Abstract
The popularity of soft tissue fillers is, in part, due to their favorable side-effect profile. However, serious complications can occur. The authors describe their extensive clinical experience with soft-tissue augmentation and the rare complication of vascular compromise, which can lead to necrosis and scarring. Over a 10-year period between January 2003 and January 2013, the authors observed a total of 12 cases of vascular compromise. Eight patients in their clinical practice showed evidence of vascular compromise out of a total of 14,355 filler injections (0.05%). In addition, four patients treated with an experimental particulate filler had vascular complications. All cases were examined for filler type, location of complication, risk factors, treatment, and outcomes. Although treatment plans differed for each patient in their series, all cases of vascular compromise resolved fully. The authors believe that an office-based protocol for both immediate and ongoing care—including a thorough individualized assessment and treatment plan for each patient—is critical to timely and effective resolution of side effects. They propose key recommendations for the prevention and management of vascular compromise to improve patient outcomes and reduce the risk of permanent complications.
Injectable fillers have become an integral part of aesthetic medicine for patients who want noninvasive rejuvenation. They are used to restore volume and to smooth and efface superficial wrinkles and deep folds of the face, among other indications. Widespread use began in the 1980s with the advent of bovine collagen. Since then, use has surged so that soft tissue augmentation is the second most popular nonsurgical aesthetic procedure in North America to botulinum toxin.1 In 2007, more than 1.5 million soft tissue filler procedures were performed in the United States, with hyaluronic acid (HA) being the most frequently used.2 As of 2010, more than 200 types of fillers were available for soft tissue augmentation worldwide.1
The popularity of soft tissue fillers is in part due to their favorable side-effect profile. Adverse effects from soft tissue filler injection are generally mild and self-limited. However, there are some well-documented serious complications. The most feared and potentially serious complications are vascular in nature. Collectively referred to as vascular compromise, these complications include partial or complete interruption of vascular supply by extravascular compression, or a complete occlusion of vascular supply from intravascular injection. Subsequent necrosis and scarring are potentially permanent sequelae.2-4
In the authors’ clinical practice, 14,355 filler injections were performed between January 2003, when they first instituted their computer database, and January 2013. Fillers that are used in their office include hyaluronic acid (HA) (Juv'derm Ultra, Ultra plus, Voluma [Allergan, Irvine, California] and Restylane [Medicis Aesthetics Inc., Scottsdale, Arizona]); poly-L-lactic acid (Sculptra, Sanofi-Aventis, Bridgewater, New Jersey); calcium hydroxylapatite (Radiesse, Merz USA, Greensboro, North Carolina); silicone oil; and collagen (Evolence Breeze, Ortho Dermatologics, Skillman, New Jersey). During this 10-year period, a total of 12 cases of vascular compromise were observed and managed, eight of which occurred in the authors’ clinical practice and four in their clinical trials practice. Those cases that developed vascular compromise after soft tissue augmentation are reviewed and treatment discussed (Appendix 1).
Over a 10-year period between January 2003 and January 2013, eight patients in the authors' clinical practice showed evidence of vascular compromise out of a total of 14,355 filler injections (0.05%). They observed four cases after injection with calcium hydroxylapatite (CaHA) (out of 1,482 total injections; 0.27%), four cases after injection with volumizing monophasic HA (Juvéderm Voluma) (out of 4,321 total injections; 0.09%), and one case resulting from treatment with biphasic HA (Restylane) (out of 3,348 injections; 0.03%). One patient was treated with both CaHA and volumizing monophasic HA, and is counted in both groups (Table 1). In all cases, the injection technique was not recorded. The authors typically use a combination of antegrade and depot injection when revolumizing. In addition to the above cases in their clinical practice, in their research practice, four patients treated with an experimental particulate filler displayed evidence of vascular compromise.
TABLE 1.
Risk of vascular compromise in the authors’ clinical practice
| FILLER | TIME FRAME EVALUATED | NUMBER OF PATIENTS WITH EVIDENCE OF VASCULAR COMPROMISE | NUMBER OF FILLER INJECTIONS OVER TIME FRAME | PERCENTAGE WITH COMPLICATION IN GROUP (%) |
|---|---|---|---|---|
| Total fillers injected in clinical practice | Jan 2003-Jan 2013 | 8 | 14,355 | 0.05 |
| CaHA* | Jan 2004-Jan 2013 | 4 | 1,482 | 0.27 |
| Volumizing monophasic HA* | Feb 2009-Jan 2013 | 4 | 4,321 | 0.09 |
| Biphasic HA | Jan 2003-Jan 2013 | 1 | 3,348 | 0.03 |
One patient with evidence of vascular compromise was treated with both CaHA and volumizing monophasic HA, and is counted in both groups
DISCUSSION
Injection of fillers is generally very well-tolerated with mild transient side effects including erythema, bruising, tenderness, and swelling lasting for a few days. Vascular compromise, occlusion, or necrosis after injection are rare, but potentially very serious adverse effects (Box 1).3,4
BOX 1. Adverse effects from soft tissue augmentation.
