SUMMARY
Three-dimensional (3D) imaging technology has been widely used to analyse facial morphology and has revealed an influence of some medical conditions on craniofacial growth and morphology.
The aim of the study is to investigate whether craniofacial morphology is different in atopic Caucasian children compared with controls.
Study design included observational longitudinal cohort study. Atopy was diagnosed via skin-prick tests performed at 7.5 years of age. The cohort was followed to 15 years of age as part of the Avon Longitudinal Study of Parents and Children (ALSPAC). A total of 734 atopic and 2829 controls were identified. 3D laser surface facial scans were obtained at 15 years of age. Twenty-one reproducible facial landmarks (x, y, z co-ordinates) were identified on each facial scan. Inter-landmark distances and average facial shells for atopic and non-atopic children were compared with explore differences in face shape between the groups.
Both total anterior face height (pg–g, pg–men) and mid-face height (Is–men, sn–men, n–sn) were longer (0.6 and 0.4mm respectively) in atopic children when compared with non-atopic children. No facial differences were detected in the transverse and antero-posterior relationships.
Small but statistically significant differences were detected in the total and mid-face height between atopic and non-atopic children. No differences were detected in the transverse and antero-posterior relationships.
Introduction
Atopy is the tendency to develop immunoglobulin E (IgE) antibodies to commonly encountered environmental allergens (Jarvis and Burney, 1998). The most common clinical manifestations of atopy are asthma, allergic rhinitis, and atopic dermatitis (Burney et al., 1989). Development of atopic responses has been linked to several genes and gene products (Steinke et al., 2008). Thus, atopic diseases represent a complex gene and environmental interaction in which environmental antigens interact with the immune system, producing atopic (IgE) responses (Peden, 2000). In epidemiological studies, skin prick testing provides an expedient test for atopy (Oryszczyn, 1991; Cookson et al., 1989; Zimmerman, 1988), and/or elevated total serum IgE level (Cookson et al., 1989; Stempel, 1980).
Coca and Cooke (1923) introduced the term ‘atopy’, which mainly indicates people with asthma, fever, eczema, urtricaria, and the food allergies. The author gave another definition of the atopy: ‘it is the abnormal condition of immunology and it would cause inflammation of organs in the body’. The combination of mast cells and basophils are used to differentiate atopy from non-atopy. The author stated that atopy is the genetic predisposition that would produce mast cells and may cause eosinophilia. The term atopy represents the group of diseases that mainly develops in allergic conditions.
The relevance of atopy in facial growth is that environmental stimuli and irritations may cause chronic swelling of the nasopharyngeal mucous membranes in atopic individuals. Obstruction of the nasal airway due to atopic allergy can therefore be associated with mouth breathing and facial anomalies, including malocclusions such as increased overjet, a higher palatal plane, narrowing of both upper and lower arches, and proclination of incisors (Jefferson, 2010; Faria, 2002; Koski and Lähdemäki, 1975; Linder-Aronson, 1974, 1970; Ricketts, 1968). The switch from nasal to oro-nasal breathing results in functional adaptation including an increase in total face height and vertical development of the lower anterior face (Tourne, 1990).
A retrospective cephalometric comparative study of 55 paediatric patients who suffered from nasal obstruction and 61 controls indicated that naso-respiratory obstruction was associated with mouth breathing. In the children with nasal obstruction there was a higher tendency for a backward rotation of the mandible associated with an increase in anterior face height by 3 degree (SD 5) of the mandibular plane angle (Harari et al., 2010).
The influence of asthma on face shape has been reported previously in a large sample indicating that the mid-face height was 0.4mm (95% CI) shorter in asthmatic when compared with non-asthmatic females (Al Ali et al., 2012).
The effect of atopy on craniofacial structures (determined cephalometrically) was studied in 100 11-year-old school children and indicated a backward rotation of the mandibular body and an anterior lowering of the nasal floor in the moderate and severe nasal allergy groups. Differences were more pronounced in children presenting with atopy and enlarged adenoids (Hannuksela, 1981).
