Abstract
Background: Demand for minimally invasive cosmetic procedures have led to an increased market of available products for facial rejuvenation. Objective: To characterize trends in the usage of aesthetic products, specifically the use of botulinum toxins and dermal fillers, by United States physicians. Methods: Data from the National Ambulatory Medical Care Survey was analyzed from 1993 to 2010 to evaluate the use of dermal fillers and neurotoxins in the United States outpatient setting. The types of physician specialties administering these products and their preferences in products were characterized. Results: There were an estimated 100,000 annual cosmetic visits at which a dermal filler was administered from 1993 to 2010. From 2002 to 2010, there were 140,000 annual cosmetic visits for a dermal filler and 440,000 visits for a neurotoxin. While collagen was the most common filler used over the entire study period, its use declined eight percent annually. Hyaluronic acid fillers were preferred from 2002 to 2010, followed by calcium hydroxylapatite filler, representing 50 percent and 16.1 percent of visits, respectively. The leading neurotoxin was onabotulinumtoxin A, used at 87.1 percent of visits. Dermatologists were the leading specialty for the cosmetic use of both dermal fillers and neurotoxins. Conclusion: Providers’ preference for cosmetic products appears to be influenced by their familiarity with them, with products that first came to market, such as the neurotoxin onabotulinumtoxin A and the hyaluronic acid fillers being used most frequently from 2002 to 2010.
The demand for minimally invasive cosmetic play a role in a provider’s preference of which specific procedures, such as the use of botulinum toxin and product(s) to use is not well studied, but is likely dermal fillers, has become increasingly popular. multifactorial and may include cost, personal experience, According to the American Society of Plastic Surgeons potential adverse effects, and patient preference. Surveyed 2012 statistics report, there has been a 680-percent experts in Asia recently reported choosing soft tissue fillers increase in the cosmetic use of botulinum toxin type A and and injection techniques based on experience, with the a 205-percent increase in soft tissue fillers from 2000 to visco-elasticity of the product being the determinant when 2012 among plastic surgeons, with 6.1 million and 2.0 choosing a filler depending on which specific facial unit is to million procedures, respectively, being performed in 2012.1 With this increased demand comes the introduction of newer injectable neurotoxins and fillers.
As newer facial rejuvenation products come to market, and as the number of minimally invasive procedures increase, it becomes increasingly important to characterize the current trends in aesthetic product usage. What factors play a role in a provider's preference of which specific product(s) to use is not well studied, but is likely multifactorial and may include cost, personal experience, potential adverse effects, and patient preference. Surveyed experts in Asia recently reported choosing soft tissue fillers and injection techniques based on experience, with the visco-elasticity of the product being the determinant when choosing a filler depending on which specific facial unit is to be augmented.2 Choice of products is also dependent on the market; the United States market is more restricted in which products are available due to United States Food and Drug Administration (FDA) regulations compared to the European market where access to greater aesthetic product selection exists.3
While the usage of aesthetic products for minimally invasive procedures has been monitored and reported by the American Society of Plastic Surgeons, characterization of usage trends by other specialists commonly performing these procedures has not been studied and may differ from plastic surgeons. The goal of this study is to characterize trends in the usage of aesthetic products, specifically the use of botulinum toxins and dermal fillers, by US physicians.
METHODS
The authors surveyed the National Ambulatory Medical Care Survey (NAMCS) to evaluate the use of dermal fillers and neurotoxins in the United States outpatient setting. The Centers for Disease Control and Prevention’s National Center for Health Statistics (NCHS) conducts this survey system yearly by sampling outpatient visits to physicians across the country.4 Data collected include patient characteristics (i.e., age, race, sex), clinical records (i.e., vital signs, diagnoses rendered, medications for patient), visit characteristics (i.e., expected payment type, type of visit, reason patient scheduled the visit), and office characteristics (i.e., use of electronic medical records, type of healthcare providers staffed, types of payments accepted). Data collected is then processed and accordingly coded. Diagnoses are classified using the International Classification of Diseases, 9th Revision, Clinical Modification system (ICD-9). Medications are coded using the National Drug Code Directory.
