Abstract
Purpose
Improvement in breast cancer survival has been observed in recent decades in the U.S., but it is unclear if similar survival gains are consistent across breast cancer subtypes, especially with regards to more advanced stages of the disease.
Methods
Data were from 13 population-based cancer registries participating in the Surveillance, Epidemiology and End Results (SEER) program, consisting of women between 20–79 years of age diagnosed with invasive breast cancer between 1992 and 2008. 2-year (1992–2008) and 5-year (1992–2006) breast cancer cause-specific survival rates were calculated and stratified by estrogen receptor (ER)/progesterone receptor (PR) status, stage and race. Annual percent changes in survival rates were assessed.
Results
From 1992 through 1998–1999, 5-year and 2-year cause specific survival rates significantly improved across ER+/PR+, ER−/PR− and ER+/PR− subtypes, with an annual increase ranging from 0.5%–1.0%. From 1998–1999 to 2006, different patterns were observed by ER/PR subtypes with survival rates slightly improving for ER+/PR+, continuing to improve at a rate of 0.5% per year for ER−/PR−, and dropping 0.3% annually for ER+/PR− No significant survival gains were experienced by patients with ER−/PR+ cancer during the study period. In terms of advanced diseases, greatest annual increases in survival rates were seen for patients with stage III–IV ER+/PR+ and ER−/PR− tumors but less progress was observed for advanced ER+/PR− breast cancers.
Conclusion
Steady improvements in survival rates for breast cancer have been achieved over the past several decades. However, 5-year survival rates for stage IV disease remained dismally below 20% for most ER/PR subtypes.
Keywords: breast cancer, survival, estrogen receptor, progesterone receptor, time trend
Introduction
Improvements in breast cancer survival rates have been observed in recent decades. While mortality rates increased 0.4% per year between 1975 and 1990, they have since steadily declined 1.8–3.2% annually [1]. Five-year relative survival rates have improved from 74.6% among women diagnosed between 1975 and1979 to 90.6% for those diagnosed in 2006 [2]. Widespread mammographic screening and improved systemic treatment, primarily adjuvant endocrine therapy, are believed to be the primary contributors to the observed reduction in breast cancer mortality rates over this period [3]. However, there is some evidence that survival improvements differ by breast cancer subtypes defined by hormone receptor status, with the greatest improvements observed for estrogen receptor positive cancers [4–8].
Tumors defined by joint estrogen receptor (ER) and progesterone receptor (PR) expression have distinct phenotypic and molecular characteristics with variations in natural history and sensitivity to adjuvant hormonal therapies observed across tumor subtypes [9, 10]. Few studies have evaluated time trends in stage specific breast cancer survival rates according to joint ER/PR status. Considering these parameters together has the potential to further elucidate areas where progress has been made and where further work is needed, as screening increases the identification of early stage breast cancer [11] and endocrine therapy only provides benefit to those with hormone receptor positive disease [12, 13]. Using data from 13 population-based cancer registries participating in the Surveillance, Epidemiology and End Results (SEER) program encompassing approximately 14% of the United States population, we evaluated trends in 2-year and 5-year breast cancer specific survival rates by stage, hormone receptor status, and race.
Materials and methods
This study used data from the National Cancer Institute’s SEER Program, which has routinely collected incidence and survival data from population-based cancer registries across the Unites States since the 1970s. Included in this study are the 13 registries that have participated in the SEER Program since 1992. These registries cover the states of Connecticut, Hawaii, Iowa, New Mexico, and Utah; the greater metropolitan areas of Atlanta, Detroit, Los Angeles, San Francisco-Oakland, San Jose-Monterey, and Seattle-Puget Sound; Alaska Natives; and an area of rural Georgia.
We identified a total of 376,719 women between 20 and 79 years of age who were diagnosed with invasive breast cancer and lived in the areas covered by these registries between 1992 and 2008. Women were excluded from the analysis if they had an unknown cancer stage (n=29,736) or an unknown/borderline ER/PR status (n=53,043), if they were not primary cases (n=41,456), if their cause of death was unknown (n=1,306), if they were alive without survival time (n=26) or dead due to other causes with no survival time (n=5). Therefore, the final analytic sample for 2-year survival rates consisted of 251,147 women including 162,242 ER+/PR+ patients, 53,004 ER−/PR− patients, 30,003 ER+/PR− patients, 5,898 ER−/PR+ patients. Five-year survival rates for women diagnosed with breast cancer more recently than 2006 are not yet available, therefore analysis on 5-year survival rates were based on data from 1992–2006, consisting of 137,689 ER+/PR+ cases, 45,851 ER−/PR− cases, 25,684 ER+/PR− cases, and 5,499 ER−/PR+ cases.
