Abstract
Objectives
The purpose of this study was to assess the correlations among the maximum standardized uptake value (SUVmax) on 18F-fluoro-2-deoxy-glucose positron emission tomography/computed tomography (FDG-PET/CT); the expressions of glucose transporter 1 (GLUT-1), glucose transporter 3, and epidermal growth factor receptor (EGFR); as well as prognosis in patients with invasive ductal carcinoma of the pancreas.
Methods
A total of 41 patients with surgically resected and histologically proven invasive ductal carcinoma of the pancreas who underwent preoperative FDG-PET/CT were assessed. The SUVmax at the primary tumor site was measured by FDG-PET/CT, and immunohistochemical staining of tumor sections was performed for GLUT-1, glucose transporter 3, and EGFR.
Results
Higher FDG uptake (SUVmax, >3.40) and GLUT-1 expression were significantly associated with shorter overall survival (P < 0.05). The SUVmax was not found to be significantly correlated with clinicopathological characteristics such as TNM classification, lymph node metastasis, and tumor differentiation. The EGFR expression was significantly correlated with the SUVmax (P = 0.024).
Conclusions
Higher FDG uptake and GLUT-1 expression in invasive ductal carcinoma of the pancreas seems to be an important prognostic factor. In addition, the EGFR expression was significantly correlated with the SUVmax.
Key Words: invasive ductal carcinoma of the pancreas, 18F-fluoro-2-deoxy-glucose positron emission tomography/computed tomography, glucose transporter 1, glucose transporter 3, epidermal growth factor receptor, maximum standardized uptake value
Pancreatic cancer is one of the most lethal diseases of the gastrointestinal tract. The disappointing outcomes of the current therapeutic approaches for pancreatic cancer represent an urgent need for the development of novel methods to control the disease. In Japan, tumor resection was performed in 36% of patients with invasive ductal carcinoma of the pancreas and the 5-year survival rate of those who underwent resection was 18.2%.1 The prognosis of affected patients is poor, even if treated by pancreatectomy combined with adjuvant therapy such as chemotherapy and radiation therapy.2,3 18F-fluoro-2-deoxy-glucose positron emission tomography/computed tomography (FDG-PET/CT) has been an important modality for detecting tumors, monitoring therapy, and evaluating the prognosis in several malignant cancers,4–7 including pancreatic cancer.8,9 The accumulation of FDG is based on the enhanced glycolysis of malignant tumor cells. Several immunohistochemical studies have demonstrated the overexpression of glucose transporters (GLUTs) in malignant tumors.9–12 This overexpression is reported to be closely related to FDG uptake.7,9,10 Some studies have shown that increased glucose uptake and overexpression of GLUT-1 lead to a worse prognosis in invasive ductal carcinoma of the pancreas.13,14 Epidermal growth factor receptor (EGFR) is overexpressed in approximately 50% to 60% of patients with invasive ductal carcinoma of the pancreas. Some studies have shown that high EGFR expression correlates with a worse prognosis.15,16
In the present study, we examined the correlations among FDG uptake; clinicopathological characteristics such as stage, lymph node metastasis, and tumor differentiation; as well as immunohistochemical expressions of GLUT-1, GLUT-3, and EGFR. In addition, we evaluated the associations among FDG uptake; GLUT-1, GLUT-3, and EGFR expressions; and prognosis.
MATERIALS AND METHODS
A total of 41 patients (21 men, 20 women) with surgically resected and histologically proven invasive ductal carcinoma of the pancreas who underwent preoperative FDG-PET/CT at our institute between March 2004 and March 2010 were included in this study. This study protocol was approved by the Kurume University institutional review board.
Whole-Body FDG-PET/CT Imaging Acquisition
An integrated full-ring PET/CT scanner (Gemini-GXL 16; Philips Medical Systems, Inc, Cleveland, Ohio) was used for data acquisition. In this system, the dimensions of the individual gadolinium silica oxide crystals are 4 × 6 × 30 mm3 and the National Electrical Manufactures Association 2001 transverse spatial resolution at 1 cm is 5.3 mm. The patients’ median blood glucose level was 102 mg/dL (range, 75–127 mg/dL). All patients were administered a mean of 6.81 mCi (range, 4.59–9.56) of 18F-FDG via the antecubital vein. All patients rested quietly for approximately 60 minutes after the 18F-FDG injection, and whole-body images were obtained. The patients were examined in the supine position. Initially, a noncontrast, full-dose CT scan was acquired, starting from the level of the head and using the following parameters: 200 mAs, 120 kV, 0.75 seconds per tube rotation, slice thickness of 3 mm, scan length of 940 mm, and data acquisition time of 40 seconds. The PET emission scans of the areas from the level of the auditory meatus to the mid-thigh were acquired with a time of 2 minutes 30 seconds per cradle position using a 3-dimensional acquisition mode. The acquisition of 8 to 9 cradle positions starting from the head and continuing to the mid-thigh resulted in an acquisition time of approximately 30 minutes. After both the transmission and emission images were obtained, they were reconstructed using the standard normal reconstruction protocol based on a 3-dimensional line-of-response row-action maximum likelihood algorithm with which the PET/CT scanner is equipped.
