SUMMARY
The nervous systems of diverse species, including worms and humans, possess mechanisms for distinguishing between sensations arising from self-generated (i.e., expected) movements from those arising from other-generated (i.e., unexpected) movements [1–3]. To make this critical distinction, animals generate copies, or corollary discharges, of motor commands [4, 5]. Corollary discharge facilitates the selective gating of reafferent signals arising from self-generated movements, thereby enhancing detection of novel stimuli [6–10]. However, for a developing nervous system, such sensory gating would be counterproductive if it impedes transmission of the very activity upon which activity-dependent mechanisms depend [11]. In infant rats during active (or REM) sleep—a behavioral state that predominates in early infancy [12–16]—neural circuits within the brainstem [17, 18] trigger hundreds of thousands of myoclonic twitches each day [19]. The putative contribution of these self-generated movements to the activity-dependent development of the sensorimotor system is supported by the observation that reafference from twitching limbs reliably and substantially triggers brain activity [20–23]. In contrast, under identical testing conditions, even the most vigorous wake movements reliably fail to trigger reafferent brain activity [21–23]. One hypothesis that accounts for this paradox is that twitches, uniquely among self-generated movements, lack corollary discharge [23]. Here, we test this hypothesis in newborn rats by manipulating the degree to which self-generated movements are expected and, therefore, their presumed recruitment of corollary discharge. We show that twitches, although self-generated, are processed as if they are unexpected.
RESULTS AND DISCUSSION
Recording sensory responses in primary motor cortex (M1)
Unanesthetized 8–10-day-old (P8–10) rats (n = 11) cycled freely between sleep and wake while head-fixed in a stereotaxic apparatus with the limbs dangling freely (Figure 1A). We used 16-channel silicon electrodes to record extracellular neural activity from the hindlimb region of M1. We chose to investigate M1 because, contrary to its designation as a motor structure, M1 also processes sensory (including proprioceptive) information [24], beginning early in development [25]. Also, because the cortical motor map develops gradually over the postnatal period, stimulation of M1 at early ages has a lower probability of producing a movement than in adults [26]. It must also be stressed that M1 appears to play no role in the production of twitches [17, 18]. For these reasons, we began this study with the primary aim of exploring the developmental foundations of sensorimotor processing within M1.
Figure 1. Hindlimb twitches, but not wake-related hindlimb movements, trigger M1 activity.
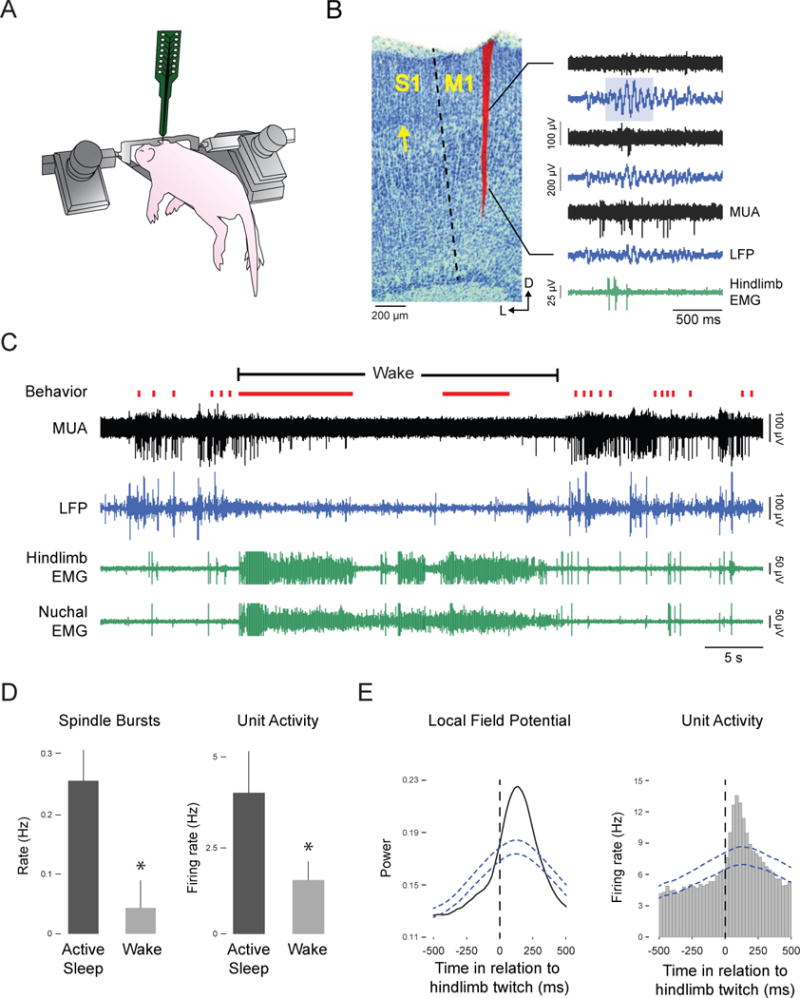