Cephalometric analysis was undertaken for 30 chronically allergic mouth-breathing subjects and 15 nonallergic nose breathers (Bresolin and co-workers in 1983) and revealed that the upper anterior face height and the total anterior face height were significantly larger in the mouth breathers. The maxilla and mandible were more retrognathic in mouth breathers. Maxillary intermolar width was narrower in the mouth breathers and was associated with a higher prevalence of posterior crossbite.
Three-dimensional (3D) analyses of facial surface anatomy are fundamental in determining deviations from normal facial morphology. 3D imaging technologies have provided both reliable and accurate data capture for facial analysis (Kau and Richmond, 2010; Kau et al., 2005; Kevin and Oleh, 1996, Toma et al., 2009, 2011; Kau et al., 2005). Quantitative analysis of facial morphology is of vital importance in determining facial discrepancies in individuals with craniofacial deformities (Kevin and Oleh, 1996). Two-dimensional techniques such as cephalometry and photographs are of limited value in describing the complex 3D topography of the facial surface.
The aim of the present study is to investigate the influence of atopy on craniofacial morphology using 3D laser scan imaging technology and to assess the null hypothesis that there is no difference in face shape in atopic and non-atopic Caucasian children.
Subjects and Methods
Sample
The children involved in this study were recruited from the Avon Longitudinal Study of Parents and Children (ALSPAC), which was designed to explore genetic and environmental factors impacting on health, behaviour, and development of children (Golding et al., 2001). Ethical approval was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees prior to the commencement of the study.
All children were invited to attend a clinic at 7.5 years of age, where skin-prick tests were performed to a panel of six allergens (house dust mite, cat, mixed grass, mixed nuts, peanut, and milk) and a positive (1 % histamine solution) and negative (diluent) controls (Henderson et al., 2008). Skin tests were carried out on the anterior surface of the left arm using new disposable sterile lancets for each allergen tested. The skin was pricked through a drop of allergen solutions, which were blotted off after five minutes, and the test were read after a further 10 minutes (Henderson et al., 2008). The maximum weal diameter was measured and a second measurement performed at 90 degree to the first, and the mean weal diameter was calculated. Atopy was defined as a positive skin-prick test to the above allergens (weal diameter ≥1mm with as negative diluents response, Henderson et al., 2008).
The cohort was re-called when the children were 15 years of age where 3D laser facial scans were acquired. Body weight and height were also measured for all children. The final sample in this study consisted of 734 atopic and 2829 non-atopic control group children.
Facial imaging
3D facial images of the children were captured using a pair of high-resolution Vivid 900 laser scanners (Konica Minolta Sensing Europe, Milton Keynes, UK), with a reported manufacturing accuracy of 0.1mm (Kau et al., 2003). The scanners were controlled with Multi-scan software (Cebas Computer GmBH, Eppelheim, Germany). Rapidform 2006 (INUS Technology, Seoul, Korea) was used to process and analyse the facial scans. The right and left facial scans of each child were registered and merged using a locally developed subroutine for above mentioned software. Quality of registration of right and left facial scans for all subjects was determined using average distance between shells and percentage of overlap between the right and left shells. The facial scan was evaluated as having good quality when the average distance between right and left facial scan is 0.3mm and below and is the most suitable for merging (Kau and Richmond, 2008). Generally, 70–100 per cent overlap of the right and left facial shells with a tolerance level set at 0.5mm indicates facial shells suitable for merging, and any non-suitable scans were excluded from the sample of the study (Toma et al., 2008; Kau and Richmond, 2010).
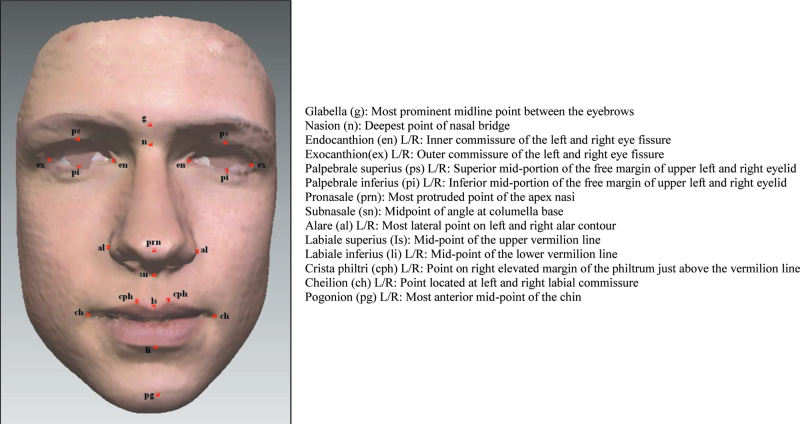
Twentyone facial soft tissue landmarks (Figure 1) were manually identified on each facial image (Toma et al., 2011, 2009), and the x, y and z co-ordinates were recorded. Landmarks reproducibility in the three dimensions has been reported previously with an error of less than 1mm for both intra- and inter-examiner assessments (Toma et al., 2009).
