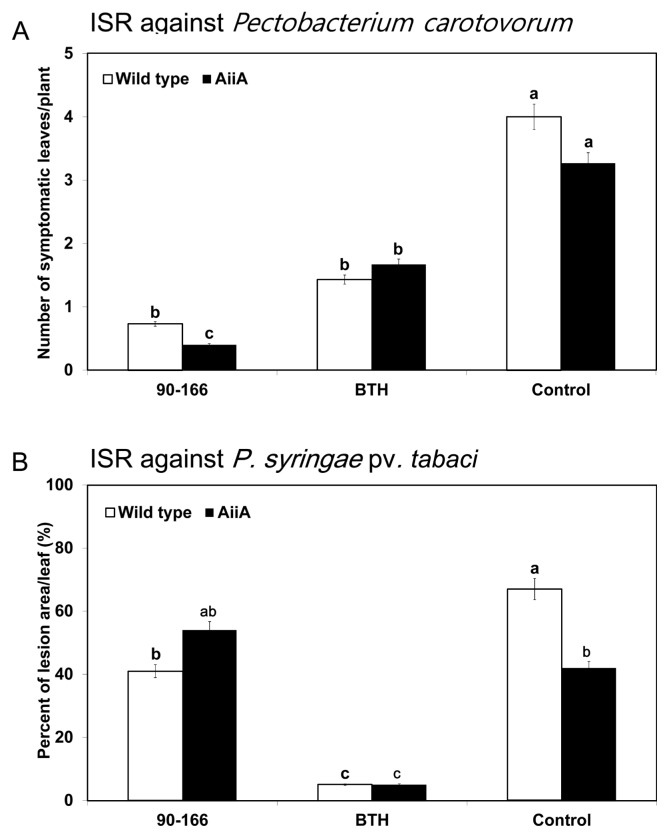
Similar experiments were conducted using AiiA plants (Fig. 4). For plants inoculated with S. marcescens 90–166 and infected with P. carotovorum subsp. carotovorum, the average number of symptomatic (soft-rot) leaves on the wild type and its AiiA plants was 0.73 and 0.4, respectively, compared with 4.0 and 3.27 for the negative control (Fig. 4A). Statistical analysis showed no difference between AiiA and wild-type plants in terms of any treatments (Fig. 4A). BTH treatment produced wild-type and AiiA plants with fewer symptomatic leaves than control treatment.
Fig. 4.
Disease severity of P. carotovorum subsp. carotovorum and P. syringae infections after treatment of acyl-homoserine lactone-degrading (AiiA) transgenic tobacco plants with S. marcescens 90–166. (A) Disease severity 24 h after drop inoculation of P. carotovorum subsp. carotovorum at 106 cfu/mL (B) Disease severity 7 days after inoculation of P. syringae pv. tabaci at 108 cfu/mL. Different letters indicate significant differences between treatments (P < 0.05 according to the least significant difference (LSD)). Different letters indicate a significant difference between wild type and AHL/AiiA plants. The experiment was repeated two times with similar results.
The ISR capacity of S. marcescens 90–166 on the wild-type plants against P. syringae pv. tabaci was observed but was not different on AiiA plants compared to the negative control (water control) (Fig. 4B). However, after the negative control treatment, disease severity was higher in wild-type plants than in AiiA plants. Positive control treatments (1 mM BTH) resulted in less lesion area than control and S. marcescens 90–166 treatments in both wild-type and AiiA plants.
ISR against CMV in the AHL and AiiA tobacco plants
To avoid effect of QS on virulence of bacterial pathogens, a viral pathogen, CMV, that does not have a QS system was used for investigation of ISR capacity. In a previous study, drench application of S. marcescens 90–166 to tobacco plants elicited ISR to CMV (Ryu et al., 2004b). Here, we evaluated ISR to CMV in tobacco plants by measuring the number of symptomatic leaves per plant and by assessing the ELISA results. The application of S. marcescens 90–166 resulted in a 28.5-fold reduction in the number of symptomatic leaves in wild-type (Samsun NN) plants but did not affect AHL plants (Fig. 5A). However, the same S. marcescens 90–166 treatment resulted in a significant reduction in the number of symptomatic leaves in both wild-type (GX3) and AiiA transgenic plants (Fig. 5B). Symptoms of viral infection were rarely detected after positive control (BTH) treatment. The application of S. marcescens 90–166 reduced the number of symptomatic leaves on wild-type (GX3) and AiiA transgenic plants (Fig. 5B). Intriguingly, there were fewer symptomatic leaves on AiiA plants inoculated with S. marcescens 90–166 than on wild-type plants.
