Abstract
Hippocampal memory formation is highly regulated by post-translational histone modifications and DNA methylation. Accordingly, these epigenetic processes play a major role in the effects of modulatory factors, such as sex steroid hormones, on hippocampal memory. Our laboratory recently demonstrated that the ability of the potent estrogen 17β-estradiol (E2) to enhance hippocampal-dependent novel object recognition memory in ovariectomized female mice requires ERK-dependent histone H3 acetylation and DNA methylation in the dorsal hippocampus. Although these data provide valuable insight into the chromatin modifications that mediate the memory-enhancing effects of E2, epigenetic regulation of gene expression is enormously complex. Therefore, more research is needed to fully understand how E2 and other hormones employ epigenetic alterations to shape behavior. This review discusses the epigenetic alterations shown thus far to regulate hippocampal memory, briefly reviews the effects of E2 on hippocampal function, and describes in detail our work on epigenetic regulation of estrogenic memory enhancement.
Keywords: Estrogen, Object recognition, Spatial memory, Histone acetylation, Hippocampus, Aging, Epigenetic
1. Introduction
What makes a memory last a lifetime? Whether a particular face, a notable event, or a remarkable location, some experiences need occur only once for their memory to persist indefinitely. The past few decades have seen an explosion of research devoted to understanding how our memories are formed and stored at the molecular and cellular levels. This work has not only provided a basic understanding of the molecular underpinnings of memory consolidation and reconsolidation, but has also provided important insight into the neural mechanisms underlying memory impairments in neurodevelopmental disorders, neurodegenerative diseases, and aging. Increasingly, epigenetic alterations have been implicated in both normal memory formation and memory dysfunction. Unlike mutations that physically alter DNA, epigenetic (“above the gene”) mechanisms alter transcriptional access to DNA. The dizzying array of epigenetic modifications that regulate the transcription of genes necessary for memory formation provides the means to regulate gene expression and enable encoding of an almost infinite number of memories. However, prior to the discovery of epigenetic regulation of memory, the role of DNA in the storage of so many distinct memories was unclear. Even Francis Crick expressed doubt that memory could be coded within specific sections of DNA (Crick, 1984). Instead, he proposed that activity-dependent chemical modifications of proteins, such as phosphorylation or methylation, could provide the increased synaptic strength hypothesized to underlie memory formation (Crick, 1984). Subsequent studies showing that DNA methylation could stably repress gene expression led to the proposal that memory formation is regulated by DNA methylation and demethylation (Holliday, 1999). In the years since, the new field of cognitive neuroepigenetics (Day and Sweatt, 2011) has demonstrated the critical importance of both DNA methylation and histone modifications for long-term memory formation.
Research from the past three decades has also highlighted the myriad of ways in which hormones can influence memory formation. Initial evidence suggesting a role for sex steroid hormones in memory came from studies in the late 1980s localizing estrogen receptors to the dorsal hippocampus and entorhinal cortex (Loy et al., 1988; Maggi et al., 1989). Not long afterwards, work by Woolley, Gould, and McEwen showing that estrogens and progesterone regulates hippocampal CA1 spine density in female rats (Gould et al., 1990; Woolley et al., 1990; Woolley and McEwen, 1992, 1993) gave birth to the field of hormones and cognition. These findings inspired hundreds of subsequent studies that have investigated the effects of estrogens and progesterone on hippocampal plasticity and memory. Although some studies have examined the influence of naturally fluctuating estrogen and progesterone levels on hippocampal function, most have administered exogenous hormones to ovariectomized female rodents to simplify interpretation of the data. The vast majority of these studies have focused on the potent estrogen, 17β-estradiol (E2), given its important role in promoting the formation of new dendritic spines and excitatory synapses in the hippocampus (Woolley, 2007). In rodents, exogenous E2 also facilitates hippocampal neurogenesis, various forms of hippocampal synaptic plasticity including long-term potentiation, and rapid hippocampal cell signaling (Woolley, 2007; Pawluski et al., 2009; Frick, 2012). Accordingly, many studies report that exogenous E2 administered to rats or mice enhances the acquisition and consolidation of several forms of hippocampal learning and memory, including spatial memory, working memory, contextual memory, and object recognition memory (for reviews, see Daniel, 2006; Frick, 2009; Gibbs, 2010; Choleris et al., 2012).
Given the importance of both E2 and epigenetics to hippocampal memory formation, it is natural to ask whether epigenetic mechanisms play a role in estrogenic regulation of hippocampal memory. As such, our laboratory began to investigate this issue in the late 2000s in ovariectomized female mice. This work arose from our studies showing that E2 rapidly (within 5 min) activates hippocampal cell signaling pathways, including the extracellular signal-regulated kinase/mitogen activated protein kinase (ERK/MAPK) pathway (Fernandez et al., 2008). Phosphorylation of ERK/MAPK increases gene transcription by activating numerous transcription factors, including cyclic AMP response element binding protein (CREB) (Roberson et al., 1999), and by promoting histone acetylation (Chwang et al., 2006). Our studies to date have demonstrated that both histone acetylation and DNA methylation are necessary for E2 to enhance the consolidation of hippocampal-dependent object recognition memory in female mice (Zhao et al., 2010, 2012). What is the functional significance of epigenetic regulation of estrogen-dependent memory? Memory dysfunction is characteristic of aging, neurodegenerative diseases such as Alzheimer’s disease, and neuropsychiatric diseases such as depression and schizophrenia (Gur and Gur, 2013; Pittenger, 2013; Ha et al., 2014), and accumulating research suggests that treatments targeting specific epigenetic modifications can reduce memory deficits in patients with Alzheimer’s disease, Parkinson’s disease, depression, schizophrenia, stroke, and traumatic brain injury (Fischer et al., 2010). Estrogen loss at menopause (surgical or natural) can accelerate the onset of age-related cognitive decline (Sherwin, 2012; Hogervorst, 2013) and is associated with an increased risk of Alzheimer’s disease in women (reviewed in Maki, 2012). Furthermore, estrogens are associated with the symptomatology of bipolar disorder, anxiety disorders, depression, schizophrenia, migraine, catamenial epilepsy, and stroke (Walf and Frye, 2006; Milad et al., 2010; Begemann et al., 2012; Marsh et al., 2012; Reddy, 2013; Sohrabji and Williams, 2013). Although E2’s neuroprotective, anti-depressant, mood-stabilizing, and memory enhancing properties (Walf and Frye, 2006; Sherwin and Henry, 2008; Kulkarni, 2009; Liu et al., 2010; Luine and Frankfurt, 2013) might support its use to reduce memory dysfunction in several brain disorders, estrogen therapy has been associated with potentially life-threatening side effects including breast and uterine cancer (Rossouw et al., 2002; Chlebowski et al., 2003). On the other hand, several histone deacetylase inhibitors have been FDA-approved to treat various cancers (Wagner et al., 2010), so epigenetic treatments might afford a mechanism for providing the cognitive benefits of estrogens without their carcinogenic effects. Therefore, understanding how epigenetic alterations regulate estrogen-dependent memory could lead to new treatments to reduce memory dysfunction in a variety of disorders in which estrogens play a role.
Thus far, our laboratory is alone in investigating the roles of epigenetic alterations in E2-induced memory enhancement. Therefore, this work will be described here in some detail. We will begin by reviewing how DNA methylation and the most well characterized histone modifications influence learning and memory. Numerous comprehensive reviews have detailed the epigenetic mechanisms involved in the neurobiology of learning and memory (e.g. Barrett and Wood, 2008; Sweatt, 2009; Day and Sweatt, 2010, 2011; Franklin and Mansuy, 2010; Lattal and Wood, 2013; Zovkic et al., 2013), so we refer the reader to these resources for more information beyond that presented here. Next, we briefly review the considerable literature on estrogenic modulation of memory and rapid cell signaling to set the stage for a more detailed description of how DNA methylation and histone acetylation regulate E2-induced memory enhancement in the hippocampus. Finally, we end by discussing future directions for this research, including how estrogenic regulation of epigenetic processes may contribute to sex differences in memory and age-related memory decline.
2. Epigenetic regulation of hippocampal memory
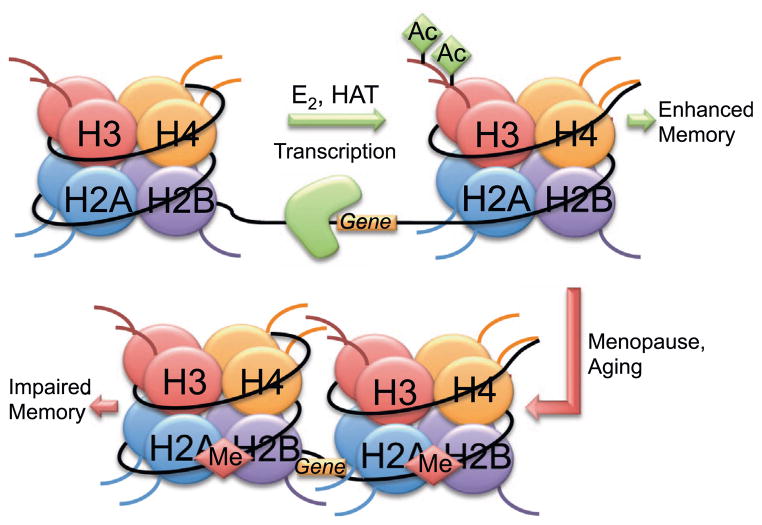
DNA is compacted in chromosomes within units called nucleosomes. Approximately 147 base pairs of DNA are wrapped around each nucleosome (Gardner et al., 2011). Each nucleosome is comprised of an octamer of four histone (H) proteins, two each of H2A, H2B, H3, and H4, all of which have an N-terminal tail that can be altered by post-translational modifications (PTMs) including acetylation, phosphorylation, methylation, ubiquitination, and SUMOylation (Fig. 1) (Eickbush and Moudrianakis, 1978; Rothbart and Strahl, 2014). Adjacent nucleosomes are connected by the linker H1 protein, and are supercoiled to form chromatin (Mazzio and Soliman, 2012). Chromatin can exist as either heterochromatin, which prohibits transcription due to its tightly compacted state, or as euchromatin, which is a more relaxed chromatin state that is permissive to transcription. Transcription of a specific segment of DNA is dependent on the methylation state of certain cytosine residues and PTMs present on histone N-terminal tails. The roles of DNA methylation and histone acetylation, phosphorylation, and methylation in regulating memory are most well studied, and so each of these modifications will be discussed in turn below. Because emerging evidence suggests a role for histone ubiquitination and SUMOylation in synaptic plasticity, these PTMs will be addressed as well.
Fig. 1.
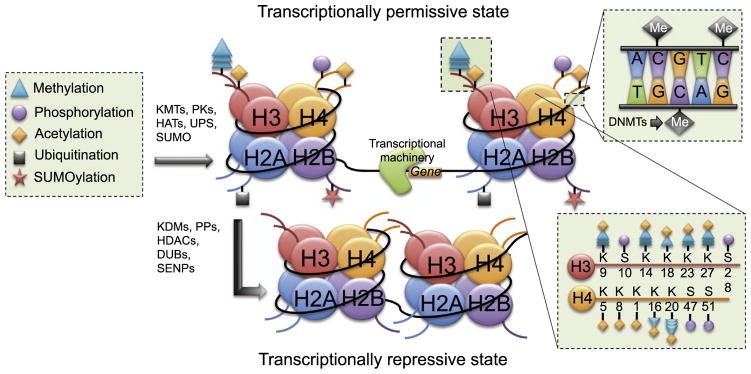
Schematic illustration of epigenetic modifications studied in relation to hippocampal memory formation. The nucleosome is represented by the colored circles (histone octamer) and curved black line (DNA). Curved lines extending from each histone protein represent N-terminal tails. Each amino acid is bidirectionally modified by enzymes controlling methylation, phosphorylation, acetylation, ubiquitination, and SUMOylation, the symbols for which are shown in the box to the left of the figure. Each N-terminal tail can receive only certain modifications at each amino acid position (see lower right box for representative post-translational modifications of H3 and H4 tails). Histone modification by KMTs, PKs, HATs, the UPS, and SUMO relaxes the chromatin structure and allows a transcriptionally permissive state where the transcriptional machinery can bind to the DNA and increase gene transcription. Removal of post-translational modifications by KDMs, PPs, HDACs, DUBs, and SENPs condenses the chromatin structure, thereby causing a transcriptionally repressive state that prevents transcriptional access. DNA methylation on cytosine residues (box in upper right) also causes transcriptional repression. Abbreviations: K, lysine; S, serine; KMTs, lysine methyltransferases; PKs, protein kinases; HATs, histone acetyltransferases; UPS, ubiquitin proteosome system; SUMO, small ubiquitin-like modifier; KDMs, lysine demethylases; PPs, protein phosphatases; HDACs, histone deacetylases; DUBs, deubiquitinating enzymes; SENPs, sentrin/SUMO-specific proteins; DNMTs, DNA methyltransferases.
2.1. DNA methylation
DNA methylation is the only epigenetic alteration that modifies the DNA itself (Robertson, 2005). This process involves the covalent addition of a methyl group from an S-adenosyl-methionine (SAM) methyl donor to the 5′ position on a cytosine, which forms 5-methylcytosine (5mC). Methylated cytosine residues are typically adjacent to a guanine nucleotide in so-called CpG islands (Fig. 1) (Day and Sweatt, 2011; Zovkic et al., 2013). Methylation within CpG islands occurs primarily at intragenic regions and in gene bodies, and to a lesser extent in promoter regions (Grayson and Guidotti, 2013). The methylation reaction is catalyzed by DNA methyltransferases (DNMTs), of which there are three in mammals: DNMT1, DNMT3A, and DNMT3B (Denis et al., 2011). DNMT1 preferentially methylates hemimethylated DNA during replication and is, therefore, regarded as a maintenance methyltransferase (Denis et al., 2011). DNMT3A and DNMT3B, however, are considered de novo methyltransferases, due to their ability to methylate sites irrespective of previous methylation status (Okano et al., 1999). Once a site has been methylated, it is ‘read’ to contain a methylation mark by methyl-CpG binding proteins (MBDs), such as MeCP2 and MBD1-4 (Robertson, 2005). MBDs recruit transcriptional co-repressors, which ultimately prevent DNA transcription by facilitating a closed heterochromatin state (Fuks et al., 2003; Robertson, 2005). Thus, DNA methylation is typically considered a transcriptionally repressive modification, although it has been shown to activate transcription (Chahrour et al., 2008). For example, mice that overexpressed MeCP2 exhibited an 85% increase in gene expression in the hypothalamus, whereas mice deficient in MeCP2 displayed an 85% decrease in expression of the same genes relative to control mice (Chahrour et al., 2008). Evidence for a dual role of DNA methylation in transcriptional regulation is also supported by studies outside of the nervous system, which suggest that DNMTs can both methylate and demethylate DNA on active promoter regions (Metivier et al., 2008). This dual functioning of DNMTs suggests that there is much to learn about the relationship between DNA methylation and gene expression.
The first evidence for a role of DNA methylation in regulating synaptic plasticity relating to memory came from reports examining in vitro regulation of brain derived neurotrophic factor (BDNF), a neurotrophin critical for hippocampal synaptic plasticity and memory (Cowansage et al., 2010). In rat cortical neurons, KCl-induced depolarization decreased MeCP2 binding to the Bdnf exon III promoter (Bdnf-pIII) and decreased methylation of CpG islands in regulatory regions of Bdnf-pIV (Chen et al., 2003; Martinowich et al., 2003). These findings suggested that synaptic activity promotes demethylation of genes important for synaptic plasticity and memory. Subsequent treatment of rat hippocampal slices with a DNMT inhibitor demethylated promoters for Bdnf exon I and reelin, another gene implicated in long-term memory formation (Levenson et al., 2006). Activation of protein kinase C also decreased reelin promoter methylation and increased mRNA levels of DNMT3A, indicating activity-dependent regulation of DNA methylation (Levenson et al., 2006). Consistent with this notion, DNMT inhibitors blocked induction of long-term potentiation (LTP) in the hippocampus of male mice and rats (Levenson et al., 2006; Miller et al., 2008), suggesting that methylation is a key regulator of hippocampal synaptic plasticity. Accordingly, hippocampal-dependent contextual fear conditioning increases the expression of DNMT3A and DNMT3B mRNA in the male rat hippocampus (Miller and Sweatt, 2007) of rats. This increased expression is necessary for fear memory consolidation, as illustrated by data showing that DNMT inhibition impairs contextual fear conditioning (Miller and Sweatt, 2007). However, memory formation appears to involve a complex balance between methylation and demethylation that is highly dependent on the function of the genes in question. For example, contextual fear conditioning in male rats decreases methylation of the memory promoting gene reelin, but increases methylation of the memory repressor gene protein phosphatase 1 (PP1) (Miller and Sweatt, 2007). Moreover, contextual fear conditioning increases Bdnf mRNA via rapid demethylation of specific Bdnf promoters (Lubin et al., 2008). Interestingly, hippocampal DNMT inhibition blocks the contextual fear conditioning-induced increase in histone H3 acetylation of hippocampal Bdnf-pIV, and prevents global H3 acetylation in the hippocampus (Lubin et al., 2008). Furthermore, the detrimental effects of DNMT inhibition on LTP and contextual fear conditioning in male rats can be reversed by histone deacetylase inhibition (Miller et al., 2008), indicating important interactions between DNA methylation and histone acetylation that regulate hippocampal memory (Lubin et al., 2008). Combined, these data suggest a complicated interplay between DNA methylation states and histone modifications that influences the expression of genes essential for hippocampal memory consolidation.