For this analysis, data from the 1993 to 2010 NAMCS surveys were used. All visits at which a dermal filler or neurotoxin was mentioned or the reason for the visit was a “collagen injection” or “botox injection” were included in the analysis. ICD-9 codes were then used to determine which visits were likely to be cosmetic. Visits to orthopedic outpatient clinics were excluded since their use of filler was likely not cosmetic. Data was also analyzed separately for 1993 to 2001 and 2002 to 2010 since the first neurotoxin approved for cosmetic use was in 2002 and the first hyaluronic acid approved for cosmetic use was in 2003. Dermal fillers included hyaluronic acid (HA), collagen, calcium hydroxylapatite, poly-L-lactic acid, platelet-rich fibrin matrix, polymethylmethacrylate microspheres, hydrogel polymers, liquid injectable silicone, and autologous fat. Neurotoxins included botulinum toxin types A and B. In total, 318 records were identified in which dermal fillers were administered and 433 records in which neurotoxins were injected.
The annual number of visits where these products were injected was estimated. These estimates were further divided by specific products used. Demographic features of patients (age, sex, race) at these visits were characterized. The visit rates per 100,000 persons were reported for each sex and racial group. These were calculated by taking the total number of visits for each group and dividing this value by the estimated population size for that group. The population estimates were extracted from the 2000 Census population estimates, as this year was an approximate midpoint of the data included. The types of physician specialties administering these products and their preferences in products were characterized, and the most common diagnoses rendered were reported.
All data management and analysis were performed using SAS 9.3 (SAS Institute Inc., Cary, North Carolina). SAS’ PROC SURVEY package was used to account for the complex survey design. Trends in the uses of specific products were calculated using PROC SURVEYREG package. To identify preferences in product use for dermatologists versus non-dermatologists, PROC SURVEYLOGISTIC was used to obtain odds ratios of product use. This study was declared exempt by the Wake Forest Baptist Hospital Institutional Review Board.
RESULTS
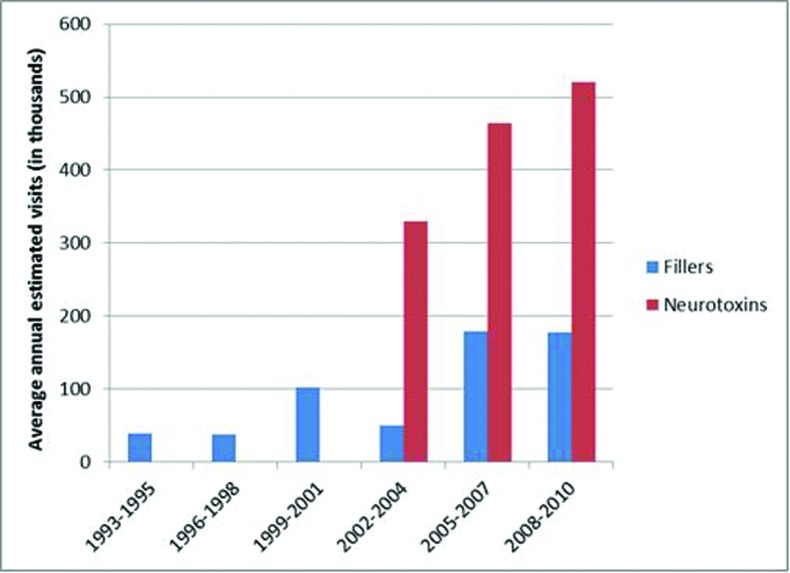
From 1993 to 2010, there were an estimated 600,000 (95% CI: 210,000-980,000) annual visits at which a filler was administered and 620,000 (95% CI: 160,000-1,100,000) annual visits at which a neurotoxin was administered. When data are restricted to visits likely to be cosmetic, there were an estimated 100,000 (95% CI: 60,000-140,000) annual visits at which a dermal filler was administered from 1993 to 2010. Neurotoxin cosmetic visits were first identified beginning in 2002 to 2004 (Figure 1). From 2002 to 2010, there were an estimated 140,000 (95% CI: 90,000-180,000) annual visits at which a dermal filler was administered for cosmetic purposes and 440,000 (95% CI: 260,000-610,000) annual visits at which a neurotoxin was administered. The most common filler was collagen (41.6% of visits) for the entire study period, with collagen representing 100 percent of visits from 1993 to 2001 and 15.9 percent of visits from 2002 to 2010 (Tables 1 and 2). However, from 2002 to 2010, HA fillers were the most common, followed by calcium hydroxylapatite filler, representing 50 and 16.1 percent of visits, respectfully (Table 2). The leading neurotoxin was onabotulinumtoxin A, used at 87.1 percent of visits (Table 2). The top diagnosis for both dermal filler and neurotoxin visits was other specified hypertrophic and atrophic conditions of skin, representing 44.3 and 50 percent of visits, respectfully (Table 3).