Figure 1.
Facial soft tissue landmarks.
Antero-posterior, vertical and transverse relationships in face shape analysis were determined using the following soft tissue parameters: exR–exL (inter-eye distance) and al–al (nose width) allows transverse analysis, pg–g, pg–men (total face height), sn–men, Is–men, n–sn (mid-face height) allows vertical relationship analysis, s–sn–pg (face convexity) relate to antero-posterior features. The statistical analysis used 95% confidence intervals (CIs) of the difference in facial parameters between the atopic and non-atopic group. The facial parameters included in the analysis were the most common parameters reported that might be influenced by different modes of breathing (Harari et al., 2010; Kerr et al., 1989; Wenzel et al., 1985; Bresolin et al., 1983).
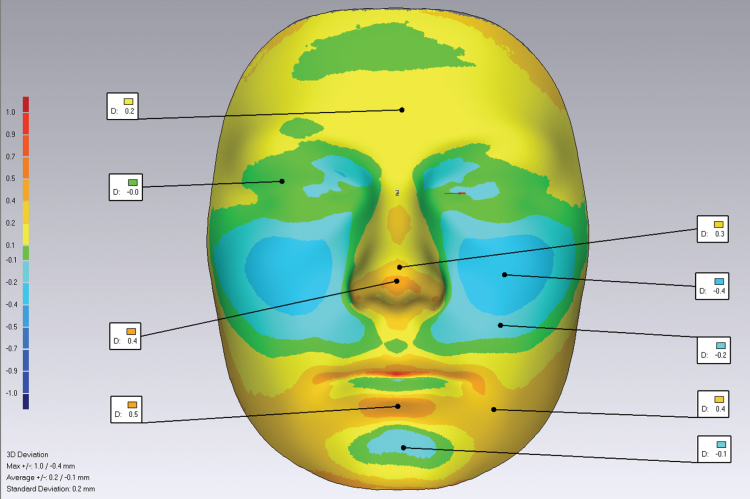
Average facial shells were created for atopic and non-atopic children using a previously validated method (Kau and Richmond, 2010; Zhurov et al., 2010). Average faces were superimposed on the mid-endocanthion point. Differences in morphology were presented using colour maps, with a tolerance level of 0.1mm to highlight significant topographical facial differences.
Body weight was measured using Tanita scales to the nearest 0.1kg. Height was measured to the nearest 0.1cm using Harpenden stadiometer (Lawlor et al., 2010). Body mass index (BMI) was then calculated as weight (kg) divided by the square of height (m2).
Statistical analysis
To compare the facial features of atopic and non-atopic children, independent t-test, 95% CI of the differences in the measured facial parameters were used.
Results
The sample represented 3563 Caucasian children (1692 male/ 1871 female) with no obvious facial dysmorphology. Of the 3563 ALSPAC children, 734 children (411 male/ 323 female) were recorded as atopic at age 7.5 years (Henderson et al., 2008). Mean BMI of the study sample was within the range (21.2kg/m²; reported normal range 17–24.2kg/m², Cole et al., 1998; Cole et al., 1995) with no significant differences between the atopic and the non-atopic group.