Like any other enzymatic reaction, DNA methylation is a bidirectional process. Although a relatively small proportion of the adult mammalian genome contains CpG islands (Saxonov et al., 2006), approximately 70–80% of these islands contain the methylated mark 5mC (Kohli and Zhang, 2013). However, the 5mC mark can be actively removed in multiple ways (for review, see Niehrs and Schafer, 2012; Baker-Andresen et al., 2013; Kohli and Zhang, 2013). Through the base excision repair (BER) pathway, the 5mC is either oxidized to 5-hydroxymethylcytosine (5hmC) by the ten-eleven translocation (TET) family of DNA hydroxylases, or undergoes deamination and subsequent removal of the mismatched T-G nucleotides. 5hmC is abundant in the hippocampal dentate gyrus (Munzel et al., 2010) and manipulations of TET1 in the dentate gyrus regulate 5hmC levels and prevent stimulation-induced demethylation of Bdnf and Fgf1B (fibroblast growth factor 1B) promoters (Guo et al., 2011). These findings suggest that active DNA demethylation via the BER pathway can regulate the expression of genes important for synaptic plasticity in the hippocampus. Alternatively, the 5mC is removed through the nucleotide excision repair (NER) pathway by the recruitment of the immediate early gene growth arrest and damage-inducible beta (Gadd45b), which regulates active DNA demethylation in a neural activity-dependent manner (Ma et al., 2009). Behavioral experience (e.g., exploration of a novel environment or exercise) increases Gadd45b expression and neurogenesis in the adult mouse dentate gyrus, and Gabb45b knockdown reduces electroconvulsive-induced demethylation of Bdnf and Fgf1B (Ma et al., 2009). However, the role of Gadd45b in hippocampal memory is unclear. Although contextual fear conditioning rapidly increases hippocampal Gadd45b mRNA levels in mice (Leach et al., 2012; Sultan et al., 2012), Gadd45b knockout has been reported to both impair (Leach et al., 2012) and enhance (Sultan et al., 2012) hippocampal-dependent memory. Nevertheless, these data support some involvement of DNA demethylation in hippocampal plasticity and memory (Sultan and Sweatt, 2013).
2.2. Post translational histone modifications
Histone modifications are the other major epigenetic alterations that regulate synaptic plasticity and memory function in the hippocampus and elsewhere. Within the nucleosome, DNA is wrapped around the four core histones, each of which has an N-terminal tail whose amino acid residues are highly amenable to post-translational modification. The amino acids most commonly modified are serine (S), lysine (K), arginine (R), threonine (T), and tyrosine (Y); some residues can be altered by multiple modifications (Fig. 1). The most well studied modifications include acetylation, phosphorylation, methylation (mono-, di-, tri-), ubiquitination, and SUMOylation. Other, less well characterized, modifications include crotonoylation, butyrylation, proprionylation, citrullination, ADP-ribosylation, and glycosylation (Musselman et al., 2012; Rothbart and Strahl, 2014). Each histone modification is regulated bidirectionally by a specific enzyme or a class of enzymes. The resulting pattern of post-translational histone modifications is often termed the “histone code”. In the sections below, the five most common post-translational modifications will be discussed in turn, with particular attention given to acetylation, phosphorylation, and methylation because of their established roles in cognitive neuroepigenetics.
2.2.1. Acetylation
Histone acetylation is the most widely studied histone modification involved in learning and memory. It is almost universally associated with activation of gene expression. Acetylation occurs on the lysine residues of each the four core histones. The addition of an acetyl group to a lysine residue decreases net positive charge, thereby weakening interactions between the histone and the negatively charged DNA. This diminished interaction produces a relaxed chromatin structure, which allows the transcriptional machinery access to the DNA. Acetylation is catalyzed by various histone acetyltransferases (HATs), which are categorized into the CBP/p300, GNAT, and MYST subfamilies (Roth et al., 2001). Acetyl groups are removed by histone deacetylases (HDACs), which are categorized into classes based on their zinc or NAD (sirtuins) dependence. Class I, II, and IV HDACs are zinc-dependent, whereas class III HDACs are NAD-dependent (Graff and Tsai, 2013a).
Because the role of histone acetylation in learning and memory has been reviewed extensively elsewhere (Vecsey et al., 2007; Fischer et al., 2010; Sharma, 2010; Graff and Tsai, 2013a, 2013b; Peixoto and Abel, 2013), our goal here is to highlight only seminal papers that have established a role for histone acetylation in hippocampal memory. The importance of histone acetylation in hippocampal memory was initially established by Sweatt and colleagues, who demonstrated that hippocampal-dependent contextual fear conditioning could increase histone H3 acetylation in the CA1 region of the male rat hippocampus in a manner dependent on NMDA receptors and ERK cell signaling (Levenson et al., 2004). Moreover, treatment with the HDAC inhibitor sodium butyrate (NaB) facilitated the induction of LTP in hippocampal slices and enhanced contextual fear memory consolidation (Levenson et al., 2004). As such, these findings provided the first evidence that hippocampal learning regulates histone acetylation and that promoting histone acetylation via HDAC inhibition could enhance hippocampal memory. Pharmacological HDAC inhibition has subsequently been shown to enhance other forms of hippocampal memory consolidation in rats and mice, including object recognition (Stefanko et al., 2009; Zhao et al., 2010) and spatial memory (Haettig et al., 2011; Hawk et al., 2011). The importance of histone acetylation in hippocampal memory formation was further supported by evidence that mice overexpressing HDAC2, but not HDAC1, exhibited impaired spatial memory and contextual fear memory (Guan et al., 2009). HDAC2 overexpression also reduced hippocampal synaptic plasticity, decreased dendritic spine density, and decreased H3 and H4 acetylation at the promoter regions of genes associated with synaptic plasticity and memory (Guan et al., 2009). In contrast, HDAC2 knockout mice displayed increased synapse number, enhanced associative memory formation, and increased H3 and H4 acetylation at the promoter regions of synaptic plasticity genes including Bdnf-pII, Egr1, and GLUR1 (Guan et al., 2009). In subsequent work, shRNA knockdown of HDAC2 in HDAC2 overexpressing mice rescued deficits in hippocampal memory and ameliorated reductions in acetyl H4 binding to gene targets important for synaptic plasticity (Graff et al., 2012). Consistent with the amnestic effects of HDAC2 expression in mice, HDAC2 expression is elevated in hippocampal CA1 and entorhinal cortex of Alzheimer’s patients (Graff et al., 2012). Combined, these seminal findings demonstrated that: (1) histone acetylation is essential for hippocampal memory consolidation, and (2) HDAC2 is a potent negative regulator of hippocampal synaptic plasticity and memory.
At least 11 HDACs have been identified, most of which are expressed in the brain. Therefore, a role for other HDACs in regulating memory has begun to emerge. For example, deletion of HDAC3 or HDAC6 in the hippocampus enhances spatial memory in mice, suggesting that HDAC3 and HDAC6 negatively regulate hippocampal memory (McQuown et al., 2011; Govindarajan et al., 2013). Moreover, knockout of HDAC6 in a mouse model of Alzheimer’s disease reversed contextual and spatial memory deficits (Govindarajan et al., 2013), suggesting that HDAC6 may contribute to cognitive impairments in Alzheimer’s disease. However, not all HDACs are detrimental to hippocampal memory. Loss of HDAC4 function impairs hippocampal memory formation and synaptic plasticity in mice (Kim et al., 2012; Sando et al., 2012), whereas evidence for the role of HDAC5 in hippocampal memory remains controversial (Kim et al., 2012; Agis-Balboa et al., 2013). Furthermore, other data suggest that HDAC1 is necessary for contextual fear memory extinction in mice (Bahari-Javan et al., 2012).
Given the important role of HDACs in regulating memory, considerable recent interest has focused on the use of HDAC inhibitors to reduce cognitive impairment in a variety of neurodegenerative and neuropsychiatric diseases (Kazantsev and Thompson, 2008; Fischer et al., 2010). The potential use of HDAC inhibitors for this purpose could be accelerated relative to other cognitive enhancing compounds because the Food and Drug Administration has already approved many HDAC inhibitors have already been approved by for the treatment of various cancers. Intracranial or systemic administration of class I and class II HDAC inhibitors such as trichostatin-A (TSA), suberoylanilide hydroxamic acid (SAHA), NaB, MS275, and RGFP136 enhances various forms of hippocampal memory in young and aged wild-type rodents, and in mouse models of neurodegenerative disease (Guan et al., 2009; Ricobaraza et al., 2009; Stefanko et al., 2009; Kilgore et al., 2010; Peleg et al., 2010; Zhao et al., 2010; Haettig et al., 2011; Hawk et al., 2011; McQuown et al., 2011). These findings generally support the use of HDAC inhibitors for treating cognitive impairment. However, not all HDACs negatively regulate memory, as described above, so specific HDACs must be carefully targeted for potential use in patients with cognitive impairment.
Although HDAC inhibition is a common approach to maintaining histone acetylation, increasing HAT activity can also augment histone acetylation. The transcriptional coactivator CREB binding protein (CBP) has been most often manipulated in rodent studies because it exhibits intrinsic HAT activity (Goodman and Smolik, 2000). Hippocampal synaptic plasticity and memory formation require CBP, as demonstrated by studies showing that mice in which the CBP protein was reduced or deleted displayed contextual fear, object recognition, and spatial memory deficits, as well as impaired hippocampal LTP (Alarcón et al., 2004; Wood et al., 2005; Chen et al., 2010). Similarly, intrahippocampal infusion of the p300/CBP inhibitor garcinol impairs object recognition memory consolidation in ovariectomized female mice (Zhao et al., 2012). Conversely, a small molecule CBP activator increases hippocampal H3 acetylation and neurogenesis, and promotes long-term spatial memory in male mice (Chatterjee et al., 2013). Together, this evidence indicates that HAT activity plays an important role in regulating hippocampal synaptic plasticity and memory, and suggests that promoting HAT activity could be a viable strategy for enhancing memory formation.
2.2.2. Phosphorylation
Phosphorylation of core histone proteins can occur on various serine, threonine, and tyrosine residues of N-terminal tails. In general, phosphorylation of histone proteins is associated with transcriptional activation (Soloaga et al., 2003; Chwang et al., 2007), although the phosphorylation of H2A on Ser 1 is a rare exception that has been associated with transcriptional repression (Zhang et al., 2004b). Phosphorylation and dephosphorylation of histone tails are regulated by kinases and phosphatases, respectively. Phosphorylation is catalyzed by numerous kinases (for review, see Banerjee and Chakravarti, 2011; Rossetto et al., 2012). Of particular relevance to memory, the phosphorylation of Ser 10 on H3 (H3pS10) is catalyzed by ERK (Adams and Sweatt, 2002). ERK is a serine/threonine kinase that is activated by phosphorylation and is necessary for hippocampal synaptic plasticity and memory formation (Sweatt, 2001). Histone dephosphorylation is regulated by the same phosphatases that act elsewhere in cells, including serine/threonine phosphatases and tyrosine phosphatases.
Histone phosphorylation, specifically phosphorylation of H3S10, was one of the first histone modifications shown to be important for hippocampal memory formation. Phosphorylation of H3S10 is coupled to the acetylation of H3 on Lys 14 (H3K14) (Walter et al., 2008), and the expression of both H3pS10 and acetyl H3K14 is associated with enhanced hippocampal synaptic plasticity and memory (Chwang et al., 2007). Contextual fear conditioning increases hippocampal H3pS10 and acetyl H3K14 in male rats in an ERK-dependent manner, as demonstrated by the fact that inhibition of ERK activation impaired contextual fear memory and prevented both H3S10 phosphorylation and H3K14 acetylation (Chwang et al., 2006). These data suggest an important role for both of these PTMs in contextual fear memory consolidation.
The importance of protein dephosphorylation in neuroepigenetics has been demonstrated in studies examining protein Ser/Thr phosphatase 1 (PP1), a phosphatase whose expression impairs hippocampal plasticity and memory in rodents (Blitzer et al., 1998; Genoux et al., 2002; Jouvenceau et al., 2006). Contextual fear conditioning increases methylation of the PP1 gene and decreases PP1 mRNA in the CA1 of male rats (Miller and Sweatt, 2007), suggesting that hippocampal learning reduces expression of this memory suppressing gene. Interestingly, PP1 appears to regulate not only histone phosphorylation, but also histone acetylation and methylation, as indicated by a mouse study in which PP1 was inhibited in forebrain areas including the hippocampus (Koshibu et al., 2009). The reduction in PP1 promoted histone phosphorylation, acetylation, and methylation in mice, which were associated with enhanced long-term object recognition and spatial memory (Koshibu et al., 2009). These findings suggest an essential role for PP1 in regulating histone modifications associated with enhanced synaptic plasticity and mnemonic processes. Although other protein phosphatases, such as PP2A, have been implicated in hippocampal synaptic plasticity and function (Liu et al., 2013; Lorrio et al., 2013), their role in histone dephosphorylation is unknown.
2.2.3. Methylation
The effects of histone methylation on gene expression vary depending on the residue affected and the number of methylation moieties expressed (Jarome and Lubin, 2013). Methylation of histones can occur on lysines or arginines of any of the four core histones (Ng et al., 2009). Arginine expresses only one (me1) or two (me2) methyl moities, including two configurations of me2 that have functionally distinct consequences (Turner, 2005). Lysine residues can express me1, me2, or me3 (three methyl moieties) on their amine group (Ng et al., 2009). Histone methylation is catalyzed by histone methyltransferases (KMTs), so named because the majority of methylated sites are lysines (K) rather than arginines. Each KMT is specific to the residue that it modifies (for reviews, see Kouzarides, 2007; Black et al., 2012; Jarome and Lubin, 2013), and typically adds only a specific number of methyl groups. Interestingly, previously added methyl groups can help catalyze the sequential addition of a subsequent methyl moiety (Zhang et al., 2003). Histones are demethylated by lysine demethylases (KDMs), which are divided into two categories: (1) those requiring flavine adenine dinucleotide (FAD) for demethylase activity (e.g., LSD1), and (2) Jumonji proteins, which contain a conserved JmjC domain (Chen et al., 2011). Methylation and demethylation of an individual histone is highly specific. For example, the G9a methyltransferase converts unmethylated H3K9 to H3K9me1 and H3K9me2, but only the MLL methyltransferase can convert H3K4 to H3K4me3 (Jarome and Lubin, 2013). This specificity of KMTs and KDMs for particular lysine residues and methyl moieties may help the hippocampus differentiate among memories that differ in type, context, and time.
