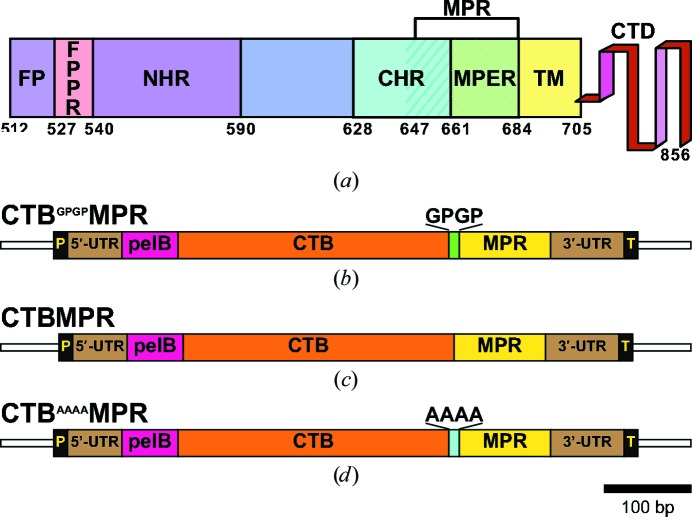
Figure 1.
(a) The architecture of gp41. FP (residues 512–527), fusion peptide; FPPR (residues 528–539), fusion peptide proximal region; NHR (residues 540–590), N-terminal heptad-repeat region; CHR (residues 628–661), C-terminal heptad-repeat region; MPER (residues 662–684), membrane-proximal external region; MPR (residues 647–684, hatched), membrane-proximal region; TM (residues 685–705), transmembrane domain; CTD (residues 706–856), cytoplasmic C-terminal domain. (b, c, d) DNA constructs for the expression in E. coli of the indicated CTB-MPR fusion proteins are based on elements of the pET-22b expression vector. P, T7 bacteriophage promoter; 5′-UTR, upstream untranslated region; pelB, the periplasmic targeting sequence of pectate lyase B of Erwinia carotovora; CTB, cholera toxin B subunit; MPR, the membrane-proximal region of the gp41 protein of HIV-1; 3′-UTR, downstream untranslated region; T, T7 terminator. The GPGP and AAAA linkers are indicated above their respective constructs. The three constructs encode the fusion proteins CTBGPGPMPR (b), CTBMPR (c) and CTBAAAAMPR (d) with expected molecular masses (after the processing of the pelB leader sequence) of 16.7, 16.4 and 16.7 kDa, respectively.