Abstract
Oxidative stress reflects an excessive accumulation of reactive oxygen species (ROS) and is a hallmark of several acute and chronic human pathologies. While many antioxidants have been investigated, the majority have demonstrated poor efficacy in clinical trials. Here, we discuss limitations of current antioxidants and describe a new class of nanoparticle antioxidants, poly(ethylene glycol)-functionalized hydrophilic carbon clusters (PEG-HCCs). PEG-HCCs show high capacity to annihilate ROS such as superoxide and hydroxyl radicals, show no reactivity toward nitric oxide, and can be functionalized with targeting moieties without loss of activity. Given these properties, we propose that PEG-HCCs offer an exciting new area of study for treatment of numerous ROS-induced human pathologies.
Keywords: antioxidant, traumatic brain injury, carbon nanoparticle, oxidative stress
Oxidative Stress as a Therapeutic Target
Oxidative stress is a state in which the equilibrium of pro-oxidants and antioxidants shifts in favor of pro-oxidant species. ROS contain unpaired electrons that are highly reactive toward other molecules such as nucleic acids, lipids and proteins. Oxidative damage to nucleic acids can lead to modifications of genetic material that contribute to mutagenesis. Lipid peroxidation is the reaction of ROS and lipids in a free-radical chain sequence also known as autoxidation. Oxidative damage to proteins can lead to alterations in their primary, secondary, and tertiary structure and of enzymes, leading to inactivation [1].
Superoxide (O2•−) is a radical anion that is considered a primary ROS and it can form secondary ROS through interaction with other molecules, metals, or enzymes [2,3]. For example, O2•− can facilitate production of the reactive hydroxyl radical (HO•) by releasing iron from iron-sulfur containing enzymes [4]. In addition, O2•− can lead to the generation of hydrogen peroxide (H2O2) through dismutation. In the presence of nitric oxide (NO•), a radical used by several tissues as a signaling molecule, the highly reactive anion peroxynitrite (ONOO−) is formed. This species is implicated in lipid peroxidation and oxidative damage [5]. Lipid oxidation generates lipid free radicals (•R).
Shortcomings of classical antioxidants
Despite the plethora of data on oxidative stress in disease, including acute ischemic injury, most large clinical trials with antioxidants have shown little to no benefit in disease treatment [6]. We propose that the critical limitations of currently available antioxidants (Table 1) include one or more of the following: (i) requirement for the presence of additional downstream enzyme(s) to detoxify the radical product of an upstream molecule, (ii) limited number of radicals removed per antioxidant moiety, (iii) antioxidant regeneration by enzymes which may be consumed in the toxic milieu, and (iv) production of additional radicals through the antioxidant’s mechanism of action.
Table 1.
Mechanism of action of various antioxidants
| Antioxidant | Target ROS | # of ROS removed per molecule | ROS generated | Detoxifying enzyme |
|---|---|---|---|---|
| SOD | •O2− | 2 | H2O2 | none |
| catalase | H2O2 | 2 | none | none |
| glutathione peroxidase | H2O2 | 1 | none | none |
| glutathione | H2O2 | 1 | none | glutathione reductase |
| vitamins E | •O2−, •R | 1 | •E | vitamin C |
| vitamin C | •E, •R | 1 | •C | dehydroascorbate reductase |
| albumin | •OH | not known | none (presumably by disulfide formation) | none |
| PBN | •O2−, •OH | 1 | nitroxide free radical | none |
| tempol | •O2−, ONOO | 1 | H2O2, •NO | none |
| fullerene derivative (C60) | •O2−, •OH | not known | none | none |
| PEG-HCCs | •O2−, •OH | estimated 106 | H2O2 | none |
The in vivo response to oxidative stress is to transfer the free radical through a chain-reaction that requires the concerted action of many antioxidants. For instance, superoxide dismutase and catalase work sequentially to convert O2•− to H2O2 and oxygen and then finally to oxygen and water [7,8]. As a result, this arrangement necessitates that both enzymes are present to effectively destroy the radical. Furthermore, the majority of classical antioxidants can remove at most two radicals per molecule of antioxidant. Most antioxidants such as glutathione, vitamin C, and vitamin E reduce an ROS by donating electrons to the free radical, and in the process generating an additional ROS (oxidized vitamin C and vitamin E) which then requires additional enzymes to regenerate the antioxidant. These limitations may provide one explanation why, even though transgenic models that overexpress antioxidants show quite robust protection against acute injury, there is little evidence for benefit of antioxidant therapy in a clinical setting when therapy begins following the injury [9,10].