Generally, the parameters describing transverse distances and the antero-posterior features in atopic children did not differ significantly from the non-atopic group (Table 1). However, all the parameters that describe vertical relationships showed significant differences between the atopic and non-atopic children. Both total anterior face height (pg–g, pg–men) and mid-face height (Is–men, sn–men, n–sn) were longer (0.6 and 0.4mm, respectively) in atopic children when compared with non-atopic children. Superimposition of average facial shells of atopic and non-atopic children showed a significant morphological difference between the groups in the z direction. The direction of the differences for the average facial shells for the atopy and non-atopic groups are shown in three presentations of colour deviation maps (Figure 2). The cheeks and chin are less prominent in the atopic group, and the atopic children showed an increased nose width (0.49mm) compared with non-atopic children.
Table 1.
Summary of the statistics of 3D co-ordinates.
| Atopic (n = 734) | Non-atopic (n = 2829) | Mean difference | 95% confidence interval | |||
|---|---|---|---|---|---|---|
| Mean | Standard deviation(SD) | Mean | SD | |||
| Eyes Distance (mm, exR–exL) | 87.4 | 3.97 | 87.5 | 4.05 | 0.06 | −0.26–0.39 |
| Nose width (mm, al–al) | 33.8 | 2.86 | 33.6 | 2.68 | −0.18 | −0.40–0.03 |
| Mid-face (mm, ls–men) | 62.0 | 4.22 | 61.6 | 3.95 | −0.45 | −0.79–−0.11 |
| Mid-face (mm, sn–men) | 48.5 | 3.69 | 48.0 | 3.43 | −0.42 | −0.71–−0.13 |
| Mid-face (mm, n–sn) | 52.6 | 3.94 | 52.3 | 3.83 | −0.34 | −0.65–−0.03 |
| Total face height (mm, pg–g) | 114.2 | 6.38 | 113.6 | 6.23 | −0.60 | −1.09–−0.07 |
| Total face height (mm, pg–men) | 94.1 | 5.85 | 93.6 | 5.66 | −0.60 | −1.03–−0.11 |
| Face convexity (angle, n–sn–pg) | 162.0 | 5.51 | 162.5 | 5.64 | 0.41 | −0.04–0.86 |
Figure 2.
The green areas represent no difference in the atopy and control groups (0mm). The blue area indicates less prominent cheeks and chin point in the atopic group (0.1–0.4mm) the deeper blue representing greater facial retrusion in the atopic group. The yellow areas are those prominent features in the atopic face—prominent forehead, nose, lower lip and wider forehead, nose and lower jaw (0.1–0.5mm).
Discussion
This study is the first to investigate the influence of atopy on facial morphology in a large cohort of 15-year-old children using 3D facial imaging. Currently available 3D soft tissue imaging technologies allow clinicians to evaluate facial differences as a result of medical and surgical interventions. The 3D laser scanning system has the advantage of being quick, non-invasive, and easy to use unlike other 3D hard tissue imaging systems, such as Cone Beam Computed Tomography (CBCT) and Magnetic Resonance Imaging (MRI), which are both expensive, time-consuming, and with CBCT presenting a radiation risk (Kau et al., 2007).
The present study revealed that laser scanner can be used in large-scale population studies and is able to detect small facial differences (0.6mm) in atopic children who had longer faces when compared with non-atopic children.
While craniofacial morphology is primarily determined by heredity (David and Carlson, 2005), environmental stimuli also markedly influence the growth of the bones, as for instance in patients with partially or totally obstructed nasal airways (Kiliç & Oktay, 2008; Dunn et al., 1973). Atopic conditions are associated with chronic swelling of the nasopharyngeal membrane that can affect normal breathing (Hannuksela, 1981). The mode of breathing and its effect on craniofacial growth has been a controversial issue within orthodontics for decades. It has been described that obstructed nasal breathing leads to mouth breathing and the so called ‘adenoidal face’ (long face syndrome) with an increased anterior face height (Chaves et al., 2010; Bresolin et al., 1983; Linder-Aronson, 1970; Subtelny, 1954). This craniofacial development has been explained by changes in the muscular balance. Mouth breathing leads to a lower tongue position in the oral cavity that will alter the force balance from the cheeks and tongue, which may be associated with an increased anterior face height, a steep mandibular plane angle resulting in a retrognathic mandible. In addition, incompetent lips, a narrow upper dental arch, retroclined mandibular incisors are also seen, in children with reported nasal breathing compared with healthy controls (Solow et al., 1984; McNamara, 1981; Linder-Aronson, 1979; Solow and Kreiborg, 1977).