The emerging role of histone methylation in memory formation has recently been reviewed (Jarome and Lubin, 2013), so just a few key studies will be described. Lysines 4 and 9 on histone H3 (H3K4 and H3K9) appear particularly important for memory regulation. For example, contextual fear conditioning increased H3K4me3 and H3K9me2 in the CA1 region of the male rat hippocampus, where H3K4me3 was associated with transcriptional activation and H3K9me2 was associated with transcriptional repression (Gupta et al., 2010; Gupta-Agarwal et al., 2012). In particular, conditioning increased H3K4me3 at the Zif268 and Bdnf Exon 1 promoters (Gupta et al., 2010), suggesting that learning-induced tri-methylation of genes involved in synaptic plasticity fosters memory consolidation. In the same study, mice deficient in Mll1, an H3K4 KMT, exhibited impaired contextual fear memory, but not cued fear memory, indicating a specific role for H3K4 methylation in hippocampal memory (Gupta et al., 2010). This notion is supported by other data showing that mice deficient in Mll2 (kmt2b) exhibited impaired object recognition and spatial memory, as well as reduced H3K4 di- and tri-methylation in promoter regions of genes associated with synaptic plasticity (Kerimoglu et al., 2013). H3K9me2 expression is also associated with enhanced memory, as indicated by evidence that the specific H3K9 G9a methyltransferase inhibitor BIX01294 blocks contextual fear memory consolidation (Gupta-Agarwal et al., 2012). Combined, these data suggest that methylation-induced silencing of genes that impair memory and expression of genes that promote memory work in concert to facilitate hippocampal memory.
2.2.4. Ubiquitination and SUMOylation
The transcriptional effects of ubiquitination and SUMOylation on gene expression in the hippocampus remain largely unknown. Outside of the nervous system, ubiquitination has been associated with transcriptional activation and repression, depending on the histone and residues affected (for review, see Zhang, 2003). Although SUMOylation can also activate or inhibit transcription, it is most often associated with transcriptional repression (for reviews, see Gill, 2005; Lyst and Stancheva, 2007). Ubiquitination involves the addition of ubiquitin to a lysine residue of a N-terminal histone tail. Ubiquitin can be attached as a single modification (monoubiquitination) or as a string of several ubiquitin marks (polyubiquitination). Polyubiquitination occurs through a series of three enzymatic steps involving specific enzymes (E): (1) activation by enzyme 1 (E1), (2) conjugation by enzyme 2 (E2), and (3) the addition of the ubiquitin by the ligase to the E2 complex by enzyme 3 (E3) (Atanassov et al., 2011; Hamilton and Zito, 2013). For histones, the most predominant form of ubiquitination is monoubiquitination of histones H2A and H2B (Zhang, 2003; Cao and Yan, 2012). Traditionally, ubiquitin marks a protein for degradation through the ubiquitin proteosome system, however this is not the case for histone ubiquitination (Zhang, 2003). The functional impact of ubiquitination on gene expression depends on which histone expresses the ubiquitin tag and which regulatory cofactors are recruited. For example, H2A ubiquitination is associated with transcriptional repressor complexes, whereas H2B ubiquitination is associated with other active histone marks such as acetylation and methylation (Zhang, 2003; Cao and Yan, 2012). Ubiquitin can be removed by peptidases called deubiquitinating enzymes (DUBs) (Atanassov et al., 2011).
Ubiquitination has received more attention of late, as it has become increasingly apparent that the ubiquitin proteosome system plays a vital role in regulating synaptic plasticity and memory in multiple brain regions (for reviews, see Tai and Schuman, 2008; Fioravante and Byrne, 2011; Jarome and Helmstetter, 2013). A role for ubiquitination in memory consolidation was first demonstrated by Medina and colleagues who showed in rats that activity of the ubiquitin proteosome system was necessary for memory consolidation in an inhibitory avoidance task (Lopez-Salon et al., 2001). Consistent with this finding, genetic deletion of the E3 ubiquitin ligase RNF13 in mice impairs hippocampal spatial learning in the Morris water maze (Zhang et al., 2013). A role for the ubiquitin proteosome system in memory consolidation has also been demonstrated in the prefrontal cortex (Reis et al., 2013) and amygdala (Jarome et al., 2014) of male rats. Interestingly, the genetic loss of the E3 ligase UBE3a causes Angelman syndrome, a disease associated with intellectual disability and impaired motor coordination (Mabb et al., 2011). Although these studies suggest an essential role for ubiquitination in hippocampal function, the specific contributions of histone ubiquitination to memory formation, or hormonal regulation of memory formation, have not yet been examined. Therefore, this is an area ripe for investigation.
Small ubiquitin-related modifier (SUMO) and ubiquitin share an 18% sequence homology, but are functionally distinct (Nathan et al., 2003). SUMOylation occurs when a SUMO protein is covalently attached to a target protein via a process analogous to ubiquitination that requires E1 (activation), E2 (conjugation), and E3 (ligase) proteins (Wilkinson et al., 2010). In mammals, there are four known SUMO proteins (SUMO 1–4) (Flotho and Melchior, 2013). Histone SUMOylation occurs only on specific lysine residues of H2A, H2B, and H4, and is associated with transcriptional repression (Ouyang and Gill, 2009). DeSUMOylation is catalyzed by enzymes in the sentrin-specific proteases (SENP) family (Drag and Salvesen, 2008; Wilkinson et al., 2010).
A role for SUMOylation in synaptic plasticity has begun to emerge in recent years (Cheng et al., 2013; Jaafari et al., 2013; Luo et al., 2013). SUMOylation occurs in hippocampal neurons, and is regulated by neuronal stimulation (Loriol et al., 2013). SUMOylation is also required for glutamate receptor trafficking in hippocampal dendritic spines (Chamberlain et al., 2012). Although no evidence yet links histone SUMOylation to memory formation, SUMOylation of transcription factors is associated with synaptic plasticity and spatial memory. For example, SUMOylation may contribute to the etiology of the neurodevelopmental disorder Rett syndrome by influencing the transcriptional repression induced by methyl CpG binding protein (MeCP2). This work shows that SUMOylation of MeCP2 at lysine 223 is necessary for its interaction with the transcriptional repression complex HDAC1/2 in cortical neurons and for synaptogenesis in hippocampal neurons (Cheng et al., 2013). Another recent report found that spatial learning in the Morris water maze reduced SUMOylation of the p300/CBP histone acetyltransferase and the binding of SUMO-1 to the Arc promoter in the rat hippocampus, suggesting that decreased SUMOylation of plasticity-related genes is associated with enhanced spatial memory (Castro-Gomez et al., 2013). Given this intriguing finding, identifying the extent to which the SUMOylation of other transcription factors and of histone proteins influences memory will become increasingly important to understanding hormonal regulation of memory formation.
3. Estrogenic regulation of hippocampal memory
Since the initial demonstration in ovariectomized female rats that 17β-estradiol (E2) increases dendritic spine density in the CA1 region of the hippocampus (Gould et al., 1990; Woolley et al., 1990; Woolley and McEwen, 1992, 1993), the field of hormones and cognition has focused almost exclusively on the hippocampus as a substrate for hormonal regulation of memory. The rationale for the field’s preoccupation with the hippocampus likely results, in part, because it was the first “cognitive” region of the rodent brain in which hormonal regulation of neuronal morphology was documented. Interest in the hippocampus has also been guided by numerous other factors, including: (1) it is essential for the formation of many types of memories, (2) is it one of the earliest brain regions to deteriorate in Alzheimer’s disease, and (3) hippocampal dysfunction is implicated in memory loss associated with aging, neurodegenerative diseases (e.g., Alzheimer’s disease, Parkinson’s disease, multiple sclerosis), and neuropsychiatric diseases (e.g., schizophrenia, depression) (Chiaravalloti and DeLuca, 2008; Whitwell, 2010; Ferreira et al., 2011; Holtzman et al., 2011; Kooi et al., 2011; Small et al., 2011). Nevertheless, effects of estrogens on other cognitive regions of the brain have been reported, most notably in the prefrontal cortex and amygdala (Tang et al., 2004; Inagaki et al., 2010). However, in light of the extensive literature on the effects of E2 on the hippocampus, and the fact that a role for epigenetic alterations in estrogenic regulation of memory has been examined only in the hippocampus thus far, the remainder of this review will focus on the hippocampus and hippocampal memory.
Because the density of CA1 dendritic spines fluctuates dramatically during the estrous cycle, with spine density increased during proestrus (high hormone levels) relative to estrus (low hormone levels) (Woolley and McEwen, 1992), it has been assumed that the primary sources of the estrogens that regulate hippocampal memory are the ovaries. As such, ovariectomy has become the standard model for studies of hormone regulation in female mammals. Although the contribution of estrogens synthesized in other tissues (e.g., heart, bone, adipose tissue Simpson and Davis, 2001; Cui et al., 2013) to hippocampal function is likely to be minimal, it is important to note that the hippocampus can make its own E2. All of the steroidogenic enzymes necessary to produce E2 are present in the rodent hippocampus (Hojo et al., 2004; Prange-Kiel et al., 2006), where this hormone influences cellular function via intracrine and/or paracrine signaling mechanisms (Inoue et al., 2012). Interestingly, E2 levels in both male and female rats are higher in the hippocampus than in plasma (Hojo et al., 2004; Kato et al., 2013), which implicates de novo hippocampal E2 synthesis in memory formation. This notion is supported by evidence that inhibition of hippocampal E2 synthesis impairs spatial memory in male zebra finches (Bailey et al., 2013). In rodents, inhibition of hippocampal E2 synthesis by the aromatase inhibitor letrozole significantly decreases hippocampal synapse number and synaptic spine density in vitro (Kretz et al., 2004), indicating an important role for local E2 synthesis in hippocampal synaptic morphology. However, letrozole impairs hippocampal LTP in female, but not male, mice (Vierk et al., 2012), suggesting a sex difference in the role of locally synthesized E2 in hippocampal plasticity. Such sex differences could contribute to the premature hippocampal memory decline observed in reproductively senescent female rodents relative to males (Markowska, 1999; Frick et al., 2000). Although the contributions of hippocampal E2 synthesis to memory formation are not yet clear, mounting evidence has demonstrated the importance of brain-derived E2 in regulating behavior (Balthazart and Ball, 2006; Saldanha et al., 2011). This work highlights the need to better understand the role of local E2 synthesis, including potential epigenetic consequences of such synthesis, in regulating hippocampal memory formation.
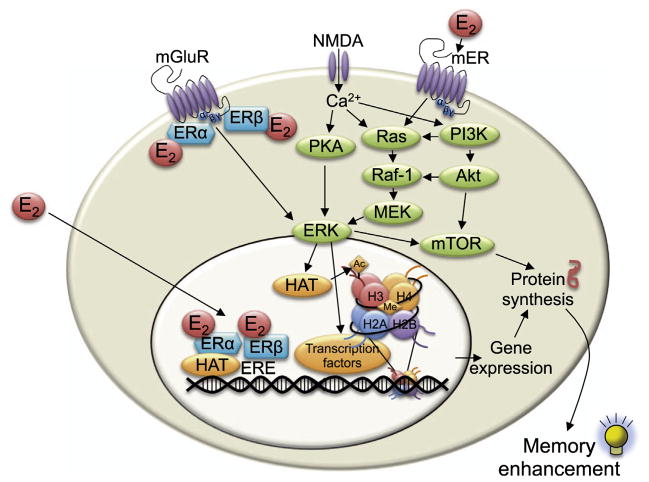
Regardless of their tissue of origin, estrogens effect change by binding to estrogen receptors (ERs), which are expressed through-out the body including brain regions such as the hypothalamus, hippocampus, amygdala, cerebral cortex, basal forebrain, striatum, raphe nuclei, and cerebellum (Cui et al., 2013). Much attention with respect to memory has focused on the classical intracellular ERs, ERα and ERβ, which are expressed in the dendritic spines, axon terminals, and nucleus of hippocampal pyramidal neurons (Milner et al., 2001, 2005; Waters et al., 2011). ERα is also expressed in the cytoplasm and nucleus of GABAergic interneurons, where it facilitates an E2-induced decrease in GABAergic tone that promotes pyramidal neuron spinogenesis (Murphy et al., 1998). The binding of estrogens to ERα and ERβ lead to either a classical “genomic” response or a non-classical “non-genomic” response. In this context, the terms “genomic” and “non-genomic” refer to the initial molecular interactions engaged in by the ERs. That is, in the genomic response, ERs interact directly with nuclear DNA, whereas in the non-genomic response, ERs interact first with other receptors and/or cell signaling molecules at the plasma membrane. Because both responses can ultimately influence gene transcription, the somewhat confusing genomic/non-genomic terminology has generally been replaced with the terms classical and non-classical, respectively. In the classical response, ERα and ERβ bind estrogens in the cytosol and form homo- or heterodimers that translocate to the nucleus and regulate expression of ER target genes by directly binding an estrogen response element (ERE) or forming protein– protein interactions to indirectly regulate other transcription factors (Fig. 2) (Cheskis et al., 2007). Many of the co-activators necessary for ERE-mediated gene transcription are HATs or interact with HATs, and some ERE-associated co-repressors exhibit HDAC activities (Spencer et al., 1997; Blanco et al., 1998; Kishimoto et al., 2006), suggesting that histone acetylation is a key element regulating estrogen-induced gene transcription. However, ERα and ERβ may also exert epigenetic effects by rapidly regulating cell-signaling pathways (e.g., ERK/MAPK; Fig. 2) that trigger histone acetylation, as is suggested by our own work with E2 (Zhao et al., 2010, 2012). Because E2 can activate ERK and related signaling molecules within seconds to minutes of treatment (Zhao and Brinton, 2007; Fernandez et al., 2008; Fan et al., 2010; Zhao et al., 2010), such non-classical responses are generally considered to be faster than classical ER responses. To facilitate non-classical effects, ERα and ERβ are thought to either translocate to the plasma membrane after binding E2 (Razandi et al., 1999; Sheldahl et al., 2008) or to reside in plasma membrane caveolae where they interact with metabotropic glutamate receptors (mGluRs) to rapidly initiate ERK/MAPK signaling and stimulate phosphorylation of the transcription factor CREB (Fig. 2) (Boulware et al., 2005, 2007). Within the dorsal hippocampus, this E2/mGluR signaling is essential for E2 and agonists of ERα and ERβ to enhance object recognition and spatial memory consolidation (Boulware et al., 2013). Rapid non-classical effects of estrogens may also be mediated by putative plasma membrane receptors, including GPER (GPR30) (Filardo et al., 2007), Gq-mER (Smith et al., 2013), and ER-X (Toran-Allerand et al., 2002). GPER is the most well studied of these receptors, and has been identified within hippocampal dendritic spines and in hippocampally-projecting basal forebrain cholinergic neurons (Hammond and Gibbs, 2011; Hammond et al., 2011; Srivastava and Evans, 2013). GPER activation reportedly facilitates spatial memory consolidation in female rats (Hammond and Gibbs, 2011; Hammond et al., 2012; Hawley et al., 2014), although the molecular mechanisms underlying this effect remain unknown.
Fig. 2.
Diagrammatic illustration of the molecular mechanisms required for E2 to enhance hippocampal memory consolidation. In the classical response, E2 binds ERα and ERβ, which then translocate into the nucleus, bind to the estrogen response element (ERE) on DNA, and interact with co-regulatory proteins (including histone acetyltransferases, HAT) to influence transcription. In a non-classical response, E2 may affect cell signaling in several ways. It can bind to ERs that interact with metabotropic glutamate receptors (mGluRs) at the membrane and activate extracellular regulated kinase (ERK) signaling. It can also interact with NMDA receptors and membrane-bound ERs (mER) to activate the protein kinase A (PKA), phosphoinositol-3-kinase (PI3K), and mammalian target of rapamycin (mTOR) signaling pathways. mTOR signaling regulates the protein synthesis necessary for memory formation. Activation of ERK increases H3 acetylation. Both H3 acetylation and DNA methylation are necessary for E2 to enhance memory consolidation. Adapted with permission from Frick (2013).