In cases of high oxidative stress, such as those following acute nervous system injuries, in conditions of ischemia or reperfusion, or during hemorrhagic shock and resuscitation, free radicals may be transferred to nearby proteins, nucleic acids or lipids, which could lead to additional biological damage. Classical antioxidant systems would be exhausted under such conditions, and regeneration of antioxidants might not occur. Therefore, antioxidants that can quench or dismutate multiple radical species or act as terminal acceptors to a large number of ROS might be more beneficial during conditions of excessive oxidative bursts than antioxidants that require regeneration.
Nano-antioxidants
The need for efficient antioxidants has led to the development of nanoparticle antioxidants, which can include many structures such as liposomes and metal. Metal oxide nanoparticles such as cerium oxide (CeO2) and yttrium oxide (Y2O3) have shown promising results in several disease models [11]. Other precious metals such as gold or platinum stabilized with pectin were able to quench O2•− and H2O2 [12]. In a similar vein, nano-jewels, composed of a diamond nanoparticle scaffold supporting either gold or platinum nanoparticles, have nearly 2-fold higher antioxidant activity than glutathione [13].
Carboxy-functionalized carbon-based buckminsterfullerenes (C60) were found to be highly reactive with ROS, and this may be mediated through their highly conjugated double bond system [14]. Several water-soluble derivatives of C60 were synthesized and tested in cells and found to be neuroprotective in cultured cortical neurons [15]. C60 and its derivatives have been reported to react with O2•− radicals, hydroxyl radical, alkylperoxyl radicals, alkoxyl radicals, and benzyl radicals [15–17]. Some C60 derivatives were found to possess SOD mimetic properties, although their rate constant was around 100-fold slower than SOD [16] and functionalization reduced activity in some circumstances. [14] Carbon nanotubes have also been found to have antioxidant activity [18,19]. However, there are concerns regarding toxicity of these structures [20].
Poly(ethylene glycol)-functionalized hydrophilic carbon clusters (PEG-HCCs): active nanovectors
The limited aqueous solubility of many new therapeutics and promising drug candidates is a long-standing challenge in the pharmaceutical industry. A safe, modular drug delivery platform consisting of small (<40 nm) poly(ethylene glycol)-functionalized hydrophilic carbon clusters (PEG-HCCs) is a possible solution [21]. Because of the presence of hydrophobic domains on the HCC core, PEG-HCCs would be excellent carriers for hydrophobic drugs such as paclitaxel, docetaxel, SN-38, prednisone, rosiglitazone, idarubicin, vinblastine, and glibenclamide. Drugs can be loaded non-covalently and the resulting aqueous solutions are stable at room temperature for at least 5 months [21].
This platform was extended into a targeted drug delivery vehicle by functionalizing the PEG-HCCs with both monoclonal antibodies and targeting peptides. PEG-HCCs can be noncovalently functionalized with the antibody to the epidermal growth factor receptor (EGFR) (Cetuximab (Cet)) for specific delivery of unmodified PTX to EGFR+ tumors but not EGFR− tumors [22,23]. Analogous delivery to tissue specific targets in glioblastoma multiforme (GBM), an aggressive human brain cancer with poor clinical outcome, has shown enhanced tumoricidal activity in multiple cell lines without evidence of toxicity to normal human astrocytes [24].
Subsequently, it was thought that the graphitic structure of the HCC core would result in antioxidant activity, as has been shown for fullerene derivatives [13–15]. Indeed, PEG-HCCs have been shown to be remarkable antioxidants, and are able to annihilate ROS such as superoxide and hydroxyl radical in vitro and in vivo [25,26]. As such, PEG-HCCs offer an exciting new area of study for treatment of numerous pathologies in which ROS are implicated, and could potentially succeed where classical antioxidants have failed.
