Abstract
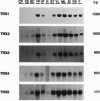
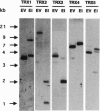
Five different clones encoding thioredoxin homologues were isolated from Arabidopsis thaliana cDNA libraries. On the basis of the sequences they encode divergent proteins, but all belong to the cytoplasmic thioredoxins h previously described in higher plants. The five proteins obtained by overexpressing the coding sequences in Escherichia coli present typical thioredoxin activities (NADP(+)-malate dehydrogenase activation and reduction by Arabidopsis thioredoxin reductase) despite the presence of a variant active site, Trp-Cys-Pro-Pro-Cys, in three proteins in place of the canonical Trp-Cys-Gly-Pro-Cys sequence described for thioredoxins in prokaryotes and eukaryotes. Southern blots show that each cDNA is encoded by a single gene but suggest the presence of additional related sequences in the Arabidopsis genome. This very complex diversity of thioredoxins h is probably common to all higher plants, since the Arabidopsis sequences appear to have diverged very early, at the beginning of plant speciation. This diversity allows the transduction of a redox signal into multiple pathways.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiychuk E., Kushnir S., Van Montagu M., Inzé D. The Arabidopsis thaliana apurinic endonuclease Arp reduces human transcription factors Fos and Jun. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3299–3303. doi: 10.1073/pnas.91.8.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugidou C., Marty I., Chartier Y., Meyer Y. The Nicotiana tabacum genome encodes two cytoplasmic thioredoxin genes which are differently expressed. Mol Gen Genet. 1993 Apr;238(1-2):285–293. doi: 10.1007/BF00279557. [DOI] [PubMed] [Google Scholar]
- Droux M., Jacquot J. P., Miginac-Maslow M., Gadal P., Huet J. C., Crawford N. A., Yee B. C., Buchanan B. B. Ferredoxin-thioredoxin reductase, an iron-sulfur enzyme linking light to enzyme regulation in oxygenic photosynthesis: purification and properties of the enzyme from C3, C4, and cyanobacterial species. Arch Biochem Biophys. 1987 Feb 1;252(2):426–439. doi: 10.1016/0003-9861(87)90049-x. [DOI] [PubMed] [Google Scholar]
- Eklund H., Cambillau C., Sjöberg B. M., Holmgren A., Jörnvall H., Hög J. O., Brändén C. I. Conformational and functional similarities between glutaredoxin and thioredoxins. EMBO J. 1984 Jul;3(7):1443–1449. doi: 10.1002/j.1460-2075.1984.tb01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein E., von Schaewen A., Scheibe R. Full-length cDNA sequences for both ferredoxin-thioredoxin reductase subunits from spinach (Spinacia oleracea L.). Biochim Biophys Acta. 1994 Apr 28;1185(2):252–254. doi: 10.1016/0005-2728(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Florencio F. J., Yee B. C., Johnson T. C., Buchanan B. B. An NADP/thioredoxin system in leaves: purification and characterization of NADP-thioredoxin reductase and thioredoxin h from spinach. Arch Biochem Biophys. 1988 Nov 1;266(2):496–507. doi: 10.1016/0003-9861(88)90282-2. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Gleason F. K., Holmgren A. Thioredoxin and related proteins in procaryotes. FEMS Microbiol Rev. 1988 Dec;4(4):271–297. doi: 10.1111/j.1574-6968.1988.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Gonnet G. H., Cohen M. A., Benner S. A. Exhaustive matching of the entire protein sequence database. Science. 1992 Jun 5;256(5062):1443–1445. doi: 10.1126/science.1604319. [DOI] [PubMed] [Google Scholar]
- Hartman H., Syvanen M., Buchanan B. B. Contrasting evolutionary histories of chloroplast thioredoxins f and m. Mol Biol Evol. 1990 May;7(3):247–254. doi: 10.1093/oxfordjournals.molbev.a040602. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Ueno Y., Okamoto T. Oxidoreductive regulation of nuclear factor kappa B. Involvement of a cellular reducing catalyst thioredoxin. J Biol Chem. 1993 May 25;268(15):11380–11388. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Höfte H., Desprez T., Amselem J., Chiapello H., Rouzé P., Caboche M., Moisan A., Jourjon M. F., Charpenteau J. L., Berthomieu P. An inventory of 1152 expressed sequence tags obtained by partial sequencing of cDNAs from Arabidopsis thaliana. Plant J. 1993 Dec;4(6):1051–1061. doi: 10.1046/j.1365-313x.1993.04061051.x. [DOI] [PubMed] [Google Scholar]
- Jacquot J. P., Rivera-Madrid R., Marinho P., Kollarova M., Le Maréchal P., Miginiac-Maslow M., Meyer Y. Arabidopsis thaliana NAPHP thioredoxin reductase. cDNA characterization and expression of the recombinant protein in Escherichia coli. J Mol Biol. 1994 Jan 28;235(4):1357–1363. doi: 10.1006/jmbi.1994.1091. [DOI] [PubMed] [Google Scholar]
- Kamo M., Tsugita A., Wiessner C., Wedel N., Bartling D., Herrmann R. G., Aguilar F., Gardet-Salvi L., Schürmann P. Primary structure of spinach-chloroplast thioredoxin f. Protein sequencing and analysis of complete cDNA clones for spinach-chloroplast thioredoxin f. Eur J Biochem. 1989 Jun 15;182(2):315–322. doi: 10.1111/j.1432-1033.1989.tb14832.x. [DOI] [PubMed] [Google Scholar]