The many effects of E2 on hippocampal morphology, physiology, and memory have been reviewed extensively elsewhere (e.g., McEwen and Alves, 1999; Daniel, 2006; Woolley, 2007; Sherwin and Henry, 2008; Spencer et al., 2008; Frick, 2009; Dumitriu et al., 2010; Gibbs, 2010; Srivastava et al., 2011; Luine and Frankfurt, 2013), so this work will be only briefly summarized here. In addition to its established effects on CA1 dendritic spines (Woolley and McEwen, 1993; Frick et al., 2004; MacLusky et al., 2005), E2 promotes neurogenesis in the dentate gyrus (see Galea et al., 2013 for a recent review), thereby providing multiple morphological substrates for encoding new memories. Physiologically, E2 rapidly facilitates excitatory synaptic transmission (Smejkalova and Woolley, 2010), decreases inhibitory synaptic transmission (Huang and Woolley, 2012), and facilitates the induction of LTP (reviewed in Baudry et al., 2013; Kramar et al., 2013). As will be discussed below, E2 also rapidly activates numerous cell-signaling cascades in the hippocampus that are essential for long-term memory formation (Zhao and Brinton, 2007; Frick, 2012). The activation of these signaling pathways influences hippocampal histone acetylation, gene expression, and protein synthesis in the hippocampus (Pechenino and Frick, 2009; Zhao et al., 2010; Fortress et al., 2013), although it remains unclear whether these changes promote synaptogenesis and neurogenesis. Such rapid changes in hippocampal morphology may be possible, given that activation of Rap/AF-6/ERK1/2 signaling by E2 promotes the formation of new dendritic spines in mature cultured cortical neurons (Srivastava et al., 2008).
Consistent with the aforementioned effects of exogenous E2 on hippocampal function, exogenous E2 administered to young ovariectomized rats and mice generally enhances memory in hippocampal-dependent tasks such as the Morris water maze, radial arm maze, T-maze, object placement/location, novel object recognition, social recognition, inhibitory avoidance, and trace eyeblink conditioning (see Daniel, 2006; Frick, 2009; Gibbs, 2010; Choleris et al., 2012 for reviews). However, the effects of E2 on hippocampal memory depend on numerous factors, including treatment dose and duration, duration of hormone loss prior to treatment, timing of treatment relative to testing, type of memory tested, and task difficulty (Frick, 2009). Perhaps the most reliable effects of E2 on hippocampal memory have come in studies of rodents using acute administration of E2 immediately post-training (Frick et al., 2010; Frick, 2013). In these studies, a single injection or infusion of E2 is administered immediately after training to eliminate confounding effects of E2 on motivation, anxiety, or sensorimotor abilities during training. Many rodent studies use a rapidly metabolized water-soluble form of E2 so that exogenous hormone levels are low or non-existent in the hippocampus during testing 4–48 h later. The post-training approach typically employs one-trial learning tasks like novel object recognition or object placement, which are ideal for linking rapid molecular events (e.g., protein phosphorylation, histone acetylation) in a causal manner to memory consolidation. Such studies have consistently reported that E2 or agonists of ERα and ERβ enhance the consolidation of spatial memory measured in the Morris water maze and object placement tasks, and of novel object recognition memory (Packard and Teather, 1997a,b; Luine et al., 2003; Gresack and Frick, 2006; Walf et al., 2006, 2008; Fernandez et al., 2008; Lewis et al., 2008; Fan et al., 2010; Jacome et al., 2010; Zhao et al., 2010; Boulware et al., 2013; Fortress et al., 2013). Findings showing that E2 administered to rats or mice two or three hours after training do not enhance spatial memory or object recognition (Packard and Teather, 1997b,a; Fernandez et al., 2008) support the temporal specificity of E2’s effects to the memory consolidation phase of memory formation. In our own work, we have used the post-training approach, combined with infusions of E2 or inhibitor drugs directly into the dorsal hippocampus, to pinpoint the molecular mechanisms in the hippocampus necessary for E2 to regulate hippocampal memory consolidation. This approach has allowed us to begin to identify cell signaling pathways, epigenetic processes, and neurotransmitter receptors that are essential for estrogenic regulation of hippocampal memory. Some of these findings will be discussed in the sections below.
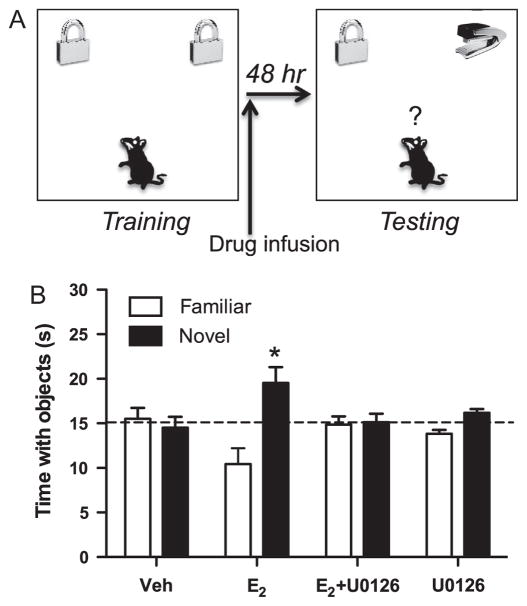
Our own research investigating the molecular underpinnings of estrogenic memory regulation began with two distinct literatures. One demonstrated that ERK phosphorylation in the hippocampus is essential for the long-term consolidation of contextual, inhibitory avoidance, spatial, and object recognition memories (Atkins et al., 1998; Blum et al., 1999; Walz et al., 2000; Bozon et al., 2003; Kelly et al., 2003; Zhang et al., 2004a). The other showed that E2 could increase ERK phosphorylation (i.e., activation) in the hippocampus within minutes, both in vivo and in vitro (Kuroki et al., 2000; Nilsen and Brinton, 2003; Yokomaku et al., 2003). We reasoned that because E2 increases ERK phosphorylation in the hippocampus, and hippocampal ERK phosphorylation is necessary for memory formation, ERK activation would be necessary for E2 to enhance hippocampal memory formation. Indeed, we demonstrated in several studies that bilateral dorsal hippocampal infusion of E2 increases hippocampal p42 ERK phosphorylation within 5 min, and that this increase was necessary for E2 to enhance novel object recognition memory consolidation in young ovariectomized mice (Fernandez et al., 2008; Fan et al., 2010; Zhao et al., 2010). In these studies, mice accumulated 30 s of time exploring two identical objects in a large square arena (Fig. 3A). Immediately after training, mice received an infusion of water-soluble E2 into the dorsal third ventricle and a bilateral infusion of the ERK activation inhibitor U0126 into the dorsal hippocampus. Importantly, this dose of U0126 does not impair object recognition on its own relative to vehicle infusion (Fernandez et al., 2008). Forty-eight hours later, mice accumulated 30 s of time exploring an object identical to that explored during training and a novel object. Because mice express an innate preference for novelty, mice that remember the familiar training objects spend more time than chance (15 s) exploring the novel object during testing (Frick and Gresack, 2003). Mice receiving E2 spend more time than chance exploring the novel object (Fig. 3B) (Fernandez et al., 2008; Lewis et al., 2008; Fan et al., 2010; Zhao et al., 2010, 2012; Boulware et al., 2013; Fortress et al., 2013), suggesting consolidation of the memory for the familiar training object. Infusion of U0126 into the dorsal hippocampus completely blocks this effect (Fig. 3B), demonstrating that ERK activation is necessary for E2-induced object recognition memory consolidation (Fernandez et al., 2008; Zhao et al., 2010; Fortress et al., 2013). In other studies using this same approach, we determined that activation of phosphatidylinositol 3-kinase (PI3K)/Akt, protein kinase A (PKA), and NMDA receptors upstream from ERK are also essential for E2 to enhance object recognition memory consolidation (Lewis et al., 2008; Fan et al., 2010; Fortress et al., 2013), as is activation of the mammalian target of rapamycin (mTOR) protein synthesis pathway downstream from ERK (Fig. 2) (Fortress et al., 2013). Supporting the importance of ERK in estrogenic regulation of hippocampal function, other labs have shown that blocking ERK activation prevents E2 from increasing hippocampal synaptic protein levels, glutamate release, and CA1 spine synapses (Yokomaku et al., 2003; Ogiue-Ikeda et al., 2008). Most recently, we reported that dorsal hippocampal ERK activation is necessary for ERα and ERβ agonists to enhance both object recognition and object placement memory in young ovariectomized mice, and showed that this effect depended on interactions at the plasma membrane between the ERs and mGluRs (Boulware et al., 2013). The central role of ERK activation in the effects of E2, ERα, and ERβ on hippocampal memory consolidation raised the possibility that epigenetic processes triggered by ERK activation may also be essential to the estrogenic regulation of memory. Given that ERK-induced regulation of hippocampal H3 acetylation facilitates contextual fear memory consolidation (Levenson et al., 2004), we reasoned that histone acetylation and other epigenetic processes downstream from ERK might be essential for E2 to enhance memory consolidation.
Fig. 3.
(A) Schematic diagram of our novel object recognition protocol. During training, mice accumulate 30 s of time exploring two identical objects. Immediately after training, mice receive an infusion of E2 or other drugs into the dorsal hippocampus or dorsal third ventricle. Twenty-four or 48 h later, mice accumulate 30 s with a novel object and an object identical to that explored during testing (familiar). Mice that remember the familiar object spend significantly more time than chance (15 s) with the novel object. Adapted with permission from Frick (2013). (B) The beneficial effects of E2 on object recognition memory consolidation depend on dorsal hippocampal ERK activation. Forty-eight hours after training, mice infused with 10 μg E2, but not vehicle, spend more time than chance with the novel object (*p < 0.05 relative to chance), demonstrating enhanced object recognition. The memory-enhancing effect of E2 is completely blocked by a dorsal hippocampal infusion of the ERK activation inhibitor U0126 (0.5 μg), which has no detrimental effect on memory on its own. Error bars represent the mean ± standard error of the mean (SEM). Reprinted with permission from Zhao et al. (2010).
4. Chromatin modifications regulating estrogenic modulation of hippocampal memory
4.1. Histone acetylation
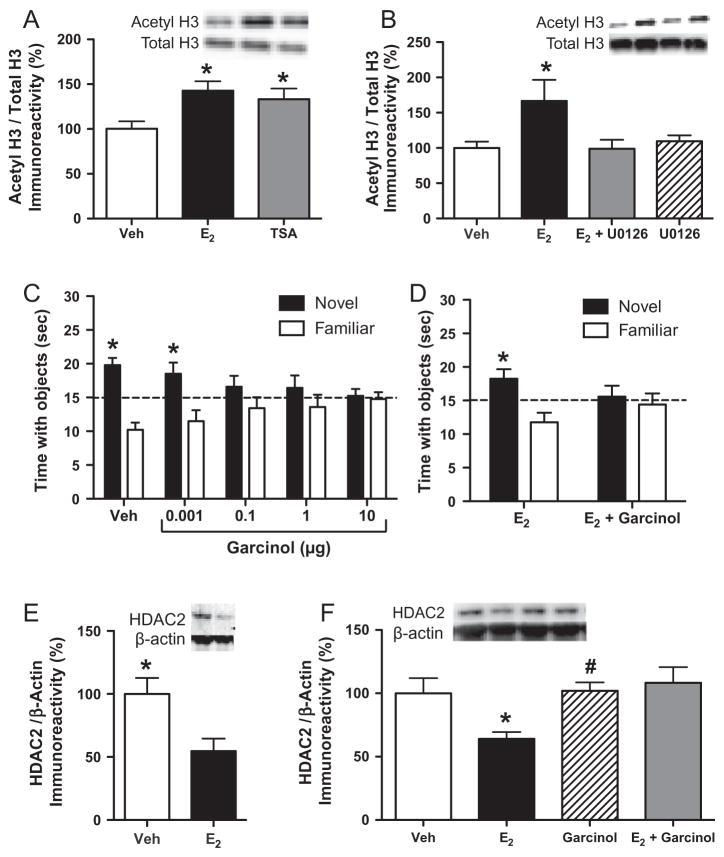
We examined the role of epigenetic alterations in estrogenic regulation of novel object recognition memory consolidation based on ample evidence demonstrating that E2 enhances this type of memory in ovariectomized rodents (Luine et al., 2003; Gresack and Frick, 2004, 2006; Walf et al., 2006, 2008; Fernandez et al., 2008; Lewis et al., 2008; Jacome et al., 2010). Furthermore, the dorsal hippocampus is essential for mediating memory consolidation in the novel object recognition protocol used by our laboratory (Baker and Kim, 2002; Cohen et al., 2013; Fernandez et al., 2008). Several studies had suggested that histone acetylation regulated novel object recognition in rodents (Alarcón et al., 2004; Korzus et al., 2004; Oliveira et al., 2007; Stefanko et al., 2009), so we first determined whether our novel object recognition protocol was sensitive to HDAC inhibition in ovariectomized female mice. In our protocol, mice receiving vehicle infusions into the dorsal hippocampus immediately after training display a significant preference for the novel object 24, but not 48, hours later (Zhao et al., 2010). However, mice receiving immediate post-training dorsal hippocampal infusions of the HDAC inhibitor TSA exhibited enhanced 48-h object recognition memory (Zhao et al., 2010), suggesting that preventing histone deacetylation doubled the length of time that information about the familiar object could be remembered. Similar to E2, TSA infusion 3 h after training had no effect on 48-h object recognition, suggesting that E2 and histone acetylation both regulate object memory within 3 h of training (Zhao et al., 2010). We then showed that dorsal hippocampal infusion of TSA significantly increased acetylation of both H3 and H4 in the dorsal hippocampus 30 min after infusion (Fig. 4A). E2, however, significantly increased acetylation of H3 (Fig. 4A), but not H4 in the dorsal hippocampus 30 min after infusion (Zhao et al., 2010, 2012). Subsequent work showed that E2 also had no effect on H2B acetylation in young female mice (Zhao et al., 2012) or on H2A and H2B acetylation in middle-aged female mice (Fortress and Frick, unpublished results). These findings suggest that genes associated with H3 are particularly important for estrogenic regulation of hippocampal memory. Alternatively, the relatively greater abundance of post-translational modifications on H3 residues compared to those on H2A, H2B, or H4 (Tweedie-Cullen et al., 2012) may provide more opportunity for genes associated with H3 to be transcribed. It is unclear at this point exactly which genes are involved, but our recent chromatin immunoprecipitation analysis showing that E2 increases H3 acetylation of Bdnf-pII and Bdnf-pIV in the dorsal hippocampus of young and middle-aged female mice (Fortress and Frick, unpublished results), suggests that BDNF may play a role.
Fig. 4.
ERK-dependent H3 acetylation in the dorsal hippocampus is necessary for E2 to enhance object recognition memory consolidation. (A) Bilateral dorsal hippocampal infusion of E2 (5 μg/hemisphere) or the HDAC inhibitor trichostatin A (TSA, 16.5 mM/hemisphere) significantly increased H3 acetylation in the dorsal hippocampus relative to vehicle 30 min after infusion (*p < 0.05). (B) Intracerebroventricular (ICV) infusion of E2 significantly increased H3 acetylation in the dorsal hippocampus relative to vehicle (*p < 0.05). This increase was blocked by dorsal hippocampal infusion of U0126 (0.5 μg/hemisphere), suggesting that the E2-induced increase in H3 acetylation is dependent on ERK activation. (C) Bilateral dorsal hippocampal infusion of the HAT inhibitor garcinol in doses of 0.1, 1.0, and 10 μg/hemisphere immediately after novel object recognition training impaired object recognition 24 h later. At this delay, vehicle-infused mice remember the familiar training object, which allows us to observe memory-impairing effects of drugs. Mice receiving 1 ng/hemisphere garcinol exhibited no memory impairment (*p < 0.05 relative to chance), suggesting that this dose does not block memory consolidation. (D) We next tested the ability of the 1 ng dose of garcinol to block the memory-enhancing effects of E2 using a 48-h delay at which vehicle-treated females do not remember the familiar object. Although the 1 ng dose of garcinol was behaviorally ineffective on its own, it blocked the memory enhancing effects of E2 at this delay (*p < 0.05 relative to chance), suggesting that histone acetylation is necessary for memory consolidation. (E) Dorsal hippocampal infusion of E2 (5 μg/hemisphere) significantly decreased HDAC2 levels in the dorsal hippocampus 4 h after infusion relative to vehicle (*p < 0.05). (F) The E2-induced decrease in HDAC2 in the dorsal hippocampus was blocked by garcinol, suggesting that histone acetylation is necessary for E2 to regulate HDAC2 protein. Error bars in all panels represent the mean ± SEM. Insets in panels A, B, E, and F illustrate representative Western blot images. Reprinted with permission from Frick (2013) and Zhao et al. (2010, 2012).
