Abstract
Background
The Androgen Receptor (AR) is a nuclear hormone receptor that functions as a critical oncogene in all stages of prostate cancer progression, including progression to castration-resistance following androgen-deprivation therapy. Thus, identifying and targeting critical AR-regulated genes is one potential method to block castration-resistant cancer proliferation. Of particular importance are transcription factors that regulate stem cell pluripotency; many of these genes are emerging as critical oncogenes in numerous tumor cell types. Of these, Nanog has been previously shown to increase the self-renewal and stem-like properties of prostate cancer cells. Thus, we hypothesized that Nanog is a candidate AR target gene that may impart castration-resistance.
Methods
We modulated AR signaling in LNCaP prostate cancer cells and assayed for Nanog expression. Direct AR binding to the NANOG promoter was tested using AR Chromatin Immunoprecipation (ChIP) and analyses of publically available AR ChIP-sequencing data-sets. Nanog over-expressing cells were analyzed for cell growth and cytotoxicity in response to the AR antagonist enzalutamide and the microtubule stabilizing agent docetaxel.
Results
AR signaling upregulates Nanog mRNA and protein. AR binds directly to the NANOG promoter, and was not identified within 75kb of the NANOGP8 pseudogene, suggesting the NANOG gene locus was preferentially activated. Nanog overexpression in LNCaP cells increases overall growth, but does not increase resistance to enzalutamide or docetaxel.
Conclusions
Nanog is a novel oncogenic AR target gene in prostate cancer cells, and stable expression of Nanog increases proliferation and growth of prostate cancer cells, but not resistance to enzalutamide or docetaxel.
Keywords: Nanog, NANOGP8, Androgen Receptor, prostate cancer, stem cell, enzalutamide, docetaxel
Introduction
Androgen Receptor (AR) signaling is critical for the development and progression of all stages of prostate cancer (1). In the normal prostate, AR is expressed in the luminal epithelial cells, and is necessary for the function, survival, and differentiation of these cells (2,3). Work in mouse models of prostate cancer, as well as analyses of human tumor samples, shows that during oncogenesis the normal function of AR in luminal epithelial cells switches from tumor suppressor to oncogene (2,4,5). This alteration occurs when AR activates an oncogenic subset of gene targets that are not normally expressed in the luminal cells; many of these genes may have an important role during embryogenesis, prostate development and/or luminal cell differentiation (4). Work on somatic reprograming to create induced pluripotent stem cells (iPSCs) demonstrates that transient expression of certain stem cell genes is capable of provoking large scale genome-wide changes in gene expression, chromatin remodeling, and ultimately cell behavior, all of which are shared properties of malignant cells (6). We therefore hypothesized that AR control of aberrantly expressed genes associated with pluripotency may play a significant role in promoting prostate cancer initiation, progression and castration resistance. Here we identify the stem cell gene NANOG as a novel AR target gene that is an oncogenic effector of AR-signaling in prostate cancer.
Revolutionary studies done by Shinya Yamanaka’s group at Kyoto University have shown that fully differentiated somatic cells virally transduced with the transcription factors Oct3/4 (Octamer-binding transcription factor 3/4), Sox2 [SRY (sex determining region Y)-box 2], c-MYC (v-myc avian myelocytomatosis viral oncogene homolog), and Klf4 (Kruppel-like factor 4) obtain a pluripotent stem cell phenotype; such cells were indistinguishable from embryonic stem cells in morphology, proliferation, gene expression, and the ability to differentiate and recapitulate all three germ layers in teratoma formation assays (6). These factors, dubbed the Yamanaka factors, generate large-scale pleiotropic genomic changes allowing for complete cellular reprogramming, rendering these cells capable of self-renewal and the ability replicate indefinitely (7). The Yamanaka factors also serve to upregulate Nanog, which is an established gatekeeper of embryonic stem cell pluripotency, and along with Sox2 and Oct4, is a critical member of the core regulatory transcriptional circuitry in embryonic stem cells (8,9).
Recent studies demonstrated an oncogenic function for Nanog within a variety of tumors cell types including colon, gastric, oral, pancreatic and ovarian (10–14). We and others identified Nanog expression in prostate cancer cell lines (15,16). Nanog is a homeodomain containing transcription factor that is essential for the establishment and maintenance of pluripotency and embryonic stem cell self-renewal (8,9). The NANOG gene has undergone 10 genomic duplications and transpositions, resulting in 11 total Nanog genes and pseudo-genes with high homology, including the NANOG gene (also known as Nanog1) found on Chromosome 12 p13.31; this specific gene encodes the Nanog protein essential for pluripotency (17). Previous work documented that proteins expressed from the NANOG gene and the NANOGP8 pseudogene impart prostate cancer cells with many of the malignant properties associated with stem cells including self-renewal, increased clonogenicity and anchorage-independence through activation of many target genes activated by Nanog to maintain stem cell pluripotency (16,18). Nanog overexpression in both androgen sensitive and insensitive prostate cancer lines also promotes increased tumor formation and growth in a castrated murine host, a commonly used model for assaying for castration-resistance (16,18). The NANOGP8 pseudogene on chromosome 15 q3.13, which differs from NANOG by 3 amino acids [99.5% homology (17)], is the only Nanog gene outside of NANOG that has been reported to produce a full-length protein (19). It is also is the most recently diverged Nanog pseudogene from the original NANOG gene, and unlike many of the other Nanog genes, it maintains high conservation of the 3’ untranslated region (UTR) (20). NANOGP8 has been shown to be dominant gene, other than NANOG, expressed in most prostate cancer cell lines under normal growth conditions (16,18,19,21), and short term overexpression of either NANOG or NANOGP8 does not increase the overall growth and proliferation of prostate cancer cells (16,18). No reported studies to date, however, have investigated the transcriptional regulation of NANOG or NANOGP8 in prostate cancer cells and the interaction of Nanog and AR-signaling; given that both Nanog (16,18,21) and AR-signaling (22) can impart self-renewal and stem-like properties to prostate cancer cells. In this work, we identify Nanog as a novel oncogenic AR target gene in prostate cancer cells, and show that stable expression of Nanog increases proliferation and growth of prostate cancer cells.
Materials and Methods
Cell Lines and Tissue Culture Reagents
R1881 and Bicalutamide were purchased from Sigma-Aldrich (St. Louis, MO), and enzalutamide (MDV3100) was purchased from Selleck Chemicals (Houston, TX), and stored at −20°C in Ethanol, and −80°C in DMSO, respectively. All human prostate cancer cell lines were grown as previously described (23). All cultures were routinely screened for the absence of mycoplasma contamination using the ATCC Universal Mycoplasma Detection Kit (Manassas, VA). All experiments were performed in phenol-red free RPMI media (Corning, Corning, NY) with 10% charcoal stripped serum (Atlanta Biologicals, Lawrenceville, GA), to decrease endogenous AR-ligands, as LNCaP cells have a mutation in the ligand binding domain (T877A) of the AR which allows for promiscuous ligand binding (24). Cells were pre-incubated in charcoal stripped serum at least 24 hours prior to any pharmacologic treatments. LNCaP cells were generously provided by Dr. John Isaacs at The Johns Hopkins University and have been previously characterized (23). The human embryonic stem cell line WA01(H1) was acquired from WiCell (Madison, WI) and cultured using the feeder-independent protocol using mTeSR1 media (Stem Cell Technologies; Vancouver, B.C.). ES cells were used within ten passages of thawing.