- Kuge S., Jones N. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 1994 Feb 1;13(3):655–664. doi: 10.1002/j.1460-2075.1994.tb06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F., Chamberlain S. H., Chu C., Masiarz F. R., Shin S., Yee B. C., Buchanan B. B. Plant thioredoxin h: an animal-like thioredoxin occurring in multiple cell compartments. Arch Biochem Biophys. 1991 May 15;287(1):195–198. doi: 10.1016/0003-9861(91)90406-9. [DOI] [PubMed] [Google Scholar]
- Marty I., Brugidou C., Chartier Y., Meyer Y. Growth-related gene expression in Nicotiana tabacum mesophyll protoplasts. Plant J. 1993 Aug;4(2):265–278. doi: 10.1046/j.1365-313x.1993.04020265.x. [DOI] [PubMed] [Google Scholar]
- Marty I., Meyer Y. Nucleotide sequence of a cDNA encoding a tobacco thioredoxin. Plant Mol Biol. 1991 Jul;17(1):143–147. doi: 10.1007/BF00036817. [DOI] [PubMed] [Google Scholar]
- Meyer M., Schreck R., Baeuerle P. A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993 May;12(5):2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller E. G. Thioredoxin deficiency in yeast prolongs S phase and shortens the G1 interval of the cell cycle. J Biol Chem. 1991 May 15;266(14):9194–9202. [PubMed] [Google Scholar]
- Natsuyama S., Noda Y., Yamashita M., Nagahama Y., Mori T. Superoxide dismutase and thioredoxin restore defective p34cdc2 kinase activation in mouse two-cell block. Biochim Biophys Acta. 1993 Mar 10;1176(1-2):90–94. doi: 10.1016/0167-4889(93)90182-o. [DOI] [PubMed] [Google Scholar]
- Nikkola M., Gleason F. K., Saarinen M., Joelson T., Björnberg O., Eklund H. A putative glutathione-binding site in T4 glutaredoxin investigated by site-directed mutagenesis. J Biol Chem. 1991 Aug 25;266(24):16105–16112. [PubMed] [Google Scholar]
- Pearson W. R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Ren X., Björnstedt M., Shen B., Ericson M. L., Holmgren A. Mutagenesis of structural half-cystine residues in human thioredoxin and effects on the regulation of activity by selenodiglutathione. Biochemistry. 1993 Sep 21;32(37):9701–9708. doi: 10.1021/bi00088a023. [DOI] [PubMed] [Google Scholar]
- Rivera-Madrid R., Marinho P., Brugidou C., Chartier Y., Meyer Y. Nucleotide sequence of a cDNA clone encoding an Arabidopsis thaliana thioredoxin h. Plant Physiol. 1993 May;102(1):327–328. doi: 10.1104/pp.102.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P., Holmgren A. Thioredoxin or glutaredoxin in Escherichia coli is essential for sulfate reduction but not for deoxyribonucleotide synthesis. J Bacteriol. 1990 Apr;172(4):1923–1929. doi: 10.1128/jb.172.4.1923-1929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz H. K., Flickinger T. W., Mittendorf E., Pellicena-Palle A., Petschek J. P., Albrecht E. B. The Drosophila maternal effect locus deadhead encodes a thioredoxin homolog required for female meiosis and early embryonic development. Genetics. 1994 Mar;136(3):1075–1086. doi: 10.1093/genetics/136.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R. Redox-modulation of chloroplast enzymes : a common principle for individual control. Plant Physiol. 1991 May;96(1):1–3. doi: 10.1104/pp.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirzadegan M., Christie P., Seemann J. R. An efficient method for isolation of RNA from tissue cultured plant cells. Nucleic Acids Res. 1991 Nov 11;19(21):6055–6055. doi: 10.1093/nar/19.21.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaya Y., Maeda Y., Mitsui A., Kondo N., Matsui H., Hamuro J., Brown N., Arai K., Yokota T., Wakasugi H. ATL-derived factor (ADF), an IL-2 receptor/Tac inducer homologous to thioredoxin; possible involvement of dithiol-reduction in the IL-2 receptor induction. EMBO J. 1989 Mar;8(3):757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonissen K., Wells J., Cock I., Perkins A., Orozco C., Clarke F. Site-directed mutagenesis of human thioredoxin. Identification of cysteine 74 as critical to its function in the "early pregnancy factor" system. J Biol Chem. 1993 Oct 25;268(30):22485–22489. [PubMed] [Google Scholar]
- Wetterauer B., Jacquot J. P., Véron M. Thioredoxins from Dictyostelium discoideum are a developmentally regulated multigene family. J Biol Chem. 1992 May 15;267(14):9895–9904. [PubMed] [Google Scholar]
- Wetterauer B., Véron M., Miginiac-Maslow M., Decottignies P., Jacquot J. P. Biochemical characterization of thioredoxin 1 from Dictyostelium discoideum. Eur J Biochem. 1992 Oct 15;209(2):643–649. doi: 10.1111/j.1432-1033.1992.tb17331.x. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lamotte-Guery F., Miginiac-Maslow M., Decottignies P., Stein M., Minard P., Jacquot J. P. Mutation of a negatively charged amino acid in thioredoxin modifies its reactivity with chloroplastic enzymes. Eur J Biochem. 1991 Mar 14;196(2):287–294. doi: 10.1111/j.1432-1033.1991.tb15816.x. [DOI] [PubMed] [Google Scholar]
- de Lamotte-Guery F., Miginiac-Maslow M., Decottignies P., Stein M., Minard P., Jacquot J. P. Mutation of a negatively charged amino acid in thioredoxin modifies its reactivity with chloroplastic enzymes. Eur J Biochem. 1991 Mar 14;196(2):287–294. doi: 10.1111/j.1432-1033.1991.tb15816.x. [DOI] [PubMed] [Google Scholar]