The observed specificity of E2 for H3 acetylation is consistent with effects of contextual fear conditioning on hippocampal H3 acetylation, and with previous data showing that H3 acetylation is ERK dependent (Levenson et al., 2004). Because the memory enhancing effects of E2 on object recognition are associated with p42 ERK phosphorylation (Fernandez et al., 2008), we next examined whether the effects of E2 on H3 acetylation were dependent on ERK activation. Young ovariectomized mice were infused with E2 and U0126, and we found that U0126 blocked the effects of E2 on both H3 acetylation (Fig. 4B) and object recognition memory consolidation (Zhao et al., 2010). These data suggested that ERK activation is necessary for E2 to increase H3 acetylation. But was H3 acetylation necessary for E2 to enhance novel object recognition? To answer this question, we turned to a naturally occurring HAT inhibitor called garcinol that is isolated from the rind of the Garcinia indica fruit. Garcinol is a potent inhibitor of the CBP/p300 and PCAF HATs (Balasubramanyam et al., 2004), and also inhibits activation of ERK and PI3K/Akt in colorectal cancer cell lines (Liao et al., 2005). At the time we conducted our study, garcinol had not yet been infused into the brain, so we first conducted a dose–response experiment to determine how post-training dorsal hippocampal infusion of this drug would affect novel object recognition. At a shorter 24-h delay between training and testing at which vehicle-treated females remember the training objects, we found that doses in the range of 0.1–10 μg impaired object recognition memory consolidation (Fig. 4C) (Zhao et al., 2012). Subsequent work from other labs has shown that garcinol also blocks fear memory consolidation and reconsolidation in rats and olfactory memory consolidation in honeybees (Merschbaecher et al., 2012; Maddox et al., 2013). In our study, a 1 ng dose did not block object recognition, so we next co-infused E2 and 1 ng garcinol into the dorsal hippocampus and tested consolidation 48 h later, a delay at which vehicle-treated mice do not remember the familiar objects. We found that garcinol blocked E2’s effects on both H3 acetylation and object recognition memory consolidation (Fig. 4D) (Zhao et al., 2012). Garcinol also blocked the E2-induced increase in dorsal hippocampal HAT activity observed 15 min after infusion (Zhao et al., 2012), confirming that E2 rapidly increases intrinsic HAT activity, and that this effect is blocked by garcinol. Combined, these data suggest that E2-induced object recognition memory consolidation requires ERK-dependent H3 acetylation in the dorsal hippocampus. It is worth noting that E2 can also increase HAT activity by binding to nuclear receptors in the traditional genomic pathway and recruiting steroid receptor coactivator complexes (Kim et al., 2001). Although our study did not specifically differentiate between these two mechanisms, the fact that garcinol blocked the E2-induced increase in HAT activity within 15 min of infusion suggests involvement of a more rapid non-classical signaling mechanism rather than a classical ERE-dependent mechanism. Therefore, we speculate that this regulation is largely epigenetic in nature.
We also reasoned that the rapid changes in ERK signaling and H3 acetylation might influence the expression of HDAC proteins, given the important role of HDAC2 and HDAC3 in regulating hippocampal memory (Guan et al., 2009; McQuown et al., 2011). Four hours after dorsal hippocampal infusion, E2 significantly reduced HDAC2, but not HDAC1, protein levels in the dorsal hippocampus of young ovariectomized mice (Fig. 4E) (Zhao et al., 2010, 2012). In middle-aged ovariectomized mice, we have replicated this finding and also shown that E2 decreases levels of HDAC3 protein in the dorsal hippocampus 4 and 6 h after infusion (Fortress and Frick, unpublished results). In young females, we found that garcinol blocks the E2-induced decrease in hippocampal HDAC2 levels (Fig. 4F) (Zhao et al., 2012), indicating that histone acetylation regulates HDAC2 protein levels, perhaps by increasing expression of another protein (e.g., a DNA methyltransferase) that decreases HDAC2 expression. The E2-induced decrease in HDAC2 and HDAC3 is consistent with previous findings that expression of HDAC2 and HDAC3, but not HDAC1, impairs hippocampal memory and synaptic plasticity (Guan et al., 2009; McQuown et al., 2011; Graff et al., 2012). Thus, E2 may facilitate hippocampal memory consolidation, at least in part, by reducing HDAC2 and HDAC 3 levels in the dorsal hippocampus. Combined, these findings suggest that E2 requires histone acetylation to facilitate memory consolidation and that intrinsic HAT activity may drive subsequent epigenetic modifications to enhance long-term memory consolidation.
4.2. DNA methylation
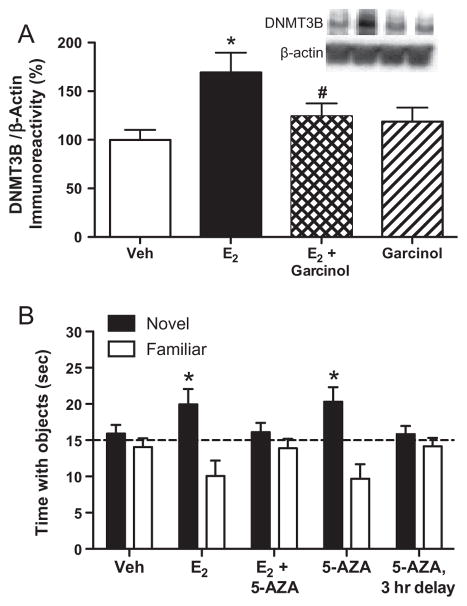
The other major chromatin alteration thus far associated with estrogenic regulation of memory is DNA methylation. Our research on this subject to date has focused on gross changes in DNA methylation and DNMT enzymes. The rationale for this work comes from the neurobiology of learning and memory literature, which illustrated that DNMT inhibition blocks learning-induced histone H3 acetylation in rats (Miller et al., 2008). We, therefore, questioned whether DNA methylation was essential for E2 to regulate hippocampal memory. We first examined the effects of a single dorsal hippocampal infusion of E2 on DNMT mRNA and protein levels in the dorsal hippocampus of young ovariectomized mice. E2 transiently increased levels of DNMT3A and DNMT3B levels 45 min after infusion, but the increase in DNMT3B (>2-fold) was more robust than that for DNMT3A (~1.5-fold) (Zhao et al., 2010). Accordingly, when DNMT protein was examined in the dorsal hippocampus 1–4 h later, only DNMT3B protein was increased 4 h after E2 infusion (Fig. 5A) (Zhao et al., 2010, 2012). Interestingly, DNMT1 mRNA and protein in the dorsal hippocampus were not affected by E2 (Zhao et al., 2010, 2012), suggesting that E2 preferentially increases expression of enzymes that catalyze de novo methylation of previously unmethylated cytosine residues in the dorsal hippocampus.
Fig. 5.
Regulation of hippocampal DNA methylation by E2. (A) Bilateral dorsal hippocampal infusion of E2 (5 μg/hemisphere) increases DNMT3B protein levels in the dorsal hippocampus 4 h after infusion (*p < 0.05 relative to vehicle). This effect is blocked by the HAT inhibitor garcinol, suggesting that histone acetylation regulates the E2-induced increase in DNMT3B (#p < 0.05 relative to E2 alone). Inset illustrates representative Western blot image. Error bars represent the mean ± SEM. Reprinted with permission from Zhao et al. (2012). (B) Post-training infusion of the DNMT inhibitor 5-AZA (100 μg/hemisphere) blocks the memory-enhancing effects of E2 measured 48 h after training, suggesting that action of DNMT enzymes is necessary for E2 to enhance object recognition memory consolidation. Dorsal hippocampal infusion of 5-AZA alone enhances 48-h object recognition memory consolidation when given immediately, but not 3 h, after training (*p < 0.05 relative to chance), suggesting that DNMT enzymes regulate object recognition memory irrespective of E2. Error bars represent the mean ± SEM. Reprinted with permission from Zhao et al. (2010).
We next asked whether DNA methylation is necessary for estrogenic regulation of memory consolidation. As with histone acetylation, we first determined the extent to which our novel object recognition task was regulated by DNA methylation in young ovariectomized female mice. Interestingly, bilateral dorsal hippocampal infusion of the DNMT inhibitor 5-aza-2-deoxycytidine (5-AZA) immediately after object recognition training enhanced memory consolidation 48 h later (Fig. 5B) (Zhao et al., 2010). This somewhat counterintuitive finding suggests that inhibiting the DNMT activity in the absence of E2 may be necessary for intact object recognition, perhaps by preventing the methylation of memory promoting genes such as reelin and Bdnf (Miller and Sweatt, 2007; Guan et al., 2009). We next co-infused E2 with 5-AZA and found that 5-AZA blocked the beneficial effects of E2 on 48 h object recognition when it was administered immediately, but not 3 h after, training (Fig. 5B) (Zhao et al., 2010). This finding suggests that, like histone acetylation, DNA methylation is also necessary for E2 to enhance object recognition memory consolidation within a 3-h critical window after training. However, more sophisticated analyses directly measuring specific E2-induced alterations in methylated DNA (e.g., bisulfite sequencing, methylated DNA immunoprecipitation (MeDIP)) will be necessary to make more definitive conclusions about the role of DNA methylation in estrogen-dependent memory consolidation.
4.3. Conclusions
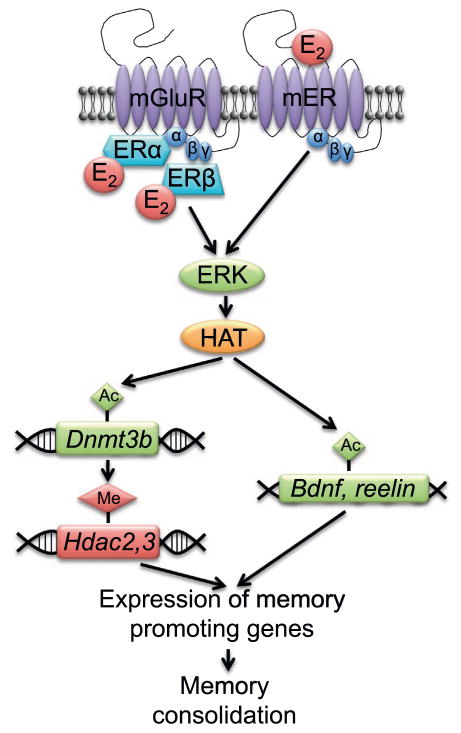
Collectively, our data suggest that dorsal hippocampal H3 acetylation and DNA methylation are essential for E2 to enhance object recognition memory consolidation in female mice. We have, thus far, shown that E2 specifically regulates H3 acetylation and protein levels of HDAC2, HDAC3, and DNMT3B in the dorsal hippocampus (Zhao et al., 2010, 2012). The fact that garcinol prevented E2 from increasing DNMT3B levels and reducing HDAC2 levels indicates that histone acetylation and DNA methylation work together to regulate the effects of E2 in the hippocampus. The findings also suggest that changes in histone acetylation may precede the changes in DNMTs and HDACs. Although it is unclear at this point whether these alterations are related to each other, it is tempting to speculate that E2 increases H3 acetylation of the Dnmt3b promoter, thereby increasing DNMT3b protein, which then methylates the Hdac2 and Hdac3 genes to reduce expression of HDAC2 and HDAC3 protein and promote long-term memory formation (Fig. 6). Our unpublished work also suggests that the E2-induced increase in histone acetylation can directly increase the expression of genes that facilitate memory formation, such as Bdnf, and we would predict that E2 would also increase expression of other memory-promoting genes like reelin that are epigenetically regulated. In future work, this hypothesis could be tested using chromatin immunoprecipitation (ChIP) to examine H3 acetylation of Dnmt3b and bisulfate sequencing or MeDIP analysis to examine methylation of Hdac2 and Hdac3 genes. These techniques could also be used to determine how E2 influences the acetylation or methylation status of other genes known to regulate hippocampal memory and synaptic plasticity. As mentioned earlier, our preliminary work suggests that E2 increases H3 acetylation of Bdnf-pII and Bdnf-pIV in young and middle-aged female mice (Fortress and Frick, unpublished results). Epigenetic regulation of Bdnf has emerged as a critical mediator of cognitive function (Lubin, 2011; Boulle et al., 2012; Gupta-Agarwal et al., 2012), so this finding supports the notion that E2 facilitates expression of genes that promote hippocampal memory formation. Other potential gene targets include reelin, Egr1, Arc, Cbp, Creb, GluR1, Nr2a, Nr2b, which are important for hippocampal memory and plasticity and regulated by histone acetylation (Guan et al., 2009).
Fig. 6.
Hypothetical model of how estrogenic regulation of epigenetic mechanisms in the dorsal hippocampus may promote memory consolidation. Activation of ERK by intracellular and membrane estrogen receptors increases HAT activity and H3 acetylation of the Dnmt3b promoter, which then increases levels of DNMT3B protein and methylation of memory repressor genes like Hdac2 and Hdac3. This, in turn, increases expression of memory promoting genes and leads to memory consolidation. E2 can also directly increase H3 acetylation of memory promoting genes, such as Bdnf and reelin, thereby, increasing expression of these genes and also leading to memory consolidation.
5. Future directions
Our work has provided the initial demonstration that histone modifications and DNA methylation play vital roles in the estrogenic regulation of memory, but these findings are merely the first pieces in what is likely to be an enormously complicated puzzle. As discussed above, we do not yet know which genes are epigenetically regulated by E2 in the hippocampus or how this regulation is accomplished. As illustrated in the first portion of this review, epigenetic regulation of learning and memory involves multiple chromatin modifications. Therefore, the roles of other histone alterations, including phosphorylation, methylation, ubiquitination, and SUMOylation, in estrogenic regulation of memory must also be addressed in future work. Histone methylation is likely to be especially interesting, given the complicated role of multiple methylation marks in memory consolidation. It will also be imperative in future studies to determine the extent to which E2-induced chromatin modifications regulate other types of hippocampal memories, and memories mediated by other brain regions. Related to this point, it should be noted that very little is known about the role of specific estrogen receptors (e.g., ERα, ERβ, GPER) in regulating memory formation, or the extent to which epigenetic alterations mediate the effects of these receptors. Because hippocampal ERα and ERβ enhance object recognition and spatial memory consolidation in ovariectomized female mice in an ERK-dependent manner (Boulware et al., 2013), there is reason to believe that activation of either receptor may trigger H3 acetylation similar to E2. However, the effects of ER-specific drugs on histone modifications or DNA methylation have not yet been examined in cognitive regions of the brain. Other important goals of this research should be to understand the extent to which epigenetic alterations influence sex differences in cognition and how hormone loss during aging influences the epigenetic landscape of the brain. As such, the sections below will briefly discuss these two issues.
5.1. Epigenetic regulation of sex differences
Organizational effects of sex steroid hormones shape male and female brains into sexually dimorphic patterns that influence behavioral responses throughout development and adulthood. Examples of sex-specific behaviors abound in endocrinology research, perhaps none better studied than the male rat mounting behavior and the female rat lordosis posture (Blaustein and Erskine, 2002; Kow and Pfaff, 2004; Etgen et al., 2006; Dewing et al., 2007; Micevych and Christensen, 2012; Lenz et al., 2013). Sex differences in cognitive function have been documented as well, most notably the reported male advantage in spatial abilities observed in humans and rodents. In humans, males have been shown to outperform females on spatial tests like the mental rotation task and virtual computer spatial navigation tasks (Astur et al., 1998; Kimura, 1999). Sex differences favoring males are observed in adulthood (Astur et al., 1998), and appear before puberty (Newhouse et al., 2007), suggesting an organizational influence of sex steroids on spatial memory. This notion is supported by observations in rats that the male advantage in spatial working memory could be reversed within the first 10 days of life by gonadectomy in males and estradiol treatment in females (Williams et al., 1990). Such treatments after the 10-day critical period have no effect on spatial abilities in adulthood, but the mechanisms governing the closing of this critical period remain unclear. Numerous subsequent rodent studies have reported a male advantage in other tests of spatial memory and in contextual fear conditioning and object recognition (e.g., (Galea et al., 1996; Perrot-Sinal et al., 1996; Frick and Gresack, 2003; Gresack et al., 2009). Various sex differences have also been reported within the hippocampus itself; relative to the adult male rat hippocampus, the adult male rat hippocampus is heavier (Madeira et al., 1991b), has a larger cross sectional area (Pfaff, 1966), contains more cells in the neurogenic granule cell layer (Madeira et al., 1991a), and has a higher density of neurons and more dendritic branching in the CA1 and dentate gyrus (Markham et al., 2005). Dendritic morphology also differs between the sexes in response to stress and environmental enrichment. For example, restraint stress increases hippocampal dendritic spine density and enhances associative learning in male rats, but decreases spine density and impairs associative learning in female rats (Wood and Shors, 1998; Shors et al., 2001). On the other hand, the female rat hippocampus is more responsive than the male rat hippocampus to environmentally enriching stimuli, given that dendritic branching of dentate granule cells is highest in male rats when raised in an isolated environment and in females when reared in an enriched environment (Juraska et al., 1985).