PEG-HCCs are antioxidants
PEG-HCCs possess a remarkable ability to quench O2•− and HO• while being inert to NO and ONOO− [25]. One potential mechanism for quenching is the catalytic dismutation of O2•− whereby the HCC core of the PEG-HCC is not destroyed:
| (1) |
| (2) |
This proposed mechanism of action, while similar to a previously described tris-malonic acid derivative of C60 (C3), does not invoke the same high entropy transition state [16]. A PEG-HCC can accept an electron from O2•− (eq 1) to form a highly delocalized electron pair on the conjugated carbon core, followed by the donation of an electron to a second molecule of O2•− (eq 2) accompanied by the rapid capture of two protons from water to complete the catalytic cycle.
PEG-HCCs are readily internalized by murine brain endothelial (bEnd.3) cells and were effective at reducing ROS levels when administered 10 minutes after induction of oxidative stress, whereas PEG-SOD and PBN, a small molecule antioxidant, required pretreatment with 10-fold or higher dose to achieve comparable ROS reduction [26]. Moreover, treatment with PEG-HCCs significantly restored cell viability whereas PEG-SOD and PBN displayed minimal cellular protective effect when administered following the onset of oxidative stress [25,26].
Synthesis, toxicity, and biodistribution of PEG-HCCs
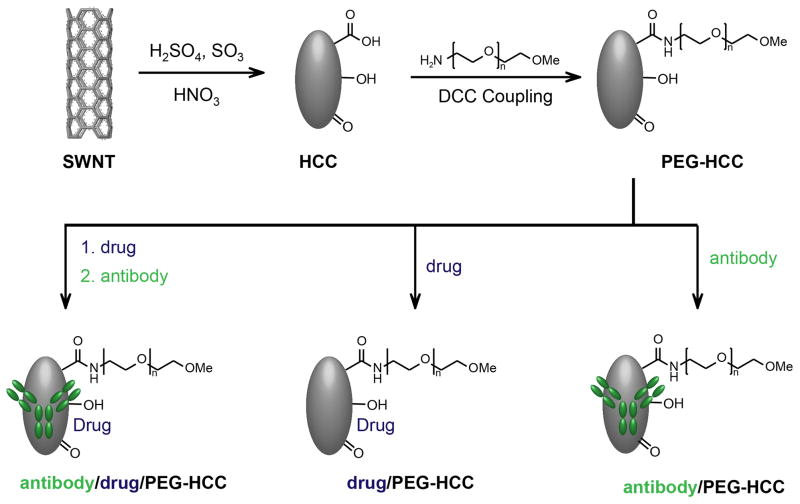
HCCs are prepared by subjecting single-walled carbon nanotubes (SWCNTs) to a harsh oxidation procedure that uses a mixture of fuming sulfuric acid and nitric acid [21]. Careful control of temperature and time yields a carbon material which bears very little resemblance to the starting SWCNTs. The surface of the HCCs harbors a variety of oxygen-containing moieties such as alcohols, ketones and carboxylic acids. To increase water solubility, poly(ethylene glycol) (PEG) is covalently attached to the HCCs via standard carbodiimide-coupling chemistry. PEG-HCCs have a hydrodynamic diameter of 35 to 40 nm, which is comparable to a medium-sized protein. The final product is a nanoparticle that can be further functionalized and used in a variety of applications (Figure 1) [27].
Figure 1. HCC synthesis and functionalization.
HCCs are prepared from SWNTs by oxidation in fuming sulfuric acid and nitric acid (top left). The carboxylic acids on the resulting HCCs are coupled to PEG to form PEG-HCCs (top right), which can be used to non-covalently sequester hydrophobic drugs, targeting antibodies or both for a variety of medicinal applications (bottom).