Lentiviral Human Nanog Homeobox and Control GFP vectors (pReceiver-Lv105) were purchased from GeneCopoeia (Rockville, MD). High-titer lentivirus was made by co-transfecting with ViraPower Lentivrial packaging mix (Invitrogen; Grand Island, NY) and Lenti-X Concentrator (Clontech; Mountain View, CA) according to manufacturer’s instructions. Pools of cells were transduced with virus in the presence of 10µM polybrene (Millipore, Billerica, MA), and then selected using 1µg/ml Puromycin (Invitrogen, Carlsbad, CA) 72 hours after 24 hour incubation with virus.
Western blotting
Whole-cell lysates collected from cells seeded at 1×106 cells per well of a 6 well plate (Becton, Dickinson and Company, Franklin Lakes, New Jersey), were lysed in RIPA-PIC buffer [150 mM sodium chloride, 1.0% Igepal CA-630 (Sigma-Aldrich), 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0, 1× protease inhibitor cocktail (Roche Molecular Biochemicals; Penzberg, Germany)], scraped, and sonicated (Fisher Scientific; Hampton, NH; model FB-120 Sonic Dismembrator). Protein was quantified by BCA assay (Thermo-Fisher Scientific), and 30µg of protein were loaded per lane. Antibodies used were: anti-Nanog (D73G4 XP, Cell Signaling Technology, Danvers, MA), anti-AR (N-20, Santa Cruz; Santa Cruz, CA); anti-Beta Actin (AC-15, Sigma-Aldrich). Secondary antibodies and Nitrocelluose membranes from Licor (Lincoln, NE) from were used and data captured using a Licor Odyssey system (Lincoln, NE) as previously described (15).
Quantitative Reverse Transcription PCR (Q-RT-PCR)
RNA was purified from similar growth conditions described above using the Qiagen RNeasy Mini Kit including the optional DNAse digestion kit (Qiagen, Valencia, CA) and quality tested using an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). For standard Q-RT-PCR, extracted RNA was converted to cDNA by reverse transcription using SuperScript® III Reverse Transcriptase (Invitrogen). Levels of Nanog, PSA and GAPDH transcript were quantified using Power SYBR® Green Master Mix (Invitrogen) using custom primers for Nanog [5′-GATTTGTGGGCCTGAAGAAA-3′ (forward), 5′-AAGTGGGTTGTTTGCCTTTG-3 (reverse)]; PSA [5′-TCATCCTGTCTCGGATTGTG-3′ (forward), 5′-ATATCGTAGAGCGGGTGTGG-3′ (reverse)]; and GAPDH [5′-GAGTCAACGGATTTGGTCGT-3′ (forward), 5′-TTGATTTTGGAGGGATCTCG-3′ (reverse)]. Standard curves were used to assess primer efficiency and average change in threshold cycle (ΔCT) values determined for each of the samples relative to endogenous GAPDH levels and compared to vehicle control (ΔΔCT). Experiments were performed in triplicate to determine mean standard error, and student's t-tests performed with normalization to control to obtain p-values.
Restriction Fragment Length Polymorphism (RFLP) analysis
RNA was purified from LNCaP cells treated with vehicle or 1nM R1881 for 3 hours, or WA01(H1) cells in growth conditions similar to above, using the Qiagen RNeasy Mini Kit including the optional DNAse digestion kit (Qiagen, Valencia, CA) and quality tested using an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). For Reverse Transcription Polymerase Chain Reaction (RT-PCR), extracted RNA was converted to cDNA by reverse transcription using SuperScript® III Reverse Transcriptase (Invitrogen). Custom primers designed to amplify regions around the unique restriction enzyme digestion site for AlwN1 in NANOGP8 but also amplifying the same sized fragments (348 base pairs) of NANOG and NANOGP7: [5′-AAAGCTTGCCTTGCTTTGAA-3′ (forward), 5′-TCTGCTGGAGGCTGAGGTAT-3 (reverse)]; and for regions around the unique restriction enzyme digestion site for Sma1 in the 3’ UTR of NANOG, primers amplifying the same sized fragment (353 base pairs) of the NANOGP8 3’ UTR: [5′-AACCACGTGTTCTGGTTTCC-3′ (forward), 5′-GATCGAGACCATCCTGGCTA-3 (reverse)]. Primers utilized for the Sma1 digestion are not capable of amplifying the NANOGP7 transcript. RT-PCR was performed using the primers and cDNA described above with Platinum® Blue PCR SuperMix (Invitrogen), and PCR products were extracted using the Wizard® SV Gel and PCR Clean-up kit (Promega, Madison, WI). Half of the purified PCR product was subjected to enzymatic digestion with either AlwN1 or Sma1 obtained from New England Biolabs (Ipswich, MA), according to manufactures protocols, and the reaction, as well as undigested PCR product, was visualized on a 3% agarose gel (Fisher Scientific) containing 10µM Ethidium Bromide (Sigma-Aldrich). Undigested PCR product was also cloned into vectors using TOPO® TA Cloning® Kit for Sequencing (Invitrogen) from isolated LNCaP cDNA, and transformed into competent bacteria and were Sanger sequenced at the University of Chicago Comprehensive Cancer Center DNA Sequencing and Genotyping Facility to screen for positive controls for NANOG, NANOGP7 and NANOGP8, and to confirm the identity of all PCR products of the Nanog genes. Lentiviral Human Nanog Homeobox (pReceiver-Lv105) vector (GeneCopoeia) was also utilized as a control.
AR Chromatin Immunoprecipitation and Quantitative PCR (AR ChIP-qPCR)
Chromatin-IP protocols were adapted from previously reported methods (15,25). Cells were grown to 70–90% confluence and treated for 24 hours with 1 nM R1881. Cells were fixed with 1% formaldehyde (Sigma-Aldrich) at room temperature for 15 minutes; the crosslinking was quenched with 0.125M Glycine in PBS for 15 minutes at room temperature and washed with ice-cold PBS. After cells were scraped off in PBS plus 1× protease inhibitor cocktail (Roche), cell pellets were collected by centrifugation and cell nuclei extracted by centrifugation at 14,000 RPM at 4°C for 15 minutes. Cell nuclei were re-suspended and washed in micrococcal nuclease buffer (Tris [pH 7.4] 10 mM, NaCl 15 mM, KCl 60 mM, Spermine 0.15 mM, Spermidine 0.5 mM, CaCl2 1 mM) then incubated with 10,000 U of micrococcal nuclease (Invitrogen) for 20 minutes at 37°C with gentle thermomixing. Nuclei were then collected and re-suspended in RIPA-PIC buffer [150 mM sodium chloride, 1.0% Igepal CA-630 (Sigma-Aldrich), 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0/1× protease inhibitor cocktail (Roche)] and sonicated (Fisher Scientific; Hampton, NH; model FB-120 Sonic Dismembrator). Sonicated chromatin extracts were then pre-cleared overnight at 4°C by incubation with protein G-agarose beads treated with Normal Rabbit IgG (Cell Signaling Technology), and chromatin fragments were immunoprecipitated with specific antibodies overnight at 4°C. For a 1-ml diluted chromatin solution, 50 µg of the following antibodies were used: Normal Rabbit IgG (Cell Signaling Technology) as a negative control; Histone H3 (Abcam; Cambridge, MA) as a positive control; and AR (N-20, Santa Cruz,) for the experimental conditions. Beads were then collected and washed in TSE I buffer [0.1% TritonX-100, 2 nM EDTA, 150 mM NaCl, 20 mM Tris-HCl (pH 8.1)], TSE II buffer [0.1% SDS, 1% TritonX-100, 2 mM EDTA, 500 mM NaCl, 20 mM Tris-HCl(pH 8.1)], Brown Buffer III [0.25 LiCl, 1.0% Igepal CA-630 (Sigma-Aldrich), 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCL(pH 8.1)], and twice with TE Buffer [1 mM EDTA, 10 mM Tris-HCL(pH 8.1)]. The immunoprecipitated chromatin fragments were eluted off of the agarose beads using Brown Elution Buffer [1% SDS, 0.1M NaHCO3, 1 mM DTT, 1× protease inhibitor cocktail (Roche)] and incubated for 10 minutes at 95°C with vigorous thermomixing. Eluted chromatin and inputs were then incubated overnight at 65°C with gentle thermomixing to reverse crosslinks. Samples were then treated with 2 µg RNaseA (Novagen; Darmstadt, Germany) at 37°C for 15 minutes, then with 2 µg Proteinase K (Invitrogen) at 55°C for 30 minutes (Cell Signaling Technology). Finally, immunoprecipitated DNA fragments were extracted by Phenol-Chloroform (Sigma-Aldrich) extraction and subjected to q PCR. For ChIP-qPCR, custom primers were designed for the NANOG promoter: primer set 1 (−1701 to −1615) [5′-TCAGCTTGTGTGGGAGCAAA-3′ (forward), 5′-TGACTCATTCTCCTCTGCACTC-3 (reverse)]; and primer set 2 (−1466 to −1413) [5′-AGAGAGACAGGAGGGCAAGT-3′ (forward), 5′- CGGTGAGTGGGGCTAGTACA-3 (reverse)]; and the PSA/KLK3 enhancer [5′-GCCTGAGGTCTTTGAGCAAG-3′ (forward), 5′-CAGTTGGTGAGTGGTCATGG-3 (reverse)], and experiments were performed similarly to the Q-RT-PCR; in triplicate to determine mean standard error, and student's t-tests performed with normalization to IgG negative control to obtain p-values.