Figure 1.
Annual number of cosmetic visits from 1993 to 2010 at which a dermal filler or neurotoxin was administered. Neurotoxins were restricted to 2002 to 2010.
TABLE 1.
Estimated cosmetic visits where specific dermal filler products were mentioned from 1993-2010
| VISITS AT WHICH A FILLER WAS MENTIONED | TOTAL ESTIMATED VISITS | PERCENT OF VISITS FOR A SPECIFIC PRODUCT |
|---|---|---|
| Collagen | 730,000 | 100 |
| Zyderm | 170,000 | 23 |
| Zyplast | 10,000 | 1 |
| Hyaluronic acid | 610,000 | 100 |
| Restylane | 410,000 | 67 |
| Juvederm | 150,000 | 25 |
| Captique | 50,000 | 8 |
| Calcium hydroxylapatite | 200,000 | 100 |
| Radiesse | 200,000 | 100 |
| Poly-L lactic acid | 160,000 | 100 |
| Sculptra | 160,000 | 100 |
The sum of all trade-names within a product may not equal the total, because for some visits a “generic neurotoxin/filler” was used to specify the use of a product.
TABLE 2.
Percent of (A) dermal filler visits and (B) neurotoxin visits at which a specific product was mentioned at cosmetic visits from 2002-2010
| TOTAL ESTIMATED VISITS | PERCENT OF VISITS† | |
|---|---|---|
| A) Visits at which a filler was mentioned | ||
| Hyaluronic acid | 610,000 | 50 |
| Calcium hydroxylapatite | 200,000 | 16.1 |
| Collagen | 190,000 | 15.9 |
| Poly-L lactic acid | 160,000 | 13.0 |
| Hydrogel | 60,000 | 5.0 |
| Unspecified “generic” filler | 50,000 | 4.1 |
| B) Visits at which a neurotoxin was mentioned* | ||
| OnabotulinumtoxinA | 3,430,000 | 87.1 |
| AbobotulinumtoxinA | 30,000 | 0.8 |
| Botulinum toxin type unspec. | 230,000 | 5.9 |
May not sum to 100% as for some visits, multiple fillers/neurotoxins were mentioned
A portion of visits for neurotoxin did not mention a product injected, but did list the reason for visit as a “botox injection.”
TABLE 3.
Top diagnoses for cosmetic dermal filler and neurotoxin visits
| DIAGNOSIS | PERCENT OF VISITS | |
|---|---|---|
| Dermal fillers | ||
| Other specified hypertrophic and atrophic conditions of skin | 44.3 | |
| Other plastic surgery for unacceptable cosmetic appearance | 29.9 | |
| Lipodystrophy | 13 | |
| Neurotoxins | ||
| Other specified hypertrophic and atrophic conditions of skin | 50 | |
| Other plastic surgery for unacceptable cosmetic appearance | 25.8 | |
| Unspecified elective surgery for purposes other than remedying health states | 13.4 | |
On average, patients receiving a dermal filler were 48 years old (95% CI: 45-51) and those receiving a neurotoxin were 50 years old (95% CI: 48-52). Most patients receiving either a dermal filler or neurotoxin were female and Caucasian (Table 4). Female patients were six times as likely as male patients to receive a dermal filler (p<0.0001) and more than 17 times likely to receive a neurotoxin (p<0.0001). Patients identified as Caucasian were seven times more likely than African American patients to receive a dermal filler, and they were 54 times as likely to receive a neurotoxin compared to African American patients.
TABLE 4.