Although reports of sex differences in hippocampal morphology and memory abound, we should note that the magnitude of these differences is considerably smaller than those associated with reproductive brain regions and behaviors (McCarthy and Konkle, 2005). Indeed, sex differences in hippocampal learning are not observed in all studies, and can be minimized or eliminated by manipulating testing parameters (McCarthy and Konkle, 2005). Nevertheless, the preponderance of evidence suggests differences in hippocampal morphology and behavioral strategies that warrant further study. Given the importance of the hippocampus to regulating the hypothalamic–pituitary–adrenal axis and the involvement of this structure in the cognitive dysfunction inherent to numerous mental illnesses, even subtle sex differences in hippocampal function could contribute to differential susceptibility to stress and psychiatric or neurodegenerative diseases. The molecular mechanisms underlying sex differences in hippocampal morphology and memory are not clear, but the emerging importance of epigenetics in the neurobiology of hippocampal memory points toward a possible role for chromatin modifications.
Unfortunately, we know very little about how epigenetic processes shape sex differences in the brain, let alone sex differences in memory. Clues linking hormonal regulation of histone acetylation to the development of sex differences in the hippocampus come from a report showing that high levels of testosterone in the hippocampus and neocortex on embryonic day 18 are associated with more H3 acetylation in males than in females (Tsai et al., 2009). Consistent with this finding, contextual fear conditioning increases ERK more in the ventral hippocampus of gonadally intact adult male rats relative to adult females (Gresack et al., 2009), raising the possibility that ERK-dependent H3 acetylation in the hippocampus may be higher in males. Several recent studies have examined epigenetic regulation of sex differences in the context of prenatal maternal stress. In one mouse study, maternal restraint stress impaired spatial memory in adult male offspring, while tending to improve spatial memory in adult female offspring (Sierksma et al., 2013). DNMT3A immunoreactivity was decreased in the dentate gyrus of females relative to males (Sierksma et al., 2013), regardless of prenatal stress, suggesting a potential association between DNMT3A levels and sex differences in spatial memory. However, much more research is clearly needed to understand possible epigenetic mechanisms underlying sex differences in cognitive function.
Such future work can take a cue from recent studies of sex differences in reproductive areas of the brain. E2 is necessary for masculinization of the brain (for reviews, see McCarthy et al., 2009; McCarthy and Nugent, 2013). The masculinizing effects of E2 require the expression and epigenetic regulation of sex hormone receptors, such as ERα, in sexually dimorphic brain areas such as the preoptic area (POA) (Kurian et al., 2010). High levels of E2 in the male rat POA increase H3 and H4 acetylation at the ERα and aromatase promoters, whereas low E2 levels are associated with decreased acetylation and increased HDAC expression at the ERα and aromatase promoters (Matsuda et al., 2011). Furthermore, post-natal infusions of the HDAC inhibitor TSA into the cerebral ventricles of male rats impair male sexual behavior in adulthood (Matsuda et al., 2011), suggesting that histone acetylation during a critical perinatal period after birth is necessary for masculinization. Additionally, the closing of this critical period in males is associated with increased methylation of the ERα promoter and reduced ERα levels (Kurian et al., 2010). HDAC inhibition also prevents masculinization of the bed nucleus of the stria terminalis (BNST), another sexually dimorphic brain region. The BNST is larger in males than females because male gonadal hormones prevent apoptosis of BNST neurons during early development (Chung et al., 2000). HDAC inhibition blocks the masculinizing effects of testosterone in mice (Murray et al., 2009), suggesting that histone acetylation is responsible for the sexual dimorphism of the BNST. Together, these data indicate that the organizational effects of E2 in the POA and BNST are mediated by epigenetic regulation of genes necessary for masculinization. These epigenetic processes early in development may provide a foundation for activational effects of hormones in adulthood that influence behavior, possibly including memory consolidation.
5.2. Epigenetic regulation of age-related memory decline
Because estrogens are vital neuroprotective factors for hippocampal neurons in adulthood (Picazo et al., 2003; Chen et al., 2006; Suzuki et al., 2006; Yang et al., 2010), the loss of ovarian-derived estrogens in reproductively senescent females is thought to contribute to age-related memory loss. Indeed, the massive loss of estrogens and progestins at menopause significantly increases the risk of memory decline and Alzheimer’s disease in middle-aged women relative to men (Zandi et al., 2002; Yaffe et al., 2007). In middle-aged female rats and mice, premature decline of spatial reference memory relative to males is associated with the loss of estrous cycling (Markowska, 1999; Frick et al., 2000). Given the importance of histone acetylation and DNA methylation to E2-induced memory enhancement, epigenetic alterations related to the loss of ovarian-derived E2 likely contribute to age-related memory decline in older females. Accumulating evidence suggests that alterations in chromatin plasticity occur during aging, although it is unclear if hormone loss contributes to these changes. For example, post-training systemic injection of the HDAC inhibitor NaB reverses object recognition memory consolidation impairments in aged male rats (Reolon et al., 2011). One of the first studies to establish a role for acetylation of specific histones in age-related cognitive decline showed that middle-aged (16 month-old) mice failed to a display learning-induced increase in hippocampal acetyl H4K12 that was evident in young (3 month-old) mice (Peleg et al., 2010). This dysregulated H4K12 acetylation was reversed by intrahippocampal infusion of the HDAC inhibitor SAHA, which also reversed deficits in contextual fear memory consolidation (Peleg et al., 2010). Support for a role of H4 acetylation in age-related cognitive decline has been suggested elsewhere. For example, spatial memory deficits in aged mice were associated with lower H4 acetylation and higher H3 acetylation in hippocampal CA1 and dentate gyrus relative to young mice with intact spatial memory (Dagnas and Mons, 2013). However, another study comparing aged cognitively impaired rats to aged non-cognitively impaired rats, reported no difference in hippocampal H4 acetylation between the groups (Castellano et al., 2012), suggesting that deficient H4 acetylation may affect memory only under certain circumstances. Recent evidence also suggests that a specific histone binding protein, RbAp48, may be critical for age-related memory decline. RbAp48 is reduced in the hippocampus of aged mice (Pavlopoulos et al., 2013). Inhibition of RbAp48 in young mice produces hippocampal-dependent memory deficits that mimic those of aged mice, whereas increasing RbAp48 expression ameliorates cognitive deficits in aged mice (Pavlopoulos et al., 2013). These findings suggest that targeting specific binding proteins such as RbAp48 may be beneficial for treating age-related cognitive decline.
Although no published studies have specifically examined histone alterations in aging females, our own unpublished data suggest that the middle-aged (16 month-old) female mouse brain retains the same epigenetic response to E2 observed in young females. As in young females, E2 enhances novel object recognition in middle-aged female mice in an ERK- and PI3K-dependent manner (Fan et al., 2010). As such, we hypothesized the E2 might influence H3 acetylation and HDAC levels in a manner similar to young females (Zhao et al., 2010, 2012). As expected, E2 increased acetylation of H3 (but not H2A, H2B, or H4) in the dorsal hippocampus 30 and 60 min after infusion, and decreased HDAC2 and HDAC3 protein levels 4 h after infusion (Fortress and Frick, unpublished results). Additional studies showed that E2 increased H3 acetylation in Bdnf-pII and Bdnf-pIV in both young and middle-aged females (Fortress and Frick, unpublished results). This evidence that histone acetylation mechanisms appear intact in the middle-aged female brain may suggest that maintaining epigenetic responsiveness to E2 is key to staving off memory decline in aging. It will be interesting to see in future work if E2 can regulate histone acetylation in the aged female mouse brain, given that post-training infusion of E2 into the dorsal hippocampus does not enhance object recognition in aged females (Fan et al., 2010).
The influence of aging on DNA methylation is likely to be as complicated as the relationship between DNA methylation and memory formation. Both aging and Alzheimer’s disease are characterized by hypermethylation of specific CpG islands in the hippocampus and neocortex (Calvanese et al., 2009; Chouliaras et al., 2012; Haberman et al., 2012; Coppieters et al., 2014). In normal aging, this hypermethylation has been associated with memory decline. For example, aged cognitively-impaired male rats exhibited hypermethylation in the CpG island of the Gabra5 gene, which is highly expressed in hippocampal pyramidal neurons and encodes for the α5 subunit of the GABAA receptor (Haberman et al., 2012). Further, aged male rats exhibit hypermethylation of the immediate early gene Arc and decreased Arc mRNA in hippocampal CA1 compared to young rats (Penner et al., 2010). Although these data suggest that age-related hypermethylation of genes involved in hippocampal synaptic plasticity is detrimental for memory consolidation, hypermethylation of DNMT genes, specifically Dnmt3a2, may be beneficial for memory in older animals. Levels of Dnmt3a2 are decreased in the hippocampus and neocortex of aged male mice, who also exhibit a deficit in learning-induced activation of Dnmt3a2 (Oliveira et al., 2012). Viral overexpression of Dnmt3a2 in aged males ameliorated age-related deficits in trace-fear conditioning and object location memory (Oliveira et al., 2012), suggesting that expression of this specific Dnmt3a transcript is essential to memory function in aged males.
However, it is unknown how DNA methylation is affected by aging in the hippocampus of females. In our unpublished work, we have found that E2 increases DNMT3B protein in middle-aged female mice as it does in young female mice (Fortress and Frick, unpublished results), which is consistent with the histone responsiveness at this age described earlier. In future work, it will be important to look for methylation in promoter regions for ERα and ERβ in the aging female hippocampus. The expression of ERα is highly regulated by methylation during early post-natal development in mice (Westberry and Wilson, 2012). More relevant for aging, methylation of the ERβ promoter is increased in the neocortex of middle-aged, but not young, female rats (Westberry et al., 2011). This increased methylation was associated with reduced ERβ mRNA. Although the behavioral consequences of this reduction are unknown, the fact that hippocampal ERβ promotes rapid ERK signaling and object recognition memory consolidation in young female mice (Boulware et al., 2013) would suggest that a reduction in ERβ could contribute to memory loss in aging females. This hypothesis will need to be addressed in future studies.
6. Conclusions
This review has highlighted the many ways in which chromatin modifications can influence hippocampal memory formation. The data, thus far, suggest that E2 facilitates a transcriptionally permissive chromatin state that leads to the transcription of genes that promote hippocampal synaptic plasticity and memory consolidation (Fig. 7). Menopause and aging may facilitate a transcriptionally repressive chromatin state that decreases the transcription of synaptic plasticity and memory genes, thereby leading to impaired memory (Fig. 7). However, much more work is needed in aging females to support this hypothesis. Although we are a long way from fully understanding the complex epigenetic code regulating memory, we are starting to appreciate the role that epigenetic processes play in hormonal modulation of memory. Our findings have thus far shed light on gross changes in histone acetylation and DNA methylation necessary for E2 to enhance hippocampal memory formation, but future studies will be needed to better pinpoint the histone residues and promoter regions that are particularly essential. As such, our work has opened up new avenues for research that should provide a much more comprehensive view of hormonal regulation of memory in the coming years. Such information should facilitate the development of treatments to reduce the incidence and severity of memory dysfunction in neuropsychiatric and neurodegenerative disorders, particularly those for which women are at increased risk.
Fig. 7.
Hypothesized general epigenetic mechanisms through which E2 and aging may regulate memory consolidation in the dorsal hippocampus. Endogenous levels of E2 from either local synthesis or from the periphery may sustain a relaxed chromatin structure and baseline level of histone acetylation by increasing HAT activity and reducing HDAC activity. Intrinsic HAT activity selectively facilitates an increase in the acetylation of H3 to promote the transcription of genes, such as Bdnf, important hippocampal memory consolidation. The loss of E2 during menopause leads to a loss of intrinsic HAT activity. The loss of HAT activity favors a reduction in histone acetylation, an increase in HDACs, and therefore, a condensed chromatin structure. With the chromatin condensed, genes important for memory are unable to be transcribed and, therefore, hippocampal memory is impaired.
Acknowledgments
The authors wish to thank Jennifer J. Tuscher and Jaekyoon Kim for critical comments on this manuscript. The empirical work described in this review was supported by a grant from the National Institute on Aging (AG022525) to KMF, an Ellison Medical Foundation/American Foundation for Aging Research Postdoctoral Fellowship in Aging to AMF, and the University of Wisconsin-Milwaukee.