Fig. 5.
Disease severity of Cucumber mosaic virus (CMV) infection after treatment of acyl-homoserine lactone-producing (AHL) and -degrading (AiiA) transgenic tobacco plants with S. marcescens 90–166. (A) CMV disease severity on a 0–5 rating scale (described in Materials and Methods) after different treatments in AHL and AiiA plants. (B) CMV accumulation in the leaves of tobacco plants (AHL and AiiA plants) subjected to different treatments as determined by an enzyme-linked immunosorbent assay (ELISA). Disease severity and ELISA values are expressed as the mean of 10 plants per treatment, arranged as a randomized complete block with each treatment within a block consisting of a row of four plants. Different letters indicate significant differences using Fisher’s LSD test at P = 0.05. Different letters indicate a significant difference between wild type and AHL/AiiA plants.
Acetoin production depending on quorum sensing in S. marcescens 90–166
To investigate whether acetoin production is dependent on QS of S. marcescens 90–166, we transformed pKPE-aiiA (Cho et al., 2007) into S. marcescens 90–166 because it was difficult to abolish individually three genes corresponding each AHL production by mutagenesis in the genome of S. marcescens 90–166. We obtained no production of AHL (Fig. 6A) and significant reduction of the acetoin production (Fig. 6B) when detected by an indicator strain CV026 while vector control (pKPEA) produce both AHL and acetoin.
Fig. 6.
2,3-butanediol/acetoin production by quorum sensing dependent manner in S. marcescens 90–166. (A) AHL detection by reporter strain C. violaceum CV026 in the S. marcescens 90–166 containing pKPE-aiiA and its vector control (pKPEA). (B) Acetoin contents were determined by the Voges-Proskauer described previously (van Houdt et al., 2006). The experiments were repeated three times with similar results. Different letters indicate significant differences using Fisher’s LSD test at P = 0.05.
Discussion
The model PGPR strain S. marcescens 90–166 significantly reduced the symptoms of infection by Colletotrichum orbiculare, P. syringae pv. lachrymans, P. carotovorum, blue mold, CMV, and even cucumber beetles in the systemic tissues of many crop plants and Arabidopsis thaliana (Enebak and Carey, 2000; Kloepper et al., 2004; Kloepper and Ryu, 2006; Press et al., 1997; Press et al., 2001; Raupach et al., 1996; Ryu et al., 2004b; Zhang et al., 2002). Even though S. marcescens 90–166 secretes SA exogenously, inoculation of a SA-negative mutant into the roots of tobacco plants maintained ISR to P. syringae pv. tabaci on the leaves (Press et al., 1997). Furthermore, transgenic salicylate hydroxylase (NahG), which renders SA inactive by converting it to catechol, did not inhibit S. marcescens 90–166-mediated ISR (Press et al., 1997), indicating that SA is not important for eliciting ISR by S. marcescens 90–166. Other ISR determinant candidates in S. marcescens 90–166 are the siderophores. The siderophoreminus mutant strain, S. marcescens 90–166–2882, reduced CMV symptoms in Arabidopsis Col-0 when compared with the control treatment but was unable to induce ISR to C. orbicularie in cucumber or P. syringae pv. tabaci in tobacco (Ryu et al, 2004; Press et al., 1997). These results indicate that S. marcescens 90–166 activates different signaling pathways depending on the pathogen. The other possible explanation is that some unknown bacterial determinants mediate ISR.