All study data were obtained from the publicly available SEER database using the SEER*Stat 8.1.5 statistical software (National Cancer Institute, Bethesda, MD). These include demographic and clinical characteristics such as age at diagnosis (grouped into less than 50 years of age, 50–64, and 65–79), race (white, black and others), hormone receptor status (ER+/PR+, ER−/PR−, ER+/PR− and ER−/PR+), and NCI’s adjusted American Joint Committee on Cancer Classification (AJCC) stage (6th edition) (I, II, III, and IV) [14]. Our primary outcome was breast cancer cause-specific survival, which describes the probability of surviving breast cancer in the absence of other causes of death. It was derived using SEER data on vital status, cause of death, and follow-up time from breast cancer diagnosis to death or dates of last follow-up whichever came first. Demographic information and cancer related data were abstracted from medical records by trained coders at each SEER registry. Vital status of patients was both actively and passively followed up by SEER through a variety of data sources (e.g., Social Security Administration, state vital records departments, the National Death Index). Using SEER*Stat, 2-year and 5-year cause-specific survival rates were calculated for breast cancer overall and by ER/PR status. For each ER/PR subtype (ER−/PR+ was not stratified by stage due to rarity of the disease), survival trends were further assessed according to stage and race. Women of other races were not included in the race-specific analysis due to small sample size. The National Cancer Institute’s Jointpoint Regression Program (Version 4.0.1) was used to calculate annual percent change in survival rates and to evaluate the statistical significance of observed time trends using a Monte Carlo permutation method [15, 16].
Results
Compared to ER+/PR+ patients, the ER−/PR− and ER−/PR+ patients were somewhat more likely to be younger, non-white, and to present with stage II-IV diseases, and the ER+/PR− patients were somewhat more likely to be older, black and to present with stage III–IV cancers (Table 1). While the numbers of cases increased over time for ER+/PR+, ER−/PR− and ER+/PR− cancers, they decreased steadily for the ER−/PR+ subtype.
Table 1.
Selected characteristics of breast cancer cases by hormone receptor status: 1992–2008
| ER+/PR+ (n=162,24 2) | ER−/PR− (n=53,00 4) | ER+/PR− (n=30,00 3) | ER−/PR+ (n=5,89 8) | |||||
|---|---|---|---|---|---|---|---|---|
| n | Column % | n | Column % | n | Colum n % | n | Colum n % | |
| Age | ||||||||
| <50 | 44,622 | 27.5 | 19,537 | 36.9 | 5,924 | 19.7 | 2,57 9 | 43.7 |
| 50–64 | 63,492 | 39.1 | 21,157 | 39.9 | 12,83 9 | 42.8 | 2,13 7 | 36.2 |
| 65–79 | 54,128 | 33.4 | 12,310 | 23.2 | 11,24 0 | 37.5 | 1,18 2 | 20.0 |
| Race | ||||||||
| White | 134,238 | 82.7 | 39,546 | 74.6 | 24,40 6 | 81.3 | 4,55 1 | 77.2 |
| Black | 10,958 | 6.8 | 8,173 | 15.4 | 2,741 | 9.1 | 675 | 11.4 |
| Other | 16,434 | 10.1 | 5,107 | 9.6 | 2,750 | 9.2 | 652 | 11.1 |