Previous data and results from the 3D study of the influence of asthma on face shape of 4747 children from Avon Longitudinal Study of Parents and children (Al Ali et al., 2012) showed shorter mid-face height in asthmatic females (0.4mm) compared with non-asthmatic children. The confliction with results in this study could be explained by the fact that the relationship between atopy and asthma is not straightforward (Carroll et al., 2006). The available epidemiological evidence suggests that the proportion of asthma cases that are attributed to atopy is less than one-half, indicating that the importance of atopy as a cause of asthma in individuals may have been overemphasized (Pearce et al., 1999).
Findings in the present study are consistent with the proposed possible contribution of environmental stimuli, such as atopic allergic conditions and breathing difficulty on craniofacial growth and morphology. Atopic diseases, therefore, should be taken into consideration when examining orthodontic patients and when planning treatment for the correction of malocclusion.
Although results have revealed an association between mouth breathing in atopic allergy and modification in craniofacial growth and morphology, there is a need to know how much nasal obstruction has to occur before an effect on facial growth is observable? Is this a reversible condition and is there a time dependent relationship? These questions required a fundamental premise of being able to define nasal obstruction its position and severity (Vig, 1998).
Conclusion
The results of this study indicate that face shape differences were present in atopic children compared with matched control. The major features included increased mid-face height and total anterior face height. The research methodology provides a framework for the investigation of environmental factors that can influence craniofacial development using 3D facial imaging.
Funding
The UK Medical Research Council, the Wellcome Trust, and the Universities of Bristol and Cardiff provided support for this ALSPAC study.
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
References
- Ali Al, et al. 2012. The influence of asthma on face shape: A three dimensional study. European Journal of Orthodontics 36: 373–380 [DOI] [PubMed] [Google Scholar]
- Bresolin D, Shapiro P A, Shapiro G G, Chapko M K, Dassel S. 1983. Mouth breathing in allergic children: its relationship to dentofacial development. American Journal of Orthodontics 83: 334–340 [DOI] [PubMed] [Google Scholar]
- Burney P G J, Anderson H R, Burrows B. 1989. Epidemiology. In: The Role of Inflammatory Processes in Airway Hyperresponsiveness. Blackwell Scientific, Oxford, pp. 222–250 [Google Scholar]
- Carroll W D, et al. 2006. Asthma severity and atopy: how clear is the relationship? Archives of Disease in Childhood 91: 405–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves T C, de Andrade e Silva T S, Monteiro S A, Watanabe P C, Oliveira A S, Grossi D B. 2010. Craniocervical posture and hyoid bone position in children with mild and moderate asthma and mouth breathing. International Journal of Pediatric Otorhinolaryngology 74: 1021–1027 [DOI] [PubMed] [Google Scholar]
- Coca A F, Cooke R A. 1923. On the classification of the phenomenon of hypersensitiveness. The Journal of Immunology 8: 163–182 [Google Scholar]
- Cole T J, Freeman J V, Preece M A. 1995. Body mass index reference curves for the UK, 1990. Archives of Disease in Childhood 73: 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T J, Freeman J V, Preece M A. 1998. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Statistics in Medicine 17: 407–429 [PubMed] [Google Scholar]
- Cookson W O, Sharp P A, Faux J A, Hopkin J M. 1989. Linkage between immunoglobulin E responses underlying asthma and rhinitis and chromosome 11q. Lancet 1: 1292–1295 [DOI] [PubMed] [Google Scholar]
- David S, Carlson 2005. Theories of Craniofacial Growth in the Postgenomic Era. Seminars in Orthodontics 11: 172–183 [Google Scholar]