Measuring Cell Growth in vitro
Cell density, growth and cytotoxicity were measured using sulforhodamine B (SRB) assay (26). Sulforhodamine B was purchased from Sigma-Aldrich. 2000 LNCaP cells per well were seeded in poly-D-lysine coated 96 well plates (Becton, Dickinson and Company), then treated with drug the following day. At days three, five, and seven the growth media was aspirated and cells were rinsed with PBS (Fisher Scientific), and then fixed in 10% Trichloroacetic acid (Sigma-Aldrich) for at least 30 min. Then plates were washed five times with water, air dried overnight, and then stained with 0.4% SRB (w/v) dissolved in 1% glacial acetic acid (Fisher Scientific), for 30min. Plates were then washed five times with 1% glacial acetic acid, air dried thoroughly overnight, and then SRB stain was solubilized for 15 minutes in 10mM Tris base (Fisher Scientific) solution pH 10, and then read on a plate spectrophotometer at 515 nm absorbance.
Statistical Analyses
Data was analyzed using SigmaPlot 11.0 software, experiments were performed in triplicate to determine mean standard error, and student's t-tests performed with normalization to control analyses to obtain p-values. Growth data was analyzed with n=6 using multiple pair-wise student t-tests.
Results
Androgen Receptor Signaling Upregulates Nanog mRNA and Protein Expression
We and others have documented Nanog expression in the LAPC-4, LNCaP and its derivative C4-2 prostate cancer cell lines (15,16). We also documented direct regulation of the stem cell transcription factor Sox2 by AR, whereby AR directly represses Sox2 expression (15). Therefore, we set out to investigate whether there was any association between AR signaling and Nanog expression. In contrast to Sox2, activation of AR by a physiologic concentration of androgen (1nM R1881) led to a statistically-significant (p<0.05) increase in Nanog mRNA expression in LNCaP cells (Figure 1A). The rapid and sustained increase in Nanog mRNA was seen after 15 minutes and up to 48 hours after treatment with R1881, with a statistically-significant (p<0.05) peak in expression compared to all other time-points at 3 hours post-treatment (Figure 1A). As a comparison, expression of the established AR target gene PSA/KLK3 in response to AR pathway activation by R1881 was similarly elevated three hours after R1881 treatment (Figure 1B). Physiologic levels of R1881 (1nM) have previously been reported to induce nuclear translocation of AR within 30 minutes of treatment (27). This increase in mRNA leads to a sustained increase of Nanog protein [which has been reported to migrate multiple bands around 35–42kD via western blot using a variety of Nanog antibodies (28)] after 24 hours, without detectable increases of AR protein by 9 hours (Figure 1C). These data document that the expression of Nanog is associated with AR pathway activation in prostate cancer cells, and suggest that Nanog is a direct target gene of ligand-bound AR.
Figure 1. Androgen Receptor (AR) activation leads to an increase in Nanog expression.
A: Increased Nanog mRNA in LNCaP cells upon AR stimulation as measured by Q-RT-PCR. Levels at 0.25 hours and beyond represent a statistically significant increase in Nanog mRNA (* indicates p<0.05). A further statistically significant increase was observed at 3 hours compared to all other conditions (** indicates p<0.05). LNCaP cells were grown in phenol-red free RPMI media (Corning, Corning, NY) with 10% charcoal stripped serum (Atlanta Biologicals, Lawrenceville, GA), to decrease endogenous AR-ligands, as LNCaP cells have a mutation in the ligand binding domain (T877A) of the AR which allows for promiscuous ligand binding (23). Cells were pre-incubated in charcoal stripped serum at least 24 hours prior to any pharmacologic treatments. B: Increased expression of the established AR target gene PSA (KLK3) in LNCaP cells within three hours. C: Increased expression of Nanog protein (35–42kD) upon AR stimulation with physiologic levels of androgen (1 nM R1881) in androgen sensitive LNCaP prostate cancer cells. Protein lysates from cells treated at defined intervals (3–72 hours) after androgen treatment were subjected to western blotting. Nanog protein migrates multiple bands around 35–42kD via western blot using a variety of Nanog antibodies (27).