Demographic characteristics of US cosmetic visits for dermal fillers and neurotoxins
| AVERAGE ANNUAL VISITS | PERCENT OF VISITS | ANNUAL VISIT RATE PER 100,000 PERSONS (95% CI) | P-VALUE* | |
|---|---|---|---|---|
| Dermal fillers | ||||
| Gender | ||||
| Female | 80,000 | 85.2 | 64 (53 - 74) | <0.0001 |
| Male | 10,000 | 14.8 | 12(0-27) | |
| Race | ||||
| Caucasian | 80,000 | 86.4 | 43 (36 - 50) | Ref |
| African American | 2,000 | 2.3 | 6(0-18) | <0.0001 |
| Other | 1,000 | 1.3 | 36 (0 - 74) | 0.27 |
| Neurotoxins | ||||
| Gender | ||||
| Female | 410,000 | 94.2 | 274 (206 - 343) | <0.0001 |
| Male | 30,000 | 5.8 | 18(5 - 30) | |
| Race | ||||
| Caucasian | 380,000 | 87.8 | 163(117 - 209) | Ref |
| African American | 1000 | 0.3 | 3(0-10) | <0.0001 |
Comparing males to females and Caucasians to Afican Americans or “other” races
Patient race was not identified at 10% of dermal filler visits and 9% of neurotoxin visits
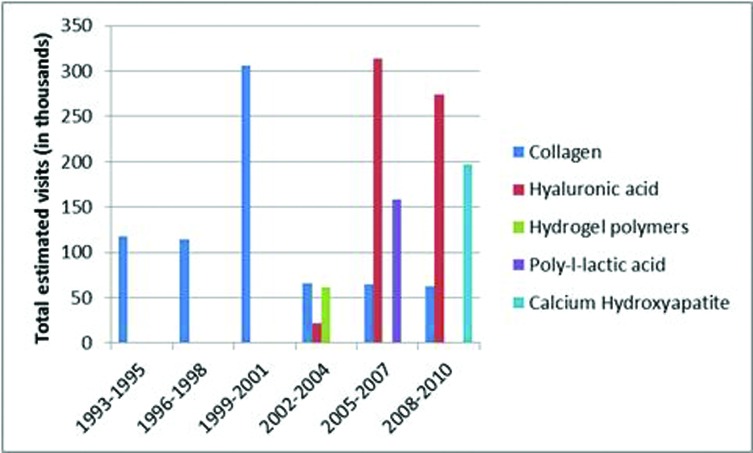
There were sufficient records to evaluate trends in use over time for HA, collagen, and onabotulinumtoxin A compared to all other dermal fillers or neurotoxins, respectively. Among dermal filler visits from 2002 to 2010, the proportion of visits at which HA was used has not significantly changed [β=0.5%/ year (95%CI: -8.0, 9.2); p=0.89]. While the proportion of dermal filler visits at which collagen was injected peaked from 1999 to 2001, it declined by five percent annually from 2002 to 2010 (95% CI: -10, -6; p<0.09; Figure 2) and by eight percent annually for the entire study period (95% CI: -10, -6; p<0.0001; Figure 2). Among visits at which the botulinum toxin type was specified, onabotulinumtoxinA (Botox®) was the leading product injected, with no change over time as to the proportion of neurotoxin visits where it was used [β=2%/year (95%CI: -2][6);p=0.37].
Figure 2.
Total estimated cosmetic visits associated with specific types of dermal fillers
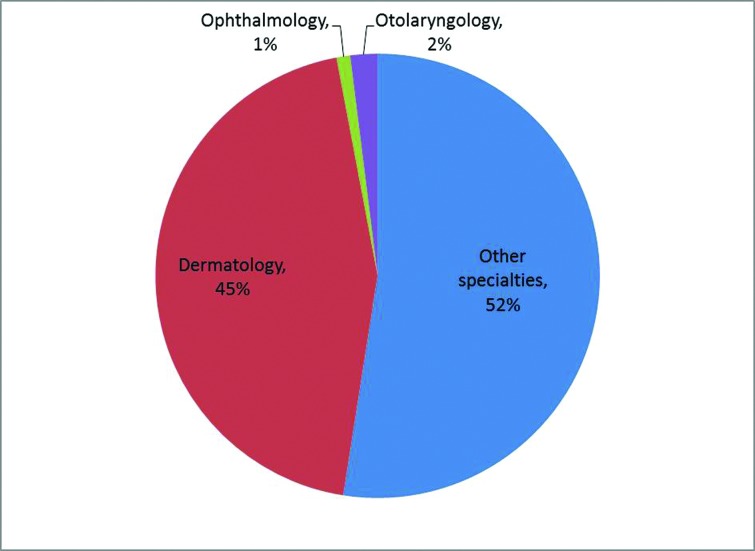
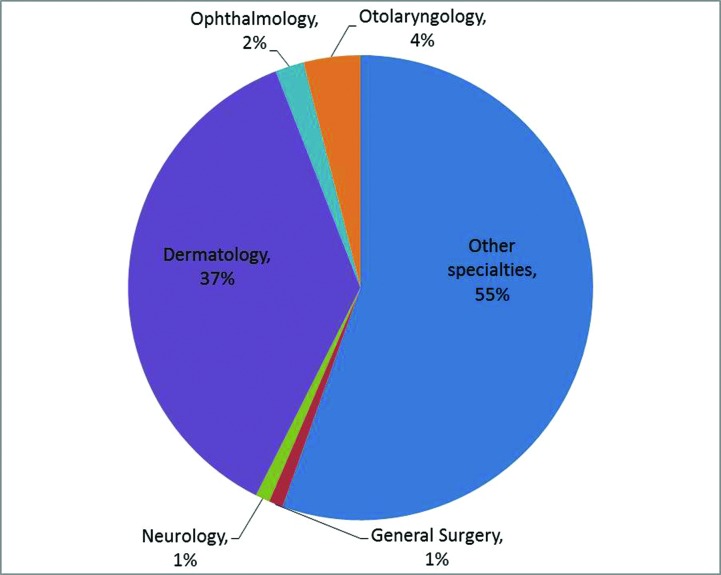