- Dunn G F, Green L J, Cunat J J. 1973. Relationships between variation of mandibular morphology and variation of nasopharyngeal airway size in monozygotic twins. The Angle Orthodontist 43: 129–135 [DOI] [PubMed] [Google Scholar]
- Faria P T, de Oliveira Ruellas A C, Matsumoto M A, Anselmo-Lima W T, Pereira F C. 2002. Dentofacial morphology of mouth breathing children. Brazilian Dental Journal 13: 129–132 [DOI] [PubMed] [Google Scholar]
- Golding J, Pembrey M, Jones R. ALSPAC Study Team. 2001. ALSPAC–the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatric and Perinatal Epidemiology 15: 74–87 [DOI] [PubMed] [Google Scholar]
- Hannuksela A. 1981. The effect of moderate and severe atopy on the facial skeleton. European Journal of Orthodontics 3: 187–193 [DOI] [PubMed] [Google Scholar]
- Harari D, Redlich M, Miri S, Hamud T, Gross M. 2010. The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patients. The Laryngoscope 120: 2089–2093 [DOI] [PubMed] [Google Scholar]
- Henderson J, Sherriff A, Farrow A, Ayres J G. 2008. Household chemicals, persistent wheezing and lung function: effect modification by atopy? The European Respiratory Journal 31: 547–554 [DOI] [PubMed] [Google Scholar]
- Jarvis D, Burney P. 1998. ABC of allergies. The epidemiology of allergic disease. BMJ (Clinical Research ed.) 316: 607–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson Y. 2010. Mouth breathing: adverse effects on facial growth, health, academics, and behavior. General Dentistry 58: 18–25; quiz 26 [PubMed] [Google Scholar]
- Kau C H, Zhurov A, Knox J, Richmond S. 2003. Validation of a portable three-dimensional laser scanner for field studies. European Craniofacial Congress: 41–45 [Google Scholar]
- Kau C H, et al. 2005. Reliability of measuring facial morphology with a 3-dimensional laser scanning system. American Journal of Orthodontics and Dentofacial Orthopedics 128: 424–430 [DOI] [PubMed] [Google Scholar]
- Kau C H, Richmond S, Incrapera A, English J, Xia J J. 2007. Three-dimensional surface acquisition systems for the study of facial morphology and their application to maxillofacial surgery. The International Journal of Medical Robotics + Computer Assisted Surgery: MRCAS 3: 97–110 [DOI] [PubMed] [Google Scholar]
- Kau C H, Richmond S. 2008. Three-dimensional analysis of facial morphology surface changes in untreated children from 12 to 14 years of age. American Journal of Orthodontics and Dentofacial Orthopedics 134: 751–760 [DOI] [PubMed] [Google Scholar]
- Kau C H, Richmond S. 2010. Three-dimensional Imaging for Orthodontics and Maxillofacial Surgery. Wiley-Blackwell, London: [Google Scholar]
- Kerr W J, McWilliam J S, Linder-Aronson S. 1989. Mandibular form and position related to changed mode of breathing–a five-year longitudinal study. The Angle Orthodontist 59: 91–96 [DOI] [PubMed] [Google Scholar]
- Kevin B, Oleh A. 1996. Three-Dimensional Facial Anthropometry Using a Laser Surface Scanner: Validation of the Technique. Plastic & Reconstructive Surgery 98: 226–235 [DOI] [PubMed] [Google Scholar]
- Kiliç N, Oktay H. 2008. Effects of rapid maxillary expansion on nasal breathing and some naso-respiratory and breathing problems in growing children: a literature review. International Journal of Pediatric Otorhinolaryngology 72: 1595–1601 [DOI] [PubMed] [Google Scholar]
- Koski K, Lähdemäki P. 1975. Adaptation of the mandible in children with adenoids. American Journal of Orthodontics 68: 660–665 [DOI] [PubMed] [Google Scholar]
- Lawlor D A, et al. 2010. Association between general and central adiposity in childhood, and change in these, with cardiovascular risk factors in adolescence: prospective cohort study. BMJ (Clinical Research ed.) 341: c6224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder-Aronson S. 1970. Adenoids. Their effect on mode of breathing and nasal airflow and their relationship to characteristics of the facial skeleton and the denition. A biometric, rhino-manometric and cephalometro-radiographic study on children with and without adenoids. Acta oto-laryngologica. Supplementum 265: 1–132 [PubMed] [Google Scholar]