FDG-PET/CT Image Analysis
Three experienced nuclear medicine physicians who were blinded to the results of other imaging modalities independently evaluated the whole-body PET images for the presence of abnormally increased uptake in the region of the pancreas. Circular regions of interest (ROIs) were manually placed over the entire area of abnormal uptake on the pancreas. On the basis of these ROIs, the maximum standardized uptake value (SUVmax) was calculated with the following formula: maximum pixel value within the ROI activity (MBq/kg)/(injected dose [MBq]/body weight [kg]).
Immunostaining
Immunohistochemical staining of tumor sections was performed for GLUT-1, GLUT-3, and EGFR. Paraffin-embedded tissue samples were cut at 4 μm, examined on a coated slide glass, and labeled with the following antibodies using the BenchMark XT (Ventana Automated Systems, Inc, Tucson, Ariz) and Bond-Max autostainer (Leica Microsystems, Newcastle, United Kingdom): GLUT-1 (×100; NeoMarkers, Fremont, Calif), GLUT-3 (ready to use; Spring Bioscience, Fremont, Calif), and EGFR (ready to use; Nichirei, Tokyo, Japan). For GLUT-1 and EGFR, BenchMark XT was used. Briefly, each slide was heat-treated using Ventana’s CC1 retrieval solution for 30 minutes, then incubated with the GLUT-1 and EGFR antibodies for 30 minutes. This automated system used the streptavidin-biotin complex method, with 3,3′-diaminobenzidine as the chromogen (Ventana iVIEW DAB Detection Kit). Immunostaining with GLUT-3 was performed on the same fully automated Bond-Max system using onboard heat-induced antigen retrieval with ER2 for 30 minutes and a Refine polymer detection system (Leica Microsystems). 3,3′-Diaminobenzidine was used as the chromogen in all immunostainings. In evaluating the expressions of GLUT-1, GLUT-3, and EGFR, we assumed that the staining intensity of the cancer cell membranes with the GLUT-1, GLUT-3, and EGFR antibodies represented GLUT-1, GLUT-3, and EGFR expressions in the cancer specimens.
Statistical Methods