Early (occurring up to one week after injection)
Injection site reactions (bruising, swelling, erythema)
Infections
Nodules/asymmetry
Hypersensitivity
Vascular compromise
Delayed (occurring weeks to years after injection)
Infection
Granuloma formation
Biofilm formation
* Adapted from Glashofer MD, Flynn TC. Complications of temporary fillers. In: Carruthers J, Carruthers A. Soft Tissue Augmentation. Toronto: Elsevier Saunders; 2013:179-187.
Mechanisms leading to tissue necrosis are not fully understood. It is felt there must be extra- and intravascular factors. Extravascular causes result from compression of the vessel from the injectable filler. Secondary inflammation and edema can further put pressure on the vessels leading to decreased skin perfusion. Intravascular causes are direct injection of the filler into the vasculature causing obstruction and damage to the wall of the blood vessels.5 Compression of the vessel from filler typically occurs immediately after injection. It can occasionally present later if there is pressure from the filler and subsequent post-injection swelling.6 Embolization of filler material has also been reported following HA injection. This presentation may be immediate and has also been reported with a delayed presentation at six hours post-injection.7
Typically, the first indication of vascular damage after filler injection is painless blanching. This can be subtle and may go unnoticed. Over the next couple of days, progression to a painful, violaceous, reticulated patch may occur. A necrotic eschar may develop on top of an ulcer with subsequent scar formation. Treatment should be instituted at first sign of this complication to prevent necrosis and scarring.2,8
Historically, the glabella has been the most commonly reported site of necrosis. This area is a watershed site with limited collateral circulation. Small-caliber vessels branch out from the supratrochlear arteries.9,10 In previous reports with injection of Zyplast (Allergan, Inc.), 50 percent of tissue necrosis occurred in the glabellar area.11 The nasolabial fold is another high-risk site. The embryonic fusion planes at the alar groove may not allow for accommodation of filler volume and the angular artery can be compromised.2 Rare, but extremely dangerous complications can occur with injection of fillers, including blindness. Lazzeri et al12 reviewed the literature and reported on 32 cases of transitory or permanent blindness following fat injection or aesthetic injections of other materials including fillers. In another case series, Park et al13 reported on 12 cases of retinal artery occlusion following injection into high-risk sites, such as the glabellar region and nasolabial folds.
To the authors’ knowledge, this is one of the largest case series published on vascular compromise with cutaneous complications secondary to soft tissue augmentation. In this series, the authors observed vascular compromise from three different commercially available fillers and an experimental particulate filler. These fillers were all placed in a deeper plane for their volumizing properties. Of note, the authors did not observe any cases of vascular compromise from fillers placed more superficially to efface rhytides.
Patients treated with CaHA and volumizing monophasic HA in the authors’ clinical practice were injected using a 28-gauge (G), ¾-inch needle. The biphasic HA was injected using a 29G, ½-inch needle. The particulate experimental filler was injected using a 27G, 1½-inch needle. Pertinent patient risk factors that may have contributed to complications include a prior rhinoplasty in a patient who developed evidence of occlusion at the nasal ala after treatment with CaHA and a positive smoking history in a patient treated with both CaHA and volumizing monophasic HA (Table 2).
TABLE 2.
Filler used and complication by site, underlying risk factors, and needle size
| FILLER | CASE# | LOCATION OF COMPLICATION | RISK FACTORS | NEEDLE SIZE |
|---|---|---|---|---|
| CaHA | 1 | Cheek | 28G, ¾ inch | |
| 3 | Nasal ala | Previous rhinoplasty | ||
| 7 | Forehead | |||
| CaHA and volumizing monophasic HA | 8 | Nasolabial fold | Smoker | 28G, ¾ inch for both |
| Volumizing monophasic HA | 2 | Nasolabial fold/lip | 28G, ¾ inch | |
| 4 | Nasolabial fold | |||
| 5 | Cheek | |||
| Biphasic HA | 6 | Nasolabial fold | 29G, ½ inch | |
| Experimental particulate filler | 9 | Nasolabial fold/lip | Study patient | 27G, 114 inch |
| 10 | Nasolabial fold/lip | Study patient | ||
| 11 | Nasolabial fold | Study patient | ||
| 12 | Nasolabial fold/lip | Study patient |
Eight patients experienced vascular compromise at the nasolabial fold or lip, two at the malar cheek, one at the nasal ala, and one at the glabella. The anatomic sites most commonly affected were at increased risk for vascular occlusion including the nasolabial folds where the angular artery or lateral nasal artery can be occluded and the glabellar region where there is minimal collateral circulation. 14-16
The incidence of vascular complications in the authors’ clinical practice was 0.05 percent. This is higher than the 0.001 percent reported in the literature with injection of collagen and HA fillers; however, it is difficult to make a direct comparison because the data includes newer synthetic fillers that are designed to be injected in a deeper plane in addition to HA fillers. It has been reported that the rate of vascular complications secondary to soft tissue augmentation is rising.17 This could parallel the rise in filler treatments performed or more likely reflects a shift in the paradigm from two-dimensional to a three-dimensional revolumization.