AR-Mediated Upregulation of Nanog Expression can be Abrogated by Treatment with the Anti-Androgen Enzalutamide
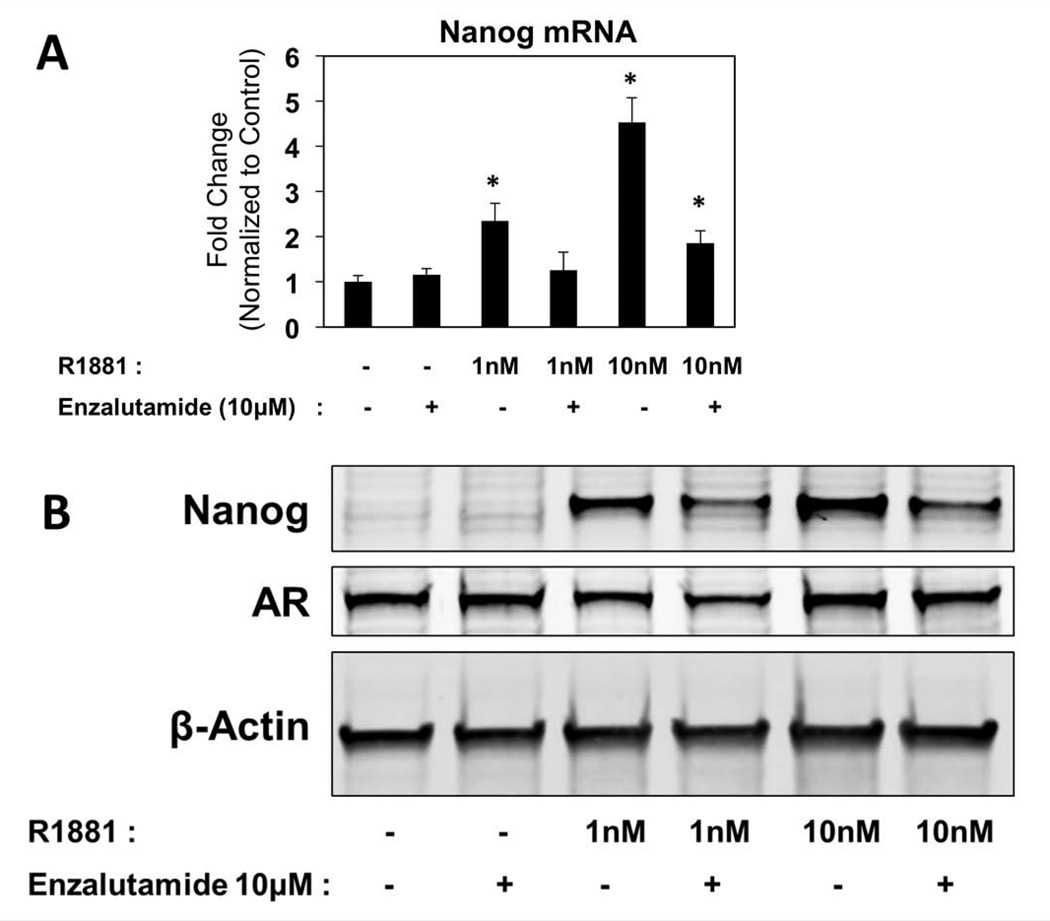
To verify that increases in Nanog expression are specific to AR activation and whether such increases were reversible, we tested whether co-treatment with the AR antagonist enzalutamide could impact AR-mediated expression of Nanog. Anti-androgens are AR antagonists that are used clinically in conjunction with androgen deprivation therapy (ADT) or as a monotherapy after prostate cancer cells acquire castration resistance (29). Enzalutamide (MDV3100) is an FDA-approved second-generation anti-androgen for the treatment of castration-resistant prostate cancer (CRPC) that decreases AR nuclear translocation, prevent DNA binding, and increase AR degradation (30,31). LNCaP cells were treated with physiologic levels of hormone (1nM R1881) and hyper-physiologic levels (10nM R1881) for 24 hours, and then cells were treated with 10mM enzalutamide for 48 hours. Indeed, enzalutamide treatment mitigates androgen induced upregulation of Nanog mRNA in LNCaP cells, where Nanog upregulation by 1nM R1881 reduced to control levels by enzalutamide (Figure 2A). We have previously reported that the effects of 1nM R1881 can be blocked by 10µM enzalutamide in CWR22rv1, CWRR1 and LAPC4 prostate cancer cell lines (15,32). A 10-fold increase in the androgen concentration (10nM R1881) was sufficient to overcome inhibition of 10µM enzalutamide with respect to Nanog expression (Figure 2A). Similarly, this dose-dependent phenomenon is observed at the protein level (Figure 2B). As expected, treatment with enzalutamide led a notable decrease in AR protein expression compared to R1881 treatment alone (Figure 2B). In sum, these data support the direct relationship between AR activation and Nanog expression, and such AR-mediated Nanog expression is partially reversible by blocking AR pathway activity.
Figure 2. Androgen Receptor (AR)-mediated up-regulation of Nanog expression can be reversed by treatment with the Anti-Androgen Enzalutamide.
A: LNCaP cells were grown in phenol-red free RPMI media (Corning, Corning, NY) with 10% charcoal stripped serum (Atlanta Biologicals, Lawrenceville, GA), to decrease endogenous AR-ligands, as LNCaP cells have a mutation in the ligand binding domain (T877A) of the AR which allows for promiscuous ligand binding (23). Cells were pre-incubated in charcoal stripped serum at least 24 hours prior to any pharmacologic treatments. To verify that the increase in Nanog mRNA and protein is specific to AR activation and can be reversed by an AR antagonist, cells were incubated 1 nm or 10 nm R1881, or vehicle for 24 hours, and then either 10 µM enzalutamide or vehicle was added to the culture medium for an additional 48 hours prior to lysis. An increase in Nanog mRNA with R1881 treatment was measured using qPCR (*p<0.05), which was brought back to control levels upon treatment with enzalutamide, in both LNCaP cells. 10nM R1881 was enough to overcome inhibition of Nanog mRNA upregulation with 10µM MDV3100 treatment in LNCaP cells. B: Western blots show an increase of Nanog protein with R1881 that can be decreased with addition of enzalutamide, with minimal changes in to AR protein levels, similar to mRNA. Nanog protein migrates multiple bands around 35–42kD via western blot using a variety of Nanog antibodies (27). β-Actin was used as a loading control.
Androgen Receptor Activation Increases both NANOG and NANOGP8 Transcripts in LNCaP Cells
Previous observations have implicated a role for the NanogP8 pseudogene in prostate cancer cells; thus it was important to discern whether increases in Nanog expression were either from the NANOG or NANOGP8 genes, especially given that the Q-RT-PCR primers utilized in Figures 1 and 2 cannot discern the identity of each individual gene (16,18,19,21). Furthermore, another Nanog pseudogene, NANOGP7 at chromosome 14 q32.12, containing 94.8% homology to NANOG, and 94.4% homology with NANOGP8, can also be identified with the primers utilized; however it is not capable of producing a full-length functional protein due to a stop codon at amino acid position 63 (17,19). To identify the origin of the increased Nanog mRNA in androgen treated cells, we utilized restriction fragment length polymorphism (RFLP) analysis. RT-PCR primers were designed around the regions of NANOG and NANOGP8 that encode for complimentary unique restriction enzyme sites in order to differentiate between the two transcripts, as outlined by Fairbanks et al. (20). As shown in Figure 3, an AlwN1 digestion site in NANOGP8 was utilized to differentiate it from NANOG and NANOGP7 at amino acid 48 (Figure 3A,B); while a Sma1 digestion site was utilized to differentiate NANOG from NANOGP8, with the unique site found 313 base pairs into the 3’ untranslated region (UTR) of NANOG (Figure 3A,B), and corresponding uncut site 316 base pairs into the 3’ UTR of NANOGP8 (Figure 3A,B).
Figure 3. Androgen Receptor Activation Increases both NANOG and NANOGP8 Transcripts in LNCaP Cells.
A: Restriction fragment length polymorphism (RFLP) approach to identify multiple Nanog transcripts. Illustration of the regions of the three genes, NANOG, NANOGP7 and NANOGP8, flanking the AlwN1 digestion site, and the regions of NANOG and NANOGP8 flanking the Sma1 site in the 3’ UTRs of these transcripts. The AlwN1 digestion site exists only in the NANOGP8 transcript, and the Sma1 digestion site exists only in NANOG. B: RT-PCR products from regions around the unique restriction enzyme digestion site for AlwN1 in NANOGP8 as well as the same sized fragments (348 base pairs) of NANOG and NANOGP7, were obtained from in LNCaP cells after 3 hours of 1nM treatment of R1881 or vehicle as previously described, and WA01(H1) cells (as a positive control for NANOG). Cloned NANOG, NANOGP7 and NANOGP8 transcripts served as controls (negative, negative and positive, respectively). A purified Nanog overexpression plasmid (Genecopia) served as an additional control. All PCR products were digested with AlwN1, and only NANOGP8 is cut into two fragments from the initial 348bp into fragments sized 230 and 118 bp. Undigested controls are also shown. For the unique restriction enzyme cut site for Sma1 in the 3’ UTR of NANOG, products the same sized fragment (353 base pairs) of the NANOGP8 3’ UTR, and as with the AlwN1 digestion, fragments were cut out of NANOG sized 221 and 132 bp. GAPDH serves as a control for cDNA loading, utilizing the same primers used for Q-RT-PCR in Figures 1 and 2. Together, these treatments illustrate an increase in both NANOG and NANOGP8.