All statistical analyses were performed using JMP for Windows (version 10.0; SAS Institute, Cary, NC). Clinicopathological findings were examined to identify significant differences in the mean SUVmax. Survival analysis for differences among the higher and lower FDG uptake, the GLUT-1 and GLUT-3, as well as EGFR expressions was performed using the Kaplan-Meier method. A P value of less than 0.05 was considered to be statistically significant.
RESULTS
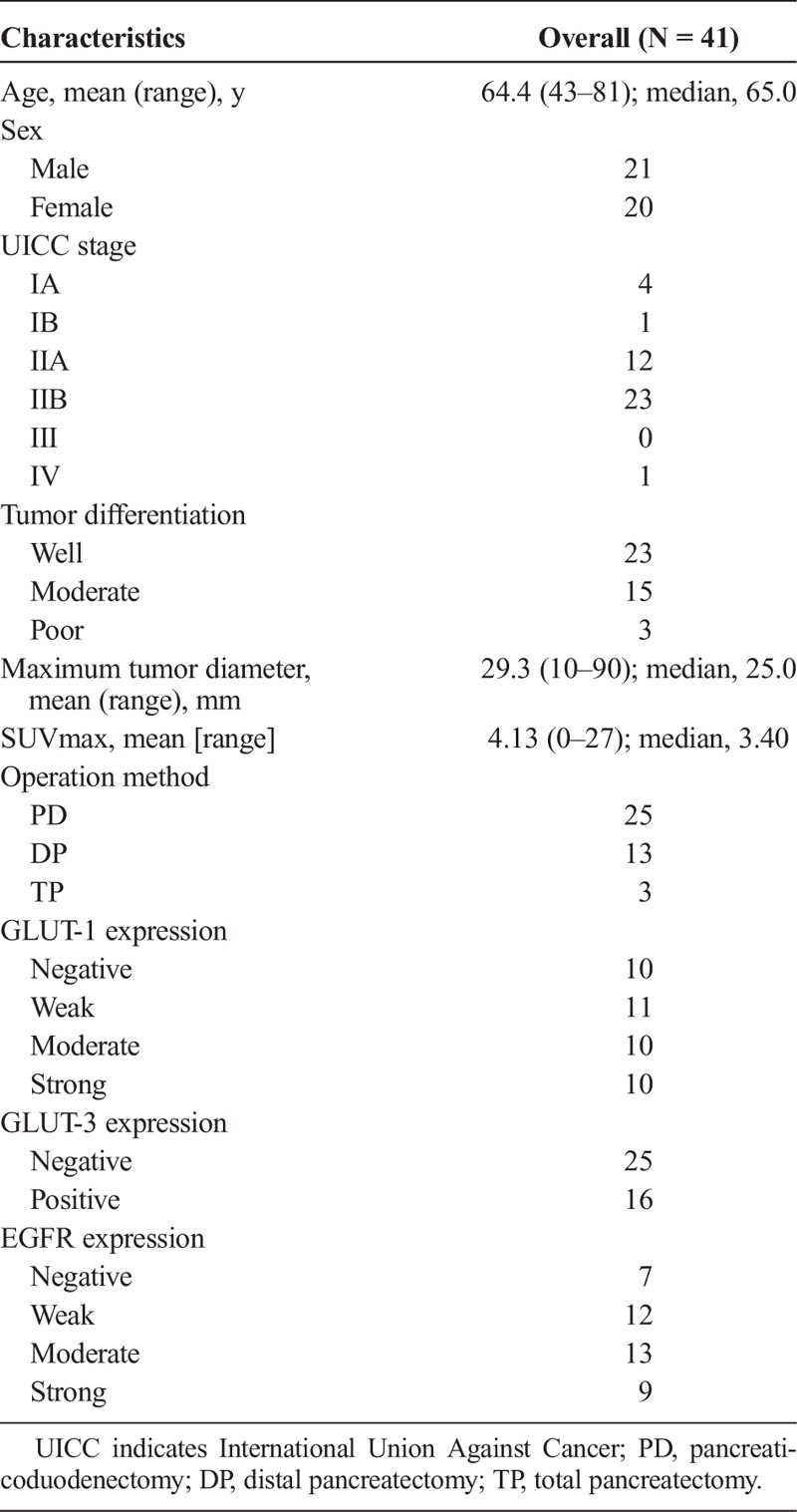
Patients’ information, characteristics, and tumor characteristics are summarized in Table 1. The age of the patients ranged from 43 to 81 years (median, 65 years). The male-to-female ratio was 21 to 20. The TNM staging categories were determined according to the criteria established by the International Union Against Cancer. In all 41 patients, the histological diagnosis was made after surgery. Twenty-five patients underwent pancreaticoduodenectomy, including pylorus-preserving pancreaticoduodenectomy and subtotal stomach-preserving pancreaticoduodenectomy; 13 underwent distal pancreatectomy; and 3 underwent total pancreatectomy. All patients were diagnosed with invasive ductal carcinoma of the pancreas by experienced pathologists of our institute. The results were as follows: well-differentiated type, n = 23; moderately differentiated type, n = 15; and poorly differentiated type, n = 3. The SUVmax at the primary tumor site was estimated by FDG-PET/CT, and the median SUVmax was 3.40 (mean, 4.13; range, 0–27). Six tumors did not demonstrate visual FDG uptake.
TABLE 1.
Patients’ Characteristics (N = 41)
Correlations between clinicopathological characteristics and FDG uptake, as measured by the mean SUVmax, at the primary tumor site are shown in Table 2. The SUVmax was not found to be significantly correlated with the clinicopathological characteristics such as TNM classification, lymph node metastasis, and tumor differentiation. However, higher GLUT-1, GLUT-3, and EGFR expressions tended to be correlated with higher FDG uptake. The mean SUVmax in cases of negative EGFR expression was significantly lower than that in cases of positive EGFR expression (P = 0.024).