(A) For these recordings, P8-10 rats were head-fixed in a stereotaxic apparatus and maintained at thermoneutrality. The torso was supported by a platform and the limbs dangled freely. (B) Left: Coronal brain section, stained with cresyl violet, depicts the electrode track for a P10 subject. M1 is medial to primary somatosensory cortex (S1) and is agranular; the granular cell layer in S1 is denoted by the arrow. Right: Recordings from six sequential electrode sites with 100 μm separation. Signals are alternately filtered for multiunit activity (MUA; black traces) and local field potentials (LFP; blue traces). The spindle burst (blue highlighting) co-occurs with a burst of action potentials after a hindlimb twitch (green trace). (C) Representative data depicting sleep and wake behavior, MUA, LFP, and hindlimb and nuchal EMG during spontaneous sleep-wake cycling. Red tick marks denote hindlimb twitches and red horizontal lines denote hindlimb wake movements. (D) Mean (+ SEM) rate of spindle burst (n = 11) and unit activity (n = 17) during active sleep and wake periods. *significant difference from other group, P < 0.05. (E) Waveform average and event correlation for LFP power and unit activity, respectively, in relation to hindlimb twitches for pooled data (4047 and 6358 twitches, respectively). The blue dashed lines denote upper and lower acceptance bands (P < 0.05).
In pilot experiments, we established the coordinates of the hindlimb region of M1 using electrical stimulation to specifically elicit contralateral hindlimb movements. Then, for every pup tested here, we verified electrode location by manually stimulating the contralateral and ipsilateral hindlimbs, as well as both forelimbs and tail, to confirm the specificity of M1 responding to the contralateral hindlimb (see Movie S1); whereas flexing the hindlimb effectively triggered M1 activity, tactile stimulation alone did not. Histology showed that electrodes were located in agranular cortex (Figure 1B, left).
The linear arrangement of the electrode sites (100 μm between sites) allowed for simultaneous recording from multiple cortical layers. Every other electrode site was filtered to identify spindle bursts in the local field potential (LFP; Figure 1B, right, blue traces) or multiunit activity (MUA; Figure 1B, right, black traces). All recorded units were located in the deep layers of M1. Spindle bursts were defined as described previously ([20], Figure 1B, blue highlight).
Twitch- But Not Wake-Related Movements Trigger M1 Activity
As shown in Figure 1C for a representative recording, both LFP and MUA activity in M1 occurred predominantly during periods of active sleep. This activity was particularly prominent during periods of hindlimb twitching (see Movie S1). In contrast, although wake-related hindlimb movements were frequent and vigorous, M1 activity was nearly absent (see Movie S1). Across all pups, there was a significant increase in mean rates of spindle bursts (t10 = 9.2, P < 0.01) and mean unit firing rates (t16 = 3.2, P < 0.01, n = 17 units, 1–2 units per pup) during sleep (Figure 1D). Moreover, LFP power and unit activity increased significantly after twitches with a latency of at least 100–125 ms (Figure 1E), consistent with previous reports of twitch-related reafference in cerebral cortex [21, 22]. Finally, these results were replicated in P4 and P12 rats, demonstrating the stability of the effect across early development (Figure S1).