Therefore, through tandem identification of NANOGP8, from NANOG and NANOGP7, and NANOG from NANOGP8, we investigated the origin of the transcripts upregulated by androgen signaling in LNCaP cells after 3 hours of 1nM treatment of R1881. Results shown in Figure 3 illustrate both NANOG and NANOGP8 transcripts are upregulated by 3 hours of 1nM R1881 treatment in LNCaP cells. Increases in total Nanog levels were seen after 3 hours of 1nM R1881 treatment, as well as cut fragments corresponding to NANOGP8 transcripts after AlwN1 digestion, and NANOG transcripts after Sma1 digestion (Figure 3B). The WA01(H1) cells were used as a positive control for their high levels of NANOG (33), and show no digestion with AlwN1 and nearly complete digestion using Sma1 (Figure 3B). These data illustrate an increase in both transcripts capable of producing a functional NANOG and NANOGP8 protein.
The Androgen Receptor Binds Directly to the NANOG Promoter
The kinetics of Nanog mRNA and protein expression in response to AR pathway activation suggested that AR may be directly binding the Nanog promoter and regulating Nanog expression. We also wanted to elucidate whether AR binding to the Nanog promoter is observed in other prostate cancer cell lines. To discern whether the Androgen Receptor directly binds the NANOG or NANOGP8 promoters, we utilized publically available ChIP-seq [Yu et al. 2010 (34)] and ChIP-chip [Wang et al. 2009 (25)] data sets to identify potential AR-binding sites in the promoter and cis-enhancer regions of NANOG within various prostate cancer cell lines, as well as ChIP-seq [Lin et al. 2009 (35)] data from PC3 prostate cancer cells transduced with AR. In VCaP, LNCaP, LNCaP-AR (LNCaP cells transduced with AR) and LNCaP-abl (LNCaP cells with active ABL1-kinase signaling) cell lines, AR binding was documented in the promoter and intragenic of NANOG, within 2kb of the transcriptional start site (TSS); while the closest binding of AR to NANOGP8 was identified >75kb away from the TSS in LNCaP-AR (Table 1) (25,34,35). While this does not necessarily rule out AR upregulating NANOGP8, it suggests that the NANOG locus on chromosome 12 is directly regulated.
Table 1.
| NANOG | Chromosome 12 p13.31 | mRNA: | Starts 7941995 | Ends 7948655 | ||
|---|---|---|---|---|---|---|
| Reference | Cell Line | Condition | Position Start | Position End | Start Distance from Nanog1 TSSa | End Distance from Nanog1 TSSa |
| Wang et al. 2009 | LNCaP-AR | DHT | 7940179 | 7941200 | −1816 | −795 |
| Wang et al. 2009 | LNCaP-AR | DHT | 7941911 | 7943014 | −84 | 1019 |
| Wang et al. 2009 | LNCaP-abl | DHT | 7838292 | 7838985 | −103703 | −103010 |
| Wang et al. 2009 | LNCaP-abl | DHT | 7941981 | 7943087 | −14 | 1092 |
| Yu et al. 2010 | LNCaP | EtOH | N/D | N/D | N/D | N/D |
| Yu et al. 2010 | LNCaP | R1881 | 7183901 | 7184050 | −758094 | −757945 |
| Yu et al. 2010 | VCaP | EtOH | 7940551 | 7940850 | −1444 | −1145 |
| Yu et al. 2010 | VCaP | EtOH | 7950926 | 7951275 | 8931 | 9280 |
| Yu et al. 2010 | VCaP | EtOH | 7976976 | 7977275 | 34981 | 35280 |
| Yu et al. 2010 | VCaP | R1881 | 7839754 | 7839813 | −102241 | −102182 |
| Yu et al. 2010 | VCaP | R1881 | 7943780 | 7943839 | 1785 | 1844 |
| NANOGP7 | Chromosome 14 q32.12 | mRNA: | Starts 91601332 | Ends 91608317 | ||
|---|---|---|---|---|---|---|
| Reference | Cell Line | Condition | Position Start | Position End | Start Distance from NanogP7 TSSa | End Distance from NanogP7 TSSa |
| Wang et al. 2009 | LNCaP-AR | DHT | 91624223 | 91625344 | 22891 | 24012 |
| Wang et al. 2009 | LNCaP-abl | DHT | 91306189 | 91306929 | −295143 | −294403 |
| Wang et al. 2009 | LNCaP-abl | DHT | 92255471 | 92256537 | 654139 | 655205 |
| Yu et al. 2010 | LNCaP | EtOH | 91601586 | 91601645 | 254 | 313 |
| Yu et al. 2010 | LNCaP | R1881 | 91624576 | 91624950 | 23244 | 23618 |
| Yu et al. 2010 | VCaP | EtOH | 91624726 | 91624850 | 23394 | 23518 |
| Yu et al. 2010 | VCaP | R1881 | 91601586 | 91601645 | 254 | 313 |
| NANOGP8 | Chromosome 15 q13.3 | mRNA: | Starts 35375427 | Ends 35377509 | ||
|---|---|---|---|---|---|---|
| Reference | Cell Line | Condition | Position Start | Position End | Start Distance from NanogP8 TSSa | End Distance from NanogP8 TSSa |
| Wang et al. 2009 | LNCaP-AR | DHT | 35298697 | 35299357 | −76730 | −76070 |
| Wang et al. 2009 | LNCaP-abl | DHT | 35810363 | 35810987 | −434936 | −435560 |
| Yu et al. 2010 | LNCaP | EtOH | N/D | N/D | N/D | N/D |
| Yu et al. 2010 | LNCaP | R1881 | N/D | N/D | N/D | N/D |
| Yu et al. 2010 | VCaP | EtOH | N/D | N/D | N/D | N/D |
| Yu et al. 2010 | VCaP | R1881 | N/D | N/D | N/D | N/D |
TSS Denotes Transcriptional Start Site
N/D – Not Detected within 1Mb of TSS
Note: No AR binding was identified within 500kb of NANOG, NANOGP7 or NANOGP8 TSS in PC3-AR cells (Lin et al. 2009)
We analyzed the ChIP-seq and ChIP-chip data set for AR binding near NANOGP7 as well, and identified AR binding at sites within the gene itself (254–313bp into the coding sequence) and 2.5kb proximal from the TSS in LNCaP and VCaP cells both with and without androgen treatment. This does not rule out the possibility that androgen signaling may regulate NANOGP7, but given that AR binds intragenically in both vehicle and androgen treated conditions and Nanog protein levels increase, it is unlikely that the increase in Nanog mRNA is coming from solely from NANOGP7 gene, which has not been reported to produce full length protein. In sum, there is a reported AR binding site within the NANOG promoter, but distally from the NANOGP8 promoter, in multiple prostate cancer cell lines.