References
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Agis-Balboa RC, Pavelka Z, Kerimoglu C, Fischer A. Loss of HDAC5 impairs memory function: implications for Alzheimer’s disease. J Alzheimers Dis. 2013;33:35–44. doi: 10.3233/JAD-2012-121009. [DOI] [PubMed] [Google Scholar]
- Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res. 1998;93:185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Atanassov BS, Koutelou E, Dent SY. The role of deubiquitinating enzymes in chromatin regulation. FEBS Lett. 2011;585:2016–2023. doi: 10.1016/j.febslet.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602– 609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Bahari-Javan S, Maddalena A, Kerimoglu C, Wittnam J, Held T, Bahr M, Burkhardt S, Delalle I, Kugler S, Fischer A, Sananbenesi F. HDAC1 regulates fear extinction in mice. J Neurosci. 2012;32:5062–5073. doi: 10.1523/JNEUROSCI.0079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DJ, Ma C, Soma KK, Saldanha CJ. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology. 2013;154:4707–4714. doi: 10.1210/en.2013-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Andresen D, Ratnu VS, Bredy TW. Dynamic DNA methylation: a prime candidate for genomic metaplasticity and behavioral adaptation. Trends Neurosci. 2013;36:3–13. doi: 10.1016/j.tins.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP, Kundu TK. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem. 2004;279:33716–33726. doi: 10.1074/jbc.M402839200. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Banerjee T, Chakravarti D. A peek into the complex realm of histone phosphorylation. Mol Cell Biol. 2011;31:4858–4873. doi: 10.1128/MCB.05631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RM, Wood MA. Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn Mem. 2008;15:460–467. doi: 10.1101/lm.917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Bi X, Aguirre C. Progesterone–estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience. 2013;239:280–294. doi: 10.1016/j.neuroscience.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann MJ, Dekker CF, van Lunenburg M, Sommer IE. Estrogen augmentation in schizophrenia: a quantitative review of current evidence. Schizophr Res. 2012;141:179–184. doi: 10.1016/j.schres.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol Cell. 2012;48:491– 507. doi: 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco JC, Minucci S, Lu J, Yang XJ, Walker KK, Chen H, Evans RM, Nakatani Y, Ozato K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998;12:1638–1651. doi: 10.1101/gad.12.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Erskine M. Feminine Sexual Behavior: Cellular Integration of Hormonal and Afferent Information in the Rodent Brain. Academic Press; San Diego, CA: 2002. [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science. 1998;280:1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J, Lesch KP, Lanfumey L, Steinbusch HW, Kenis G. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatr. 2012;17:584–596. doi: 10.1038/mp.2011.107. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozon B, Kelly A, Josselyn SA, Silva AJ, Davis S, Laroche S. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philos Trans R Soc Lond B. 2003;358:805–814. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvanese V, Lara E, Kahn A, Fraga MF. The role of epigenetics in aging and age-related diseases. Ageing Res Rev. 2009;8:268–276. doi: 10.1016/j.arr.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Cao J, Yan Q. Histone ubiquitination and deubiquitination in transcription, DNA damage response, and cancer. Front Oncol. 2012;2:26. doi: 10.3389/fonc.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JF, Fletcher BR, Kelley-Bell B, Kim DH, Gallagher M, Rapp PR. Age-related memory impairment is associated with disrupted multivariate epigenetic coordination in the hippocampus. PLoS ONE. 2012;7:e33249. doi: 10.1371/journal.pone.0033249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Gomez S, Barrera-Ocampo A, Machado-Rodriguez G, Castro-Alvarez JF, Glatzel M, Giraldo M, Sepulveda-Falla D. Specific de-SUMOylation triggered by acquisition of spatial learning is related to epigenetic changes in the rat hippocampus. NeuroReport. 2013;24:976–981. doi: 10.1097/WNR.0000000000000025. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SE, Gonzalez-Gonzalez IM, Wilkinson KA, Konopacki FA, Kantamneni S, Henley JM, Mellor JR. SUMOylation and phosphorylation of GluK2 regulate kainate receptor trafficking and synaptic plasticity. Nat Neurosci. 2012;15:845–852. doi: 10.1038/nn.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Mizar P, Cassel R, Neidl R, Selvi BR, Mohankrishna DV, Vedamurthy BM, Schneider A, Bousiges O, Mathis C, Cassel JC, Eswaramoorthy M, Kundu TK, Boutillier AL. A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice. J Neurosci. 2013;33:10698–10712. doi: 10.1523/JNEUROSCI.5772-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zou X, Watanabe H, van Deursen JM, Shen J. CREB binding protein is required for both short-term and long-term memory formation. J Neurosci. 2010;30:13066–13077. doi: 10.1523/JNEUROSCI.2378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Nilsen J, Brinton RD. Dose and temporal pattern of estrogen exposure determines neuroprotective outcome in hippocampal neurons: therapeutic implications. Endocrinology. 2006;147:5303–5313. doi: 10.1210/en.2006-0495. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Chen X, Hu Y, Zhou DX. Epigenetic gene regulation by plant Jumonji group of histone demethylase. Biochim Biophys Acta. 2011;1809:421–426. doi: 10.1016/j.bbagrm.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Cheng J, Huang M, Zhu Y, Xin YJ, Zhao YK, Huang J, Yu JX, Zhou WH, Qiu Z. SUMOylation of MeCP2 is essential for transcriptional repression and hippocampal synapse development. J Neurochem. 2013;128:798–806. doi: 10.1111/jnc.12523. [DOI] [PubMed] [Google Scholar]
- Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;213:610–617. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch, McTiernan A. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Valsecchi P, Kavaliers M. Estrogenic involvement in social learning, social recognition and pathogen avoidance. Front Neuroendocrinol. 2012;33:140–159. doi: 10.1016/j.yfrne.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Chouliaras L, van den Hove DL, Kenis G, Keitel S, Hof PR, van Os J, Steinbusch HW, Schmitz C, Rutten BP. Prevention of age-related changes in hippocampal levels of 5-methylcytidine by caloric restriction. Neurobiol Aging. 2012;33:1672–1681. doi: 10.1016/j.neurobiolaging.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WC, Swaab DF, De Vries GJ. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J Neurobiol. 2000;43:234–243. [PubMed] [Google Scholar]
- Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci. 2007;27:12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters N, Dieriks BV, Lill C, Faull RL, Curtis MA, Dragunow M. Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiol Aging. 2014;35:1334–1344. doi: 10.1016/j.neurobiolaging.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- Crick F. Memory and molecular turnover. Nature. 1984;312:101. doi: 10.1038/312101a0. [DOI] [PubMed] [Google Scholar]
- Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013;19:197–209. doi: 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnas M, Mons N. Region- and age-specific patterns of histone acetylation related to spatial and cued learning in the water maze. Hippocampus. 2013;23:581–591. doi: 10.1002/hipo.22116. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. DNA methylation and memory formation. Nat Neurosci. 2010;13:1319–1323. doi: 10.1038/nn.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Cognitive neuroepigenetics: a role for epigenetic mechanisms in learning and memory. Neurobiol Learn Mem. 2011;96:2–12. doi: 10.1016/j.nlm.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis H, Ndlovu MN, Fuks F. Regulation of mammalian DNA methyltransferases: a route to new mechanisms. EMBO Rep. 2011;12:647–656. doi: 10.1038/embor.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drag M, Salvesen GS. DeSUMOylating enzymes–SENPs. IUBMB Life. 2008;60:734–742. doi: 10.1002/iub.113. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Ann NY Acad Sci. 2010;1204:104–112. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickbush TH, Moudrianakis EN. The histone core complex: an octamer assembled by two sets of protein-protein interactions. Biochemistry. 1978;17:4955–4964. doi: 10.1021/bi00616a016. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Gonzalez-Flores O, Todd BJ. The role of insulin-like growth factor-I and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Front Neuroendocrinol. 2006;27:363–375. doi: 10.1016/j.yfrne.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, Diniz BS, Forlenza OV, Busatto GF, Zanetti MV. Neurostructural predictors of Alzheimer’s disease: a meta-analysis of VBM studies. Neurobiol Aging. 2011;32:1733–1741. doi: 10.1016/j.neurobiolaging.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- Fioravante D, Byrne JH. Protein degradation and memory formation. Brain Res Bull. 2011;85:14–20. doi: 10.1016/j.brainresbull.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–385. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in dorsal hippocampus. Learn Mem. 2013;20:147–155. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Mansuy IM. The prevalence of epigenetic mechanisms in the regulation of cognitive functions and behaviour. Curr Opin Neurobiol. 2010;20:441– 449. doi: 10.1016/j.conb.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Building a better hormone therapy? How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Epigenetics, oestradiol and hippocampal memory consolidation. J Neuroendocrinol. 2013;25:1151–1162. doi: 10.1111/jne.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Harburger LL. A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim Biophys Acta. 2010;1800:1045–1055. doi: 10.1016/j.bbagen.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety, and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur J Neurosci. 2004;19:3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem. 2003;278:4035–4040. doi: 10.1074/jbc.M210256200. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. J Exp Biol. 1996;199:195–200. doi: 10.1242/jeb.199.1.195. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013;25:1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- Gardner KE, Allis CD, Strahl BD. Operating on chromatin, a colorful language where context matters. J Mol Biol. 2011;409:36–46. doi: 10.1016/j.jmb.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. Something about SUMO inhibits transcription. Curr Opin Genet Dev. 2005;15:536–541. doi: 10.1016/j.gde.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schluter OM, Bradke F, Lu J, Fischer A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol Med. 2013;5:52–63. doi: 10.1002/emmm.201201923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Tsai LH. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013a;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- Graff J, Tsai LH. The potential of HDAC inhibitors as cognitive enhancers. Annu Rev Pharmacol Toxicol. 2013b;53:311–330. doi: 10.1146/annurev-pharmtox-011112-140216. [DOI] [PubMed] [Google Scholar]
- Graff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, Nieland TJ, Fass DM, Kao PF, Kahn M, Su SC, Samiei A, Joseph N, Haggarty SJ, Delalle I, Tsai LH. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Guidotti A. The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology. 2013;38:138–166. doi: 10.1038/npp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Schafe GE, Frick KM. Sex differences in contextual fear conditioning are associated with differential ventral hippocampal ERK activation. Neuroscience. 2009;159:451–467. doi: 10.1016/j.neuroscience.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011;145:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD. Histone methylation regulates memory formation. J Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta-Agarwal S, Franklin AV, Deramus T, Wheelock M, Davis RL, McMahon LL, Lubin FD. G9a/GLP histone lysine dimethyltransferase complex activity in the hippocampus and the entorhinal cortex is required for gene activation and silencing during memory consolidation. J Neurosci. 2012;32:5440–5453. doi: 10.1523/JNEUROSCI.0147-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gur RE. Memory in health and in schizophrenia. Dialogues Clin Neurosci. 2013;15:399–410. doi: 10.31887/DCNS.2013.15.4/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TH, Chang JS, Oh SH, Kim JS, Cho HS, Ha K. Differential patterns of neuropsychological performance in the euthymic and depressive phases of bipolar disorders. Psychiatry Clin Neurosci. 2014 doi: 10.1111/pcn.12158. http://dx.doi.org/10.1111/pcn.12158. [DOI] [PubMed]
- Haberman RP, Quigley CK, Gallagher M. Characterization of CpG island DNA methylation of impairment-related genes in a rat model of cognitive aging. Epigenetics. 2012;7:1008–1019. doi: 10.4161/epi.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem. 2011;18:71–79. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AM, Zito K. Breaking it down: the ubiquitin proteasome system in neuronal morphogenesis. Neural Plast. 2013;2013:196848. doi: 10.1155/2013/196848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res. 2011;1379:53–60. doi: 10.1016/j.brainres.2010.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology. 2011;36:182–192. doi: 10.1016/j.psyneuen.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Kline E, Gibbs RB. Chronic treatment with a GPR30 antagonist impairs acquisition of a spatial learning task in young female rats. Horm Behav. 2012;62:367–374. doi: 10.1016/j.yhbeh.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk JD, Florian C, Abel T. Post-training intrahippocampal inhibition of class I histone deacetylases enhances long-term object-location memory. Learn Mem. 2011;18:367–370. doi: 10.1101/lm.2097411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Moody NM, Dohanich GP, Vasudevan N. Activation of G-protein-coupled receptor 30 is sufficient to enhance spatial recognition memory in ovariectomized rats. Behav Brain Res. 2014;262:68–73. doi: 10.1016/j.bbr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Hogervorst E. Effects of gonadal hormones on cognitive behavior in elderly men and women. J Neuroendocrinol. 2013;25:1182–1195. doi: 10.1111/jne.12080. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R. Is there an epigenetic component in long-term memory? J Theor Biol. 1999;200:339–341. doi: 10.1006/jtbi.1999.0995. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Trans Med. 2011;3:77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Miki Y, Abe K, Hatori M, Hosaka M, Kariya Y, Kakuo S, Fujimura T, Hachiya A, Honma S, Aiba S, Sasano H. Sex steroid synthesis in human skin in situ: the roles of aromatase and steroidogenic acute regulatory protein in the homeostasis of human skin. Mol Cell Endocrinol. 2012;362:19–28. doi: 10.1016/j.mce.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Jaafari N, Konopacki FA, Owen TF, Kantamneni S, Rubin P, Craig TJ, Wilkinson KA, Henley JM. SUMOylation is required for glycine-induced increases in AMPA receptor surface expression (ChemLTP) in hippocampal neurons. PLoS ONE. 2013;8:e52345. doi: 10.1371/journal.pone.0052345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Lubin FD. Histone lysine methylation: critical regulator of memory and behavior. Rev Neurosci. 2013:1–13. doi: 10.1515/revneuro-2013-0008. [DOI] [PubMed] [Google Scholar]
- Jarome TJ, Helmstetter FJ. The ubiquitin-proteasome system as a critical regulator of synaptic plasticity and long-term memory formation. Neurobiol Learn Mem. 2013;105:107–116. doi: 10.1016/j.nlm.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Kwapis JL, Hallengren JJ, Wilson SM, Helmstetter FJ. The ubiquitin-specific protease 14 (USP14) is a critical regulator of long-term memory formation. Learn Mem. 2014;21:9–13. doi: 10.1101/lm.032771.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenceau A, Hedou G, Potier B, Kollen M, Dutar P, Mansuy IM. Partial inhibition of PP1 alters bidirectional synaptic plasticity in the hippocampus. Eur J Neurosci. 2006;24:564–572. doi: 10.1111/j.1460-9568.2006.04938.x. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Fitch JM, Henderson C, Rivers N. Sex differences in the dendritic branching of dentate granule cells following differential experience. Brain Res. 1985;333:73–80. doi: 10.1016/0006-8993(85)90125-8. [DOI] [PubMed] [Google Scholar]
- Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circ. 2013;7:149. doi: 10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;12:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerimoglu C, Agis-Balboa RC, Kranz A, Stilling R, Bahari-Javan S, Benito-Garagorri E, Halder R, Burkhardt S, Stewart AF, Fischer A. Histone-methyltransferase MLL2 (KMT2B) is required for memory formation in mice. J Neurosci. 2013;33:3452–3464. doi: 10.1523/JNEUROSCI.3356-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Akhtar MW, Adachi M, Mahgoub M, Bassel-Duby R, Kavalali ET, Olson EN, Monteggia LM. An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. J Neurosci. 2012;32:10879–10886. doi: 10.1523/JNEUROSCI.2089-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Hsiao SJ, Kraus WL. A role for coactivators and histone acetylation in estrogen receptor alpha-mediated transcription initiation. EMBO J. 2001;20:6084–6094. doi: 10.1093/emboj/20.21.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D. Sex and Cognition. The MIT Press; Cambridge, MA: 1999. [Google Scholar]
- Kishimoto M, Fujiki R, Takezawa S, Sasaki Y, Nakamura T, Yamaoka K, Kitagawa H, Kato S. Nuclear receptor mediated gene regulation through chromatin remodeling and histone modifications. Endocr J. 2006;53:157–172. doi: 10.1507/endocrj.53.157. [DOI] [PubMed] [Google Scholar]
- Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502:472–479. doi: 10.1038/nature12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooi EJ, Prins M, Bajic N, Belien JA, Gerritsen WH, van Horssen J, Aronica E, van Dam AM, Hoozemans JJ, Francis PT, van der Valk P, Geurts JJ. Cholinergic imbalance in the multiple sclerosis hippocampus. Acta Neuropathol. 2011;122:313–322. doi: 10.1007/s00401-011-0849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshibu K, Graff J, Beullens M, Heitz FD, Berchtold D, Russig H, Farinelli M, Bollen M, Mansuy IM. Protein phosphatase 1 regulates the histone code for long-term memory. J Neurosci. 2009;29:13079–13089. doi: 10.1523/JNEUROSCI.3610-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci USA. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Babayan AH, Gall CM, Lynch G. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience. 2013;239:3–16. doi: 10.1016/j.neuroscience.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni J. Oestrogen – a new treatment approach for schizophrenia? Med J Aust. 2009;190:S37–38. doi: 10.5694/j.1326-5377.2009.tb02373.x. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151:2297–2305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol. 2000;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Wood MA. Epigenetics and persistent memory: implications for reconsolidation and silent extinction beyond the zero. Nat Neurosci. 2013;16:124–129. doi: 10.1038/nn.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach PT, Poplawski SG, Kenney JW, Hoffman B, Liebermann DA, Abel T, Gould TJ. Gadd45b knockout mice exhibit selective deficits in hippocampus-dependent long-term memory. Learn Mem. 2012;19:319–324. doi: 10.1101/lm.024984.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. J Neurosci. 2013;33:2761–2772. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Roth TL, Lubin FD, Miller CA, Huang I-C, Desai P, Malone LM, Sweatt JD. Evidence that DNA (Cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CH, Sang S, Ho CT, Lin JK. Garcinol modulates tyrosine phosphorylation of FAK and subsequently induces apoptosis through down-regulation of Src, ERK, and Akt survival signaling in human colon cancer cells. J Cell Biochem. 2005;96:155–169. doi: 10.1002/jcb.20540. [DOI] [PubMed] [Google Scholar]