Hindlimb Exafference Triggers M1 Activity Regardless of State
It is possible that the data in Figure 1 resulted from global gating of all wake-related sensory input. If true, then manual stimulation of the hindlimb (i.e., exafference) should be able to trigger M1 activity during sleep but not during wake. To rule out this possibility, we manually flexed the hindlimb contralateral to M1 as pups cycled between sleep and wake over a period of 10 min. Figure 2A depicts representative stimulations (arrows) performed during each state and the neural responses that follow these stimulations. Across all pups tested (n = 11), we observed significant increases in both LFP power and unit activity in response to stimulations regardless of behavioral state (Figure 2B). Importantly, because exafference was transmitted to M1 during periods of high muscle tone (see Figure 2A, right), muscle tone alone cannot account for the wake-related gating of reafference. Finally, there was no significant difference in maximum LFP power between sleep and wake (t10 = 1.3); in contrast, there was a small (<10%) but significant difference in maximum unit firing rate (t16 = 4.1, P < 0.005). In any event, it is clear that there is no global gating of sensory input to M1 during wake.
Figure 2. Exafferent hindlimb stimulation triggers M1 activity regardless of behavioral state.
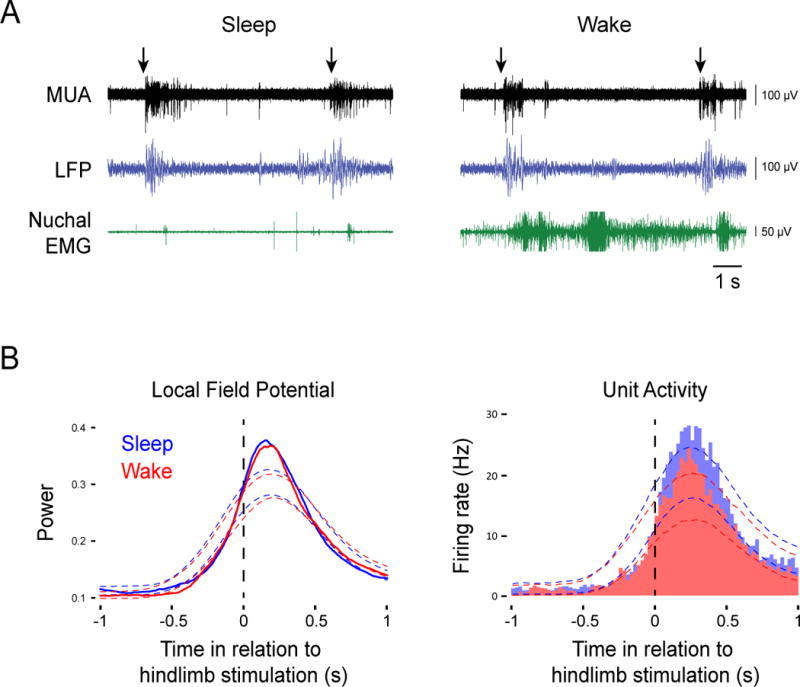
(A) Representative recordings in P8-10 subjects depicting multiunit activity (MUA; black traces) and local field potential (LFP; blue traces) responses to hindlimb stimulation (arrows) during sleep (left) or wake (right). Nuchal EMG (green traces) is also shown. (B) Left: Waveform averages for LFP power in relation to hindlimb stimulation during sleep (blue line) and wake (red line) for data pooled across all subjects (n = 11). Thresholds for statistical significance are indicated by the color-coded dotted lines. Right: Event correlations for unit activity in relation to hindlimb stimulation during sleep (blue histogram; 417 stimulations) and wake (red histogram; 418 stimulations) for data pooled across all units (n = 17). Color-coded dotted lines denote upper and lower acceptance bands (P < 0.05).
“Unexpected” Self-Generated Movements Trigger M1 Activity
By design, because pups’ limbs dangled freely in the apparatus (see Figure 1A), there was no opportunity for unexpected reafference from hindlimb movements. Consequently, the lack of M1 activity after wake-related hindlimb movements is consistent with the idea that corollary discharge gates or cancels the expected reafference from self-generated movements [1, 3] (Figure S2A). In contrast, exafferent stimulation of the hindlimb cannot, by definition, be accompanied by corollary discharge and is therefore unexpected (s), thus explaining the findings presented in Figure 2. We next evoked self-generated movements that differ in their expectancy so as to provide insight into the mechanisms by which twitches trigger M1 activity. If corollary discharge is involved in the processing of reafference from self-generated movements, we predicted that only unexpected movements (i.e., movements not accompanied by corollary discharge) would trigger M1 activity.