| Unknown | 612 | 0.4 | 178 | 0.3 | 106 | 0.4 | 20 | 0.3 |
| Stage | ||||||||
| I | 84,191 | 51.9 | 18,781 | 35.4 | 14,06 1 | 46.9 | 2,43 7 | 41.3 |
| II | 53,151 | 32.8 | 21,504 | 40.6 | 9,867 | 32.9 | 2,13 9 | 36.3 |
| III | 19,869 | 12.2 | 10,215 | 19.3 | 4,539 | 15.1 | 1,06 0 | 18.0 |
| IV | 5,031 | 3.1 | 2,504 | 4.7 | 1,536 | 5.1 | 262 | 4.4 |
| Year of diagnosis | ||||||||
| 1992–1995 | 28,080 | 17.3 | 10,100 | 19.1 | 5,668 | 18.9 | 1,94 6 | 33.0 |
| 1996–1999 | 35,926 | 22.1 | 11,746 | 22.2 | 6,555 | 21.8 | 1,67 5 | 28.4 |
| 2000–2003 | 40,743 | 25.1 | 12,913 | 24.4 | 7,521 | 25.1 | 1,14 2 | 19.4 |
| 2004–2008 | 57,493 | 35.4 | 18,245 | 34.4 | 10,25 9 | 34.2 | 1,13 5 | 19.2 |
| SEER registry | ||||||||
| San Francisco-Oakla nd, CA | 20,889 | 12.9 | 5,996 | 11.3 | 3,830 | 12.8 | 642 | 10.9 |
| Connecticut | 16,479 | 10.2 | 5,532 | 10.4 | 3,132 | 10.4 | 615 | 10.4 |
| Detroit, MI | 16,002 | 9.9 | 6,528 | 12.3 | 3,458 | 11.5 | 649 | 11.0 |
| Hawaii | 6,521 | 4.0 | 1,709 | 3.2 | 1,033 | 3.4 | 306 | 5.2 |
| Iowa | 14,217 | 8.8 | 4,464 | 8.4 | 2,502 | 8.3 | 700 | 11.9 |
| New Mexico | 6,394 | 3.9 | 2,066 | 3.9 | 1,297 | 4.3 | 255 | 4.3 |
| Seattle, WA | 22,773 | 14.0 | 6,053 | 11.4 | 3,517 | 11.7 | 463 | 7.9 |
| Utah | 7,127 | 4.4 | 2,236 | 4.2 | 1,075 | 3.6 | 209 | 3.5 |
| Atlanta, GA | 10,965 | 6.8 | 4,536 | 8.6 | 2,065 | 6.9 | 475 | 8.1 |
| San Jose, CA | 9,850 | 6.1 | 2,816 | 5.3 | 1,864 | 6.2 | 289 | 4.9 |
| Los Angeles, CA | 30,293 | 18.7 | 10,751 | 20.3 | 6,088 | 20.3 | 1,26 4 | 21.4 |
| Alaska Natives | 316 | 0.2 | 115 | 0.2 | 65 | 0.2 | 9 | 0.2 |
| Rural Georgia | 416 | 0.3 | 202 | 0.4 | 77 | 0.3 | 22 | 0.4 |
From 1992 through 1998–1999, 5-year (Fig 1a) and 2-year (Fig 1b) cause specific survival rates significantly improved for patients with ER+/PR+, ER−/PR− and ER+/PR− breast cancers, with an annual increase ranging from 0.5% to 1.0%. From 1998–1999 to 2006, different patterns were observed across the ER/PR subtypes as 5-year survival rates slightly improved for ER+/PR+ disease, continued to improve at a rate of 0.5% per year for ER−/PR− disease, and dropped 0.3% annually for ER+/PR− disease. No significant survival gains were experienced by patients with ER−/PR+ cancer from 1992–2006. In absolute terms, the 5-year survival for ER−/PR− tumors remained considerably poorer compared to other types of breast cancer across all years.
Fig 1.
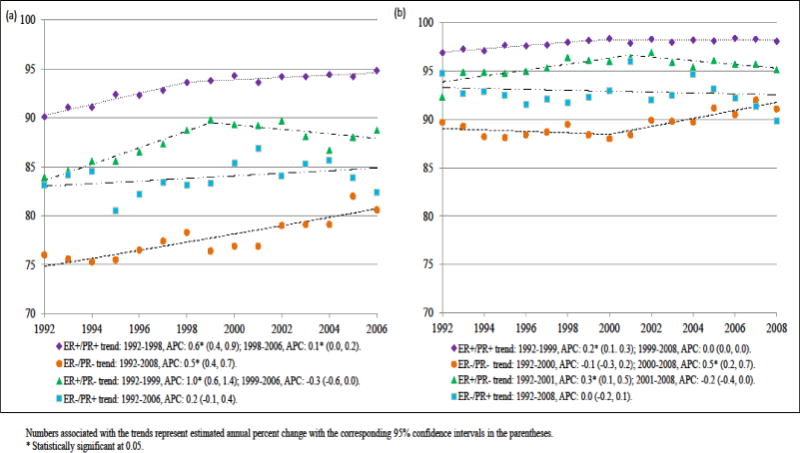
(a) 5-year (1992–2008), (b) 2-year (1992–2006) breast cancer specific survival rates overall and by ER/PR status
Both white and black women experienced improvements in 5-year survival rates for ER+/PR+, ER+/PR−, and ER−/PR− subtypes from 1992–2006, but rates were consistently lower among black women (Fig 2). On an absolute scale these disparities narrowed somewhat for ER+/PR+ and ER+/PR− subtypes, as 5–year survival rates plateaued in recent years for whites but continued to improve for blacks, but the gap held constant for ER−/PR− breast cancer and widened somewhat for ER−/PR+ disease.