Our previous study revealed that bacterial QS played an important role in eliciting ISR by S. marcescens 90–166 (Wilson et al., 1997). In the current study, we aimed to provide new evidence that QS from S. marcescens 90–166 was responsible for eliciting ISR to both a bacterial pathogen, P. syringae pv. tabaci, and a viral pathogen, CMV, in tobacco. To validate the involvement of QS, we employed two transgenic tobacco plants, one which could produce a Gram-negative QS signal and one which could degrade it (referred to as AHL and AiiA tobacco plants, respectively). First, we confirmed that S. marcescens 90–166 produced BHL, OHL, OHHL (Fig. 1). Second, we evaluated the effect of different tobacco plants (AHL, AiiA and their parental strains) on rhizosphere bacterial dynamics. Gram-negative bacterial populations were increased on AHL plants and Gram-positive bacterial populations were increased on AiiA plants (Fig. 2). These results indicate that plant production of a Gram-negative bacterial QS signal recruited Gram-negative bacteria (Morello et al., 2004; Pierson et al., 1998). By contrast, AiiA plant produces AHLactonase and cleavage of the lactone ring of AHL resulted in signal interference of QS for Gram-negative bacteria, which caused a decrease of Gram-negative bacterial ratio. Furthermore, the increase of the Gram-positive bacterial ratio in AiiA plants can result from competition in the rhizosphere (Fray, 2002; Pierson et al., 1998). These results suggest that “chemical warfare” between Gram-negative and Gram-positive bacteria occurs in the rhizosphere and significantly affects bacterial population dynamics.
Intriguingly, our previous data also showed that the total populations of S. marcescens 90–166 and an entA mutant did not change at any time point in the rhizoplane, while only the endophytic population density of the entA mutant was significantly lower (Press et al., 2001). The lack of enterobactin production by the siderophoreminus mutant strain, S. marcescens 90–166–2882, may render this strain more susceptible to reactive oxygen species and result in reduced internal populations probably due to loss of self-protection from plant-oriented hydroxyl radicals. In addition, the loss of ISR capacity by mutant strain 90–166–2882 is likely attributable to the lack of endophytic colonization, leading to a failure to reach a sufficiently high density to activate the QS system. It remains to be determined if changes in internal colonization by the entA mutant contribute to changes in the ISR phenotype of this strain.
Similarly, it was reported that a critical bacterial volatile signal, 2,3-butanediol, was regulated by a QS system in the many species of Serratia (Kovacikova et al., 2005; van Loudt et al., 2006). Specifically, QS can affect 2,3-butanediol fermentation in S. plymuthica RVH1 and S. marcescens MG1 (Van Houdt et al., 2006; 2007). Among diverse bacterial metabolites, bacterial VOCs such as 2,3-butanediol elicited ISR in Arabidopsis and tobacco (Cortes-Barco et al., 2010a; 2010b; Ryu et al., 2004a). The increasing level of ISR in the AHL plants inoculated with S. marcescens 90–166 in the current study (Fig. 3) may be explained by an overproduction of 2,3-butanediol. In contrast, the AiiA tobacco plants inoculated with S. marcescens 90–166 showed reduced ISR, perhaps indicating that the breakdown of the AHL produced by S. marcescens 90–166 resulted in a lower level of 2,3-butanediol production. In addition, direct evidence shows that acetoin production is dependent on quorum sensing of strain 90–166 (Fig. 6).