We first considered the possibility that with direct activation of lumbar spinal motoneurons we could trigger self-generated hindlimb movements while bypassing corollary discharge mechanisms that originate in the brain [27] (Figure S2C). In P8-10 rats, we injected a nonselective 5-HT agonist, quipazine (3.0 mg/kg ip), which activates lumbar motoneurons and, as a consequence, produces limb movements [28] (see Movie S1). We recorded M1 activity before and after quipazine or saline injection (Figure 3A). Hindlimb movements, rate of spindle burst activity, and unit firing rate all increased significantly after quipazine administration. Specifically, for hindlimb movements, we found significant main effects of group (F1,10 = 59.7, P < 0.001) and time (F1,10 = 184.8, P < 0.001), and a significant group × time interaction (F1,10 = 271.8, P < 0.001; Figure 3B). For spindle bursts, we found significant main effects of group (F1,10 = 24.5, P < 0.01) and time (F1,10 = 14.0, P < 0.01), and a significant group × time interaction (F1,10 = 29.2, P < 0.001; Figure 3C). Finally, only 4 pups in each group yielded clear M1 units; nonetheless, for unit activity, we found a significant main effect of group (F1,6 = 7.3, P < 0.05), a non-significant main effect of time (F1,6 = 4.8, P = 0.07), and a marginally significant group × time interaction (F1,6 = 5.9, P = 0.05; Figure 3C). These results suggest that reafference from unexpected self-generated movements are conveyed to M1. They also suggest that spinal motoneurons and associated local circuitry are downstream from the generators of corollary discharge that suppress reafference associated with wake-related limb movements.
Figure 3. Pharmacological induction of hindlimb movements triggers M1 activity.
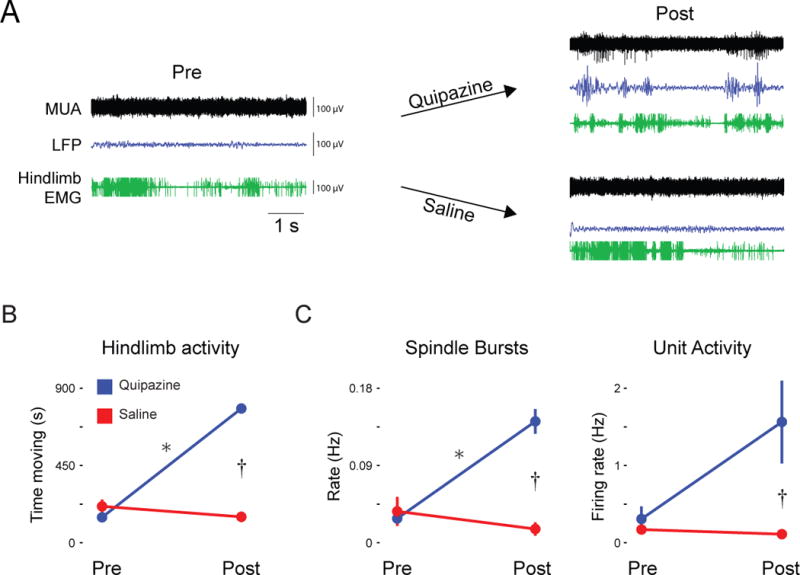
(A) Representative recordings in P8-10 subjects depicting multiunit activity (MUA; black traces) and local field potentials (LFP; blue traces) before and after intraperitoneal injection of the serotonin agonist, quipazine, or saline. Hindlimb EMG (green traces) is also shown. (B) Mean (± SEM) time that the hindlimb moved before and after quipazine or saline injection across all subjects (n = 6 per group). (C) Mean (± SEM) rate of spindle bursts (left; n = 6 per group) and unit activity (right; n = 4 per group) before and after quipazine or saline injection across all subjects. * within-subjects significant difference, P < 0.05; † between-subjects significant difference, P < 0.05.
Because quipazine was injected systemically, we wanted to ensure that the M1 activity we observed was due to effects on spinal motoneurons. Therefore, in two additional P8-10 rats, we performed mid-thoracic spinal transections, thereby severing communication between the lumbar spinal cord and brain (s). We immediately noticed that, consistent with previous findings in somatosensory cortex after spinal transection [20], spindle bursts in M1 were much less prevalent (although not eliminated), thereby indicating that M1 activity is driven by limb reafference. Critically, injection of quipazine in the transected pups evoked hindlimb movements, similar to those in the non-transected pups (Figure S3B, top row). However, unlike the non-transected pups, spindle burst activity in the transected pups did not increase after quipazine injection, thus suggesting that the earlier results arose from quipazine’s direct effects on spinal circuits.