When stratified by specialty, dermatologists (45%) most commonly used dermal fillers, followed by otolaryngology (2%), ophthalmology (1%), and all other specialties that are not distinguished by NAMCS (53%; all other specialties includes plastic surgery; Figure 3). Dermatologists (37%) were also the most common specialty using neurotoxin products for cosmetic purposes, followed by physicians specializing in otolaryngology (4%), ophthalmology (2%), neurology (1%), general surgery (1%), and all other specialties that are not distinguished by NAMCS (56%; Figure 4). Compared to all other physician specialties, dermatologists were more likely to use collagen as a filler [OR: 2.7 (95% CI: 1.1, 6.6); p=0.03]. There was no difference in the likelihood of using HA [OR: 2.7 (95%CI: 0.8][9.4); p=0.12] for dermatologists versus non-dermatologists. There were insufficient records to make comparisons for hydrogel polymers, calcium hydroxylapatite, poly-L lactic acid, and silicone. At cosmetic visits where a neurotoxin was injected, dermatologists were as likely as non-dermatologists to choose onobotulinum toxinA rather than any other botulinum [OR 0.9 (95% CI: 0.3][2.4);p=0.80]. There were insufficient records to make comparisons of use for abobotulinumtoxinA and rimabotulinumtoxinB.
Figure 3.
Distribution of physician specialties for cosmetic dermal filler visits
Figure 4.
Distribution of physician specialties for cosmetic neurotoxin visits.
When a HA filler was used, dermatologists were as likely as non-dermatologists to use Restylane (Medicis, Scottsdale, Arizona) [OR: 1.4 (95%CI: 0.4][4.9);p=0.56] or Juvederm (Allergan, Inc., Irvine, California) [OR: 2.2 (95%CI: 0.4][13.3)][p=0.39] rather than an unspecified brand of hyaluronic product. When collagen was used as a filler, there was no evidence of differing preferences for dermatologists versus non-dermatologists in the use of Zyderm compared to other collagen products [OR: 0.3 (95% CI: 0.1][1.5);p=0.15]. There were insufficient data to make comparisons for the less commonly used products.