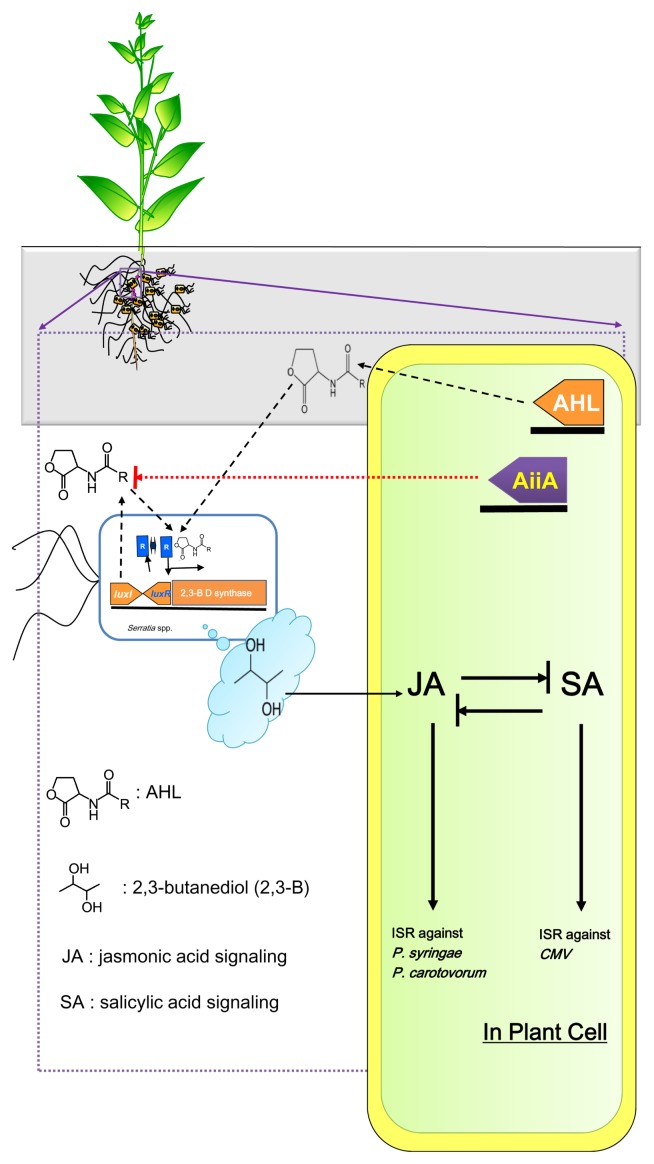
ISR to CMV by treatment with S. marcescens 90–166 in AHL and AiiA tobacco plants was unexpected. We do not have any clear hypotheses to explain the results. S. marcescens 90–166 has previously been shown to cause strong ISR to fungal, bacterial and viral pathogens in tomato, cucumber, tobacco, Arabidopsis and loblolly pine (Enebak and Carey, 2000; Press et al., 1997, 2001; Raupach et al., 1996; Zhang et al., 2002; Zhang et al., 2004). Seed treatment with S. marcescens 90–166 significantly and consistently reduced or delayed symptom development caused by CMV in cucumber and tomato (Raupach et al., 1996). The area under the disease progress curve (AUDPC) was significantly lower for S. marcescens 90–166 than for other PGPR strains and controls. A detailed mechanistic study of ISR to CMV elicited by S. marcescens 90–166 was undertaken using A. thaliana and its defense signaling mutants. ISR was shown to require the jasmonic acid (JA) signaling pathway, but not SA signaling. The involvement of the JA signaling pathway was confirmed by detection of the PDF1.2::GUS plant reporter and expression of the PDF1.2 gene. There are two hypotheses. First, production of AHL in the root system induces a change in the metabolism of S. marcescens 90-166. The unknown QS-dependent bacterial determinant results in the increased susceptibility of AHL tobacco plants against bacterial and viral pathogen infection. For instance, a previous study indicated that QS in S. marcescens controlled 2,3-butanediol production by regulating the production of the precursor acetoin (Rao et al., 2012). Previously 2,3-butanediol as a bacterial determinant induced to up-regulate JA signaling-related genes such as PDF1.2 (Ryu et al., 2004). The antagonistic action between JA and SA in plant defense responses has been well documented (Robert-Seilaniantz et al., 2011, Ryals et al., 1996). The bacterial volatile 2,3-butanediol can turn on JA signaling, resulting in suppression of SA signaling (Fig. 7). Besides signal antagonism, it is common that tobacco plants require SA signaling to elicit systemic resistance to viruses, such as Tobacco mosaic virus and CMV (Naylor et al., 1998). Second, the inhibition of SA signaling decreases plant defense mechanisms against CMV (Fig. 7). These hypothesis may explain the increase observed for ISR against CMV in AiiA tobacco plants.
Fig. 7.