We next devised two behavioral methods that, although different in their presumed recruitment of corollary discharge mechanisms, allowed us to precisely trigger the onset of self-generated hindlimb movements. Moreover, because the two methods could be performed in the same subjects, we were able to directly assess differences in the processing of proprioceptive reafference arising from expected and unexpected movements. First, to produce unexpected reafference, we flicked the tail, thereby engaging local spinal circuits to cause reflexive hindlimb movements (Figure 4A, red trace; see also Figure S2C and Movie S1). Second, to produce expected reafference, we applied a cold stimulus to the snout [29], thereby causing brain-mediated arousal and associated activation of hindlimb movements (Figure 4A, blue trace; see also Figure S2A and Movie S1). Figure 4B presents representative data for the two manipulations. Both tail flick and application of the arousing stimulus (black arrows) elicited self-produced hindlimb movements (green arrows). As predicted, hindlimb movements elicited by tail flick, but not those elicited by the arousing stimulus, triggered significant increases in LFP power and unit activity in M1 (Figure 4C). Moreover, maximum values for both LFP and unit activity were significantly greater in response to tail flick than to the arousing stimulus (LFP: t5 = 4.1, P < 0.01; MUA: t5 = 65.3, P < 0.001).
Figure 4. Effects of expected and unexpected reafference on M1 activity.
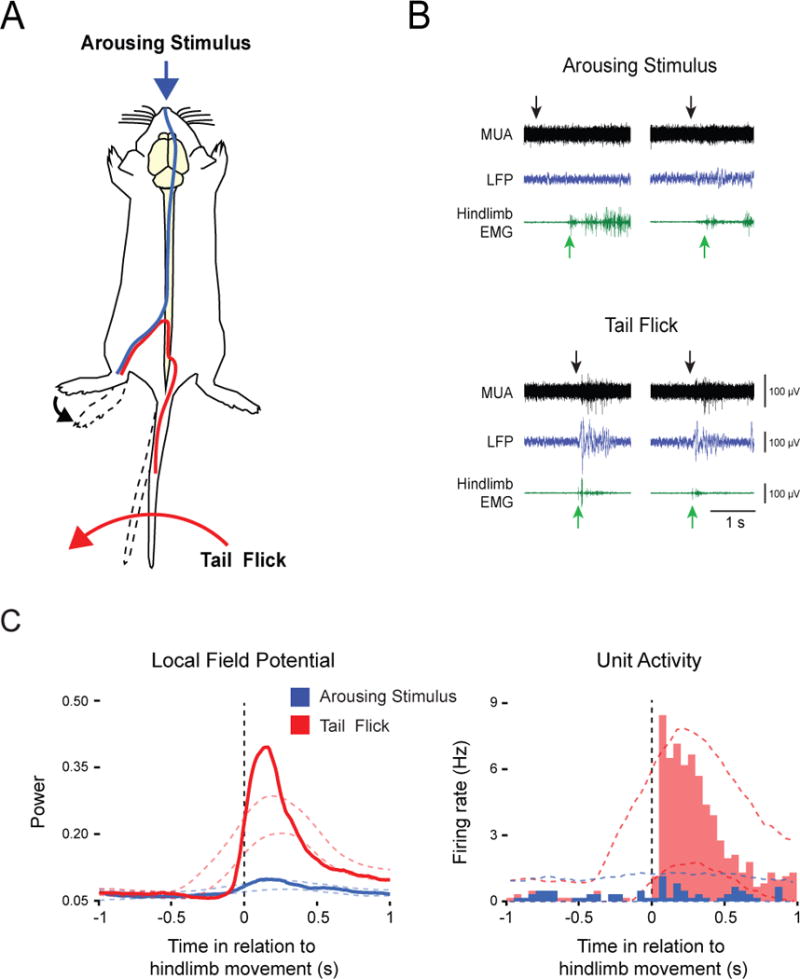
(A) Schematic diagram representing the two types of self-generated movements. Application of a cold stimulus to the snout produced generalized arousal and elicited vigorous hindlimb movements, thereby producing expected reafference (blue pathway). A tail flick engaged local spinal circuits to cause a reflexive movement of the hindlimb, thereby producing unexpected reafference (red pathway). (B) Representative recordings in P8-10 subjects depicting multiunit activity (MUA; black traces) and local field potentials (LFP; blue traces) in response to the arousing stimulus (top) or tail flick (bottom). Black arrows denote stimulus presentation and green arrows denote onset of hindlimb activity. (C) Waveform averages for LFP power (left) and event correlations for unit activity (right) in relation to onset of hindlimb movement for data pooled across all animals (n = 6) and units (n = 6), respectively (arousing stimulations = 102; tail flicks = 87). Color-coded dashed lines denote upper and lower acceptance bands (P < 0.05).