PREVENTION
There are a number of preventative strategies that can reduce the risk of occlusion. The key strategies the authors’ group has implemented are outlined in Box 2.
BOX 2. Authors’ key prevention strategies.
Before injecting, a detailed history should be obtained including any previous cosmetic procedures or surgeries. Exercise caution in the setting of nasal augmentation post-rhinoplasty
The injector should be well-trained and have a firm understanding of the facial vascular anatomy and the depth at which the filler should be implanted
Choosing a reversible filler (HA filler) increases the likelihood of resolution without sequelae as hyaluronidase can be used to remove the product
Using the cannula technique may reduce risk of vascular compromise
Extreme caution should be observed when injecting filler in anatomically sensitive areas such as the glabellar region and nasolabial folds
In high-risk areas, use low volumes of product in two or more treatment sessions
Inject product slowly. Discontinue the injection immediately if blanching occurs
Use a smaller gauge needle if practical
If possible, aspirate before injecting to ensure filler is not placed in the intravascular space
Preventative measures include taking a detailed history to assess for previous treatment with fillers or cosmetic surgeries in the area to be treated. Choosing a reversible HA filler allows for treatment with hyaluronidase and possible reversal of vascular occlusion if used early.5 It is important to be cautious when injecting high-risk anatomic areas, such as the glabella or nasolabial folds.2,14,15 Aspirating before injecting if possible and using low volumes of product in two or more treatment sessions reduces the risk of vascular occlusion.10,12 Other measures to reduce vascular complications include injecting with a small gauge needle,2,12 injecting slowly,12,18,19 and use of the cannula technique.14
TREATMENT
It is important to recognize that the management of vascular compromise from soft tissue fillers is not based on a large body of evidence. A treatment protocol has been developed at the authors’ office to be implemented if vascular complications are suspected (Box 3).
BOX 3. Authors’ key treatment strategies.
Warm compresses, 10 minutes every 1-2 hours
Vigorous massage, nerve block if necessary, no epinephrine
Hyaluronidase if filler is HA filler
Consider topical nitroglycerin paste 2%. Can be applied as frequently as every 1-2 hours initially. Once at home, patient can apply up to 3 times a day to the affected area provided he/she does not develop symptoms of dizziness
Consider administering aspirin, 325mg under tongue immediately and 81 mg daily thereafter
Consider oral prednisone 20-40mg daily for 3-5 days
Consider hyperbaric oxygen
Follow patient daily until improvement. Provide him/her with clear written instructions about management at home and contact phone numbers
If blanching occurs while injecting, immediately discontinue the injection and begin warm compresses. The authors suggest application for 10 minutes every one to two hours. This encourages quick vasodilation to restore blood supply to the area.4,15 Massage of the treated area should be instituted.20 A nerve block may be necessary to facilitate vigorous massage. In the authors’ experience, massage can dramatically, albeit temporarily, reverse the blanched or dusky appearance of the skin and should be repeated regularly for several days to improve the likelihood of tissue viability.
If the complication occurs with an HA filler, hyaluronidase is recommended.19 Hyaluronidase works to break down and hydrolyze hyaluronic acid. One can consider skin testing to evaluate risk of allergy.7 Dayan et al17 proposed treating impending necrosis with 10 to 30 units of hyaluronidase regardless of the filler type, including for non-HA fillers. Hyaluronidase has been shown to have edema-reducing benefits and theoretically reduces occluded vessel pressure.16 Some authors have experience canalizing an occluded vessel and injecting hyaluronidase with immediate reversal of signs of occlusion.
Nitroglycerin paste application can allow for more significant vasodilation.4,14,15,17 The authors have used topical 2% nitroglycerin paste applied initially every one to two hours. This can be continued at home three times per day providing the patient does not experience dizziness. This treatment recommendation is controversial because it may cause superficial shunting of blood and disruption of deeper vascular supply or may allow further migration and compromise blood flow downstream in the case of particulate fillers.
Aspirin is used to block platelet aggregation and has moderate anti-inflammatory properties. It can help prevent blood clotting within a partially occluded vessel.14,15 The authors suggest a dose of 325mg under the tongue immediately and 81mg daily thereafter.