TABLE 2.
Correlationship Between the Mean SUV Value and the Clinicopathological Findings
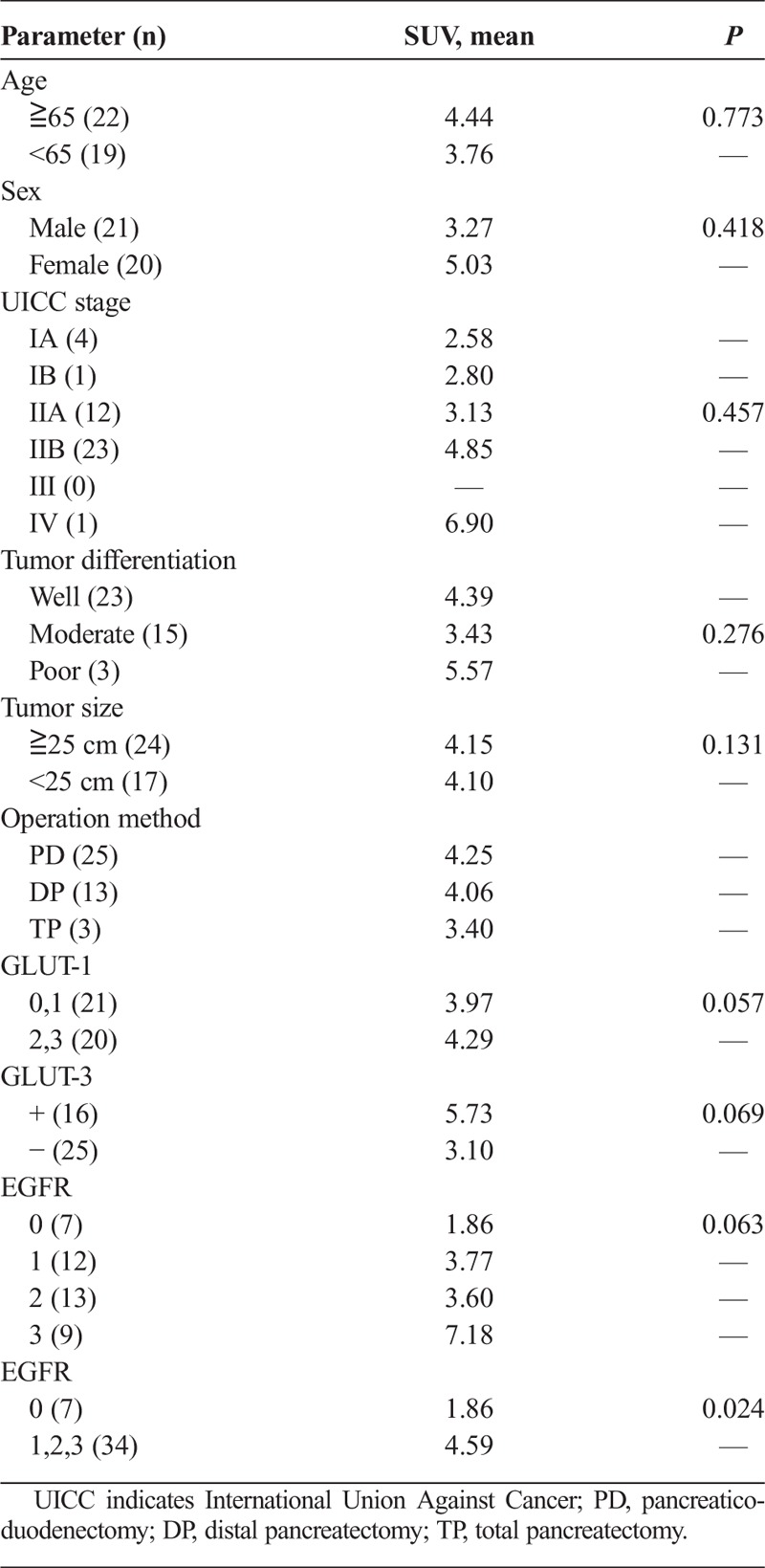
Figure 1 shows the relationship between overall survival and SUVmax. The median SUVmax was 3.40. Thus, lower FDG uptake was defined as the uptake below the median SUVmax and higher FDG uptake was defined as the uptake above the median SUVmax. Higher FDG uptake (SUVmax, >3.40) was significantly associated with shorter overall survival (P = 0.0314). The 1-, 2-, and 4-year overall survival rates in patients with lower FDG uptake (SUVmax, <3.40) were 100%, 63.2%, and 41.3%, respectively, and those in patients with higher FDG uptake (SUVmax >3.40) were 71.4%, 38.1%, and 0%, respectively. Figure 2 shows the relationship between disease-free survival and the SUVmax. Higher FDG uptake (SUVmax >3.40) was significantly associated with shorter disease-free survival (P = 0.0432). Figure 3 shows that higher (moderate and strong) GLUT-1 expression was significantly associated with shorter overall survival (P = 0.0102). The median survival time was 1149 days for patients with lower (negative and weak) GLUT-1 expression and 585 days for patients with higher GLUT-1 expression. The 1-, 2-, and 4-year overall survival rates in patients with lower GLUT-1 expression were 90.5%, 70.4%, and 44.0%, respectively, and those in patients with higher GLUT-1 expression were 80.0%, 30.0%, and 10.0%, respectively. However, higher GLUT-3 and EGFR expressions were not associated with shorter overall survival (data not shown).
FIGURE 1.
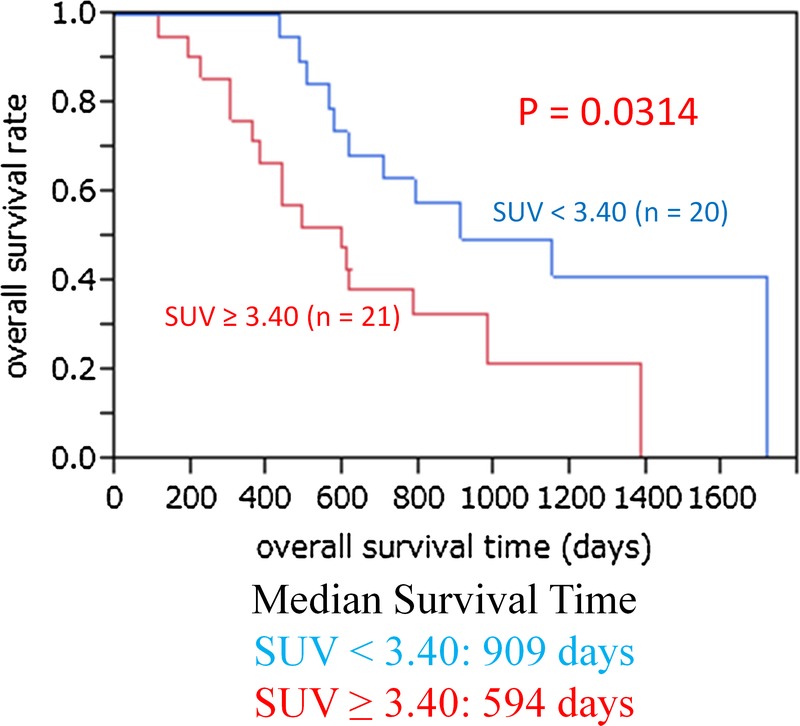
The relationship between overall survival and SUVmax. Higher FDG uptake (SUVmax, >3.40) was significantly associated with shorter overall survival (P = 0.0314). The 1-, 2-, and 4-year overall survival rates in patients with lower FDG uptake (SUVmax, <3.40) were 100%, 63.2%, and 41.3%, respectively, and those in patients with higher FDG uptake (SUVmax, >3.40) were 71.4%, 38.1%, and 0%, respectively.