As with any newly designed therapeutic compound, toxicity considerations are of utmost importance. Carbon nanotubes have been suggested to be hazardous due to their superficial resemblance to asbestos [20]. Although derived from SWCNTs, PEG-HCCs are highly functionalized, well dispersed, hydrophilic, small in size, and contain little to no contamination with metals. Thus, they bear no resemblance to asbestos, and these characteristics should allay fears of toxicity [28–30]. Mice treated with PEG-HCCs weekly for 10 weeks showed no signs of discomfort, fatigue, or weight loss; histology revealed no toxicity to heart, lungs, spleen, kidneys, liver, or brain; renal and liver markers were unchanged and hematology was normal [21]. PEG-HCCs accumulated mostly in the spleen, liver, and kidneys, were excreted through the urine, and their blood half-life was estimated to be about 2 to 3 hours [21]. Although PEG-HCCs did not accumulate in large quantities in the brain parenchyma, functionalizing them with a lipophilic moiety such as adamantane [31] might enable biodistribution to the brain.
While differing in nearly all major features from carbon nanotubes, the conjugated carbon framework may make it possible for PEG-HCCs to be eliminated in the same fashion as smaller carboxylated or oxidized carbon nanotubes, which can be degraded by enzymes such as horseradish peroxidase [32] and myeloperoxidase [33] in the presence of radicals such as H2O2 or by conditions created to simulate the phagolysosomal environment.
PEG-HCCs as Potential Therapeutic Agents in Experimental Models
Traumatic brain injury is a bi-phasic injury in which the first phase is the mechanical damage to the brain and the second phase is the activation of various biochemical, physiological, and molecular cascades that lead to exacerbation of the initial insult. Not only are ROS produced immediately after the primary mechanical injury to the brain, but also during secondary events initiated by TBI and during treatment such as resuscitation and reperfusion of blood that had been lost by a secondary insult [34,35]. Secondary insults such as significant blood loss (hemorrhagic shock), hypotension or hypoxia significantly worsen outcomes in animal models of TBI and are associated with increased mortality in TBI patients [36]. There are currently no FDA-approved treatments proven to mitigate the effects of TBI.
Many therapeutic agents have entered clinical trials for TBI and, unfortunately, the majority of them showed no significant improvement in patient outcome and survival, other than perhaps some specific subgroups. Because ROS production is correlated with the second phase of damage in TBI [35], it is possible that the damage could be due to oxidative stress, thus antioxidants have been a major point for consideration. The antioxidant compounds PEG-SOD and tirilazad, an inhibitor of lipid peroxidation, have both been tested in multicenter phase III trials with no significant benefit in the overall TBI population, although post hoc analysis demonstrated potential benefit in certain subsets of patients [34,37,38]. In a small study, vitamin C and vitamin E each had mixed results [39]. Dexanabinol, a mixed glutamate antagonist and antioxidant drug, also failed to show therapeutic benefit in a randomized, placebo-controlled clinical trial in severe TBI [40].
Given the great potential of PEG-HCCs to remove O2•−, we postulate that these carbon nanoparticles could inhibit oxidative damage and possibly restore autoregulation in experimental TBI models. Indeed, PEG-HCCs restored cerebral blood flow in rats to pre-injury levels temporarily (consistent with their blood half-life) and reduced O2•− levels compared to vehicle-treated animals [26]. With this result, it is conceivable that native or targeted PEG-HCCs might be therapeutically useful in other cases, such as for acute and chronic diseases, including: nonalcoholic fatty liver disease (NAFLD), chemotherapy induced neurotoxicity, multiple sclerosis, and rheumatoid arthritis, as oxidative stress has been implicated in each [41,42].
Concluding remarks and future perspectives
The body is inevitably subjected to oxidative stress and, if not adequately resolved, various resulting pathologies could culminate in disease. Although traditional antioxidants would seem to offer a remedy, their success has been limited, possibly due to their mechanism of action. Most antioxidants possess low radical capacity (i.e. they can detoxify 1 to 2 radicals per molecule of antioxidant), and must depend on other enzymes to complete the detoxification and regenerate the antioxidant. PEG-HCCs are unique due to their high antioxidant capacity, the possibility of catalytic behavior, and non-reliance on detoxifying enzymes. Additional advantages include the potential to become targetable via simple mixing with antibodies and carry additional drug payloads. PEG-HCCs thus offer an exciting new area of study for treatment of numerous ROS-induced human pathologies.
Box 1. Outstanding Questions.