Working model of the modulation of S. marcescens 90–166-elicited induced systemic resistance (ISR) in acyl-homoserine lactone-producing (AHL) or -degrading (AiiA) tobacco plants. In AHL plants, AHL produced by tobacco induces 2,3-butanediol synthesis after binding to the budB (luxR homologue) product. The 2,3-butanediol elicits jasmonic acid (JA)-dependent plant systemic defense. The JA defense mechanism in tobacco confers systemic resistance to P. carotovorum. Based on salicylic acid (SA) and JA antagonism, induction of JA signaling may suppress SA signaling leading to a reduction in ISR to CMV.
Acknowledgments
We thank Dr. Rupert G. Fray for AHL tobacco and Dr. Lian-Hui Zhang for AiiA tobacco. This research was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0011655), the Industrial Source Technology Development Program of the Ministry of Knowledge Economy (TGC0281011) of Korea, the Next-Generation BioGreen 21 Program (SSAC grant #PJ008170), Rural Development Administration, and the KRIBB initiative program, South Korea.
References
- Audenaert K, Pattery T, Cornelis P, Höfte M. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: Role of salicylic acid, pyochelin, and pyocyanin. Mol Plant-Microbe Interact. 2002;15:1147–1156. doi: 10.1094/MPMI.2002.15.11.1147. [DOI] [PubMed] [Google Scholar]
- Cho HS, Park SY, Ryu C-M, Kim JF, Kim JG, Park SH. Interference of quorum sensing and virulence of the rice pathogen Burkholderia glumae by an engineered endophytic bacterium. FEMS Microbiol Ecol. 2007;60:14–23. doi: 10.1111/j.1574-6941.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- Cortes-Barco AM, Goodwin PH, Hsiang T. Comparison of induced resistance activated by benzothiadiazole, (2R,3R)-butanediol and an isoparaffin mixture against anthracnose of Nicotiana benthamiana. Plant Pathol. 2010a;59:643–653. [Google Scholar]
- Cortes-Barco AM, Hsiang T, Goodwin PH. Induced systemic resistance against three foliar diseases of Agrostis stolonifera by (2R,3R)-butanediol or an isoparaffin mixture. Ann Appl Biol. 2010b;157:179–189. [Google Scholar]
- De Vleesschauwer D, Hofte M. Rhizobacteria-induced systemic resistance. Adv Bot Res. 2009;51:223–281. [Google Scholar]
- Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH. Quenching quorum-sensing-dependent bacterial infection by an N-acylhomoserine lactonase. Nature. 2001;411:813–817. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- Enebak SA, Carey WA. Evidence for induced systemic protection to fusiform rust in loblolly pine by plant growth-promoting rhizobacteria. Plant Dis. 2000;84:306–308. doi: 10.1094/PDIS.2000.84.3.306. [DOI] [PubMed] [Google Scholar]
- Fray RG, Throup JP, Wallace A, Daykin M, Williams P, Stewart GSAB, Grierson D. Plants genetically modified to produce N-acylhomoserine lactones communicate with bacteria. Nature Biotechnol. 1999;17:1017–1020. doi: 10.1038/13717. [DOI] [PubMed] [Google Scholar]
- Fray RG. Altering plant-microbe interaction through artificially manipulating bacterial quorum sensing. Ann Bot. 2002;89:245–53. doi: 10.1093/aob/mcf039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm MS, Sumayo M, Hwang YJ, Jeon SA, Park SJ, Lee JY, Ahn JH, Kim BS, Ryu CM, Ghim SY. Biological control and plant growth promoting capacity of rhizobacteria on pepper under greenhouse and field conditions. J Microbiol. 2012;50:380–385. doi: 10.1007/s12275-012-1477-y. [DOI] [PubMed] [Google Scholar]
- Kloepper JW, Tuzun S, Kuc JA. Proposed definitions related to induced disease resistance. Biocontrol Sci Technol. 1992;2:349–351. [Google Scholar]
- Kloepper JW, Ryu C-M, Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94:1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- Kloepper JW, Ryu C-M. Bacterial endophytes as elicitors of induced systemic resistance. In: Schulz BS, Boyle C, Sieber TN, editors. Soil Biology. Microbial Root Endophytes. Vol. 9. Berlin Heidelberg: Springer-Verlag. Germany; 2006. pp. 33–52. [Google Scholar]