- Liu GP, Wei W, Zhou X, Shi HR, Liu XH, Chai GS, Yao XQ, Zhang JY, Peng CX, Hu J, Li XC, Wang Q, Wang JZ. Silencing PP2A inhibitor by lentishRNA interference ameliorates neuropathologies and memory deficits in tg2576 mice. Mol Ther. 2013;21:2247–2257. doi: 10.1038/mt.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Kelley MH, Herson PS, Hurn PD. Neuroprotection of sex steroids. Minerva Endocrinol. 2010;35:127–143. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Salon M, Alonso M, Vianna MR, Viola H, Mello e Souza T, Izquierdo I, Pasquini JM, Medina JH. The ubiquitin-proteasome cascade is required for mammalian long-term memory formation. Eur J Neurosci. 2001;14:1820–1826. doi: 10.1046/j.0953-816x.2001.01806.x. [DOI] [PubMed] [Google Scholar]
- Loriol C, Khayachi A, Poupon G, Gwizdek C, Martin S. Activity-dependent regulation of the sumoylation machinery in rat hippocampal neurons. Biol Cell. 2013;105:30–45. doi: 10.1111/boc.201200016. [DOI] [PubMed] [Google Scholar]
- Lorrio S, Romero A, Gonzalez-Lafuente L, Lajarin-Cuesta R, Martinez-Sanz FJ, Estrada M, Samadi A, Marco-Contelles J, Rodriguez-Franco MI, Villarroya M, Lopez MG, de los Rios C. PP2A ligand ITH12246 protects against memory impairment and focal cerebral ischemia in mice. ACS Chem Neurosci. 2013;4:1267–1277. doi: 10.1021/cn400050p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy R, Gerlach JL, McEwen BS. Autoradiographic localization of oestradiol binding neurons in the hippocampal formation and the entorhinal cortex. Dev Brain Res. 1988;39:245–251. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- Lubin FD. Epigenetic gene regulation in the adult mammalian brain: multiple roles in memory formation. Neurobiol Learn Mem. 2011;96:68–78. doi: 10.1016/j.nlm.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luo J, Ashikaga E, Rubin PP, Heimann MJ, Hildick KL, Bishop P, Girach F, Josa-Prado F, Tang LT, Carmichael RE, Henley JM, Wilkinson KA. Receptor trafficking and the regulation of synaptic plasticity by SUMO. Neuromol Med. 2013;15:692–706. doi: 10.1007/s12017-013-8253-y. [DOI] [PubMed] [Google Scholar]
- Lyst MJ, Stancheva I. A role for SUMO modification in transcriptional repression and activation. Biochem Soc Trans. 2007;35:1389–1392. doi: 10.1042/BST0351389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabb AM, Judson MC, Zylka MJ, Philpot BD. Angelman syndrome: insights into genomic imprinting and neurodevelopmental phenotypes. Trends Neurosci. 2011;34:293–303. doi: 10.1016/j.tins.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287– 293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Maddox SA, Watts CS, Doyere V, Schafe GE. A naturally-occurring histone acetyltransferase inhibitor derived from Garcinia indica impairs newly acquired and reactivated fear memories. PLoS ONE. 2013;8:e54463. doi: 10.1371/journal.pone.0054463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MD, Cadete-Leite A, Andrade JP, Paula-Barbosa MM. Effects of hypothyroidism upon the granular layer of the dentate gyrus in male and female adult rats: a morphometric study. J Comp Neurol. 1991a;314:171–186. doi: 10.1002/cne.903140116. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Cadete-Leite A, Sousa N, Paula-Barbosa MM. The supraoptic nucleus in hypothyroid and undernourished rats: an experimental morphometric study. Neuroscience. 1991b;41:827–839. doi: 10.1016/0306-4522(91)90373-v. [DOI] [PubMed] [Google Scholar]
- Maggi A, Susanna L, Bettini E, Mantero G, Zucchi I. Hippocampus: a target for estrogen action in mammalian brain. Mol Endocrinol. 1989;3:1165–1170. doi: 10.1210/mend-3-7-1165. [DOI] [PubMed] [Google Scholar]
- Maki PM. Minireview: effects of different HT formulations on cognition. Endocrinology. 2012;153:3564–3570. doi: 10.1210/en.2012-1175. [DOI] [PubMed] [Google Scholar]
- Markham JA, McKian KP, Stroup TS, Juraska JM. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005;15:97–103. doi: 10.1002/hipo.20034. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh WK, Ketter TA, Crawford SL, Johnson JV, Kroll-Desrosiers AR, Rothschild AJ. Progression of female reproductive stages associated with bipolar illness exacerbation. Bipolar Disord. 2012;14:515–526. doi: 10.1111/j.1399-5618.2012.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M. Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology. 2011;152:2760–2767. doi: 10.1210/en.2011-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzio EA, Soliman KF. Basic concepts of epigenetics: impact of environmental signals on gene expression. Epigenetics. 2012;7:119–130. doi: 10.4161/epi.7.2.18764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Konkle ATM. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM. Epigenetic contributions to hormonally mediated sexual differentiation of the brain. J Neuroendocrinol. 2013;25:1133–1140. doi: 10.1111/jne.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Wright CL, Schwarz JM. New tricks by an old dogma: mechanisms of the organizational/activational hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm Behav. 2009;55:655–665. doi: 10.1016/j.yhbeh.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, Wood MA. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merschbaecher K, Haettig J, Mueller U. Acetylation-mediated suppression of transcription-independent memory: bidirectional modulation of memory by acetylation. PLoS ONE. 2012;7:e45131. doi: 10.1371/journal.pone.0045131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metivier R, Gallais R, Tiffoche C, Le Peron C, Jurkowska RZ, Carmouche RP, Ibberson D, Barath P, Demay F, Reid G, Benes V, Jeltsch A, Gannon F, Salbert G. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- Micevych P, Christensen A. Membrane-initiated estradiol actions mediate structural plasticity and reproduction. Front Neuroendocrinol. 2012;33:331–341. doi: 10.1016/j.yfrne.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Munzel M, Globisch D, Bruckl T, Wagner M, Welzmiller V, Michalakis S, Muller M, Biel M, Carell T. Quantification of the sixth DNA base hydroxymethylcytosine in the brain. Angew Chem Int Ed Engl. 2010;49:5375–5377. doi: 10.1002/anie.201002033. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EK, Hien A, de Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150:4241–4247. doi: 10.1210/en.2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman CA, Lalonde ME, Cote J, Kutateladze TG. Perceiving the epigenetic landscape through histone readers. Nat Struct Mol Biol. 2012;19:1218–1227. doi: 10.1038/nsmb.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D, Sterner DE, Berger SL. Histone modifications: now summoning sumoylation. Proc Natl Acad Sci USA. 2003;100:13118–13120. doi: 10.1073/pnas.2436173100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse P, Newhouse C, Astur RS. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res. 2007;183:1–7. doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Ng SS, Yue WW, Oppermann U, Klose RJ. Dynamic protein methylation in chromatin biology. Cell Mol Life Sci. 2009;66:407–422. doi: 10.1007/s00018-008-8303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C, Schafer A. Active DNA demethylation by Gadd45 and DNA repair. Trends Cell Biol. 2012;22:220–227. doi: 10.1016/j.tcb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci USA. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, Takata N, Kimoto Y, Kawato S. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res Rev. 2008;57:363–375. doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Hemstedt TJ, Bading H. Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities. Nat Neurosci. 2012;15:1111–1113. doi: 10.1038/nn.3151. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Wood MA, McDonough CB, Abel T. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn Mem. 2007;14:564–572. doi: 10.1101/lm.656907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J, Gill G. SUMO engages multiple corepressors to regulate chromatin structure and transcription. Epigenetics. 2009;4:440–444. doi: 10.4161/epi.4.7.9807. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997a;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. NeuroReport. 1997b;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Pavlopoulos E, Jones S, Kosmidis S, Close M, Kim C, Kovalerchik O, Small SA, Kandel ER. Molecular mechanism for age-related memory loss: the histone-binding protein RbAp48. Sci Trans Med. 2013;5:200ra115. doi: 10.1126/scitranslmed.3006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30:343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Pechenino AS, Frick KM. The effects of acute 17β-estradiol treatment on gene expression in the young female mouse hippocampus. Neurobiol Learn Mem. 2009;91:315–322. doi: 10.1016/j.nlm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Penner MR, Roth TL, Barnes CA, Sweatt JD. An epigenetic hypothesis of aging-related cognitive dysfunction. Front Aging Neurosci. 2010;2:9. doi: 10.3389/fnagi.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav Neurosci. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Morphological changes in the brains of adult male rats after neonatal castration. J Endocrinol. 1966;36:415–416. doi: 10.1677/joe.0.0360415. [DOI] [PubMed] [Google Scholar]
- Picazo O, Azcoitia I, Garcia-Segura LM. Neuroprotective and neurotoxic effects of estrogens. Brain Res. 2003;990:20–27. doi: 10.1016/s0006-8993(03)03380-8. [DOI] [PubMed] [Google Scholar]
- Pittenger C. Disorders of memory and plasticity in psychiatric disease. Dialogues Clin Neurosci. 2013;15:455–463. doi: 10.31887/DCNS.2013.15.4/cpittenger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange-Kiel J, Fester L, Zhou L, Lauke H, Carrétero J, Rune GM. Inhibition of hippocampal estrogen synthesis causes region-specific downregulation of synaptic protein expression in hippocampal neurons. Hippocampus. 2006;16:464–471. doi: 10.1002/hipo.20173. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Neuroendocrine aspects of catamenial epilepsy. Horm Behav. 2013;63:254–266. doi: 10.1016/j.yhbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis DS, Jarome TJ, Helmstetter FJ. Memory formation for trace fear conditioning requires ubiquitin-proteasome mediated protein degradation in the prefrontal cortex. Front Behav Neurosci. 2013;7:150. doi: 10.3389/fnbeh.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reolon GK, Maurmann N, Werenicz A, Garcia VA, Schroder N, Wood MA, Roesler R. Posttraining systemic administration of the histone deacetylase inhibitor sodium butyrate ameliorates aging-related memory decline in rats. Behav Brain Res. 2011;221:329–332. doi: 10.1016/j.bbr.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricobaraza A, Cuadrado-Tejedor M, Perez-Mediavilla A, Frechilla D, Del Rio J, Garcia-Osta A. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacology. 2009;34:1721–1732. doi: 10.1038/npp.2008.229. [DOI] [PubMed] [Google Scholar]
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- Rossetto D, Avvakumov N, Cote J. Histone phosphorylation: a chromatin modification involved in diverse nuclear events. Epigenetics. 2012;7:1098–1108. doi: 10.4161/epi.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperbert C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- Rothbart SB, Strahl BD. Interpreting the language of histone and DNA modifications. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbagrm.2014.03.001. http://dx.doi.org/10.1016/j.bbagrm.2014.03.001. [DOI] [PMC free article] [PubMed]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando R, 3rd, Gounko N, Pieraut S, Liao L, Yates J, 3rd, Maximov A. HDAC4 governs a transcriptional program essential for synaptic plasticity and memory. Cell. 2012;151:821–834. doi: 10.1016/j.cell.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc Natl Acad Sci USA. 2006;103:1412–1417. doi: 10.1073/pnas.0510310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK. Protein acetylation in synaptic plasticity and memory. Neurosci Biobehav Rev. 2010;34:1234–1240. doi: 10.1016/j.neubiorev.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induced rapid translocation of estrogen receptor β, but not estrogen receptor α, to the neuronal plasma membrane. Neuroscience. 2008;153:751–761. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women: lessons we have learned. Behav Neurosci. 2012;126:123–127. doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierksma AS, Prickaerts J, Chouliaras L, Rostamian S, Delbroek L, Rutten BP, Steinbusch HW, van den Hove DL. Behavioral and neurobiological effects of prenatal stress exposure in male and female APPswe/PS1dE9 mice. Neurobiol Aging. 2013;34:319–337. doi: 10.1016/j.neurobiolaging.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis–some new perspectives. Endocrinology. 2001;142:4589–4594. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AW, Bosch MA, Wagner EJ, Ronnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17beta-estradiol. Am J Physiol Endocrinol Metab. 2013;305:E632–E640. doi: 10.1152/ajpendo.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Williams M. Stroke neuroprotection: estrogen and IGF-1 interactions and the role of microglia. J Neuroendocrinol. 2013;25:1173–1181. doi: 10.1111/jne.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, Mahadevan LC, Arthur JS. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J. 2003;22:2788– 2797. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, O’Malley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Evans PD. GPER 1: trials and tribulations of a membrane oestrogen receptor. J Neuroendocrinol. 2013 doi: 10.1111/jne.12071. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Waters EM, Mermelstein PG, Kramar EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31:16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci USA. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci USA. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan FA, Sweatt JD. The role of the gadd45 family in the nervous system: a focus on neurodevelopment, neuronal injury, and cognitive neuroepigenetics. Adv Exp Med Biol. 2013;793:81–119. doi: 10.1007/978-1-4614-8289-5_6. [DOI] [PubMed] [Google Scholar]
- Sultan FA, Wang J, Tront J, Liebermann DA, Sweatt JD. Genetic deletion of Gadd45b, a regulator of active DNA demethylation, enhances long-term memory and synaptic plasticity. J Neurosci. 2012;32:17059–17066. doi: 10.1523/JNEUROSCI.1747-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai HC, Schuman EM. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci. 2008;9:826–838. doi: 10.1038/nrn2499. [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ESJ, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HW, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. doi: 10.4161/epi.4.1.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BM. Reading signals on the nucleosome with a new nomenclature for modified histones. Nat Struct Mol Biol. 2005;12:110–112. doi: 10.1038/nsmb0205-110. [DOI] [PubMed] [Google Scholar]
- Tweedie-Cullen RY, Brunner AM, Grossman J, Mohanna S, Sichau D, Nanni P, Panse C, Mansuy IM. Identification of combinatorial patterns of post-translational modifications on individual histones in the mouse brain. PLoS ONE. 2012;7:e36980. doi: 10.1371/journal.pone.0036980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB: CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, Graser L, Schwarz J, Rune GM. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci. 2012;32:8116–8126. doi: 10.1523/JNEUROSCI.5319-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JM, Hackanson B, Lubbert M, Jung M. Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin Epigenetics. 2010;1:117–136. doi: 10.1007/s13148-010-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter W, Clynes D, Tang Y, Marmorstein R, Mellor J, Berger SL. 14-3-3 interaction with histone H3 involves a dual modification pattern of phosphoacetylation. Mol Cell Biol. 2008;28:2840–2849. doi: 10.1128/MCB.01457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz R, Roesler R, Quevedo J, Sant’Anna MK, Madruga M, Rodrigues C, Gottfried C, Medina JH, Izquierdo I. Time-dependent impairment of inhibitory avoidance retention in rats by posttraining infusion of a mitogen-activated protein kinase kinase inhibitor into cortical and limbic structures. Neurobiol Learn Mem. 2000;73:11–20. doi: 10.1006/nlme.1999.3913. [DOI] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberry JM, Wilson ME. Regulation of estrogen receptor alpha gene expression in the mouse prefrontal cortex during early postnatal development. Neurogenetics. 2012;13:159–167. doi: 10.1007/s10048-012-0323-z. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor beta expression in the rat cortex during aging. NeuroReport. 2011;22:428–432. doi: 10.1097/WNR.0b013e328346e1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL. Progression of atrophy in Alzheimer’s disease and related disorders. Neurotox Res. 2010;18:339–346. doi: 10.1007/s12640-010-9175-1. [DOI] [PubMed] [Google Scholar]
- Wilkinson KA, Nakamura Y, Henley JM. Targets and consequences of protein SUMOylation in neurons. Brain Res Rev. 2010;64:195–212. doi: 10.1016/j.brainresrev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci. 1990;104:84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem. 2005;12:111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Lindquist K, Cauley J, Simonsick EM, Penninx B, Satterfield S, Harris T, Cummings SR. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging. 2007;28:171–178. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Yang LC, Zhang QG, Zhou CF, Yang F, Zhang YD, Wang RM, Brann DW. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS ONE. 2010;5:e9851. doi: 10.1371/journal.pone.0009851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomaku D, Numakawa T, Numakawa Y, Suzuki S, Matsumoto T, Adachi N, Nishio C, Taguchi T, Hatanaka H. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol Endocrinol. 2003;17:831–844. doi: 10.1210/me.2002-0314. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS. Hormone replacement therapy and incidence of Alzheimer disease in older women. J Am Med Assoc. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Zhao Y, Huang Y, Dorairaj NR, Chandler LJ, O’Donnell JM. Inhibition of the phosphodiesterase 4 (PDE4) enzyme reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CA1 subregion of the rat hippocampus. Neuropsychopharmacology. 2004a;29:1432–1439. doi: 10.1038/sj.npp.1300440. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Zhang L, Yang N, Meng J, Zuo P, Zhang Y, Chen J, Wang L, Gao X, Zhu D. E3 ubiquitin ligase RNF13 involves spatial learning and assembly of the SNARE complex. Cell Mol Life Sci. 2013;70:153–165. doi: 10.1007/s00018-012-1103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang Z, Khan SI, Horton JR, Tamaru H, Selker EU, Cheng X. Structural basis for the product specificity of histone lysine methyltransferases. Mol Cell. 2003;12:177–185. doi: 10.1016/s1097-2765(03)00224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Transcriptional regulation by histone ubiquitination and deubiquitination. Genes Dev. 2003;17:2733–2740. doi: 10.1101/gad.1156403. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Griffin K, Mondal N, Parvin JD. Phosphorylation of histone H2A inhibits transcription on chromatin templates. J Biol Chem. 2004b;279:21866–21872. doi: 10.1074/jbc.M400099200. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Estrogen receptor α and β differentially regulate intracellular Ca2+ dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate the estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA. 2010;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci. 2012;32:2344–2351. doi: 10.1523/JNEUROSCI.5819-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovkic IB, Guzman-Karlsson MC, Sweatt JD. Epigenetic regulation of memory formation and maintenance. Learn Mem. 2013;20:61–74. doi: 10.1101/lm.026575.112. [DOI] [PMC free article] [PubMed] [Google Scholar]