What features of PEG-HCC structure are responsible for the catalytic antioxidant activity and the preferred reaction produces, e.g. oxygen vs hydrogen peroxide?
To what extent are these antioxidant properties generic to oxidized carbon nanoparticles?
What is the in-vivo duration of action?
Is the metabolic fate of PEG-HCCs similar to other carbon nanomaterials in relation to breakdown by endogenous peroxidases?
Highlights.
Most antioxidants show little efficacy following trauma such as brain injury or stroke.
Carbon nanoparticles quench superoxide affording no downstream radicals.
Each nontoxic nanoparticle can annihilate thousands of superoxide molecules; the same nanoparticles are selective, being inert to nitric oxide.
Prospects are shown for treatment of numerous superoxide-induced human pathologies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fucci L, et al. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci USA. 1983;80:1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valko M, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Miller DM, et al. Transition metals as catalysts of ‘autoxidation’ reactions. Free Radic Biol Med. 1990;8:95–108. doi: 10.1016/0891-5849(90)90148-c. [DOI] [PubMed] [Google Scholar]
- 4.Liochev SI, Fridovich I. The role of O2- in the production of HO.: in vitro and in vivo. Free Radic Biol Med. 1994;16:29–33. doi: 10.1016/0891-5849(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 5.Radi R, et al. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 6.Deng-Bryant Y, et al. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J Cereb Blood Flow Metab. 2008;28:1114–1126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- 7.Fridovich I. Superoxide Dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- 8.Evans CAL. On the Catalytic Decomposition of Hydrogen Peroxide by the Catalase of Blood. Biochem J. 1907;2:133–155. doi: 10.1042/bj0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokubo Y, et al. Transgenic mice expressing human copper-zince superoxide disumtase exhibit attenuated apparent diffusion coefficient reduction during reperfusion following focal cerebral ischemia. Brain Res. 2002;947:1–8. doi: 10.1016/s0006-8993(02)02899-8. [DOI] [PubMed] [Google Scholar]
- 10.Slemmer JE, et al. Antioxidants and Free Radical Scavengers for the Treatment of Stroke, Traumatic Brain Injury and Aging. Curr Med Chem. 2008;15:404–414. doi: 10.2174/092986708783497337. [DOI] [PubMed] [Google Scholar]
- 11.Schubert D, et al. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun. 2006;342:86–91. doi: 10.1016/j.bbrc.2006.01.129. [DOI] [PubMed] [Google Scholar]
- 12.Kajita M, et al. Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radic Res. 2007;41:615–626. doi: 10.1080/10715760601169679. [DOI] [PubMed] [Google Scholar]
- 13.Martínez A, Galano A. Free Radical Scavenging Activity of Ultrashort Single-Walled Carbon Nanotubes with Different Structures through Electron Transfer Reactions. J Phys Chem C. 2010;114:8184–8191. [Google Scholar]
- 14.Krusic PJ, et al. Radical reactions of C60. Science. 1991;254:1183–1185. doi: 10.1126/science.254.5035.1183. [DOI] [PubMed] [Google Scholar]
- 15.Dugan LL, et al. Buckminsterfullerenol free radical scavengers reduce excitotoxic and apoptotic death of cultured cortical neurons. Neurobiol Dis. 1996;3:129–135. doi: 10.1006/nbdi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 16.Ali SS, et al. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radic Biol Med. 2004;37:1191–1202. doi: 10.1016/j.freeradbiomed.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Bensasson RV, et al. Reactions of e(−)(aq), CO(2)(*)(−), HO(*), O(2)(*)(−) and O(2)((1)delta(g)) with a dendro[60]fullerene and C(60)[C(COOH)(2)](n) (n = 2–6) Free Radic Biol Med. 2000;29:26–33. doi: 10.1016/s0891-5849(00)00287-2. [DOI] [PubMed] [Google Scholar]
- 18.Lucente-Schultz RM, et al. Antioxidant Single-Walled Carbon Nanotubes. J Am Chem Soc. 2009;131:3934–3941. doi: 10.1021/ja805721p. [DOI] [PubMed] [Google Scholar]