FIGURE 2.
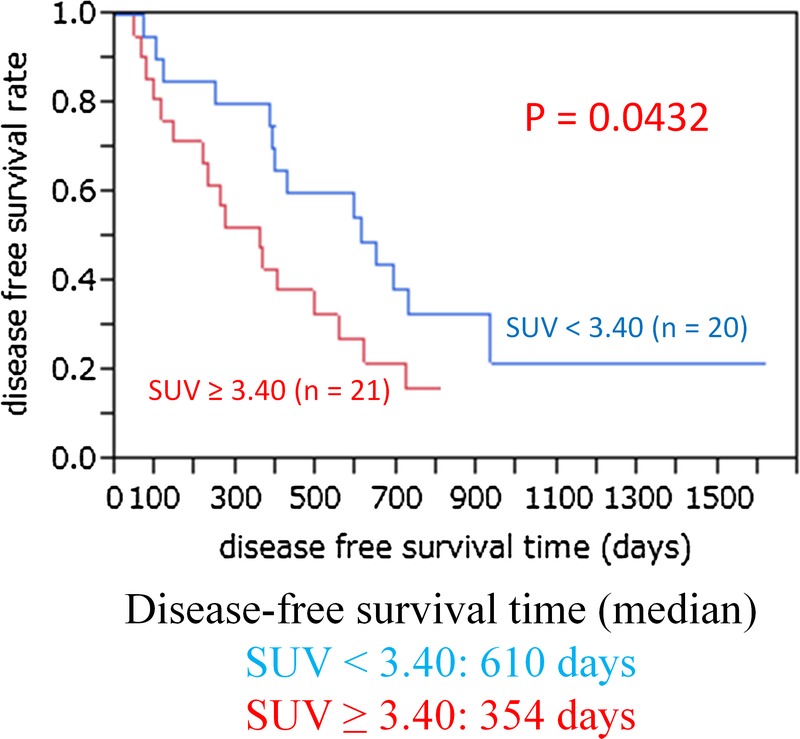
The relationship between disease-free survival and the SUVmax. Higher FDG uptake (SUVmax, >3.40) was significantly associated with shorter disease-free survival (P = 0.0432).
FIGURE 3.
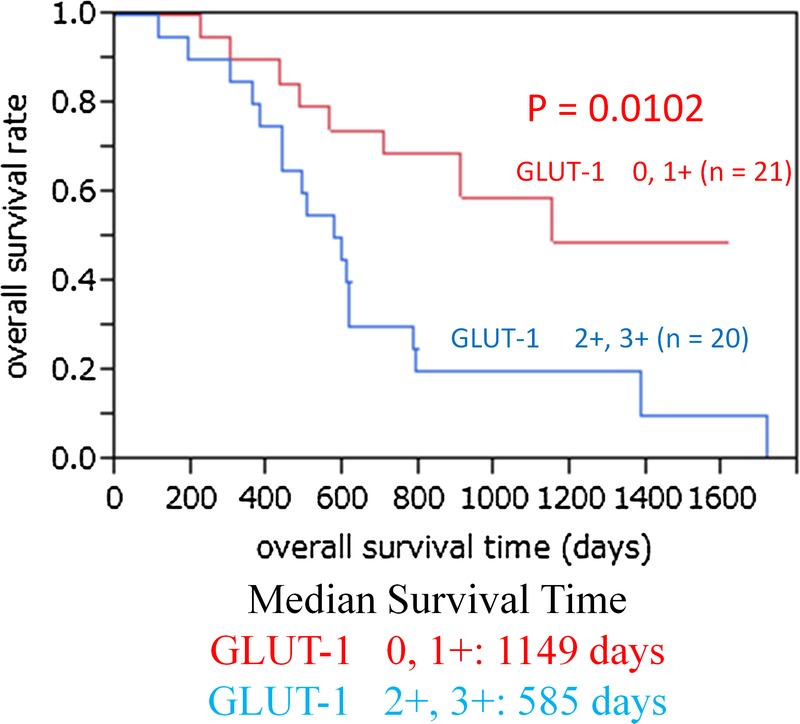
The relationship between overall survival and GLUT-1 expression. Higher (moderate and strong) GLUT-1 expression was significantly associated with shorter overall survival (P = 0.0102). The 1-, 2-, and 4-year overall survival rates in patients with lower GLUT-1 expression were 90.5%, 70.4%, and 44.0%, respectively, and those in patients with higher GLUT-1 expression were 80.0%, 30.0%, and 10.0%, respectively.
Figure 4 shows the immunohistochemical staining of GLUT-1. The intensity of staining was scored using the following scale: no staining, 0; weak staining, 1+; moderate staining, 2+; and strong staining, 3+ in more than 10% of cancer cells. Figure 5 shows the immunohistochemical staining of EGFR. The intensity of staining was scored using the following scale: no staining, 0; weak staining, 1+; moderate staining, 2+; and strong staining, 3+ in more than 10% of cancer cells.