- Kovacikova G, Lin W, Skorupski K. Dual regulation of genes involved in acetoin biosynthesis and motility/biofilm formation by the virulence activator AphA and the acetate-responsive LysR-type regulator AlsR in Vibrio Cholerae. Mol Microbiol. 2005;57:420–433. doi: 10.1111/j.1365-2958.2005.04700.x. [DOI] [PubMed] [Google Scholar]
- Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anolles G, Rolfe BG, Bauer WD. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc. Natl. Acad. Sci. USA. 2003;100:1444–1449. doi: 10.1073/pnas.262672599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- Moons P, Van Houdt R, Vivijs B, Michiels CM, Aertsen A. Integrated regulation of acetoin fermentation by quorum sensing and pH in Serratia plymuthica RVH1. Appl Environ Microbiol. 2011;77:3422–3427. doi: 10.1128/AEM.02763-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello JE, Pierson EA, Pierson LS., 3rd Negative cross-communication among wheat rhizosphere bacteria: effect on antibiotic production by the biological control bacterium Pseudomonas aureofaciens. Appl Environ Microbiol. 2004;70:30–84. 3103–3109. doi: 10.1128/AEM.70.5.3103-3109.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JF, Reddy MS, Ryu C-M, Kloepper JW, Li R. Rhizobacteria-mediated growth promotion of tomato leads to protection against Cucumber mosaic virus. Phytopathology. 2003;93:1301–1307. doi: 10.1094/PHYTO.2003.93.10.1301. [DOI] [PubMed] [Google Scholar]
- Naylor M, Murphy AM, Berry JO, Carr JP. Salicylic acid can induce resistance to plant virus movement. Mol Plant-Microbe Interact. 1998;11:860–868. [Google Scholar]
- Niu DD, Liu HX, Jiang CH, Wang YP, Wang QY, Jin HL, Guo JH. The plant growth-promoting rhizo-bacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. Mol Plant-Microbe Interact. 2011;24:533–542. doi: 10.1094/MPMI-09-10-0213. [DOI] [PubMed] [Google Scholar]
- Pang Y, Liu X, Ma Y, Chernin L, Berg G, Gao K. Induction of systemic resistance, root colonisation and biocontrol activities of the rhizospheric strain of Serratia plymuthica are dependent on N-acyl homoserine lactones. Eur J Plant Pathol. 2009;124:261–268. [Google Scholar]
- Pierson LS, 3rd, Wood DW, Pierson EA. Homoserine lactone-mediated gene regulation in plant-associated bacteria. Annu Rev Phytopathol. 1998;36:207–25. doi: 10.1146/annurev.phyto.36.1.207. [DOI] [PubMed] [Google Scholar]
- Press CM, Loper JE, Kloepper JW. Role of iron in rhizobacteria-mediated induced systemic resistance of cucumber. Phytopathology. 2001;91:593–598. doi: 10.1094/PHYTO.2001.91.6.593. [DOI] [PubMed] [Google Scholar]
- Press CM, Wilson M, Tuzun S, Kloepper JW. Salicylic acid produced by Serratia marcescens 90–166 is not the primary determinant of induced systemic resistance in cucumber or tobacco. Mol Plant-Microbe Interact. 1997;10:761–768. [Google Scholar]
- Rao B, Zhang LY, Sun J, Su G, Wei D, Chu J, Zhu J, Shen Y. Characterization and regulation of the 2,3-butanediol pathway in Serratia marcescens. Appl Microbiol Biotechnol. 2012;93:2147–2159. doi: 10.1007/s00253-011-3608-5. [DOI] [PubMed] [Google Scholar]
- Raupach GS, Liu L, Murphy JF, Tuzun S, Kloepper JW. Induced systemic resistance in cucumber and tomato against cucumber mosaic cucumovirus using plant growth-promoting rhizobacteria (PGPR) Plant Dis. 1996;80:891–894. [Google Scholar]