DISCUSSION
Increasingly patients are opting for less invasive options when considering cosmetic procedures. Physicians have more products available to them today to meet this demand, with newer aesthetic products for facial rejuvenation coming to market. Of the approved neurotoxins during the study period of 2002 to 2010, onabotulinumtoxinA, the first neurotoxin approved for the treatment of rhytides in 2002, was used at the majority of visits, 87.1 percent, with abobotulinumtoxinA used at only 0.8% of visits. While abobotulinumtoxinA became FDA-approved in 2009, onabotulinumtoxinA continues to be the neurotoxin of choice; however, this study’s results only include one year of data after abobotulinumtoxinA was approved for use. Provider’s preference of neurotoxin may simply be one of familiarity with one product over the other. Providers may have become comfortable using onabotulinumtoxinA, which was the sole neurotoxin available for the treatment of wrinkles for several years. Additionally, when abobotulinumtoxinA became available, providers had to familiarize themselves with how it differed from its counterpart, which is primarily a difference in dose equivalences. Interestingly, there is some evidence that abobotulinumtoxinA may have more longevity compared to onabotulinumtoxinA.5,6 Other studies between the two products have generally been inconclusive in favoring one over the other, with diffusion and spread of product specifically compared and appearing to be dose-dependent. 7-9
The use of dermal fillers for soft tissue augmentation has evolved over the years as newer products have become available. Historically, the use of collagen and fat were the primary favored options for fillers, with collagen used for the majority of procedures.1 From the NAMCS data, collagen was the only filler identified from 1993 to 2001 for use in soft tissue augmentation. However, with the introduction of HA fillers in 2003, the use of collagen has declined at a rate of eight percent annually, data that is in line with statistics reports by the American Society of Plastic Surgeons (6% decline from 2011-2012, and 88% decline from 2000-2012).1 HA fillers instead have become the favored filler product, used at 50 percent of visits since its introduction. In comparison studies, HA fillers lasted longer and required less product to achieve the same cosmetic effect as collagen in the treatment of nasolabial folds, likely leading to them gaining preference among providers and patients. 10-14 Similarly, calcium hydroxylapatite filler, the second most common filler used from 2002 to 2010 (approved in 2006, but used prior to this time off-label for soft tissue augmentation15), was superior to collagen in terms of longevity and the need for less volume of product and less injections to achieve the same amount of correction in treating nasolabial folds.16 Dermatologists were more likely to use collagen as a filler than other specialties during the study period of 1993 to 2010.
Several HA fillers are available today in the United States and the market continues to grow rapidly. Unlike the neurotoxins, HA fillers can differ significantly in the product themselves, as well as in injection techniques. Differences, such as concentration of HA, amount of cross-linking, the modulus (G′, or viscoelasticity), and differences in particle sizes, lead to a variety of products to choose from that may be advantageous over another depending on which facial area is to be treated and the desired cosmetic outcome.17 Restylane, the first approved HA filler, was most commonly used, followed by Juvederm. Again, it may be that Restylane has been available longest and providers are most familiar with it. The idea that a combination of fillers (either an HA with another filler or two different HAs with different properties) may result in the best cosmetic outcome, as well as the expanding options of fillers since 2010, will likely lead to changes in preference trends in the future as providers become more proficient with the various products and the injection techniques.18
Limitations to this study include small sample size for some of the NAMCS records, which restrict subgroup analysis for some of the products. In addition, the majority of the products were not available for the entire study period (s) and as a result the proportion of visits for some individual products may be under-represented. Further, since 2010, several new products including a botulinum toxin type A and several dermal fillers have been approved. These results, therefore, may not reflect current usage trends.
Today, providers have more products than ever to choose from when providing cosmetic services to their patients. It appears that familiarity with a product influences providers’ preference of product, with products that first came to market being used more frequently than newer products. As the newer products are around longer, and providers become more familiar with each individual product and how a combination of different products may best achieve a desired cosmetic outcome, the preference among products may change over time.
Footnotes
DISCLOSURE:This study was funded by a grant from Merz Pharmaceuticals. The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories, LP. Dr. Feldman is a consultant and speaker for Galderma, Stiefel/GlaxoSmithKline, Abbott Labs, Warner Chilcott, Janssen, Amgen, Photomedex, Genentech, BiogenIdec, and Bristol Myers Squibb. He has received grants from Galderma, Astellas, Abbott Labs, Warner Chilcott, Janssen, Amgen, Photomedex, Genentech, BiogenIdec, Coria/Valeant, Pharmaderm, Ortho Pharmaceuticals, Aventis Pharmaceuticals, Roche Dermatology, 3M, Bristol Myers Squibb, Stiefel/GlaxoSmithKline, Novartis, Medicis, Leo, HanAll Pharmaceuticals, Celgene, Basilea, and Anacor and has received stock options from Photomedex. He is the founder and holds stock in Causa Research. Drs. Huang, Davis, and Feldman have consulted for Merz Pharmaceuticals. Drs. Sandoval and Taylor report no relevant conflicts of interest
REFERENCES
- 1. [5-15-14]. http://www.plasticsurgery.org/news/plastic-surgery-statistics/2012-plastic-surgery-statistics.html 2012 Plastic Surgery Procedural Statistics. 2012.