Fig 2.
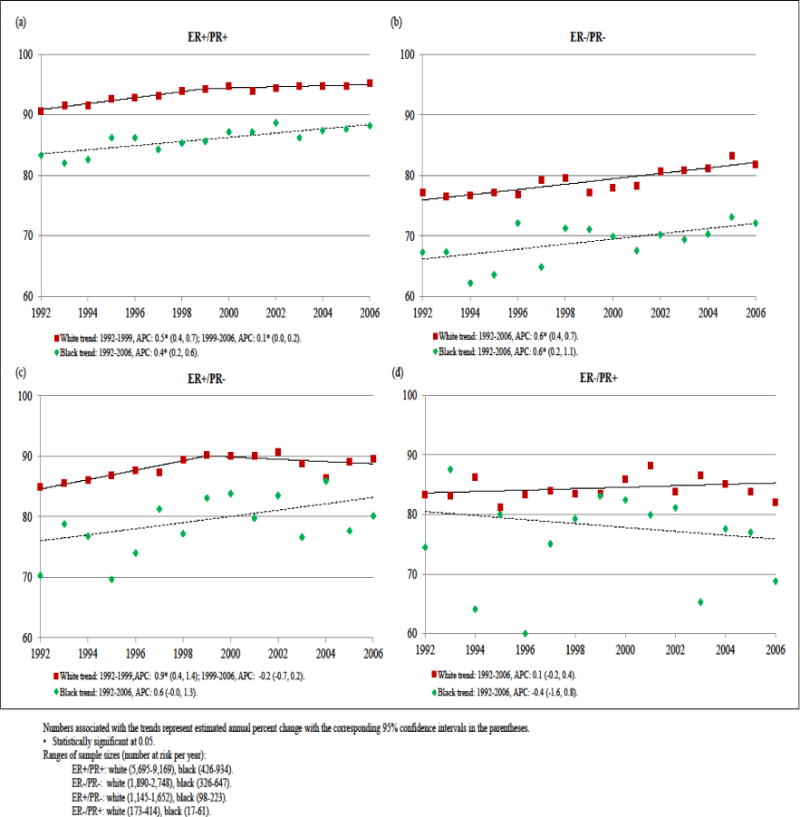
Trends in 5-year breast cancer specific survival rates by race for women with (a) ER+/PR+, (b) ER−/PR−, (c) ER+/PR− and (d) ER−/PR+ diseases, 1992–2006
Notable differences in stage-specific survival trends were observed by hormone receptor status (Fig 3). Patients with ER+/PR+ breast cancer experienced significant survival gains across all stages, and the greatest annual increases in survival rates occurred for women diagnosed with stage III and IV disease (rising 1.0% and 2.3% per year, respectively). Similarly, improvements were consistently seen across all stages for ER−/PR− disease with rates rising 3.8% per year from 2000–2006 for stage III and 3.6% per year for stage IV ER−/PR− cancers from 1992–2006. However, in absolute terms the 5-year survival rates for stage IV ER+/PR+ and ER−/PR− were still only 38.0% and 17.4%, respectively, among cases diagnosed in 2006. Among ER+/PR− patients, survival rates for stage I cancer held essentially constant, improved at a rate of 1.0% per year for stage II cancer only from 1992–1999, steadily increased 1.5% per year for stage III cancer from 1992–2006, and increased 8.0% per year for stage IV cancer from 1992–2000 after which they declined a non-statistically significant 7.3% per year through 2006. Further examination on trends of stage IV disease across ER/PR subtypes using 2-year breast cancer specific survival rates revealed similar patterns with stage IV ER+/PR+ and ER−/PR− patients experienced rapid survival improvements (rates rising 1.2% and 3.0% respectively, data not shown) from 1992–2008 while rates of stage IV ER+/PR− had a non-statistically significant increase at 1.0% per year (data not shown). In absolute terms, 2-year survival rates were 70.2% for patients diagnosed with stage IV ER+/PR+ cancer in 2008, compared to 44.8% and 56.4% for those diagnosed with stage IV ER−/PR− and ER+/PR− diseases in 2008, respectively.
Fig 3.