Oral prednisone, 20 to 40mg daily, for three to five days is recommended. Oral corticosteroids prevent further vascular compromise by decreasing the inflammatory component of the injury.14
Treatment with hyperbaric oxygen can be considered. It has the potential to deliver oxygen deep into the skin to keep tissues viable. Its use is controversial though and the risks, benefits, and inconvenience must be weighed when considering this treatment.14,17
Other treatments outside of the authors’ protocol have been recommended. Low molecular weight heparin has been used to prevent thrombosis and embolization.4 Drugs designed for treatment of erectile dysfunction, such as sildenafil and tadalafil, have been utilized; these medications cause smooth muscle relaxation, dilation of the blood vessels, and increased blood flow.14
If necrosis does occur, diligent wound care is critical.9 Treatment of the resulting scar may involve silicone gel sheeting and intralesional triamcinolone acetonide. Long-term scar management can include dermabrasion, surgical excision, or laser resurfacing.4
While the exact treatment plan differed for each patient in the authors’ series, the end result for all patients was full resolution of symptoms. The authors believe that a thorough individual assessment and treatment plan should be instituted for each patient. Having an office-based protocol for both prevention and treatment of vascular-related complications is critical to timely and effective resolution of side effects. Immediate and ongoing care ensures optimal outcomes and decreases the risk of permanent complications.
SUMMARY
With the increased use of soft tissue augmentation for revolumization, it is imperative to be aware of potential complications. Vascular compromise is one of the most serious side effects. Herein the authors report on their extensive collective experience with soft tissue augmentation and describe 12 cases of vascular compromise. They propose key recommendations for prevention and management of this side effect to improve patient outcomes.
APPENDIX 1. Case summaries filler complications, management, and outcomes
| PATIENT | AGE | DATE OF INJECTION | FILLER* AND LOCATION | TIME OF ASSESSMENT POST-INJECTION | COMPLICATIONS | MANAGEMENT | OUTCOME |
|---|---|---|---|---|---|---|---|
| 1 | 54 | 10/14/2009 | 1.3mL CaHA into nasolabial folds (NLF) and malar cheeks | Day 5 | Violaceous reticulated painful patch to right cheek with pustules | Massage, clindamycin, intralesional triamcinolone acetonide (ILTAC) | Complete resolution |
| 2 | 69 | 12/14/2009 | 1ml monophasic HA to pre-jowl sulcus, lip corners, right lateral cheek | Day 2 | Violaceous reticulated painful patch to right NLF. Right upper lip pain, numbness and ecchymosis | Massage, warm compresses, patient declined treatment with hyaluronidase and prednisone | Complete resolution |
| 3 | 26 | 01/1½010 | 0.3ml CaHA to nasal dorsum | Day 4 | Violaceous reticulated patch to right nasal ala | Warm compresses, conservative management | Complete resolution |
| 4 | 65 | 06/15/2010 | 1ml monophasic HA to NLF | 2 hours | Violaceous reticulated painful patch to left NLF | Massage, hyaluronidase | Complete resolution |
| 5 | 31 | 11/16/2011 | 2ml monophasic HA to nasojugal folds | Day 5 | Violaceous reticulated painful patch to right central cheek (Figure 1, left) | Massage, warm compresses, hyaluronidase | Complete resolution (Figure 1, right) |
| 6 | 50 | 12/6/2011 | 2ml biphasic HA to NLF (no anesthetic) | 24 hours | Violaceous reticulated painful patch to left NLF and alar crease | Massage, warm compresses, hyaluronidase, prednisone | Complete resolution |
| 7 | 56 | 06/1/2012 | 1.5mLCaHA to nasal bridge and glabella | Day 4 | Violaceous reticulated painful patch to forehead (Figure 2, left) | Massage, warm compresses, nitroglycerin paste, prednisone | Complete resolution (Figure 2, right) |
| 8 | 58 | 08/1/2012 | 3mL CaHA to inferior NLF and malar cheeks, 0.5 cc monophasic HA to superior NLF | Immediate | Violaceous reticulated patch and blanching to left NLF (Figure 3, left) | Massage, warm compresses, nitroglycerin paste, hyaluronidase, prednisone, aspirin | Complete resolution (Figure 3, right) |
| 9 | 57 | 08/18/2009 | STUDY: Particulate experimental filler | Day 3 | Violaceous reticulated painful patch and edema to right upper lip and nasolabial fold | Massage, warm compresses, nitroglycerin paste, prednisone, ILTAC | Complete resolution |
| 10 | 55 | 03/15/2010 | STUDY: Particulate experimental filler | Day 3 | Violaceous reticulated painful patch to left NLF; ecchymosis to left NLF and petechiae to lip mucosa | Massage, prednisone | Complete resolution |
| 11 | 38 | 03/15/2010 | STUDY: Particulate experimental filler | Day 1 | Violaceous reticulated patch, swelling, and sluggish capillary refill to left NLF; erosion on post-injection Day 6 | Prednisone, fucidin 2% ointment to erosion | Complete resolution |
| 12 | 36 | 03/16/2010 | STUDY: Particulate experimental filler | Day 3 | Violaceous reticulated patch, swelling, and sluggish capillary refill to left nasolabial fold | Massage, prednisone | Complete resolution |
For all patie its seen in th e authors’ office the filler is reconstituted with 2% lidocaine and 20mg/mL, 1:200,000 epinephrine unless stated otherwise
Figure 1.