To confirm the NANOG promoter as a robust AR binding site, we conducted AR ChIP to detect and validate the two Nanog promoter binding sites reported in both Yu et al. and Wang et al. (25,34). These sites are located between −1816 and −795 upstream of the NANOG TSS by directed AR ChIP (outlined in Figure 4A). AR ChIP followed by qPCR validation of the chromatin enrichment demonstrates that in the presence of R1881 AR immunoprecipitates with DNA at regions both at −1701 to −1615 and −1466 to −1413 on the Nanog promoter (Figure 4B). Robust AR binding to the NANOG promoter was evident since the promoter was as enriched as the Histone H3 positive control. Binding of AR to the Nanog promoter in the absence of ligand does not show enrichment of DNA over the IgG negative control (Figure 4B). The known AR-target gene, PSA/KLK3, was used to validate the AR-ChIP; interrogation of AR binding at the PSA/KLK3 enhancer region documents a similar pattern of DNA enrichment over IgG as the NANOG promoter sites in LNCaP cells (Figure 4B). Together, these data support the direct AR-mediated upregulation of NANOG in prostate cancer cells.
Figure 4. Androgen Receptor (AR) directly binds to the NANOG promoter.
A: Schematic of AR binding sites identified at the promoter and cis-enhancer regions of NANOG (within 2kb of the Nanog transcriptional start site) in published ChIP-seq (Yu et al. 2010) and ChIP-chip (Wang et al. 2009) data sets. Primer sequences were designed to investigate AR binding to Androgen Response Elements (ARE) and verify published binding via ChIP-qPCR. B: AR Chromatin Immunoprecipitation (ChIP) documents direct binding of ligand-activated AR to the Nanog promoter region in response to AR stimulation by R1881. LNCaP cells were treated with vehicle control or 1 nM R1881, and enrichment of the Nanog promoter after AR-ChIP was verified by primer-directed qPCR. Normal Rabbit IgG and Histone H3 served as negative and positive controls, respectively. When compared to the IgG negative control, both the positive control Histone H3 and ligand-activated AR significantly enriched for the Nanog enhancer (p<0.05). Known AR recruitment to the PSA/KLK3 promoter served to validate the AR ChIP. Cells were grown in phenol-red free RPMI media (Corning, Corning, NY) with 10% charcoal stripped serum (Atlanta Biologicals, Lawrenceville, GA), to decrease endogenous AR-ligands, as LNCaP cells have a mutation in the ligand binding domain (T877A) of the AR which allows for promiscuous ligand binding (23). Cells were pre-incubated in charcoal stripped serum at least 24 hours prior to any pharmacologic treatments.
Nanog Overexpression in LNCaP Cells Leads to Increased Cell Proliferation, But Cells Remain Sensitive to Androgen, Anti-Androgen, and Docetaxel
Previous studies have implicated an oncogenic function for Nanog in prostate cancer, as overexpression imparts cancer cell self-renewal, anchorage-independence, and enhanced tumor formation and growth in a castrate murine host (16). However, the role of Nanog in promoting resistance to anti-androgens and taxanes has not been investigated. Thus, we hypothesized that expression of Nanog would be sufficient to impart resistance to AR-targeted or cytotoxic therapies used clinically in prostate cancer. To test this, we examined the ability of Nanog overexpression to impart resistance to these drugs in vitro. Stable expression of lentiviral Nanog (LV-Nanog), or control vector (LV-GFP) was documented by western blotting (Figure 5A). Cells were grown in 1 nM or 10 nM R1881 for 24 hours, and as an additional control we added 10 µM enzalutamide added to some of the cultures for an additional 48 hours. Nanog levels increased further in LV-Nanog cells with R1881 treatment, indicative of increases in endogenous Nanog expression; as expected treatment with enzalutamide decreased Nanog expression to levels comparable to the vehicle treated cells (Figure 5A). LV-GFP transduced cells behaved similarly to parental cells examined in Figure 2; Nanog expression increases with R1881 in a dose dependent manner, and enzalutamide mitigates these effects (Figure 5A). A schematic illustrates the infection, selection, pharmacologic treatment and analysis of growth of the transduced cells (Figure 5B). To assess sensitivity to pharmacologic modulators of the androgen receptor, Nanog over-expressing cells were analyzed for growth upon treatment with 1nM R1881, 10 µM enzalutamide, or vehicle for 7 days (Figure 5C). Previous work from our group has shown that sensitivity to these treatments, changes in cellular viability and growth kinetics can be reliably assayed for after 7 days of drug treatment (32). Nanog overexpression imparts an overall additive growth advantage in all conditions; however cells remain sensitive to both androgens and anti-androgens, with R1881 increasing growth and enzalutamide decreasing growth in both GFP and Nanog overexpressed cells (Figure 5C).
Figure 5. Nanog overexpression in LNCaP cells leads to an increase in growth, but cells remain sensitive to androgen, anti-androgen and chemotherapeutic drug treatments.
A: Immunoblot validation of lentiviral overexpression of Nanog or GFP control. LNCaP cells were transduced with lentivirus for stabile overexpression of Nanog (LV-Nanog) or control GFP (LV-GFP), and treated with R1881 and enzalutamide, and Nanog and AR protein levels were analyzed. Cells were grown in 1 nm or 10 nm R1881, or vehicle for 24 hours, and then either 10 µM enzalutamide or vehicle was added to the culture medium for an additional 48 hours. β-Actin was used as a loading control. Cells were grown in phenol-red free RPMI media (Corning, Corning, NY) with 10% charcoal stripped serum (Atlanta Biologicals, Lawrenceville, GA), to decrease endogenous AR-ligands, as LNCaP cells have a mutation in the ligand binding domain (T877A) of the AR which allows for promiscuous ligand binding (23). Cells were pre-incubated in charcoal stripped serum at least 24 hours prior to any pharmacologic treatments in all experiments. Nanog protein migrates multiple bands around 35–42kD via western blot using a variety of Nanog antibodies (27). B: A schematic illustrates the infection, selection, pharmacologic treatment and analysis of growth of the transduced cells. C: LNCaP cells transduced with lentivirus for stabile overexpression of Nanog or control GFP were assayed for cell density, growth and cytotoxicity using a Sulforhodamine B (SRB) assay (calculated by change in absorbance) over 7 day treatment with 10 µM enzalutamide, 1nM R1881 or vehicle control (* indicates p<0.05, when compared to GFP control, † indicates p<0.05, when compared to vehicle). D: LNCaP GFP or Nanog overexpressing cells were assayed for sensitivity to Docetaxel included cytotoxicity using SRB assay over 7 day treatment with 1nM, 5nM or 10nM Docetaxel or vehicle control (* indicates p<0.05, when compared to GFP control, † indicates p<0.05, when compared to vehicle).
Nanog over-expressing cells were then tested for sensitivity to the chemotherapeutic agent docetaxel. Cells were again cultured for 7 days in the presence 1nM, 5nM or 10nM concentrations of docetaxel, consistent with inducing a range of cytotoxicity in these lines (36,37). Nanog overexpression did increase growth when compared to control cells in all concentrations of docetaxel (Figure 5D). However cells remain sensitive to the cytotoxic effects of the drug (Figure 5D) when compared to their respective vehicle controls, as docetaxel treatment of Nanog over-expressing cells decreases growth back to levels comparable with untreated GFP-control cells at all concentrations (Figure 5D). The Nanog overexpressing cells seem to actually be relatively more sensitive to the effects of docetaxel treatment when compared to the GFP control cells (Figure 5D); which is potentially a byproduct of their overall increase in proliferation, as more proliferative cells are more sensitive to the effects of taxane chemotherapeutics (38). Together these data further support an oncogenic role for Nanog, and its role as an AR-target gene in prostate cancer cells to promote cancer cell proliferation, but does not support a role for Nanog in promoting AR pathway independence or resistance to taxane therapies.