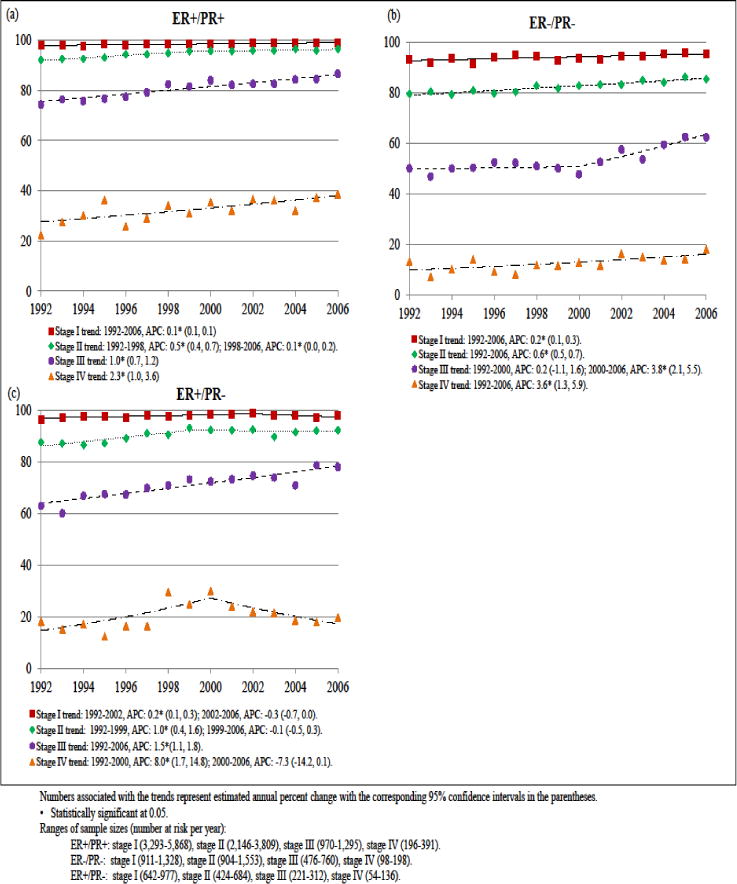
Trends in 5-year breast cancer specific survival rates by stage of cancer for women with (a) ER+/PR+, (b) ER−/PR−, and (c) ER+/PR− diseases, 1992–2006
Focusing on 2-year breast cancer specific survival rates for stage IV breast cancers among the two most common tumor subtypes, ER+/PR+ and ER−/PR−, notable differences in trends by race were observed (Fig 4). There was an appreciable narrowing of the disparity in 2-year survival rates between blacks and white for ER+/PR+ breast cancer, but a widening of this disparity for ER−/PR− breast cancer such that by 2006 these rates were near equal for ER+/PR+ breast cancer, but for ER−/PR− disease these rates were 44.0% for whites and 39.2% for blacks, although the sample sizes for black women were relatively small (black patients at risk at each year ranged from 16–65 for stage IV ER+/PR+ and 13–57 for ER−/PR−).
Fig 4.
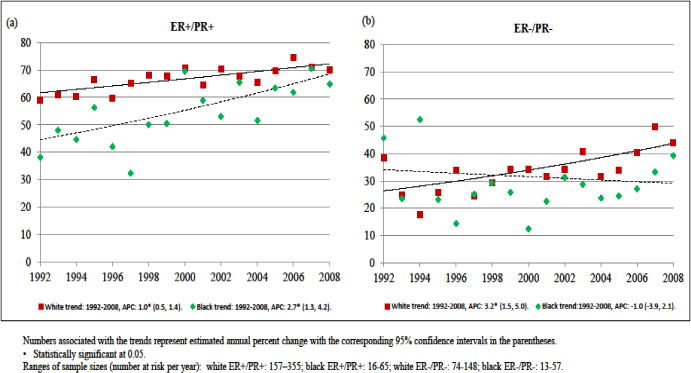
Trends in 2-year breast cancer specific survival rates of stage IV disease by race for women with (a) ER+/PR+ and (b) ER−/PR− cancers, 1992–2008
Discussion
Consistent with prior studies, we observed that both 2-year and 5-year cause-specific survival rates for breast cancer overall have improved since 1992 [1, 5, 17]. While earlier studies observed that improvements in breast cancer survival were largely limited to women with ER+ disease [7, 8, 18], our more recent data indicate that ER−/PR− patients have now also experienced rapid survival gains. What has not changed though are consistent differences in overall survival rates by subtype with ER+/PR+ patients having the highest survival rates and ER−/PR− patients have the lowest rates. These differences are likely largely driven by the less aggressive nature of hormone receptor positive tumors as well as the availability of effective adjuvant hormonal therapies that only benefit these patients [19–21].