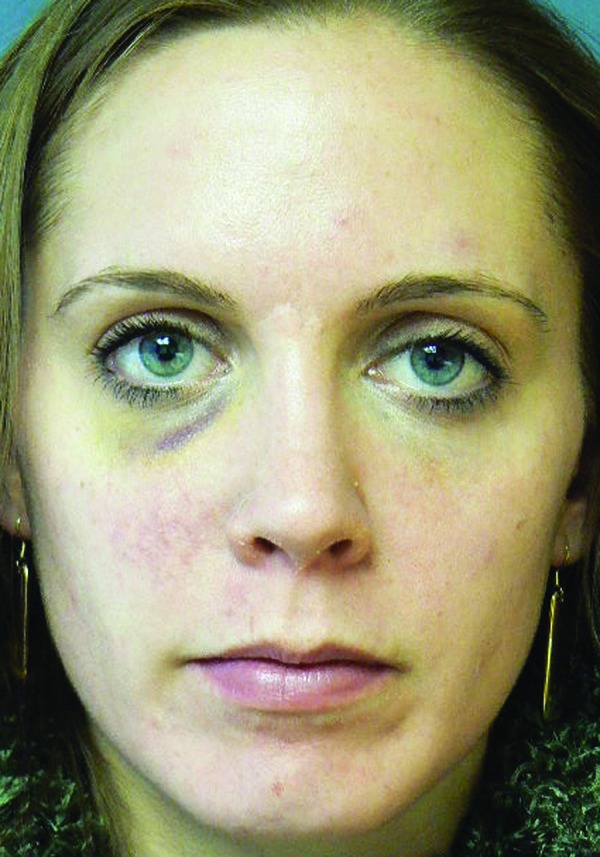
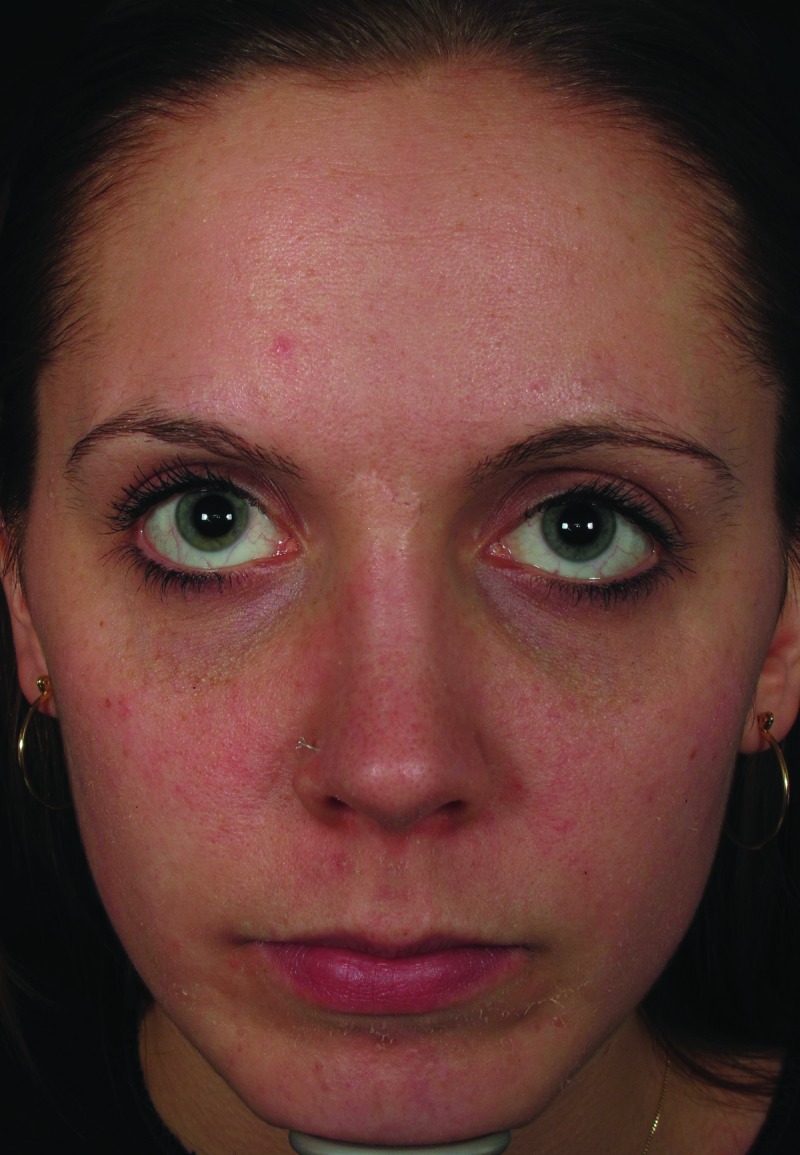
Mottled erythema to right cheek, five days post-injection (left). Resolution of vascular compromise with no sequelae, two months post-injection (right)
Figure 2.