Discussion
In the present study, we show the specificity of ligand-bound AR to induce Nanog expression, direct binding of AR to the NANOG promoter by chromatin-immunoprecipiation, and the ability of Nanog overexpression to increase cell proliferation, but not resistance to anti-androgen enzalutamide or the chemotherapeutic docetaxel. These data continue to raise questions about the role of stem cell transcription factors in prostate cancer. For example, previous data suggested that Nanog activates within prostate cancer cells many of the same genes it activates in embryonic stem cells (16); however, the full range of common and unique Nanog target genes in prostate cancer cells is unknown as prostate cancer cells appear to lack the expression of partnering pluripotency factors. Furthermore, Nanog1 and NanogP8, although 99.5% homologous, do exhibit differential regulation of known pluripotency genes in prostate cancer cells (16). Given Nanog1 and NanogP8’s differential regulation and expression in prostate cancer cells under different androgen conditions, it is important to determine the shared and different target genes of Nanog1 and NanogP8, as well as the biological outcome and clinical relevance of their differential regulation. Recent advances in CRISPR and ChIP-seq technologies (39) would help elucidate this differential regulation, given that their significant homology presents a challenge toward creating isoform-specific antibodies. Moreover, Nanog generally functions as a homodimer to bind DNA (40), and to date no studies have identified whether Nanog1 and NanogP8 can heterodimerize, and if these dimers could regulate similar or differential target genes.
Additionally, the role of pseudogene expression in post-translational and post-transcriptional regulation of Nanog is intriguing, and can account for the unusual kinetics of Nanog mRNA and protein. Pseudogene expression has been shown to influence the transcription and translation of normal protein coding genes in both positively and negatively. Pseudogenes can positively regulate normal gene mainly as acting as “microRNA sponges” by competitively inhibition of microRNAs which stabilizes and upregulates coding gene expression, and negatively, by enhancing degradation of transcripts through being processed like a small interfering RNA or double stranded RNAs (41,42). The interaction between the transcripts expressed off of the different pseudogenes may account for the unique mRNA and protein kinetics of Nanog upregulation. The peak of Nanog mRNA seen at 3 hours post-R1881 treatment in LNCaP cells, and the increase in NANOGP8 transcripts seen with androgen treatment even when AR binding is so distal from the promoter, could all do to the expression of the different Nanog gens. The kinetics of Nanog protein as well could be influenced by this phenomenon, given how protein levels of Nanog do not seem to be drastically increased until 24 hours, and continue to increase even more after 48 hours even after transcription has peaked. Also this may account for how the Nanog protein seems to be further stabilized with androgens even when Nanog is overexpressed. The regulation of Nanog protein, particularly in the context of AR signaling, is being uncovered as increasingly complex, given the homology of NANOG and NANOGP8 in both the coding region and the 3’ UTR, as well as the high expression of NANOGP7 in LNCaP cells. Given that our data suggests that both NANOG and NANOGP8 transcripts are upregulated, the relative contribution of these proteins to the observed phenotype is unknown and will be the subject of future work.
Here we show that AR can upregulate the pluripotency factor Nanog. We previously documented, however, that AR activation down-regulates another essential pluripotency factor, Sox2 (15). This interesting dichotomy, and the role of AR in regulating the expression both Sox2 and Nanog, points to unique functions for each transcription factor. Work in embryonic stem cells documents that variations in the expression levels of pluripotency factors govern cell fate and lineage specific differentiation of cells and tissues by exhibiting differential binding, occupancy, and regulation of co-activators/co-repressor proteins in specific promoters (43,44). In our previous work, we over-expressed Sox2 in Nanog-positive LAPC-4 cells, and saw no increase in 83 genes associated with pluripotency and early differentiation (15). However, we did not see Sox2 expressed without its canonical partner in embryonic stem cells, Oct4, or Nanog in normal prostate epithelial cells and the castration-resistant CWR cell lines (15). Thus, the lack of detectable co-expression of pluripotency factors could impact occupation of available promoters and lead to the expression of novel target genes that our previous data suggests could be quite different than those regulated during pluripotency in embryonic stem cells (15,45,46). The complexity of the regulation of these factors in prostate cancer, and their target genes, and role and connections in AR-signaling has yet to be fully elucidated.
Supplementary Material
Acknowledgments
We wish to acknowledge the support of the University Of Chicago Section Of Urology led by Dr. Arieh Shalhav, and the Director of Urologic Research Dr. Carrie Rinker-Schaeffer. We would also like to acknowledge the support of the University of Chicago Comprehensive Cancer Center (UCCCC) led by Dr. Michelle Le Beau. We also thank Masis Isikbay, Dr. Jacob Kach, and Paul Volden for assistance with data analyses and manuscript preparation. Finally, we wish to thank Dr. John Isaacs at Johns Hopkins for generously providing many of the cancer cell lines used.
Funding: RO1CA178431 (Vander Griend); a Pilot Award from the NCI P50 CA090386 SPORE in Prostate Cancer at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University and the Cancer Research Center of the University of Chicago; The Brinson Foundation; the Alvin Baum Family Fund; The University of Chicago Cancer Research Foundation Women's Board; An Anonymous Foundation; S. Kregel was supported by an HHMI: Med-into-Grad Fellowship (56006772) and a Cancer Biology Training Grant (T32-CA09594).
Abbreviations
- ADT
Androgen Deprivation Therapy
- AR
Androgen Receptor
- ARE
Androgen Response Elements
- BP
base pairs
- CRPC
Castration-Resistant Prostate Cancer
- ChIP
Chromatin Immunoprecipation
- c-MYC
v-myc avian myelocytomatosis viral oncogene homolog
- CRPC
Castration-Resistant Prostate Cancer
- hESCs
human Embryonic Stem Cells
- iPSCs
induced Pluripotent Stem Cells
- GFP
Green Fluorescent Protein
- Klf4
Kruppel-like factor 4
- Oct3/4
Octamer-binding transcription factor 3/4
- PCR
Polymerase Chain Reaction
- PSA
Prostate-Specific Antigen
- qPCR
Quantitative PCR
- Q-RT-PCR
Quantitative Reverse Transcription PCR
- RFLP
Restriction Enzyme Length Polymorphism
- RT-PCR
Reverse Transcription PCR
- Sox2
[SRY sex determining region Y-box 2]
- SRB
Sulforhodamine B
- TSS
Transcriptional Start Site
- UTR
Untranslated Region
Footnotes
Conflicts of Interest: None
References Cited
- 1.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. Journal of carcinogenesis. 2011;10:20. doi: 10.4103/1477-3163.83937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Marzo AM, Meeker AK, Epstein JI, Coffey DS. Prostate stem cell compartments: expression of the cell cycle inhibitor p27Kip1 in normal, hyperplastic, and neoplastic cells. The American journal of pathology. 1998;153(3):911–919. doi: 10.1016/S0002-9440(10)65632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isaacs JT, Furuya Y, Berges R. The role of androgen in the regulation of programmed cell death/apoptosis in normal and malignant prostatic tissue. Seminars in cancer biology. 1994;5(5):391–400. [PubMed] [Google Scholar]
- 4.Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. International journal of cancer Journal international du cancer. 2007;120(4):719–733. doi: 10.1002/ijc.22365. [DOI] [PubMed] [Google Scholar]