Consistent survival improvements across all stages were observed for ER−/PR− breast cancer. However significant variations by stage were seen with rates rising most rapidly for stage III cancer starting in 2000, while they rose consistently for stage IV disease during the entire 1992–2006 study period. The more recent improvements in survival rates for advanced stage ER−/PR− breast cancer are likely due to enhanced therapeutic agents and options for women with ER−/PR− disease. Notable is the introduction of anti-HER2 agents such as trastuzumab (approved for use in 1998) which reduce mortality among patients with HER2+ breast cancer regardless of stage [22]. Unfortunately data on HER2 status and use of anti-HER2 therapy are not available from the SEER program over this time period, but approximately a third of ER−/PR− tumors are known to overexpress HER2 [23].
Similar to a prior study [7], rapid survival improvements for patients with ER+/PR− cancer were seen across all stages before 1999–2002. Our data suggest though that the survival improvements slowed across all stages except stage III in the more recent time period and that among stage IV ER+/PR− tumors survival rates have actually worsened since 2000. However this reversal was not apparent when examined using 2-year survival rates. Given the small number of cases with stage IV ER+/PR− in our data, this reversal could simply be a chance finding. Another issue is the enhanced reporting of weakly ER+ tumors as ER+ rather than ER− over time. In the past >10% ER expression was used to define ER positivity, however, this has shifted in recent years as current consensus guidelines mandate a lower threshold of >1% [24]. The tools used to detect ER and PR expression have also improved in their sensitivity and robustness (e.g., the use of rabbit monoclonal antibodies) resulting in the classification of more patients as ER positive [25–27]. Regardless, survival rates of ER+/PR− cancer remained markedly lower than those for ER+/PR+ disease from 1992 through 2006. It is well established that ER+/PR− breast cancer have more aggressive features (e.g., larger tumor sizes, more extensive axillary node involvement, more frequent HER2 positivity) likely accounting for this gap [28, 29]. Although the increased use of aromatase inhibitors has improved outcomes somewhat for ER+ tumors [30]. adjuvant hormonal therapies provide greater benefit to ER+/PR+ patients than to ER+/PR− patients [9, 10].
In terms of racial disparities, as documented in numerous studies [31–34]. we found that black women had consistently poorer survival rates compared to whites overall and across all ER/PR subtypes. Recent studies indicate that this disparity has either held constant [35, 36] or widened somewhat overall and for patients with advanced stage diseases [18, 37, 38]. Our study further evaluated this issue according to ER/PR subtype, and found that the gap has actually narrowed for patients with ER+ tumors while holding constant for ER−/PR− tumors. There is some evidence that use of adjuvant hormonal therapy is now similar among both black and white ER+ patients [39], which may have contributed to the narrowing of this disparity. On the other hand, the persistent racial differences in ER− breast cancer survival remains a concern and may be driven by ongoing differences in the utilization of other adjuvant breast cancer treatments including both chemotherapy and trastuzumab [40–42].
Given the ecologic design of this study, individual level data on a number of factors potentially relevant to these trends were not available including screening history, receipt of adjuvant treatment regimens (chemotherapy, endocrine therapy, and HER2-targetted therapy), and other personal and lifestyle factors such as body mass index, co-morbid conditions, and smoking history. A limitation of focusing on 5-year cause specific survival rates is lead time bias resulting from differential performance of screening mammography by hormone receptor status [43], and the possibility that 5-year follow-up may not be sufficient to assess any long-term survival differences by hormone receptor status [19]. However, these factors do not impact comparisons of time trends across or within each of the ER/PR subtypes assessed. Some of our results, specifically the race-specific trends, were based on small sample sizes and thus should be interpreted with caution. Lastly, given the demographics of the registries included in the SEER program, there are limitations in generalizing these results to the whole US population. In particular, the populations covered by the SEER program tend to have a higher proportion of ethnic minority and foreign-born persons than the general U.S. population [44].