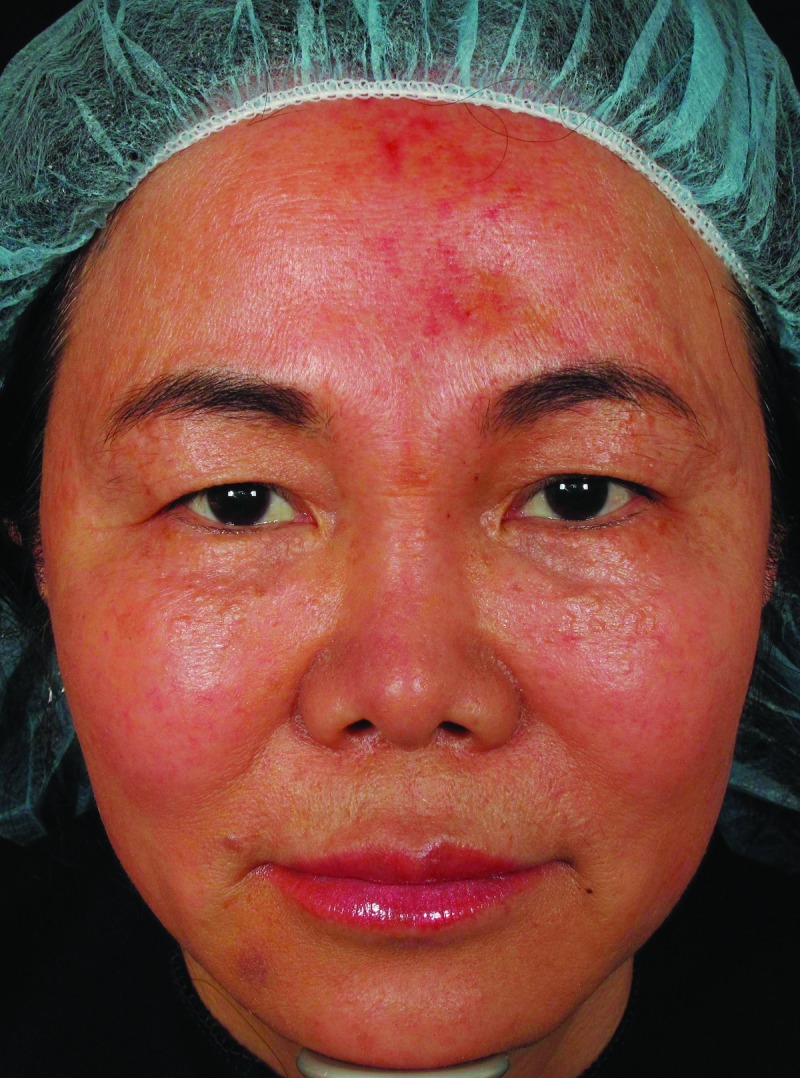
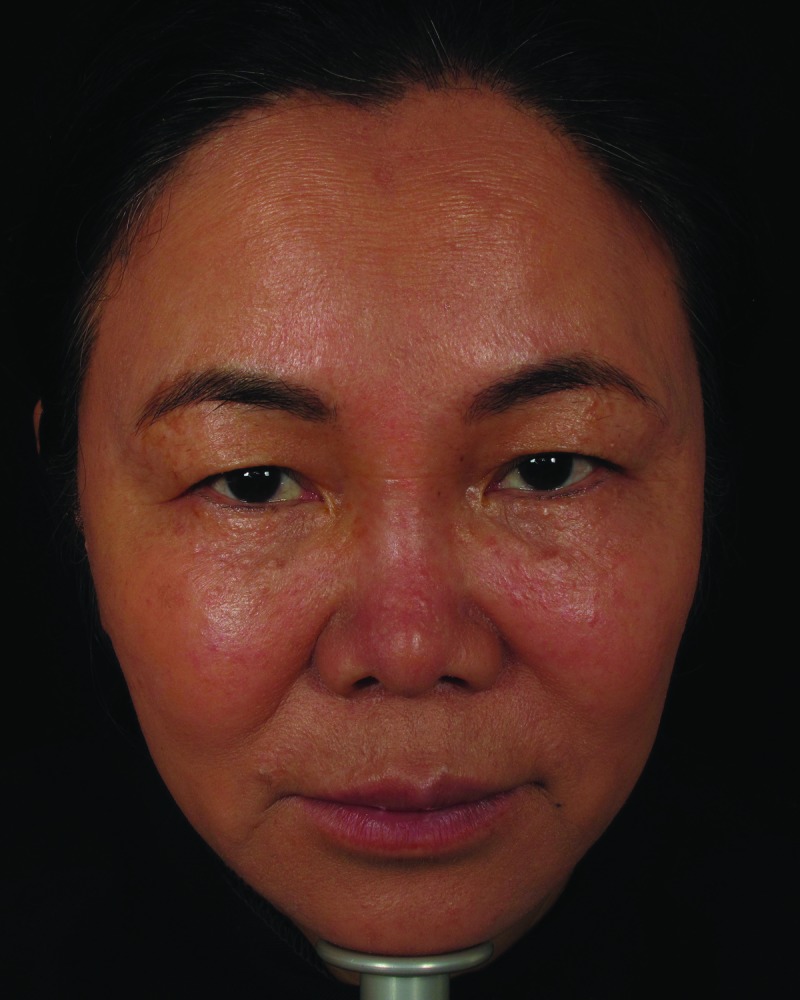
Mottled erythema to forehead, five days post-injection (left). Resolution of vascular compromise with no sequelae, 2.5 months post-injection (right)
Figure 3.