- 2.Lee SK, Kim HS. Recent trend in the choice of fillers and injection techniques in Asia: a questionnaire study based on expert opinion. J Drugs Dermatol. 2014;13:24–31. [PubMed] [Google Scholar]
- 3.Gilbert E, Calvisi L. Midface and perioral volume restoration: a conversation between the US and Italy. J Drugs Dermatol. 2014;13:67–74. [PubMed] [Google Scholar]
- 4.National Center for Health Statistics. [5-27-2014]. http://www.cdc.gov/nchs/about/major/ahcd/ahcdl.htm National Ambulatory Medical Care Survey (NAMCS). 2010.
- 5.Kassir R, Kolluru A, Kassir M. Triple-blind, prospective, internally controlled comparative study between abobotulinumtoxinA and onabotulinumtoxinA for the treatment of facial rhytids. Dermatol Ther (Heidelb). 2013;3:179–189. doi: 10.1007/s13555-013-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn TC. Botulinum toxin: examining duration of effect in facial aesthetic applications. Am J Clin Dermatol. 2010;11:183–199. doi: 10.2165/11530110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Hexsel D, Brum C, do Prado DZ, et al. Field effect of two commercial preparations of botulinum toxin type A: a prospective, double-blind, randomized clinical trial. J Am Acad Dermatol. 2012;67:226–232. doi: 10.1016/j.jaad.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Hexsel D, Hexsel C, Siega C, et al. Fields of effects of 2 commercial preparations of botulinum toxin type A at equal labeled unit doses: a double-blind randomized trial. JAMA Dermatol. 2013;149:1386–1391. doi: 10.1001/jamadermatol.2013.6440. [DOI] [PubMed] [Google Scholar]
- 9.Hexsel D, Dal’Forno T, Hexsel C, et al. A randomized pilot study comparing the action halos of two commercial preparations of botulinum toxin type A. Dermatol Surg. 2008;34:52–59. doi: 10.1111/j.1524-4725.2007.34008.x. [DOI] [PubMed] [Google Scholar]
- 10.Kontis TC. Contemporary review of injectable facial fillers. JAMA Facial Plast Surg. 2013;15:58–64. doi: 10.1001/jamafacial.2013.337. [DOI] [PubMed] [Google Scholar]
- 11.Narins RS, Brandt F, Leyden J, et al. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–595. doi: 10.1046/j.1524-4725.2003.29150.x. [DOI] [PubMed] [Google Scholar]
- 12.Lindqvist C, Tveten S, Bondevik BE, Fagrell D. A randomized, evaluator-blind, multicenter comparison of the efficacy and tolerability of Perlane versus Zyplast in the correction of nasolabial folds. Plast Reconstr Surg. 2005;115:282–289. [PubMed] [Google Scholar]
- 13.Baumann LS, Shamban AT, Lupo MP, et al. Comparison of smooth-gel hyaluronic acid dermal fillers with cross-linked bovine collagen: a multicenter, double-masked, randomized, within-subject study. Dermatol Surg. 2007;33(Suppl 2):S128–S135. doi: 10.1111/j.1524-4725.2007.33352.x. [DOI] [PubMed] [Google Scholar]
- 14.Lupo MP, Smith SR, Thomas JA, et al. Effectiveness of Juvederm Ultra Plus dermal filler in the treatment of severe nasolabial folds. Plast Reconstr Surg. 2008;121:289–297. doi: 10.1097/01.prs.0000294968.76862.83. [DOI] [PubMed] [Google Scholar]
- 15.Berlin A, Cohen JL, Goldberg DJ. Calcium hydroxylapatite for facial rejuvenation. Semin Cutan Med Surg. 2006;25:132–137. doi: 10.1016/j.sder.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Smith S, Busso M, McClaren M, Bass LS. A randomized, bilateral prospective comparison of calcium hydroxylapatite microspheres versus human-based collagen for the correction of nasolabial folds. Dermatol Surg. 2007;33(Suppl 2):S112–S121. doi: 10.1111/j.1524-4725.2007.33350.x. [DOI] [PubMed] [Google Scholar]
- 17.Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(Suppl 1):302–312. doi: 10.1111/j.1524-4725.2008.01046.x. [DOI] [PubMed] [Google Scholar]
- 18.Beer K. Dermal fillers and combinations of fillers for facial rejuvenation. Dermatol Clin. 2009;27:427–432, v. doi: 10.1016/j.det.2009.08.011. [DOI] [PubMed] [Google Scholar]