- 5.Niu Y, Altuwaijri S, Lai K-P, Wu C-T, Ricke WA, Messing EM, Yao J, Yeh S, Chang C. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proceedings of the National Academy of Sciences. 2008;105(34):12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2(4):333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138(4):722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14(13):4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi N, Ishii H, Nagai K, Hoshino H, Mimori K, Tanaka F, Nagano H, Sekimoto M, Doki Y, Mori M. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc Natl Acad Sci U S A. 2010;107(1):40–45. doi: 10.1073/pnas.0912407107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng HM, Zheng P, Wang XY, Liu C, Sui HM, Wu SJ, Zhou J, Ding YQ, Li JM. Overexpression of nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol Ther. 2010;9(4) doi: 10.4161/cbt.9.4.10666. [DOI] [PubMed] [Google Scholar]
- 13.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65(8):3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 14.Wen J, Park JY, Park KH, Chung HW, Bang S, Park SW, Song SY. Oct4 and Nanog expression is associated with early stages of pancreatic carcinogenesis. Pancreas. 2010;39(5):622–626. doi: 10.1097/MPA.0b013e3181c75f5e. [DOI] [PubMed] [Google Scholar]
- 15.Kregel S, Kiriluk KJ, Rosen AM, Cai Y, Reyes EE, Otto KB, Tom W, Paner GP, Szmulewitz RZ, Vander Griend DJ. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PloS one. 2013;8(1):e53701. doi: 10.1371/journal.pone.0053701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeter CR, Liu B, Liu X, Chen X, Liu C, Calhoun-Davis T, Repass J, Zaehres H, Shen JJ, Tang DG. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011;30(36):3833–3845. doi: 10.1038/onc.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booth HA, Holland PW. Eleven daughters of NANOG. Genomics. 2004;84(2):229–238. doi: 10.1016/j.ygeno.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K, Fowler M, Glass J, Yin H. Activated 5'flanking region of NANOGP8 in a self-renewal environment is associated with increased sphere formation and tumor growth of prostate cancer cells. The Prostate. 2014;74(4):381–394. doi: 10.1002/pros.22759. [DOI] [PubMed] [Google Scholar]
- 19.Ambady S, Malcuit C, Kashpur O, Kole D, Holmes WF, Hedblom E, Page RL, Dominko T. Expression of NANOG and NANOGP8 in a variety of undifferentiated and differentiated human cells. The International journal of developmental biology. 2010;54(11–12):1743–1754. doi: 10.1387/ijdb.103192sa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairbanks DJ, Fairbanks AD, Ogden TH, Parker GJ, Maughan PJ. NANOGP8: evolution of a human-specific retro-oncogene. G3. 2012;2(11):1447–1457. doi: 10.1534/g3.112.004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeter CR, Badeaux M, Choy G, Chandra D, Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ, Tang DG. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem cells. 2009;27(5):993–1005. doi: 10.1002/stem.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vander Griend DJ, D'Antonio J, Gurel B, Antony L, Demarzo AM, Isaacs JT. Cell-autonomous intracellular androgen receptor signaling drives the growth of human prostate cancer initiating cells. The Prostate. 2010;70(1):90–99. doi: 10.1002/pros.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litvinov IV, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL, Isaacs JT. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer research. 2006;66(17):8598–8607. doi: 10.1158/0008-5472.CAN-06-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobel RE, Sadar MD. Cell lines used in prostate cancer research: a compendium of old and new lines--part 1. The Journal of urology. 2005;173(2):342–359. doi: 10.1097/01.ju.0000141580.30910.57. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M, Wu T, Regan MM, Meyer CA, Carroll JS, Manrai AK, Janne OA, Balk SP, Mehra R, Han B, Chinnaiyan AM, Rubin MA, True L, Fiorentino M, Fiore C, Loda M, Kantoff PW, Liu XS, Brown M. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138(2):245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature protocols. 2006;1(3):1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 27.Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung JK, Watt K, Tam T, Yang YC, Banuelos CA, Williams DE, McEwan IJ, Wang Y, Sadar MD. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer cell. 2010;17(6):535–546. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Liu B, Badeaux MD, Choy G, Chandra D, Shen I, Jeter CR, Rycaj K, Lee CF, Person MD, Liu C, Chen Y, Shen J, Jung SY, Qin J, Tang DG. Nanog1 in NTERA-2 and Recombinant NanogP8 from Somatic Cancer Cells Adopt Multiple Protein Conformations and Migrate at Multiple M.W Species. PloS one. 2014;9(3):e90615. doi: 10.1371/journal.pone.0090615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Clegg NJ, Scher HI. Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target. The lancet oncology. 2009;10(10):981–991. doi: 10.1016/S1470-2045(09)70229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semenas J, Dizeyi N, Persson JL. Enzalutamide as a second generation antiandrogen for treatment of advanced prostate cancer. Drug design, development and therapy. 2013;7:875–881. doi: 10.2147/DDDT.S45703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isikbay M, Otto K, Kregel S, Kach J, Cai Y, Vander Griend DJ, Conzen SD, Szmulewitz RZ. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Hormones & cancer. 2014;5(2):72–89. doi: 10.1007/s12672-014-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444(7118):481–485. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- 34.Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, Wu L, Li J, Hu M, Gong Y, Cheng H, Laxman B, Vellaichamy A, Shankar S, Li Y, Dhanasekaran SM, Morey R, Barrette T, Lonigro RJ, Tomlins SA, Varambally S, Qin ZS, Chinnaiyan AM. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer cell. 2010;17(5):443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin B, Wang J, Hong X, Yan X, Hwang D, Cho JH, Yi D, Utleg AG, Fang X, Schones DE, Zhao K, Omenn GS, Hood L. Integrated expression profiling and ChIP-seq analyses of the growth inhibition response program of the androgen receptor. PloS one. 2009;4(8):e6589. doi: 10.1371/journal.pone.0006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ting HJ, Hsu J, Bao BY, Lee YF. Docetaxel-induced growth inhibition and apoptosis in androgen independent prostate cancer cells are enhanced by 1alpha,25-dihydroxyvitamin D3. Cancer letters. 2007;247(1):122–129. doi: 10.1016/j.canlet.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Zhu Y, Lou W, Nadiminty N, Chen X, Zhou Q, Shi XB, deVere White RW, Gao AC. Functional p53 determines docetaxel sensitivity in prostate cancer cells. The Prostate. 2013;73(4):418–427. doi: 10.1002/pros.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchison TJ. The proliferation rate paradox in antimitotic chemotherapy. Molecular biology of the cell. 2012;23(1):1–6. doi: 10.1091/mbc.E10-04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154(2):442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullin NP, Yates A, Rowe AJ, Nijmeijer B, Colby D, Barlow PN, Walkinshaw MD, Chambers I. The pluripotency rheostat Nanog functions as a dimer. The Biochemical journal. 2008;411(2):227–231. doi: 10.1042/BJ20080134. [DOI] [PubMed] [Google Scholar]
- 41.Johnsson P, Ackley A, Vidarsdottir L, Lui WO, Corcoran M, Grander D, Morris KV. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nature structural & molecular biology. 2013;20(4):440–446. doi: 10.1038/nsmb.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pink RC, Wicks K, Caley DP, Punch EK, Jacobs L, Carter DR. Pseudo-genes: pseudo-functional or key regulators in health and disease? Rna. 2011;17(5):792–798. doi: 10.1261/rna.2658311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomson M, Liu SJ, Zou LN, Smith Z, Meissner A, Ramanathan S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145(6):875–889. doi: 10.1016/j.cell.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell. 2012;10(4):440–454. doi: 10.1016/j.stem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 45.Christophersen NS, Helin K. Epigenetic control of embryonic stem cell fate. J Exp Med. 2010;207(11):2287–2295. doi: 10.1084/jem.20101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.