To ensure that tail flicks did indeed activate local spinal circuitry, we performed tail flicks and arousing stimulations in the same two pups with mid-thoracic spinal transections described above. In the transected pups, flicks of the tail triggered hindlimb reflexes without affecting M1 LFP power (Figure S3B, bottom row). In contrast, stimulation of the snout was still able to arouse the transected pups (e.g., as seen by forelimb movements); however, as expected, we did not observe hindlimb movements or increases in M1 LFP power (data not shown).
Conclusions
The absence of M1 activity during self-generated wake-related movements, as observed here, is consistent with earlier reports describing differential sleep- and wake-related neural activity in the thalamus, somatosensory cortex, hippocampus, and cerebellum [21–23, 30]. This absence of M1 activity, coupled with the reliable activation of M1 by exafferent stimulation, suggests the operation of corollary discharge during wake-related movements (Figures S2A and S2B). Similarly, in primates, passive head movements drive neural activity in the vestibular nuclei whereas active head movements do not, suggesting the selective cancelling of reafference by corollary discharge signals [31]. To further test the hypothesis that corollary discharge is functioning early in development, we manipulated the expectancy of the reafference from self-generated movements (Figure S2C). Only unexpected reafference reliably drove M1 activity, similar to what we observed with twitches (Figure S2D). To our knowledge, this is the first demonstration of a self-generated movement that is processed as if it were an other-generated movement and, therefore, unexpected.
Taken together, our results indicate that proprioceptors are sufficient to trigger the reafferent activity observed in M1. Recent evidence suggests that corollary discharge mechanisms originating in the brain suppress proprioceptive reafference from the hindlimbs that are processed by Clarke’s column neurons [27]. At this time, however, little is known about the neural sources of twitches, especially early in development, thus preventing identification of the neural sources of corollary discharge or the sites where it modulates reafference. Therefore, an important next step is to determine whether the brainstem mechanisms that trigger twitches do not simultaneously generate corollary discharge or, alternatively, whether corollary discharge is generated but its effects are somehow inhibited. Regardless of the exact mechanism, the downstream effects on M1 activity would be the same.
Under normal waking conditions, corollary discharge makes it possible to account for expected reafferent signals triggered by one’s own movements so that one is able to detect and respond appropriately to unexpected stimuli in the environment. Such accounting entails the gating or cancelling of reafference from self-generated movements. However, for the development and maintenance of precise, integrated, and hierarchically organized sensorimotor maps [32], infants likely depend upon the conveyance of high-fidelity sensory information from self-generated limb movements to developing brain structures [33, 34]. Twitch movements may be particularly well suited to this task because, unlike wake movements, they are produced discretely against a background of muscle atonia, both of which enhance signal-to-noise ratio [19]. Our results further suggest that the high fidelity of twitching depends upon the suspension of corollary discharge mechanisms, providing the infant with ideal conditions for activity-dependent development of the spinal cord [35], cerebellum [23], and forebrain [20–22, 36]. The information provided by twitching limbs may also enable the construction and calibration of internal models and predictive codes, which are thought to be essential for flexible and efficient sensorimotor control throughout the lifespan [37–39].