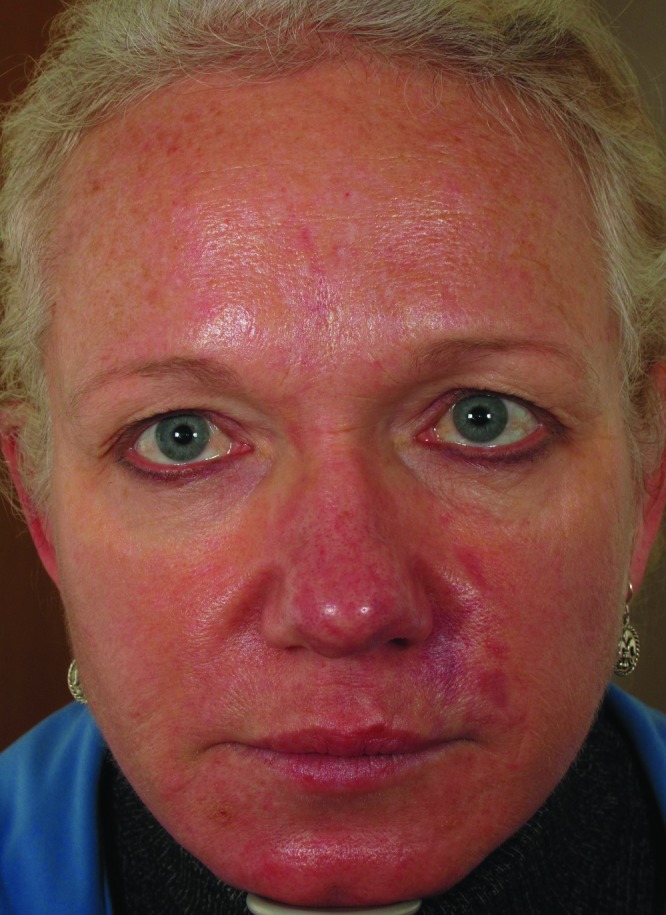
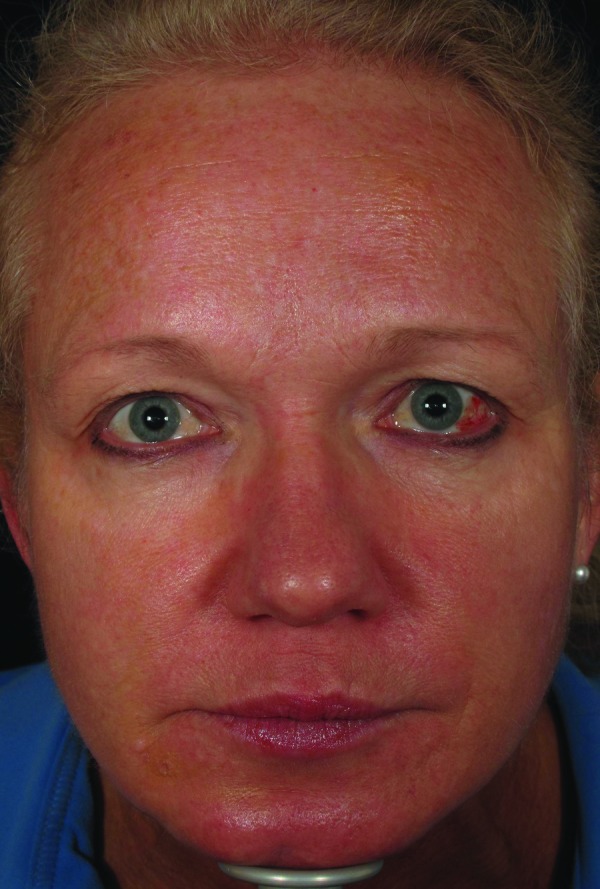
Dusky erythema to left nasolabial fold, five days post-injection (left). Resolution of vascular compromise with no sequelae, 3.5 months post-injection (right)
Footnotes
DISCLOSURE:The authors report no relevant conflicts of interest.