FIGURE 4.
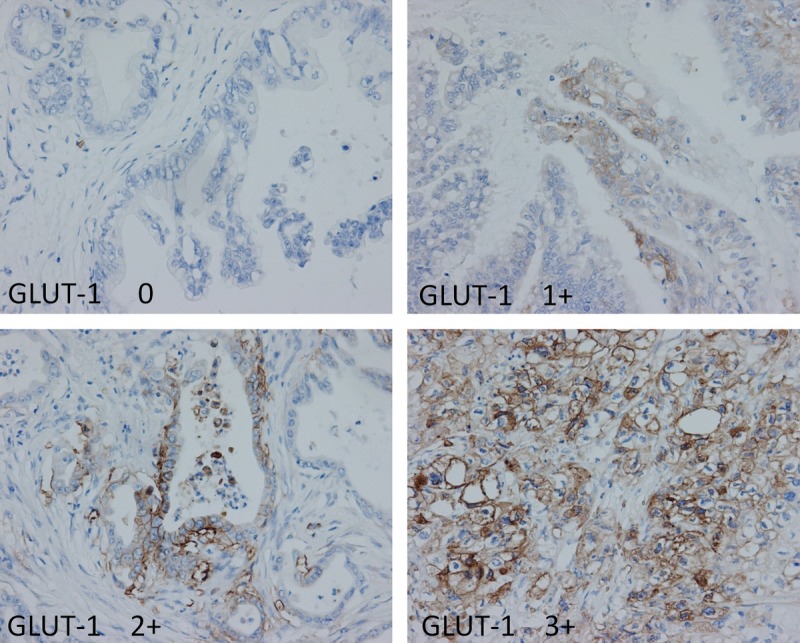
Immunohistochemical staining of GLUT-1. The intensity of staining was scored using the following scale: no staining, 0; weak staining, 1+; moderate staining, 2+; and strong staining, 3+ in more than 10% of cancer cells.
FIGURE 5.
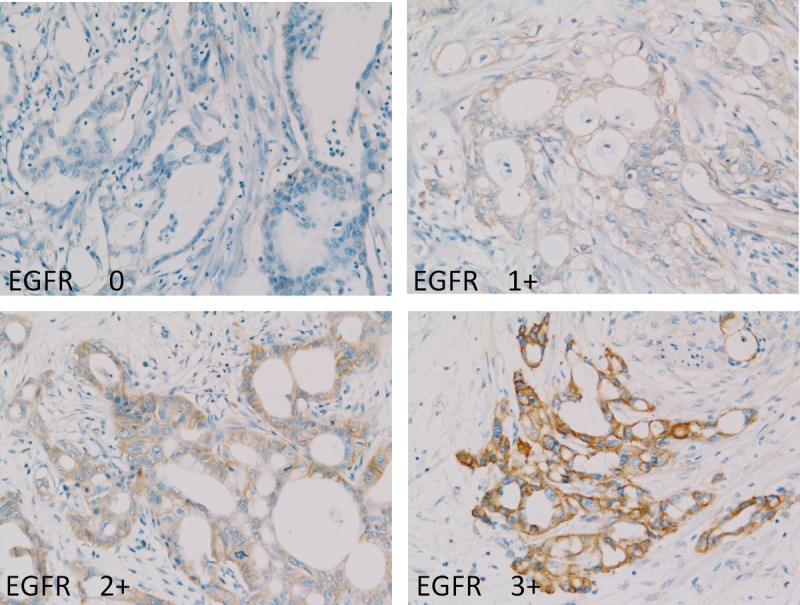
Immunohistochemical staining of EGFR. The intensity of staining was scored using the following scale: no staining, 0; weak staining, 1+; moderate staining, 2+; and strong staining, 3+ in more than 10% of cancer cells.
DISCUSSION
Pancreatic cancer is considered to be one of the most aggressive human cancers. Only a minority of patients with pancreatic cancer present with resectable disease at the time of first diagnosis.1 Approximately 15% to 20% of affected patients have locally advanced, nonmetastatic pancreatic cancer. Thus, the prognosis of pancreatic ductal adenocarcinoma remains dismal; nevertheless, substantial improvements have been made in pancreatectomy and chemoradiotherapy.2,3 Pancreatic resection remains the only curative treatment in patients with resectable and nonmetastatic pancreatic cancer. Although the treatment strategy is controversial,17,18 surgery should be considered only in resectable disease and neoadjuvant chemoradiation therapy plus surgery should be considered in cases of borderline resectable disease.19,20 Therefore, patient selection is important to ensure that surgery is only offered to those patients who are likely to benefit from it. Furthermore, preoperatively predicting the prognosis of pancreatic ductal adenocarcinoma is important in the determination of the most appropriate treatment strategy.