Conclusion
Overall, our finding suggest that while overall steady improvements in 5 year survival rates for breast cancer have been achieved in the U.S. over the past several decades, there are subsets of breast cancer for which little progress in reducing mortality has been made. Despite the encouraging rapid improvements in survival rates for stage IV ER+/PR+ and ER−/PR− breast cancer in recent years, these improvements have been largely incremental as survival rates for stage IV breast cancer of all subtypes except ER+/PR+ remain below 20%. On-going efforts to reduce the incidence of or improve the outcomes for advanced stage breast cancer remain an important priority.
Acknowledgments
Funding/Support
This work was supported in part by NCI (N01-PC95001). The data set used for this analysis was obtained from the Surveillance, Epidemiology, and End Results (SEER) Program Public-Use Data (1992–2005), NCI, Department of Cancer Control and Population Sciences, Surveillance Systems Branch, released April 2013, based on the November 2012 submission. The NCI and SEER program were not involved in the design and conduct of this study, in the analysis or interpretation of the data, or in the preparation and review of this article.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Lu Chen, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington; Department of Epidemiology, University of Washington, Seattle, WA.
Hannah M. Linden, Clinical Division, Fred Hutchinson Cancer Research Center, Seattle, Washington; School of Medicine, University of Washington, Seattle.
Benjamin O. Anderson, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington; School of Medicine, University of Washington, Seattle.
Christopher I. Li, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington; Department of Epidemiology, University of Washington, Seattle, WA.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. 2013 http://seer.cancer.gov/csr/1975_2010/. Accessed 14 August 2014.
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. 2014 http://seer.cancer.gov/csr/1975_2010/. Accessed 14 August 2014.
- 3.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 4.Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Cancer. 2004;100:44–52. doi: 10.1002/cncr.11859. [DOI] [PubMed] [Google Scholar]
- 5.Holleczek B, Brenner H. Trends of population-based breast cancer survival in Germany and the US: decreasing discrepancies, but persistent survival gap of elderly patients in Germany. BMC Cancer. 2012;12:317. doi: 10.1186/1471-2407-12-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25:1683–1690. doi: 10.1200/JCO.2006.09.2106. [DOI] [PubMed] [Google Scholar]
- 7.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ademuyiwa FO, Groman A, Hong C-C, et al. Time-trends in survival in young women with breast cancer in a SEER population-based study. Breast Cancer Res Treat. 2013;138:241–248. doi: 10.1007/s10549-013-2425-1. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett JMS, Brookes CL, Robson T, et al. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J Clin Oncol. 2011;29:1531–1538. doi: 10.1200/JCO.2010.30.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowsett M, Allred C, Knox J, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol. 2008;26:1059–1065. doi: 10.1200/JCO.2007.12.9437. [DOI] [PubMed] [Google Scholar]
- 11.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 12.Boccardo F, Rubagotti A, Guglielmini P, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann Oncol. 2006;17(Suppl 7):vii10–4. doi: 10.1093/annonc/mdl941. [DOI] [PubMed] [Google Scholar]
- 13.Smith RE, Good BC. Chemoprevention of breast cancer and the trials of the National Surgical Adjuvant Breast and Bowel Project and others. Endocr Relat Cancer. 2003;10:347–357. doi: 10.1677/erc.0.0100347. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Adjusted AJCC 6th ed. T, N, M, and Stage – SEER Documentation. http://seer.cancer.gov/seerstat/variables/seer/ajcc-stage/6th/. Accessed 23 Jun 2014.
- 15.Statistical Research and Applications Branch NCI Jointpoint Regression Program, Version 4.0.1.