REFERENCES
- 1.Prather CL, Wiest LG. Complications of permanent fillers. In: Carruthers J, Carruthers A, editors. Soft Tissue Augmentation. Toronto: Elsevier Saunders; 2013. pp. 188–199. [Google Scholar]
- 2.Kassir R, Kolluru A, Kassir M. Extensive necrosis after injection of hyaluronic acid filler: case report and review of the literature. J Cosmet Dermatol. 2011;10:224–231. doi: 10.1111/j.1473-2165.2011.00562.x. [DOI] [PubMed] [Google Scholar]
- 3.Glashofer MD, Flynn TC. Complications of temporary fillers. In: Carruthers J, Carruthers A, editors. Soft Tissue Augmentation. Toronto: Elsevier Saunders; 2013. pp. 179–187. [Google Scholar]
- 4.Glaich AS, Cohen JL, Goldberg LH. Injection necrosis of the glabella: protocol for prevention and treatment after use of dermal fillers. Dermatol Surg. 2005;32:285–290. doi: 10.1111/j.1524-4725.2006.32052.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim DW, Yoon ES, Ji YH, et al. Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. JPlastReconstrAesthetSurg. 2011;64:1590–1595. doi: 10.1016/j.bjps.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch RJ, Lupo M, Cohen JL, Duffy D. Delayed Presentation of impending necrosis following soft tissue augmentation with hyaluronic acid and successful management with hyaluronidase. J Drugs Dermatol. 2007;6:325–328. [PubMed] [Google Scholar]
- 7.Hirsch RJ, Cohen JL, Carruthers JD. Successful management of an unusual presentation of impending necrosis following a hyaluronic acid injection embolus and proposed algorithm for management with hyaluronidase. Dermatol Surg. 2007;33:357–360. doi: 10.1111/j.1524-4725.2007.33073.x. [DOI] [PubMed] [Google Scholar]
- 8.Georgescu D, Jones Y, McCann JD, Anderson RL. Skin necrosis after calcium hydroxylapatite injection into the glabellar and nasolabial folds. Ophthal Plast Reconstr Surg. 2009;25:498–499. doi: 10.1097/IOP.0b013e3181b81082. [DOI] [PubMed] [Google Scholar]
- 9.Bachman F, Erdmann R, Hartmann V, Wiest L, et al. The spectrum of adverse reactions after treatment with injectable fillers in the glabellar region: results from the injectable filler study. Dermatol Surg. 2009;35:1629–1634. doi: 10.1111/j.1524-4725.2009.01341.x. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JL. Understanding, avoiding, and managing dermal filler complications. Dermatol Surg. 2008;34(Suppl 1):S92–S99. doi: 10.1111/j.1524-4725.2008.34249.x. [DOI] [PubMed] [Google Scholar]
- 11.Grunebaum LD, Allemann IB, Dayan S, et al. The risk of alar necrosis associated with dermal filler injection. Dermatol Surg. 2009;35:1635–1640. doi: 10.1111/j.1524-4725.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 12.Lazzeri D, Agostini T, Figus M, et al. Blindness following cosmetic injections ofthe face. Plast Reconstr Surg. 2011;129:995–1012. doi: 10.1097/PRS.0b013e3182442363. [DOI] [PubMed] [Google Scholar]
- 13.Park SW, Woo SJ, Park KH, et al. Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol. 2012;154:653–662. doi: 10.1016/j.ajo.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Beer K, Downie J, Beer J. A treatment protocol for vascular occlusion from particulate soft tissue augmentation. J Clin Aesthet Dermatol. 2012;5:44–47. [PMC free article] [PubMed] [Google Scholar]
- 15.Kleydman K, Cohen JL, Marmur E. Nitroglycerin: a review of its use in the treatment of vascular occlusion after soft tissue augmentation. Dermatol Surg. 2012;38:1889–1897. doi: 10.1111/dsu.12001. [DOI] [PubMed] [Google Scholar]
- 16.Carruthers J, Rzany B, Sattler G, Carruthers A. Anatomic guidelines for augmentation ofthe cheek and infraorbital hollow. Dermatol Surg. 2012;38:1223–1233. doi: 10.1111/j.1524-4725.2012.02478.x. [DOI] [PubMed] [Google Scholar]
- 17.Dayan S, Arkins JP, Mathison CC. Management of impending necrosis associated with soft tissue filler injections. J Drugs Dermatol. 2011;10:1007–1012. [PubMed] [Google Scholar]
- 18.Cohen JL, Dayan SH, Brandt FS, et al. Systematic review of clinical trials of small- and large-gel-particle hyaluronic acid injectable fillers for aesthetic soft tissue augmentation. Dermatol Surg. 2013;39:205–231. doi: 10.1111/dsu.12036. [DOI] [PubMed] [Google Scholar]
- 19.Kang MS, Park ES, Shin HS, et al. Skin necrosis ofthe nasal ala after injection of dermal fillers. Dermatol Surg. 2011;37:375–380. doi: 10.1111/j.1524-4725.2011.01891.x. [DOI] [PubMed] [Google Scholar]
- 20.Narins RS, Jewell M, Rubin M, et al. Clinical conference: management of rare events following dermal fillers-focal necrosis and angry red bumps. Dermatol Surg. 2006;32:426–434. doi: 10.1111/j.1524-4725.2006.32086.x. [DOI] [PubMed] [Google Scholar]