18F-fluoro-2-deoxy-glucose PET/CT allows for the visualization of the metabolic activity of tissue and provides information about the rate of glucose metabolism.9,12 Accumulation of FDG reflects the rate of carbohydrate metabolism, which is an index of the metabolic activity of the cells.9,12 Carbohydrate metabolism is more active in malignant cells, resulting in significant accumulation of FDG.4–7,11 These changes in FDG uptake in the tumor may arise from the expression of GLUTs and glycolytic enzymes in tumor cells. A variety of studies using FDG-PET/CT have been reported.4–9,11,12 Many investigators have suggested that higher FDG uptake in the primary tumor is associated with poor survival among patients with pancreatic cancer and other malignancies.4–6,10 Okamoto et al21 reported that the preoperative SUVmax was higher in the recurrence group during the early postoperative period and that a high SUVmax was a risk factor for early postoperative recurrence. Carbohydrate metabolism is more active in malignant cells, resulting in strong accumulation of FDG. Thus, the accumulation of FDG is believed to indicate the activity of the cancer. Our results revealed significant relationships between prognosis and FDG uptake. On the basis of our results, we speculate that significant improvements in pancreatic cancer treatment may be possible if we can preoperatively reveal the prognosis.
Malignant cells demonstrate increased glucose uptake because of the expression of GLUTs.6,7,9–14 The present results indicate that cellular uptake of FDG is mediated by GLUT-1. Higher GLUT-1 expression has also been proven to be associated with pancreatic cellular invasiveness.22 Glucose transporter-1 is undetectable in normal pancreatic tissue but is expressed in pancreatic cancer.23 Younes et al23 hypothesized that the expression of GLUT-1 promotes the use of energy in cancer cells, contributing to their aggressive behavior. Glucose transporter-1 is considered to be the predominant upregulated GLUT in malignant epithelial tissues and has been found to correlate with biological behavior in various malignancies.13,14,23 Basturk et al13 reported that GLUT-1 may be a potential therapeutic target to limit glucose uptake and metabolism, thereby limiting the proliferative potential of malignant cells. Further investigation is needed. Our results revealed significant relationships between prognosis (overall survival and disease-free survival) and SUVmax. The overexpression of GLUT-1 was also associated with enhanced tumor aggressiveness and poor survival.13,14,22,23 There were also significant relationships between overall survival and FDG uptake as well as GLUT-1 expression. Thus, the standardized uptake value and GLUT-1 expression are predictors of survival in patients with pancreatic ductal adenocarcinoma.
Epidermal growth factor receptor is overexpressed in approximately 50% to 60% of pancreatic adenocarcinomas.24 It has been extensively studied in pancreatic ductal adenocarcinoma, and erlotinib was recently approved in combination with gemcitabine for the treatment of metastatic disease.25 The overexpression of EGFR is reported to be significantly correlated with survival in patients with pancreatic cancer.15,16,26,27 However, our study showed that EGFR overexpression was not associated with survival. Other studies have shown similar results.28,29 The actual prognostic significance of EGFR overexpression is controversial. In nonsmall cell lung cancer, higher FDG uptake correlates with higher EGFR expression.30 The relationship between erlotinib and EGFR expression has not been reported. Our results showed a correlation between SUVmax and EGFR expression. Higher FDG uptake predicts higher expression of EGFR. 18F-fluoro-2-deoxy-glucose PET/CT is useful for predicting a patient’s prognosis and the overexpression of EGFR after surgery for pancreatic cancer.
Our data indicate that molecular and metabolic imaging technology provides useful clinical information. Current imaging technology could help to guide treatment recommendations. The SUVmax is believed to indicate the activity of the cancer. Our results revealed significant relationships between prognosis and SUVmax as well as between prognosis and GLUT-1 expression. On the basis of our results, we believe that significant improvements in pancreatic cancer treatment may be possible if we can preoperatively reveal the prognosis.
There are several limitations to this study. First, this was a retrospective study involving a single institution. Second, the number of cases in this study was small. Studies involving a greater number of cases are necessary.