- 16.Kim HJ, Fay MP, Feuer EJMD. Permutation tests for jointpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Bérubé S, Provencher L, Robert J, et al. Quantitative exploration of possible reasons for the recent improvement in breast cancer survival. Breast Cancer Res Treat. 2007;106:419–431. doi: 10.1007/s10549-007-9503-1. [DOI] [PubMed] [Google Scholar]
- 18.Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;26:4891–4898. doi: 10.1200/JCO.2007.14.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer recurrence and 15 year survival:an overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 20.Colozza M, de Azambuja E, Personeni N, et al. Achievements in systemic therapies in the pregenomic era in metastatic breast cancer. Oncologist. 2007;12:253–70. doi: 10.1634/theoncologist.12-3-253. [DOI] [PubMed] [Google Scholar]
- 21.Riley GF, Warren JL, Harlan LC, Blackwell SA. Endocrine therapy use among elderly hormone receptor-positive breast cancer patients enrolled in Medicare Part D. Medicare Medicaid Res Rev. 2011;1:E1–E25. doi: 10.5600/mmrr.001.04.a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitri Z, Constantine T, O’Regan R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother Res Pract. 2012;2012:743193. doi: 10.1155/2012/743193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 24.Hammond MEH, Hayes DF, Wolff AC, et al. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cano G, Milanezi F, Leitão D, et al. Estimation of hormone receptor status in fine-needle aspirates and paraffin-embedded sections from breast cancer using the novel rabbit monoclonal antibodies SP1 and SP2. Diagn Cytopathol. 2003;29:207–211. doi: 10.1002/dc.10365. [DOI] [PubMed] [Google Scholar]
- 26.Cheang MCU, Treaba DO, Speers CH, et al. Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J Clin Oncol. 2006;24:5637–5644. doi: 10.1200/JCO.2005.05.4155. [DOI] [PubMed] [Google Scholar]
- 27.Welsh AW, Harigopal M, Wimberly H, et al. Quantitative analysis of estrogen receptor expression shows SP1 antibody is more sensitive than 1D5. Appl Immunohistochem Mol Morphol. 2013;21:139–147. doi: 10.1097/PAI.0b013e31825d73b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arpino G, Weiss H, Lee AV, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 29.Yu K-D, Liu G-Y, Di G-H, et al. Progesterone receptor status provides predictive value for adjuvant endocrine therapy in older estrogen receptor-positive breast cancer patients. Breast. 2007;16:307–315. doi: 10.1016/j.breast.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Carlson RW, Hudis CA, Pritchard KI. Adjuvant endocrine therapy in hormone receptor-positive postmenopausal breast cancer: evolution of NCCN, ASCO, and St Gallen recommendations. J Natl Compr Canc Netw. 2006;4:971–979. doi: 10.6004/jnccn.2006.0082. [DOI] [PubMed] [Google Scholar]
- 31.Hunt BR, Whitman S, Hurlbert MS. Increasing Black:White disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol. 2014;38:118–123. doi: 10.1016/j.canep.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Menashe I, Anderson WF, Jatoi I, Rosenberg PS. Underlying causes of the black-white racial disparity in breast cancer mortality: a population-based analysis. J Natl Cancer Inst. 2009;101:993–1000. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96:1311–1321. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 34.Gerend MA, Pai M. Social Determinants of Black-White Disparities in Breast Cancer Mortality: A Review. Cancer Epidemiol Biomarkers Prev. 2008;17:2913–2923. doi: 10.1158/1055-9965.EPI-07-0633. [DOI] [PubMed] [Google Scholar]
- 35.Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310:389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 36.Lepeak L, Tevaarwerk A, Jones N, et al. Persistence in breast cancer disparities between African Americans and whites in Wisconsin. WMJ. 2011;110:21–25. [PMC free article] [PubMed] [Google Scholar]
- 37.Jatoi I, Anderson WF, Rao SR, Devesa SS. Breast cancer trends among black and white women in the United States. J Clin Oncol. 2005;23:7836–7841. doi: 10.1200/JCO.2004.01.0421. [DOI] [PubMed] [Google Scholar]
- 38.DeLancey JOL, Thun MJ, Jemal A, Ward EM. Recent trends in Black-White disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 2008;17:2908–2912. doi: 10.1158/1055-9965.EPI-08-0131. [DOI] [PubMed] [Google Scholar]
- 39.Livaudais JC, Lacroix A, Chlebowski RT, et al. Racial/ethnic differences in use and duration of adjuvant hormonal therapy for breast cancer in the women’s health initiative. Cancer Epidemiol Biomarkers Prev. 2013;22:365–373. doi: 10.1158/1055-9965.EPI-12-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–006. J Clin Oncol. 2010;28:4135–4141. doi: 10.1200/JCO.2009.27.2427. [DOI] [PubMed] [Google Scholar]
- 41.Sail K, Franzini L, Lairson D, Du X. Differences in treatment and survival among African-American and Caucasian women with early stage operable breast cancer. Ethn Health. 2012;17:309–323. doi: 10.1080/13557858.2011.628011. [DOI] [PubMed] [Google Scholar]
- 42.Freedman RA, Hughes ME, Ottesen RA, et al. Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer. 2013;119:839–846. doi: 10.1002/cncr.27831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehtimäki T, Lundin M, Linder N, et al. Long-term prognosis of breast cancer detected by mammography screening or other methods. Breast Cancer Res. 2011;13:R134. doi: 10.1186/bcr3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Population Characteristics – SEER Registries. http://seer.cancer.gov/registries/characteristics.html. Accessed 31 Jan 2013.