EXPERIMENTAL PROCEDURES
All experiments were approved by the Institutional Animal Care and Use Committee of The University of Iowa. The apparatus and methods for recording and analyzing neural and muscle activity in head-fixed pups have been described previously [18, 21, 22]. All surgeries were performed under isoflurane anesthesia and data were collected from unanesthetized subjects; brain temperature was maintained at 36–37°C. As described previously [23], spike sorting was performed in Spike2 (Cambridge Electronic Design, Cambridge, UK). Sleep and wake states were determined by analyzing the nuchal EMG in conjunction with behavioral scoring [18, 21, 22]. Active sleep was characterized by the occurrence of myoclonic twitches against a background of muscle atonia [40]. State-related differences in M1 activity were tested within each subject (Wilcoxon matched-pairs signed-ranks test) and across subjects (paired t test). Spikes of EMG activity with amplitudes greater than 3× baseline were considered twitches. For testing the relations between events (e.g., twitches) and M1 activity, twitch-triggered event correlations and waveform averages were constructed [22]. We tested statistical significance for event correlations and waveform averages using a jitter protocol [41, 42] implemented in Matlab (MathWorks, Natick, MA). We corrected for multiple comparisons using the method of Amarasingham et al. [43]; this method produces upper and lower confidence bands for each event correlation and waveform average. ANOVA was used to evaluate the influence of quipazine administration on hindlimb movements, spindle bursts, and unit activity.
Supplementary Material
HIGHLIGHTS.
Reafference from REM sleep twitches, but not wake movements, triggers M1 activity.
Only “unexpected” self-generated movements trigger M1 activity.
Twitches are processed as if they are unexpected.
Acknowledgments
We are very grateful to Matthew Harrison for statistical assistance and Adam Hantman, Kathleen Cullen, Robert Wurtz, Greta Sokoloff, and Karen Adolph for many helpful comments on the manuscript. This work was supported by a grant from the National Institutes of Health (HD63071) to M.S.B. The Fulbright Foreign Student Program supported C.D.R.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes three figures, Supplemental Experimental Procedures, and one movie.
AUTHOR CONTRIBUTIONS
A.T., C.D.R.B., and M.S.B. designed the study, A.T. and C.D.R.B. collected and analyzed the data, and A.T. and M.S.B. wrote the paper.
References
- 1.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poulet JFA, Hedwig B. New insights into corollary discharges mediated by identified neural pathways. Trends Neurosci. 2007;30:14–21. doi: 10.1016/j.tins.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Cullen KE. Sensory signals during active versus passive movement. Curr Opin Neurobiol. 2004;14:698–706. doi: 10.1016/j.conb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Von Holst E, Mittelstaedt H. The principle of reafference: interactions between the central nervous system and the peripheral organs. Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- 5.Sperry R. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psych. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- 6.Brooks JX, Cullen KE. The primate cerebellum selectively encodes unexpected self-motion. Curr Biol. 2013;23:947–955. doi: 10.1016/j.cub.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell CC. Memory-based expectations in electrosensory systems. Curr Opin Neurobiol. 2001;11:481–487. doi: 10.1016/s0959-4388(00)00238-5. [DOI] [PubMed] [Google Scholar]
- 8.Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- 9.Poulet J, Hedwig B. The cellular basis of a corollary discharge. Science. 2006;311:518–522. doi: 10.1126/science.1120847. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy A, Wayne G, Kaifosh P, Alviña K, Abbott L, Sawtell NB. A temporal basis for predicting the sensory consequences of motor commands in an electric fish. Nature Neuro. 2014;17:416–422. doi: 10.1038/nn.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkby LA, Sack GS, Firl A, Feller MB. A role for correlated spontaneous activity in the assembly of neural circuits. Neuron. 2013;80:1129–1144. doi: 10.1016/j.neuron.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 13.Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 14.Thurber A, Jha S, Coleman T, Frank M. A preliminary study of sleep ontogenesis in the ferret (Mustela putorius furo) Behav Brain Res. 2008;189:41–51. doi: 10.1016/j.bbr.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scriba MF, Ducrest AL, Henry I, Vyssotski AL, Rattenborg NC, Roulin A. Linking melanism to brain development: expression of a melanism-related gene in barn owl feather follicles covaries with sleep ontogeny. Front Zool. 2013;10:42. doi: 10.1186/1742-9994-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Dev Psychobiol. 