- Reimmann C, Ginet N, Michel L, Keel C, Michaux P, Krishnapillai V, Zala M, Heurlier K, Triandafillu K, Harms H, Defago G, Haas D. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology. 2002;148:923–932. doi: 10.1099/00221287-148-4-923. [DOI] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Grant M, Jones JD. Hormone crosstalk in plant disease and defense: more than just jas-monate-salicylate antagonism. Annu. Rev. Phytopathol. 2011;49:317–43. doi: 10.1146/annurev-phyto-073009-114447. 2011. [DOI] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu C-M, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004a;134:1017–1026. doi: 10.1104/pp.103.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu C-M, Murphy JF, Mysore KS, Kloepper JW. Plant growth-promoting rhizobacteria systemically protect Arabidopsis thaliana against Cucumber mosaic virus by a salicylic acid and NPR1-independent and jasmonic acid-dependent signaling pathway. Plant J. 2004b;39:381–392. doi: 10.1111/j.1365-313X.2004.02142.x. [DOI] [PubMed] [Google Scholar]
- Schaefer AL, Hanzelka BL, Parsek MR, Greenberg EP. Detection, purication, and structural elucidation of the acylhomoserine lactone inducer of Vibrio scheri luminescence and other related molecules. Methods Enzymol. 2000;305:288–301. doi: 10.1016/s0076-6879(00)05495-1. [DOI] [PubMed] [Google Scholar]
- Shaw PD, Ping G, Daly SL, Cha C, Cronan JE, Jr, Rinehart KL, Farrand SK. Detecting and characterizing N-acylhomoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikora A, Schenk ST, Stein E, Molitor A, Zuccaro A, Kogel KH. N-acyl-homoserine lactone confers resistance toward biotrophic and hemibiotrophic pathogens via altered activation of AtMPK6. Plant Physiol. 2011;157:1407–1418. doi: 10.1104/pp.111.180604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhegger R, Ihring A, Gantner S, Bahnweg G, Knappe C, Vogg G, Hutzler P, Schmid M, Van Breusegem F, Eberl L, Hartmann A, Langebartels C. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006;29:909–918. doi: 10.1111/j.1365-3040.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- Teplitski M, Robinson JB, Bauer WD. Plants secrete substances that mimic bacterial N-acyl homoserine lac-tone signal activities and affect population density-dependent behaviors in associated bacteria. Mol Plant-Microbe Interact. 2000;13:637–648. doi: 10.1094/MPMI.2000.13.6.637. [DOI] [PubMed] [Google Scholar]
- Van Houdt R, Moons P, Hueso Buj M, Michiels CW. N-acyl-L-homoserine lactone quorum sensing controls butanediol fermentation in Serratia plymuthica RVH1 and Serratia marcescens MG1. J Bacteriol. 2006;188:4570–4572. doi: 10.1128/JB.00144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houdt R, Moons P, Aertsen A, Jansen A, Vanoirbeek K, Daykin M, Williams P, Michiels CW. Characterization of a luxI/luxR-type quorum sensing system and N-acyl-homoserine lactone-dependent regulation of exo-enzyme and antibacterial component production in Serratia plymuthica RVH1. Res Microbiol. 2007;158:150–158. doi: 10.1016/j.resmic.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Whiteley M, Lee KM, Greenberg EP. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Ezelle JE, Press CM, Park KS, Farrand SK, Pierson LS, III, Kloepper JW. Autoinducer production in Serratia marcescens 90–166. Phytopathology. 1997;87:S103. [Google Scholar]
- Yang JW, Yi H-S, Kim H, Lee B, Lee S, Ghim S-Y, Ryu C-M. Whitefly infestation elicits defense responses against bacterial pathogens on the leaf and root and below-ground dynamic change of microflora in pepper. J Ecol. 2011;99:46–56. [Google Scholar]
- Zhang S, Moyne A-L, Reddy MS, Kloepper JW. The role of salicylic acid in induced systemic resistance elicited by plant growth-promoting rhizobacteria against blue mold of tobacco. Biol. Control. 2002;25:288–296. [Google Scholar]
- Zhang S, Reddy MS, Kloepper JW. Tobacco growth enhancement and blue mold disease protection by rhizobacteria: Relationship between plant growth promotion and systemic disease protection by PGPR strain 90–166. Plant Soil. 2004;262:277–288. [Google Scholar]