- Linder-Aronson S. 1974. Effects of adenoidectomy on dentition and nasopharynx. American Journal of Orthodontics 65: 1–15 [PubMed] [Google Scholar]
- Linder-Aronson S. 1979. Respiratory function in relation to facial morphology and the dentition. British Journal of Orthodontics 6: 59–71 [DOI] [PubMed] [Google Scholar]
- McNamara J A. 1981. Influence of respiratory pattern on craniofacial growth. The Angle Orthodontist 51: 269–300 [DOI] [PubMed] [Google Scholar]
- Oryszczyn M P, Annesi I, Neukirch F, Dore M F, Kauffmann F. 1991. Relationships of total IgE level, skin prick test response, and smoking habits. Annals of Allergy 67: 355–358 [PubMed] [Google Scholar]
- Pearce N, Pekkanen J, Beasley R. 1999. How much asthma is really attributable to atopy? Thorax 54: 268–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden D B. 2000. Development of atopy and asthma: candidate environmental influences and important periods of exposure. Environmental Health Perspectives 108 Suppl 3: 475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts R M. 1968. Respiratory obstruction syndrome. American Journal of Orthodontics 54: 495–507 [DOI] [PubMed] [Google Scholar]
- Solow B, Kreiborg S. 1977. Soft-tissue stretching: a possible control factor in craniofacial morphogenesis. Scandinavian Journal of Dental Research 85: 505–507 [DOI] [PubMed] [Google Scholar]
- Solow B, Siersbaek-Nielsen S, Greve E. 1984. Airway adequacy, head posture, and craniofacial morphology. American Journal of Orthodontics 86: 214–223 [DOI] [PubMed] [Google Scholar]
- Steinke J W, Rich S S, Borish L. 2008. 5. Genetics of allergic disease. The Journal of Allergy and Clinical Immunology 121: S384–7; quiz S416 [DOI] [PubMed] [Google Scholar]
- Stempel D A, Clyde W A, Jr, Henderson F W, Collier A M. 1980. Serum IgE levels and the clinical expression of respiratory illnesses. The Journal of Pediatrics 97: 185–190 [DOI] [PubMed] [Google Scholar]
- Subtelny J D. 1954. The significance of adenoid tissue in orthodontia. The Angle Orthodontist 24: 59–69 [Google Scholar]
- Toma A M, Zhurov A, Playle R, Richmond S. 2008. A three-dimensional look for facial differences between males and females in a British-Caucasian sample aged 151/2 years old. Orthodontics & Craniofacial Research 11: 180–185 [DOI] [PubMed] [Google Scholar]
- Toma A M, Zhurov A, Playle R, Ong E, Richmond S. 2009. Reproducibility of facial soft tissue landmarks on 3D laser-scanned facial images. Orthodontics & Craniofacial Research 12: 33–42 [DOI] [PubMed] [Google Scholar]
- Toma A M, Zhurov A I, Playle R, Marshall D, Rosin P L, Richmond S. 2011. The assessment of facial variation in 4747 British school children. European Journal of Orthodontics 34: 655–664 [DOI] [PubMed] [Google Scholar]
- Tourne L P. 1990. The long face syndrome and impairment of the nasopharyngeal airway. The Angle Orthodontist 60: 167–176 [DOI] [PubMed] [Google Scholar]
- Vig K W. 1998. Nasal obstruction and facial growth: the strength of evidence for clinical assumptions. American Journal of Orthodontics and dentofacial orthopedics 113: 603–611 [DOI] [PubMed] [Google Scholar]
- Wenzel A, Höjensgaard E, Henriksen J M. 1985. Craniofacial morphology and head posture in children with asthma and perennial rhinitis. European Journal of Orthodontics 7: 83–92 [DOI] [PubMed] [Google Scholar]
- Zimmerman B, et al. 1988. Allergy in asthma. I. The dose relationship of allergy to severity of childhood asthma. The Journal of Allergy and Clinical Immunology 81: 63–70 [DOI] [PubMed] [Google Scholar]
- Zhurov A I, Richmond S, Kau C H, Toma A. 2010. Averaging facial images. In: Kau C H, Richmond S. (eds.) Three-dimensional Imaging for Orthodontics and Maxillofacial Surgery. Wiley-Blackwell, London: [Google Scholar]