In conclusion, higher SUVmax and GLUT-1 expression in invasive ductal carcinoma of the pancreas seems to be an important prognostic factor and higher SUVmax predicts EGFR expression. Combining these findings can improve their prognostic value and clinical utility. The findings of this study must be validated in larger cohorts.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Egawa S, Toma H, Ohigashi H, et al. Japan pancreatic cancer registry; 30th year anniversary. Pancreas. 2012; 41: 985– 992 [DOI] [PubMed] [Google Scholar]
- 2.Audrey V, Joseph H, Rich S, et al. Pancreatic cancer. Lancet. 2011; 378: 607– 62021620466 [Google Scholar]
- 3.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996; 223: 273– 279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert JC, Ayesha SB, Buddhiwardhan O, et al. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg. 2005; 130: 151– 159 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Hasegawa Y, Terada A, et al. FDG-PET predicts survival and distant metastasis in oral squamous cell carcinoma. Oral Oncol. 2009; 45: 569– 573 [DOI] [PubMed] [Google Scholar]
- 6.Kaira K, Serizawa M, Koh Y, et al. Relationship between 18F-FDG uptake on positron emission tomography and molecular biology in malignant pleural mesothelioma. Eur J Nucl Med. 2012; 48: 1244– 1254 [DOI] [PubMed] [Google Scholar]
- 7.Park SG, Lee JH, Lee WA, et al. Biologic correlation between glucose transporters, hexokinase-II, Ki-67 and FDG uptake in malignant melanoma. Nucl Med Biol. 2012; 39: 1167– 1172 [DOI] [PubMed] [Google Scholar]
- 8.Cem P, Erkan T, Cem O, et al. Prognostic value of gross tumor volume delineated by FDG-PET-CT based radiotherapy treatment planning in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Radiat Oncol. 2012; 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higashi T, Saga T, Nakamoto Y, et al. Relationship between retention index in dual-phase 18F-FDG-PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J Nucl Med. 2002; 43: 173– 180 [PubMed] [Google Scholar]
- 10.Baardwijk AV, Dooms C, Suylen RJ, et al. The maximum uptake of 18F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1α and GLUT-1 in non-small cell lung cancer. Eur J Cancer. 2007; 43: 1392– 1398 [DOI] [PubMed] [Google Scholar]
- 11.Kaira K, Endo M, Abe M, et al. Biologic correlation of 2-18F-fluoro-2-deoxy-d-glucose uptake on positron emission tomography in thymic epithelial tumors. J Clin Oncol. 2010; 28: 3746– 3753 [DOI] [PubMed] [Google Scholar]
- 12.Higashi T, Tamaki N, Torizuka T, et al. FDG uptake, GLUT-1 glucose transporter and cellularity in human pancreatic tumors. J Nucl Med. 1988; 39: 1727– 1735 [PubMed] [Google Scholar]
- 13.Basturk O, Singh R, Kaygusuz E, et al. GLUT-1 expression in pancreatic neoplasia. Pancreas. 2011; 40: 187– 192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun HC, Qiu ZJ, Liu J, et al. Expression of hypoxia-inducible factor-1 alpha and associated proteins in pancreatic ductal adenocarcinoma and their impact on prognosis. Int J Oncol. 2007; 30: 1359– 1367 [PubMed] [Google Scholar]
- 15.Uegaki K, Nio Y, Inoue S, et al. Clinicopathological significance of epidermal growth factor and receptor in human pancreatic cancer. Anticancer Res. 1997; 17: 3841– 3847 [PubMed] [Google Scholar]
- 16.Dong M, Nio Y, Guo KJ, et al. Epidermal growth factor and its receptor as prognostic indicators in Chinese patients with pancreatic cancer. Anticancer Res. 1998; 18: 4613– 4619 [PubMed] [Google Scholar]
- 17.Gong Y, Zhang L, He T, et al. Pancreaticoduodenectomy combined with vascular resection and reconstruction for patients with locally advanced pancreatic cancer: a multicenter, retrospective analysis. PLoS One. 2013; 8: e70340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullar SA, Hartel M, Mehrabi A, et al. Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg. 2009; 13: 784– 792 [DOI] [PubMed] [Google Scholar]
- 19.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006; 13: 1035– 1046 [DOI] [PubMed] [Google Scholar]
- 20.Papalezova KT, Tyler DS, Blazer DG, et al. Does preoperative therapy optimize outcomes in patients with resectable pancreatic cancer? J Surg Oncol. 2012; 106: 111– 118 [DOI] [PubMed] [Google Scholar]
- 21.Okamoto K, Koyama I, Miyazawa M, et al. Preoperative 18F-fluorodeoxyglucose positron emission tomography/computed tomography predicts early recurrence after pancreatic cancer resection. Int J Clin Oncol. 2011; 16: 39– 44 [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Duxbury M, Zinner MJ, et al. Glucose transporter-1 gene expression is associated with pancreatic cancer invasiveness and MMP-2 activity. Surgery. 2004; 136: 548– 556 [DOI] [PubMed] [Google Scholar]
- 23.Younes M, Lechago LV, Somoano JR, et al. Wide expression of the human erythrocyte glucose transporter GLUT-1 in human cancers. Cancer Res. 1996; 56: 1164– 1167 [PubMed] [Google Scholar]
- 24.Friess H, Wang L, Zhu Z, et al. Growth factor receptors are differentially expressed in cancers of the papilla of vater and pancreas. Ann Surg. 1999; 230: 767– 774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007; 25: 1960– 1966 [DOI] [PubMed] [Google Scholar]
- 26.Ueda S, Ogata S, Tsuda H, et al. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004; 29: e1– e9 [DOI] [PubMed] [Google Scholar]
- 27.Valsecchi ME, McDonald M, Brody JB, et al. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer. 2012; 118: 3484– 3493 [DOI] [PubMed] [Google Scholar]
- 28.Gansauge F, Gansauge S, Schmidt E, et al. Prognostic significance of molecular alternations in human pancreatic carcinoma: an immunological study. Langenbecks Arch Surg. 1988; 383: 152– 155 [DOI] [PubMed] [Google Scholar]
- 29.Kuniyasu H, Ellis LM, Evans DB, et al. Relative expression of E-cadherin and type IV collagenase genes predicts disease outcome in patients with resectable pancreatic carcinoma. Clin Cancer Res. 1999; 5: 25– 33 [PubMed] [Google Scholar]
- 30.Mak RH, Digumarthy SR, Muzikansky A, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor mutations in non-small cell lung cancer. Oncologist. 2011; 16: 319– 326 [DOI] [PMC free article] [PubMed] [Google Scholar]