1970;3:267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- 17.Kreider J, Blumberg MS. Mesopontine contribution to the expression of active “twitch” sleep in decerebrate week-old rats. Brain Res. 2000;872:149–159. doi: 10.1016/s0006-8993(00)02518-x. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson KÆ, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biol. 2005;3:e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Curr Biol. 2013;23:R532–R537. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 21.Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Curr Biol. 2012;22:2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohns EJ, Blumberg MS. Neocortical activation of the hippocampus during sleep in newborn rats. J Neurosci. 2010;30:3438–3449. doi: 10.1523/JNEUROSCI.4832-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sokoloff G, Uitermarkt BD, Blumberg MS. REM sleep twitches rouse nascent cerebellar circuits: Implications for sensorimotor development. Dev Neurobiol. 2014 doi: 10.1002/dneu.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatsopoulos NG, Suminski AJ. Sensing with the motor cortex. Neuron. 2011;72:477–487. doi: 10.1016/j.neuron.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An S. Doctoral Thesis. University of Mainz; 2013. Long-term potentiation and neural network activity in the neonatal rat cerebral cortex; pp. 1–111. [Google Scholar]
- 26.Martin JH. The corticospinal system: From development to motor control. Neuroscientist. 2005;11:161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- 27.Hantman AW, Jessell TM. Clarke’s column neurons as the focus of a corticospinal corollary circuit. Nature Neurosci. 2010;13:1233–1239. doi: 10.1038/nn.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brumley MR, Robinson SR. The serotonergic agonists quipazine, CGS-12066A, and α-methylserotonin alter motor activity and induce hindlimb stepping in the intact and spinal rat fetus. Behav Neurosci. 2005;119:821–833. doi: 10.1037/0735-7044.119.3.821. [DOI] [PubMed] [Google Scholar]
- 29.Todd WD, Gibson J, Shaw C, Blumberg MS. Brainstem and hypothalamic regulation of sleep pressure and rebound in newborn rats. Behav Neurosci. 2010;124:69–78. doi: 10.1037/a0018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohns EJ, Blumberg MS. Synchronous bursts of neuronal activity in the developing hippocampus: Modulation by active sleep and association with emerging gamma and theta rhythms. J Neurosci. 2008;28:10134–10144. doi: 10.1523/JNEUROSCI.1967-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: Neural mechanisms in the vestibular nuclei. J Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleinfeld DD, Berg RWR, O’Connor SMS. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens Mot Res. 1999;16:69–88. doi: 10.1080/08990229970528. [DOI] [PubMed] [Google Scholar]
- 33.Marques HG, Imtiaz F, Iida F, Pfeifer R. Self-organization of reflexive behavior from spontaneous motor activity. Biol Cybern. 2013;107:25–37. doi: 10.1007/s00422-012-0521-7. [DOI] [PubMed] [Google Scholar]
- 34.Blumberg MS, Coleman CM, Gerth AI, McMurray B. Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Curr Biol. 2013;23:2100–2109. doi: 10.1016/j.cub.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersson P, Waldenström A, Fåhraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- 36.McVea DA, Mohajerani MH, Murphy TH. Voltage-sensitive dye imaging reveals dynamic spatiotemporal properties of cortical activity after spontaneous muscle twitches in the newborn rat. J Neurosci. 2012;32:10982–10994. doi: 10.1523/JNEUROSCI.1322-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolpert DM, Miall CR, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 38.Shipp S, Adams RA, Friston KJ. Reflections on agranular architecture: predictive coding in the motor cortex. Trends Neurosci. 2013;36:706–716. doi: 10.1016/j.tins.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frith CD, Blakemore SJ, Wolpert DM. Explaning the symptoms of schizophrenia: Abnormalities in the awareness of action. Brain Res Rev. 2000;31:357–363. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 40.Seelke AMH, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep. 2008;31:691–699. doi: 10.1093/sleep/31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amarasingham A, Harrison MT, Hatsopoulos NG, Geman S. Conditional modeling and the jitter method of spike resampling. J Neurophysiol. 2012;107:517–531. doi: 10.1152/jn.00633.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrison MT, Geman S. A rate and history-preserving resampling algorithm for neural spike trains. Neural Comput. 2009;21:1244–1258. doi: 10.1162/neco.2008.03-08-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amarasingham A, Harrison MT, Hatsopoulos NG. Conditional modeling and the jitter method of spike re-sampling: Supplement. arXiv. 2011 doi: 10.1152/jn.00633.2011. Available at: http://arxiv.org/abs/1111.4296. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