- 19.Galano A. Carbon nanotubes: promising agents against free radicals. Nanoscale. 2010;2:373–380. doi: 10.1039/b9nr00364a. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, et al. Understanding the Toxicity of Carbon Nanotubes. Acc Chem Res. 2013;46:702–713. doi: 10.1021/ar300028m. [DOI] [PubMed] [Google Scholar]
- 21.Berlin JM, et al. Effective Drug Delivery, In Vitro and In Vivo, by Carbon-Based Nanovectors Noncovalently Loaded with Unmodified Paclitaxel. ACS Nano. 2010;4:4261–1636. doi: 10.1021/nn100975c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berlin JM, et al. Noncovalent Functionalization of Carbon Nanovectors with an Antibody Enables Targeted Drg Delivery. ACS Nano. 2011;5:6643–6650. doi: 10.1021/nn2021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sano D, et al. Noncovalent Assembly of Targeted Carbon Nanovectors Enables Synergistic Drug and Radiation Cancer Therapy in Vivo. ACS Nano. 2012;6:2497–2505. doi: 10.1021/nn204885f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharpe MA, et al. Antibody-targeted nanovectors for the treatment of brain cancers. ACS Nano. 2012;6:3114–3120. doi: 10.1021/nn2048679. [DOI] [PubMed] [Google Scholar]
- 25.Marcano DC, et al. Design of poly(ethylene glycol)-functionalized hydrophilic carbon clusters for targeted therapy of cerebrovascular dysfunction in mild traumatic brain injury. J Neurotrauma. 2013;30:789–796. doi: 10.1089/neu.2011.2301. [DOI] [PubMed] [Google Scholar]
- 26.Bitner BR, et al. Antioxidant carbon particles improve cerebrovasculate dysfunction following traumatic brain injury. ACS Nano. 2012;6:8007–8014. doi: 10.1021/nn302615f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berlin JM, Tour JM. Development of novel drug delivery vehicles. Nanomedicine (Lond) 2010;5:1487–1489. doi: 10.2217/nnm.10.121. [DOI] [PubMed] [Google Scholar]
- 28.Hurt RH, et al. Toxicology of carbon nanomaterials: Status, trends, and perspectives on the special issue. Carbon. 2006;44:1028–1033. [Google Scholar]
- 29.Sayes CM, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161:135–142. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Schipper ML, et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 31.Wanka L, et al. The Lipophilic Bullet Hits the Targets: Medicinal Chemistry of Adamantane Derivatives. Chem Rev. 2013;113:3516–3604. doi: 10.1021/cr100264t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen BL, et al. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008;8:3899–3903. doi: 10.1021/nl802315h. [DOI] [PubMed] [Google Scholar]
- 33.Kagan VE, et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol. 2010;5:354–359. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall ED, et al. Antioxidant therapies for traumatic brain injury. Nanotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabian RH, et al. In vivo detection of superoxide anion production by the brain using a cytochrome c electrode. J Cereb Blood FLow Metab. 1995;15:242–247. doi: 10.1038/jcbfm.1995.30. [DOI] [PubMed] [Google Scholar]
- 36.Chesnut RM, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Marshall LF, et al. A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J Neurosurg. 1998;89:519–525. doi: 10.3171/jns.1998.89.4.0519. [DOI] [PubMed] [Google Scholar]
- 38.Muizelaar JP, et al. PEG-SOD after head injury. J Neurosurg. 1995;83:942. doi: 10.3171/jns.1995.83.5.0942. [DOI] [PubMed] [Google Scholar]
- 39.Razmkon A, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133–137. doi: 10.1227/neu.0b013e3182279a8f. [DOI] [PubMed] [Google Scholar]
- 40.Maas AIR, et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III double-blind controlled trial. Lancet Neurol. 2006;5:38–45. doi: 10.1016/S1474-4422(05)70253-2. [DOI] [PubMed] [Google Scholar]
- 41.Phillips DC, et al. Aberrant reactive oxygen and nitrogen species generation in rheumatoid arthritis (RA): causes and consequences for immune function, cell survival, and therapeutic intervention. Antioxid Redox Signal. 2010;12:743–785. doi: 10.1089/ars.2009.2607. [DOI] [PubMed] [Google Scholar]
- 42.Uttara B, et al. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]